Abstract
Objectives
More than half of head and neck squamous cell carcinoma (HNSCC) patients are initially treated with curative intent, but will relapse over the course of their disease and have poor prognosis with a median survival of approximately 6 months. Novel therapeutic approaches are in desperate need for this patient population. The anti-apoptotic BCL-2 family proteins such as BCL-2, BCL-XL, and MCL-1 are involved in oncogenesis and chemoresistance and are overexpressed in HNSCC. Obatoclax is a small-molecule antagonist of the BH3-binding groove of anti-apoptotic BCL-2 family. We evaluated the activity of obatoclax against 4 HNSCC cell lines (UMSCC-1, Cal33, 1483, UMSCC-22A).
Methods
Cell viability was determined by MTT assay, cell cycle status by propidium iodide staining, and apoptosis by Annexin-V staining and immunoblotting. Autophagy was assessed by immunofluorescence and immunoblotting.
Results
All four HNSCC cell lines were highly sensitive to single-agent obatoclax with IC50’s ranging from 46-177 nM. Obatoclax induced apoptosis in all four HNSCC cell lines as evidenced by increases in sub-G1 DNA content, Annexin-V staining, and PARP cleavage. In addition, obatoclax induced autophagy in all 4 cell lines, and the addition of the autophagy inhibitor chloroquine enhanced obatoclax cytotoxicity.
Conclusion
Our findings demonstrate potent monotherapeutic activity of obatoclax against HNSCC cells, and enhancement of this activity in the presence of chloroquine. This preclinical study suggests that obatoclax might have therapeutic value in the treatment of HNSCC, either alone or in combination with inhibitors of autophagy.
Keywords: obatoclax, BCL-2, head and neck cancer, apoptosis, autophagy
Introduction
Approximately 40,000 new cases of head and neck cancer are diagnosed annually in the United States (1). Patients with head and neck squamous cell carcinoma (HNSCC) usually present with locoregionally advanced disease. However, recurrence of disease either in local or distant sites, after potentially curative treatment with surgery, radiation, and/or chemotherapy occurs in more than 50% of patients. These patients have a dismal prognosis with a median survival of only 6 months (2-4). Current treatment consists of platinum-based therapy that is difficult to tolerate and is indicated only in patients with good performance status. Therefore, new therapeutic approaches are needed, particularly in patients with recurrent and metastatic (R/M) HNSCC. The epidermal growth factor receptor inhibitor cetuximab is the last agent approved by the United States Food and Drug Administration for HNSCC, but exhibits an overall response rate of only 13% as a single agent for R/M HNSCC patients who failed platinum-based therapy (5). Targeting anti-apoptotic proteins represents a promising strategy either alone or in combination with other therapies.
The anti-apoptotic members of the BCL-2 protein family, particularly MCL-1, BCL-2 and BCL-XL, are overexpressed in HNSCC and correlate with chemotherapy resistance (6) and poor clinical outcome (7). Targeting these proteins might allow the restoration of apoptosis in HNSCC cells, while increasing the cytotoxicity of conventional chemotherapy or other targeted therapies. We previously reported the activity of ABT-737, a small-molecule inhibitor of BCL-XL and BCL-2 in HNSCC cells (8). As a single agent, ABT-737 had minimal activity in HNSCC cell lines, although it showed potent synergy in combination with conventional chemotherapy such as cisplatin. However, ABT-737 fails to inhibit MCL-1, an anti-apoptotic BCL-2 family member that is expressed in approximately 90% of head and neck tumors (9). Obatoclax is a small-molecule antagonist of the BH3-binding groove of anti-apoptotic members of the BCL-2 protein family, including MCL-1. Currently, gossypol and obatoclax are the only BCL-2 inhibitors in phase II clinical trials that are capable of inhibiting MCL-1 (10). Although the activity of obatoclax has been reported in several hematological malignancies (11-13) and solid tumors (14-16), its activity in HNSCC cells remain unknown.
In view of the paucity of effective targeting agents currently available for the treatment of HNSCC, we undertook an evaluation of the impact of obatoclax on HNSCC cells. We report that obatoclax potently inhibited the growth of 4 different HNSCC cell lines. Obatoclax treatment led to induction of HNSCC cell apoptosis and autophagy. Treatment with the autophagy inhibitor chloroquine potentiated the cytotoxicity of obatoclax, demonstrating that the induced autophagy exerts a pro-survival effect. These findings support the clinical evaluation of obatoclax as a single agent or in combination with an autophagy inhibitor in HNSCC patients.
2. Materials and methods
2.1. Cell cultures and reagents
Four human HNSCC cell lines UMSCC-1, Cal33, 1483 and UMSCC-22A were maintained in Dulbecco’s Modified Eagles’ Media (DMEM) from Cellgro (Manassas, VA), supplemented with 10% heat-inactivated fetal bovine serum (Clontech, Mountain View, CA), 100 units/ml penicillin, and 100 μg/ml streptomycin (Gibco, Grand Island, NY) at 37°C with 5% CO2 in a humidified atmosphere. UMSCC-22A cells stably expressing GFP-LC3 have been previously described (17), and were maintained in medium containing 500 μg/ml G418 (Cellgro, Manassas, VA). Obatoclax was purchased from Selleck Chemicals (Houston, TX), while chloroquine was purchased from Sigma (St. Louis, MO).
2.2 Assessment of cell viability
Cell proliferation was assessed by MTT (3-(4,5-dimethyl-thiazolyl-2)-2,5-diphenyltetrazolium bromide) assays, according to the manufacturer’s instructions (Sigma, St. Louis, MO). Briefly, cells were seeded on 96-well plates at 5-10×103 cells per 100 μl per well and incubated overnight. After treatment with 0.1% DMSO (vehicle control), or obatoclax for 48 hrs, 15 μl MTT (5 mg/ml) was added to each well, and the cells were incubated for 2-4 hrs at 37°C. The cell supernatant was then replaced with 100 μl DMSO was added per well and incubation continued for 30 minutes at room temperature. The absorbance was recorded at 570 nm with a plate reader (Molecular Devices, Sunnyvale, CA). Data were normalized to vehicle treatment, and the half inhibitory concentrations (IC50) were calculated using GraphPad Prism software (version 4.03; La Jolla, CA).
