Abstract
Pattern recognition receptors (PRRs) are essential sentinels for pathogens or tissue damage and integral components of the innate immune system. Recent structural studies have provided unprecedented insights into the molecular mechanisms of ligand recognition and signal transduction by several PRR families at distinct subcellular compartments. Here we highlight some of the recent discoveries and summarize the common themes that are emerging from these exciting studies. Better mechanistic understanding of the structure and function of the PRRs will improve future prospects of therapeutic targeting of these important innate immune receptors.
Introduction
The innate immune system senses foreign or dangerous substances through families of germline-encoded pattern recognition receptors (PRRs) in the extracellular environment and various subcellular compartments [1]. Activation of PRRs such as the Toll-like receptors (TLRs), RIG-I-like receptors (RLRs), NOD-like receptors (NLRs) and PYD and HIN domain-containing protein (PYHIN) family of receptors triggers immune responses that contain or eliminate invading pathogens, but may also elicit serious damage to the host. Recent developments in the structural studies of these PRRs families have provided exciting new insights into how these receptors recognize their respective ligands and initiate distinctive signal transduction events. A number of common regulatory mechanisms have emerged from studies of different PRR families, including receptor autoinhibition, receptor oligomeric signaling platform, and post-translational modifications. It is likely that these common themes are applicable to other less well-characterized PRRs, particularly those that may elicit tissue damage through highly proinflammatory responses. Here we focus on insights gained from recent structural studies of the above four PRR families and their implications on ligand recognition, signaling and regulatory mechanisms.
Toll-like receptors
TLRs are glycosylated type I membrane proteins with an N-terminal extracellular domain (ECD), a single transmembrane helix, and a cytosolic Toll/IL-1 receptor (TIR) domain responsible for downstream signaling to other TIR domain-containing proteins. The ECD is composed of leucine-rich repeats (LRRs) that contain binding sites for pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs). Recognition of PAMPs or DAMPs by TLRs is critical for the activation of transcription factor NF-κB or IRFs (interferon response factors), leading to the production of pro-inflammatory cytokines or type I interferons (IFNs). Structural studies have revealed the general architecture of the TLR ECDs. The TLR ECDs are composed of ~25 LRRs of ~25 residues each. The N- and C-terminal cysteine-rich capping modules (LRRNT and LRRCT) shield the hydrophobic core of the LRRs from solvent. The LRRs are characterized by a consensus sequence motif LxxLxLxxN [2]. The conserved leucines or asparagines play important roles in forming the hydrophobic core and maintaining the overall horseshoe shape of the LRRs, whereas residues from variable regions confer distinct convex surface features relevant to ligand binding and receptor association.
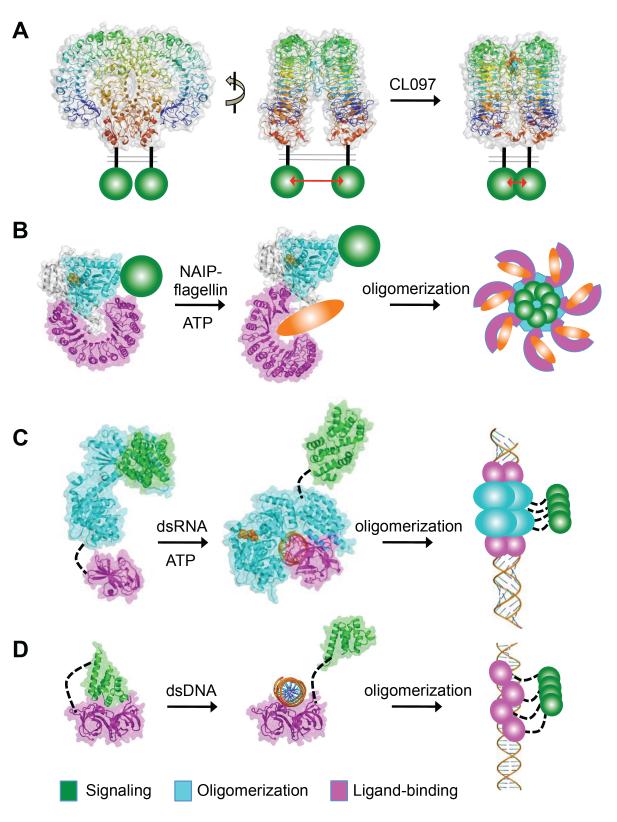
Recent structural studies of the TLR5 and TLR8 ECDs provided insights into their unique structural features and specific modes of ligand engagement. The structure of the TLR5 ECD:flagellin complex is a 2:2 hetero-tetramer with each TLR5 molecule engaging both flagellin molecules using its lateral and convex surface [3••]. A loop at its highly conserved LRR9 makes extensive contact with the N- and C-terminal helices of the flagellin D1 domain. Importantly, these TLR5-contacting flagellin residues are highly conserved and mediate flagellar protofilament assembly. In comparison, the TLR8 ECD adopts a more