2.3 Assessment of apoptosis and cell cycle analysis
Cells were seeded at 0.05-0.1× 106 cells/ml in a 6-well plate and treated 24 hours later with vehicle or obatoclax (100-200 nM). For cell cycle evaluation, treated cells were harvested, washed in cold PBS, fixed in 70% ethanol and stored at 4°C. The cells were then treated with RNase-A solution (final concentration 0.2 mg/ml) at 37°C for one hour, and stained with propidium iodide (final concentration 10 μg/ml) prior to analysis. For apoptosis evaluation, similarly treated cells were washed with cold PBS prior to resuspension in 1× Annexin V binding buffer. Cell suspensions (100 μl) were stained with 5 μl of Annexin V for 15 minutes in the dark (BD Biosciensces, San Diego, CA), as previously described (8). Flow cytometric determination of Annexin V staining and analysis of DNA content was performed using an Accuri C6 flow cytometer (Ann Arbor, MI). The percentage of apoptotic cells from three independent experiments was determined and presented as mean ± standard error of the mean (SEM).
2.4 Assessment of autophagy
UMSCC-22A cells stably expressing GFP-LC3 (18) were seeded on microscope coverslips at 0.35 × 106 cells/ml in 24-well plates and treated 24 hours later with vehicle or obatoclax (100-200 nM). Following 24-48 hrs of treatment, cells were fixed in 4% paraformaldehyde, rinsed twice with cold PBS, and briefly dried. Cells were then stained with Hoechst 33258 (Sigma, St. Louis, MO), followed by rinsing with PBS and sealing with mounting medium. A confocal Olympus Flueview 1000 microscope was used to visualize GFP-LC3 punctate dots. The average number of GFP-LC3 puncta per cell was determined by counting 5 random fields, with a minimum of 25 cells per field for each treatment group. Results were presented as the mean number of puncta per cell from 3 independent experiments ± SEM.
2.5 Protein extraction and Western blot analysis
Cells were seeded at 0.05-0.1 × 106 cells/ml in 6-well plates and treated 24 hours later with vehicle or obatoclax (100-200 nM). After treatment, non-adherent and adherent cells were harvested and combined, and cells were centrifuged at 1,300 rpm for 5 min at 4°C. The cells were then washed once in cold PBS, and lysed for 10 min on ice in lysis buffer (150 mM NaCl, 50 mM Tris pH 8.0, 0.1% SDS, 1% Nonidet P-40) containing protease inhibitor cocktail (1 tablet/10 ml; Roche Applied Sciences). Extracts were clarified by centrifugation and protein concentrations were determined using Bio-Rad DC Kits (Bio-Rad, Hercules, CA). Equivalent amounts of proteins were electrophoresed on 12% SDS-polyacrylamide gels and transferred to nitrocellulose membranes which were blocked for 1 h in TBST buffer containing 5% nonfat milk. The membranes were probed overnight at 4°C with primary antibodies, followed by incubation with secondary antibody for 1 hr before development using enhanced chemiluminescence reagent (PerkinElmer Inc., Waltham, MA). The membranes were probed with antibodies specific for BCL-XL (sc-8392), MCL-1 (sc-819), Beclin-1 (sc-11427) from Santa Cruz Biotechnology Inc. (Dallas, TX); BCL-2 (M0887; Dako Corp., Carpinteria, CA), PARP (9542) from Cell Signaling Technology (Danvers, MA), LC3 (NB100-2220) from Novus Biologicals Inc. (Littleton, CO) and β-actin (A5441) from Sigma.
2.6 Statistical analysis
The data are presented as the mean of three independent experiments ± SEM. One-way ANOVA was used to assess differences in the means between the groups, followed by Tukey’s multiple comparison test. P values less than 0.05 were considered as statistically significant. All statistical analyses were performed using Prism software (version4; GraphPad Software, Inc., San Diego, CA).
3. Results
3.1 Potent single-agent activity of obatoclax on HNSCC cell growth
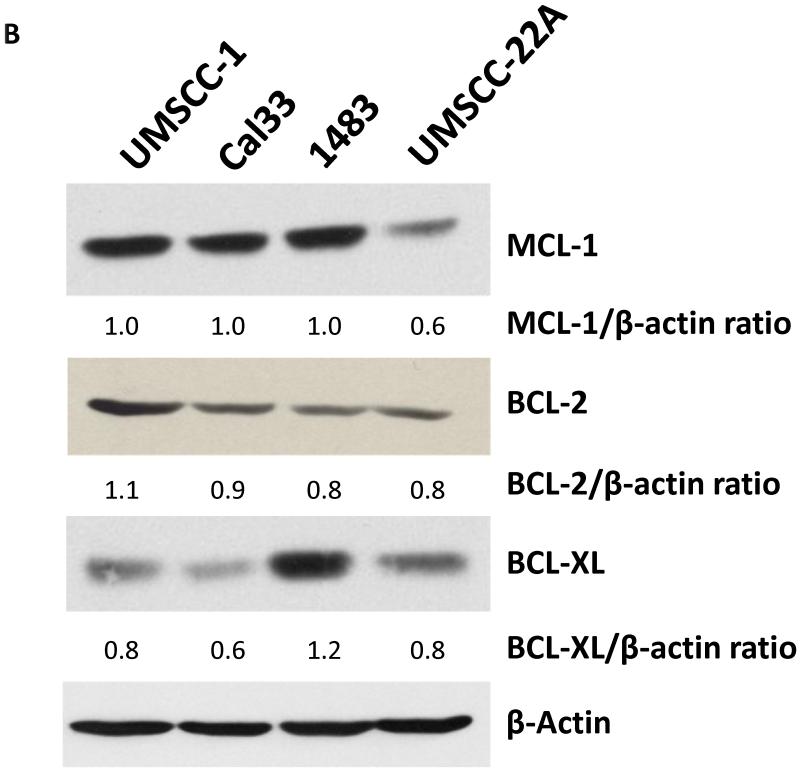
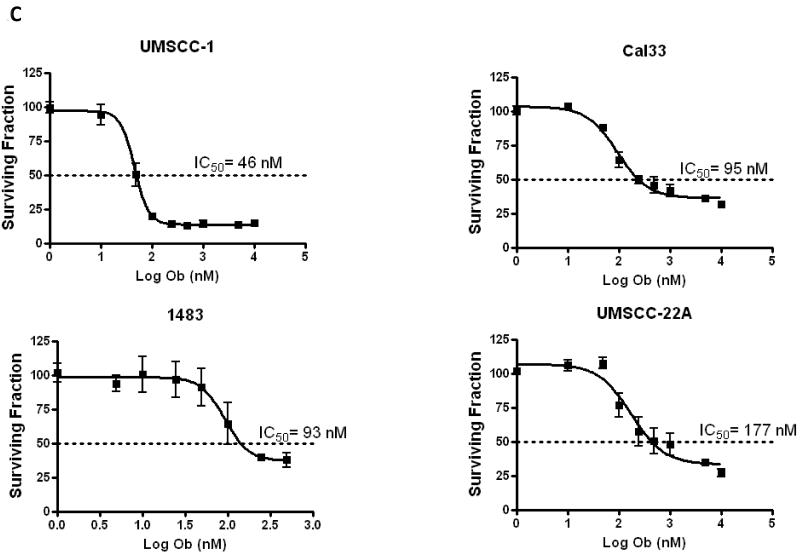
In order to assess the impact of obatoclax (Fig. 1A) treatment on HNSCC cells four HNSCC cell lines were employed: UMSCC-1, Cal33, 1483 and UMSCC-22A. Initially, the endogenous expression levels of the three major anti-apoptotic BCL-2 family members, BCL-2, BCL-XL, and MCL-1 was assessed (Fig. 1 B). Notably, MCL-1 expression was readily detectable in all cell lines, but was lowest in UMSCC-22A. We then treated cells with varying concentration of obatoclax, followed by measurement of cell growth inhibition using MTT assays and determination of IC50 values. Obatoclax showed potent single-agent activity with IC50’s ranging from 46-177 nM in the four HNSCC cell lines (Fig. 1C). The impact of obatoclax was dose-dependent, and UMSCC-22A cells, with the lowest MCL-1 expression levels, were found to be the least sensitive to obatoclax. Importantly, the recommended phase II dose for obatoclax is 28 mg/m2, given via intravenous infusion over 3 hours (19). At this dose, a maximal concentration of 176 nM (coefficient of variation of 44%) can be achieved. Thus, concentrations of obatoclax sufficient for single-agent activity against HNSCC cells can be reached in patients.
Figure 1. Obatoclax inhibits growth activity of HNSCC cells.
A: Chemical structure of obatoclax
B: UMSCC-1, Cal33, 1483, and UMSCC-22A were grown in 6-well plates for 48 hours. Whole cell lysates were prepared and subjected to immunoblotting for MCL-1, BCL-2 and BCL-XL; β-actin was used as a loading control. Ratios were calculated by densitometric analysis.
C: HNSCC cell lines were treated with 0.1% DMSO or increasing concentrations of obatoclax (1 nM -10 000 nM) for 48 hours. Growth inhibition was assessed by MTT assays. Each experiment was performed at least three independent times, and the values represent the mean (± SEM).
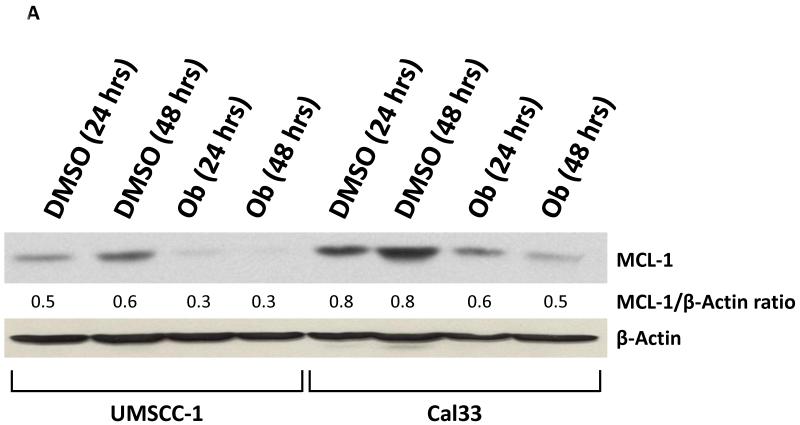
Obatoclax has been shown to decrease the expression level of several anti-apoptotic gene products including MCL-1 (20). Therefore, we examined the effects of obatoclax treatment on MCL-1 in the HNSCC cells. As shown in Fig. 2A, obatoclax treatment for 48 hours resulted in a decrease in the MCL-1 expression levels in both UMSCC-1 and Cal33 cells. By contrast, no changes in MCL-1 expression were observed in UMSCC-22A (not shown).
Figure 2. Obatoclax decreases MCL-1 protein expression in HNSCC cells.
A: UMSCC-1 and Cal33 cells were incubated with 0.1% DMSO or obatoclax (100 or 200 nM) for 48 hours. The levels of MCL-1 expression were determined by immunoblotting. Ratios were calculated by densitometric analysis. Data shown are representative of those obtained in three independent experiments.
3.2 Obatoclax induces apoptosis signaling in HNSCC cells
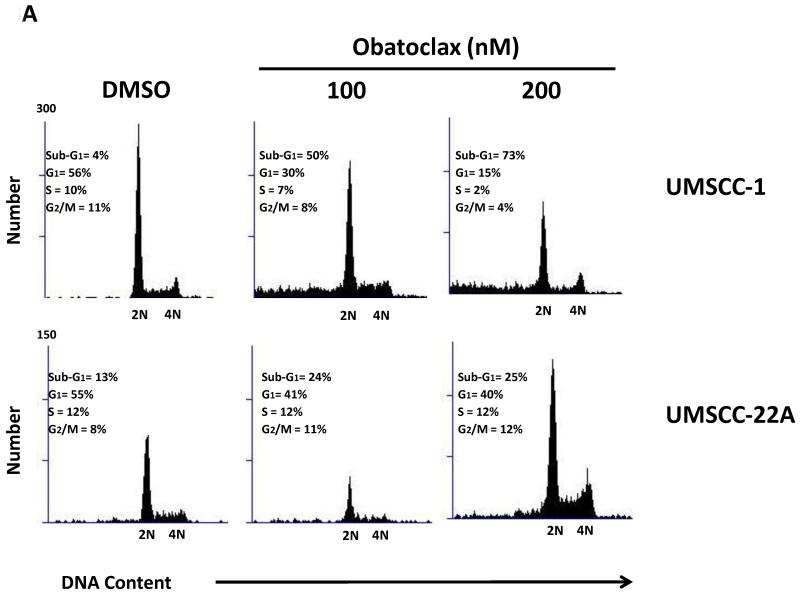
To determine the impact of obatoclax on cell cycle status, treated cells were permeabilized and the DNA was stained with propidium iodide. Flow cytometric analysis demonstrated induction of a sub-G1 population of cells in all 4 HNSCC lines, consistent with an induction of apoptotic cell death (Fig. 3A). The appearance of sub-G1 cells was accompanied in UMSCC-1 by decreased cells in G1, S and G2/M phases and in UMSCC-22A by decreased cells in G1-phase (Fig. 3A).
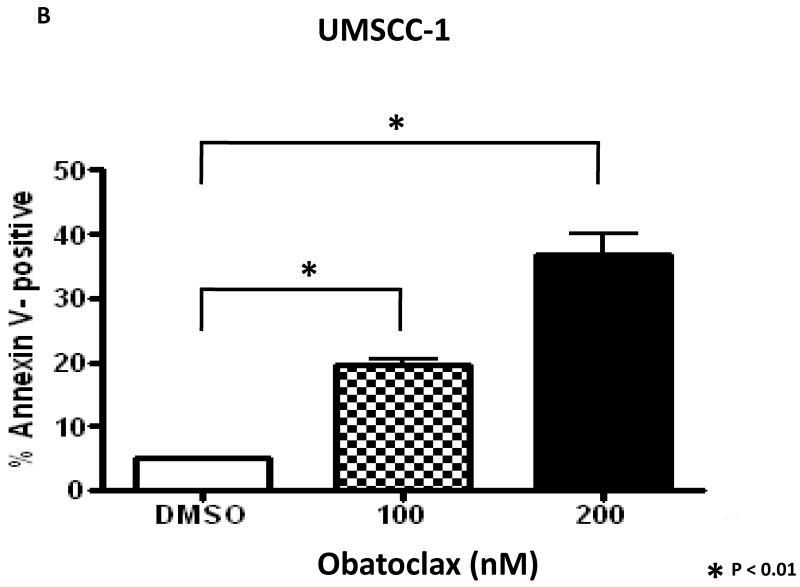
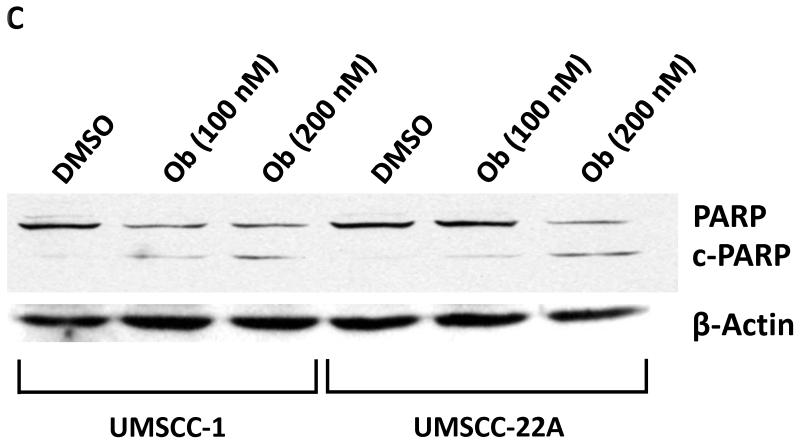
Figure 3. Obatoclax induces apoptosis in HNSCC cells.
A: UMSCC-1 and UMSCC-22A were treated for 48 hours with 0.1% DMSO or obatoclax (100 or 200 nM. Cell cycle distributions were determined by flow cytometric analyses, as described in Materials and Methods. The data shown is representative of 3 independent experiments.
B: UMSCC-1 cells were treated for 48 hours with 0.1% DMSO or obatoclax, followed by flow cytometric determination of Annexin V staining. Columns represent the average percentage of Annexin-V positive cells from three independent experiments; error bars represent SEM.
C: UMSCC-1 and UMSCC-22A were treated with 0.1% DMSO or obatoclax for 48 hours, followed by immunoblotting with anti-PARP. β-actin was used as a loading control. Full-length PARP (PARP) and cleaved PARP (c-PARP) are depicted. Data shown are similar to those obtained in three independent experiments.
In view of the increase in sub-G1 cells following obatoclax treatment, we investigated apoptosis induction. As shown in Fig. 3B, flow cytometry detected dose-dependent increases in Annexin-V binding in UMSCC-1. In addition, treatment with obatoclax resulted in cleavage of poly(ADP-ribose) polymerase (PARP) protein (Fig. 3C), indicative of caspase protease activation and apoptosis induction. Similar results were obtained for the other cell lines (not shown).
3.3 Obatoclax induces pro-survival autophagy in HNSCC cells
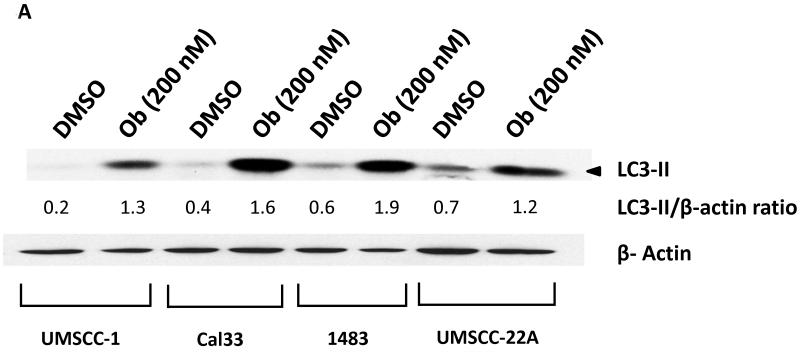
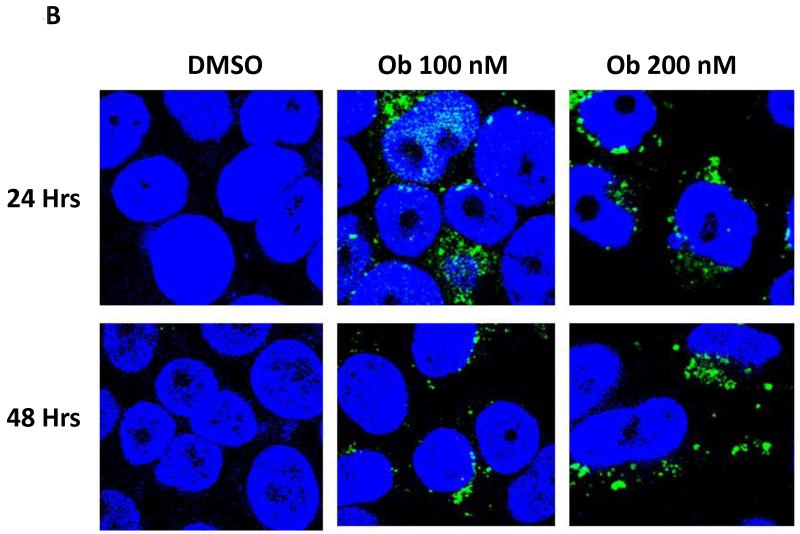
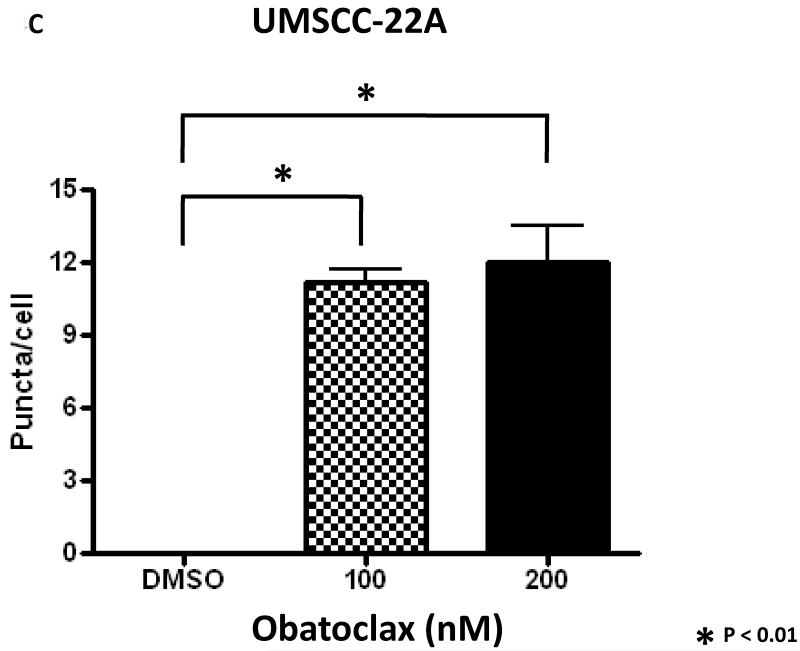
We next explored the impact of obatoclax on autophagy in the HNSCC cell lines. In initial experiments immunoblotting was used to measure expression levels of LC3-II protein. The expression levels of LC3-II are known to increase during autophagy induction, and the LC3-II protein associates with newly forming autophagasomes (21). As shown in Fig. 4A, treatment with obatoclax markedly induced the expression of LC3-II in all 4 cell lines, suggestive of autophagy induction. To confirm the induction of autophagy by obatoclax, we utilized fluorescence microscopy to examine autophagosome formation in UMSCC-22A cells stably expressing GFP-LC3 protein (Fig. 4B). Treatment of these cells for 24 or 48 hours with obatoclax (100 or 200 nM) resulted in relocalization of the GFP-LC3 protein to punctate cytoplasmic dots, an indicator of autophagosome formation (22). Treatment with obatoclax resulted in an approximately 10-fold increase in the average number of puncta per cell at 48 hrs (Fig. 4C) as well as 24 hours (not shown).
Figure 4. Obatoclax induces prosurvival autophagy in HNSCC cells.
A: HNSCC cells were treated with 0.1% DMSO or 200 nM of obatoclax for 48 hours, followed by immunoblotting for LC3-II. β-actin was used as a loading control. Ratios were calculated by densitometric analysis. Data shown are representative of those obtained in three independent experiments.
B: UMSCC-22A cells stably expressing GFP-LC3 were treated with 0.1% DMSO or obatoclax for 24 or 48 hrs. Relocalization of GFP-LC3-II to a punctuate cytoplasmic distribution was assessed via confoncal microscopy.
C: The average number of puncta per cell was determined as described in Materials and Methods. Columns represent the means from 3 independent experiments. Error bars represent SEM.
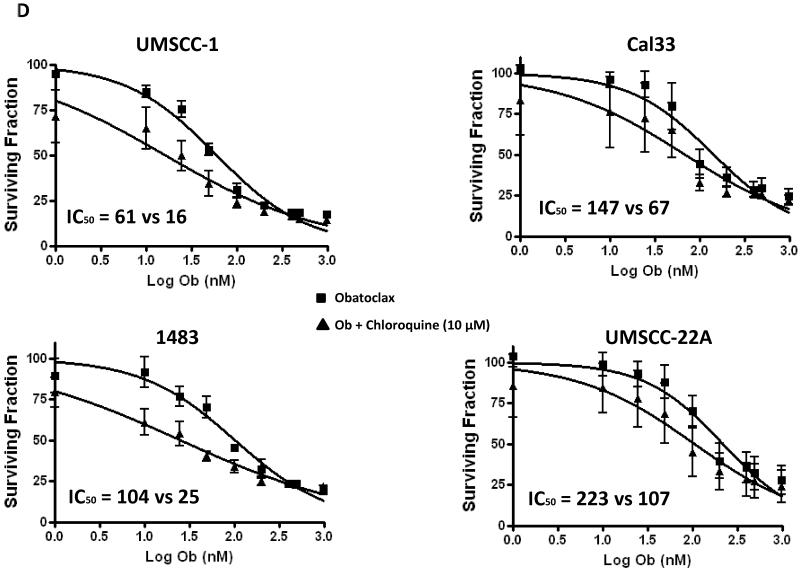
D: HNSCC cells treated with increasing concentrations of obatoclax (1-1000 nM) in the absence (squares) or presence (triangles) of 10 μmol/L chloroquine. Cell growth was assessed by MTT assay at 48 hours. Each experiment was performed at least three times, and the values represent the mean ± SEM.
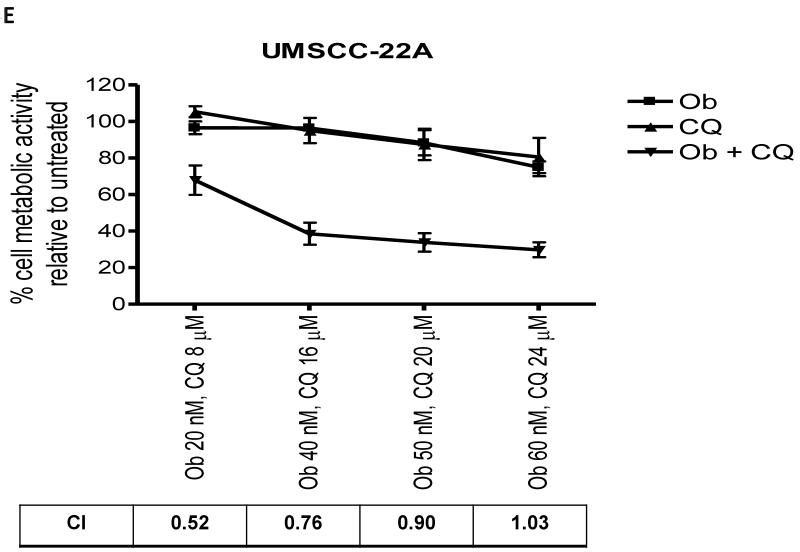
E: Constant ratios of obatoclax (Ob) and chloroquine (CQ) were used to treat UMSCC-22A for 48 hours, followed by performance of MTT assays. The data represent the means from 3 independent experiments, and error bars the SEM. Combination indexes (CI) were determined according to the method of Chou and Talalay (25).
Prior studies have shown that induction of autophagy can act to either promote cell survival, (17, 23) or promote cell death (24). To determine whether the autophagy induced by obatoclax was exerting a pro-survival or a pro-death activity, we inhibited autophagy using chloroquine. While treatment with chloroquine alone (10 μg/ml; (18)) had minimal impact on the cells, co-treatment with chloroquine and obatoclax led to a reduction in the IC50’s values for obatoclax in all 4 HNSCC cell lines (Fig. 4D). UMSCC-1 exhibited the greatest increase in sensitivity to obatoclax (IC50s of 61 nM and 16 nM in the absence and presence of chloroquine, respectively), while UMSCC-22A exhibited the least (223 nM vs 107 nM). Given that chloroquine is a known late-stage inhibitor of autophagy, these findings suggest that obatoclax induces pro-survival autophagy which when inhibited potentiates the cytotoxicity of the drug.
Potential synergism between obatoclax and chloroquine was assessed in UMSCC-22A and UMSCC-1 cells, according to the method of Chou and Talalay (25). At low concentrations, obatoclax and chloroquine synergized to induce cell death in UMSCC-22A cells, as evidenced by combination index (CI) values less than 1.0 (Fig. 4E). However, at higher concentrations, the effects were only additive (CI values approximately 1.0; Fig. 4E). In addition, synergism was not observed at any concentration in UMSCC-1 cells (not shown).
4. Discussion
Increased expression of the anti-apoptotic members of the BCL-2 protein family particularly MCL-1, BCL-2 and BCL-XL in HNSCC correlates with chemotherapy resistance (6) and poor outcome (7). These three major anti-apoptotic proteins are known to inhibit cell apoptosis, and therefore targeting them may restore the capacity of HNSCC tumor cells to undergo apoptosis. In contrast to ABT-737, which inhibits BCL-2 and BCL-XL, obatoclax is capable of inhibiting MCL-1, in addition to BCL-2 and BCL-XL (26). In our study, we observed potent single-agent activity of obatoclax against four HNSCC cell lines. At the clinically achievable concentration (176 nM), all currently studied HNSCC cell lines were obatoclax-sensitive as demonstrated by their respective IC50’s. However, the level of sensitivity varied between these cell lines. MCL-1 protein expression levels appeared to correlate with obatoclax sensitivity. In fact, UMSCC-22A harboring the lowest levels of MCL-1, was the least sensitive to obatoclax. Whether MCL-1 protein level can be used as a biomarker for obatoclax sensitivity in HNSCC requires further studies.
MCL-1 has been shown to confer resistance to several molecular targeting agents, including ABT-737 and the proteasome inhibitor bortezomib (26). Obatoclax was initially designed as a BH3-mimetic that liberates pro-apoptotic BAK from its association with MCL-1, leading to activation of the intrinsic apoptotic pathway (26). In addition, obatolcax has been found to inhibit the NFκB and other anti-apoptotic signaling pathways leading to decreased MCL-1 expression in lymphoma cell lines (20). We observed that obatoclax decreased MCL-1 expression in UMSCC-1 and Cal33, while expression levels were not affected in UMSCC-22A. The mechanism(s) responsible for obatoclax-induced downregulation of MCL-1 expression merits further evaluation. In this regard, bortezomib is known to increase MCL-1 levels by inhibiting its degradation via the proteasome (26). Interestingly, bortezomib liberates p53 activity in human papilloma virus (HPV)-positive HNSCC cells, while obatolcax has been demonstrated to inhibit p53 activity in lymphoma cell lines (27). All four HNSCC cell lines used in our study are HPV-negative, where p53 is either mutated or absent (28). Thus, the effects we observed with obatoclax were p53-independent. The impact of obatoclax on NFκB signaling in HNSCC cells, as well as the impact of obatoclax on HPV-positive HNSCC cells remains to be determined. Moreover, it is possible that obatoclax will exhibit synergism in HNSCC cells with compounds such as bortezomib or ABT-737, whose activities are blunted due to MCL-1 expression.
The anti-apoptotic BCL-2 proteins regulate autophagy through binding and inhibition of Beclin-1, a key BH3 domain-containing protein that is essential for autophagy initiation (29). The role of cellular autophagy has been somewhat controversial, with both cytoprotective and cytotoxic roles having been reported (30). In esophageal carcinoma cells, inhibition of autophagy has been reported to enhance obatoclax activity (23). We observed autophagy induction in all four HNSCC cell lines following treatment with obatoclax, as evidenced by upregulation of LC3-II protein and autophagasome formation. The addition of the autophagy inhibitor chloroquine increased obatoclax cytotoxicity in all HNSCC cells, suggesting a potential avenue for achieving enhanced therapeutic activity.
Novel targeted therapies with an improved toxicity profile, and the potential to improve outcome are in desperate need for patients with R/M HNSCC. Taken together, our results showed that obatoclax potently inhibited the growth activity of HNSCC cells, while inducing apoptotic cell death and prosurvival autophagy. Given its broad and potent preclinical activity, further evaluation of obatoclax, or similarly acting compounds, as a single-agent or in combination with an autophagy inhibitor is warranted in HNSCC.
Acknowledgements
This work was supported by 2T32CA060397, NIH/NCI, Postdoctoral Research Training in Head and Neck Oncology and NIH grants R01 CA137260 and P50 CA097190. This project also used UPCI shared resources supported in part by P30CA047904.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
None declared
References
- 1.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005 Jan-Feb;55(1):10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Forastiere AA, Metch B, Schuller DE, Ensley JF, Hutchins LF, Triozzi P, et al. Randomized comparison of cisplatin plus fluorouracil and carboplatin plus fluorouracil versus methotrexate in advanced squamous-cell carcinoma of the head and neck: a Southwest Oncology Group study. Journal of Clinical Oncology. 1992;10(8):1245–51. doi: 10.1200/JCO.1992.10.8.1245. [DOI] [PubMed] [Google Scholar]
- 3.Gibson MK, Li Y, Murphy B, Hussain MH, DeConti RC, Ensley J, et al. Randomized phase III evaluation of cisplatin plus fluorouracil versus cisplatin plus paclitaxel in advanced head and neck cancer (E1395): an intergroup trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2005 May 20;23(15):3562–7. doi: 10.1200/JCO.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs C, Lyman G, Velez-Garcia E, Sridhar KS, Knight W, Hochster H, et al. A phase III randomized study comparing cisplatin and fluorouracil as single agents and in combination for advanced squamous cell carcinoma of the head and neck. J Clin Oncol. 1992;10(2):257–63. doi: 10.1200/JCO.1992.10.2.257. [DOI] [PubMed] [Google Scholar]
- 5.Vermorken JB, Trigo J, Hitt R, Koralewski P, Diaz-Rubio E, Rolland F, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007 Jun 1;25(16):2171–7. doi: 10.1200/JCO.2006.06.7447. [DOI] [PubMed] [Google Scholar]
- 6.Trask DK, Wolf GT, Bradford CR, Fisher SG, Devaney K, Johnson M, et al. Expression of Bcl-2 family proteins in advanced laryngeal squamous cell carcinoma: correlation with response to chemotherapy and organ preservation. Laryngoscope. 2002 Apr;112(4):638–44. doi: 10.1097/00005537-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Nichols AC, Finkelstein DM, Faquin WC, Westra WH, Mroz EA, Kneuertz P, et al. Bcl2 and human papilloma virus 16 as predictors of outcome following concurrent chemoradiation for advanced oropharyngeal cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010 Apr 1;16(7):2138–46. doi: 10.1158/1078-0432.CCR-09-3185. [DOI] [PubMed] [Google Scholar]
- 8.Li R, Zang Y, Li C, Patel NS, Grandis JR, Johnson DE. ABT-737 synergizes with chemotherapy to kill head and neck squamous cell carcinoma cells via a Noxa-mediated pathway. Mol Pharmacol. 2009 May;75(5):1231–9. doi: 10.1124/mol.108.052969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hotz MA, Bosq J, Zbaeren P, Reed J, Schwab G, Krajewski S, et al. Spontaneous apoptosis and the expression of p53 and Bcl-2 family proteins in locally advanced head and neck cancer. Archives of otolaryngology--head & neck surgery. 1999 Apr;125(4):417–22. doi: 10.1001/archotol.125.4.417. [DOI] [PubMed] [Google Scholar]
- 10.Zhai D, Jin C, Satterthwait AC, Reed JC. Comparison of chemical inhibitors of antiapoptotic Bcl-2-family proteins. Cell Death Differ. 2006 Aug;13(8):1419–21. doi: 10.1038/sj.cdd.4401937. [DOI] [PubMed] [Google Scholar]
- 11.Konopleva M, Watt J, Contractor R, Tsao T, Harris D, Estrov Z, et al. Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15-070 (obatoclax) Cancer research. 2008 May 1;68(9):3413–20. doi: 10.1158/0008-5472.CAN-07-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez-Galan P, Roue G, Villamor N, Campo E, Colomer D. The BH3-mimetic GX15-070 synergizes with bortezomib in mantle cell lymphoma by enhancing Noxa-mediated activation of Bak. Blood. 2007 May 15;109(10):4441–9. doi: 10.1182/blood-2006-07-034173. [DOI] [PubMed] [Google Scholar]
- 13.Trudel S, Li ZH, Rauw J, Tiedemann RE, Wen XY, Stewart AK. Preclinical studies of the pan-Bcl inhibitor obatoclax (GX015-070) in multiple myeloma. Blood. 2007 Jun 15;109(12):5430–8. doi: 10.1182/blood-2006-10-047951. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Viallet J, Haura EB. A small molecule pan-Bcl-2 family inhibitor, GX15-070, induces apoptosis and enhances cisplatin-induced apoptosis in non-small cell lung cancer cells. Cancer chemotherapy and pharmacology. 2008 Mar;61(3):525–34. doi: 10.1007/s00280-007-0499-3. [DOI] [PubMed] [Google Scholar]
- 15.Chiappori AA, Schreeder MT, Moezi MM, Stephenson JJ, Blakely J, Salgia R, et al. A phase I trial of pan-Bcl-2 antagonist obatoclax administered as a 3-h or a 24-h infusion in combination with carboplatin and etoposide in patients with extensive-stage small cell lung cancer. British journal of cancer. 2012 Feb 28;106(5):839–45. doi: 10.1038/bjc.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005 Jun 2;435(7042):677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 17.Li C, Johnson DE. Bortezomib induces autophagy in head and neck squamous cell carcinoma cells via JNK activation. Cancer Lett. 2012 Jan 1;314(1):102–7. doi: 10.1016/j.canlet.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zang Y, Thomas SM, Chan ET, Kirk CJ, Freilino ML, DeLancey HM, et al. Carfilzomib and ONX 0912 inhibit cell survival and tumor growth of head and neck cancer and their activities are enhanced by suppression of Mcl-1 or autophagy. Clin Cancer Res. 2012 Oct 15;18(20):5639–49. doi: 10.1158/1078-0432.CCR-12-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urtishak KA, Edwards AY, Wang LS, Hudome A, Robinson BW, Barrett JS, et al. Potent obatoclax cytotoxicity and activation of triple death mode killing across infant acute lymphoblastic leukemia. Blood. 2013 Apr 4;121(14):2689–703. doi: 10.1182/blood-2012-04-425033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Paniagua MA, Baritaki S, Huerta-Yepez S, Ortiz-Navarrete VF, Gonzalez-Bonilla C, Bonavida B, et al. Mcl-1 and YY1 inhibition and induction of DR5 by the BH3-mimetic Obatoclax (GX15-070) contribute in the sensitization of B-NHL cells to TRAIL apoptosis. Cell Cycle. 2011 Aug 15;10(16):2792–805. doi: 10.4161/cc.10.16.16952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cook KL, Shajahan AN, Clarke R. Autophagy and endocrine resistance in breast cancer. Expert review of anticancer therapy. 2011 Aug;11(8):1283–94. doi: 10.1586/era.11.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008 Feb 16;4(2):151–75. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan J, Cheng C, Verstovsek S, Chen Q, Jin Y, Cao Q. The BH3-mimetic GX15-070 induces autophagy, potentiates the cytotoxicity of carboplatin and 5-fluorouracil in esophageal carcinoma cells. Cancer letters. 2010 Jul 28;293(2):167–74. doi: 10.1016/j.canlet.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Tang Y, Hamed HA, Cruickshanks N, Fisher PB, Grant S, Dent P. Obatoclax and lapatinib interact to induce toxic autophagy through NOXA. Molecular pharmacology. 2012 Apr;81(4):527–40. doi: 10.1124/mol.111.076851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, Murthy Madiraju SR, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A. 2007 Dec 4;104(49):19512–7. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brem EA, Thudium K, Khubchandani S, Tsai PC, Olejniczak SH, Bhat S, et al. Distinct cellular and therapeutic effects of obatoclax in rituximab-sensitive and -resistant lymphomas. British journal of haematology. 2011 Jun;153(5):599–611. doi: 10.1111/j.1365-2141.2011.08669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li C, Johnson DE. Liberation of functional p53 by proteasome inhibition in human papilloma virus-positive head and neck squamous cell carcinoma cells promotes apoptosis and cell cycle arrest. Cell cycle. 2013 Mar 15;12(6):923–34. doi: 10.4161/cc.23882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005 Sep 23;122(6):927–39. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Hippert MM, O’Toole PS, Thorburn A. Autophagy in cancer: good, bad, or both? Cancer research. 2006 Oct 1;66(19):9349–51. doi: 10.1158/0008-5472.CAN-06-1597. [DOI] [PubMed] [Google Scholar]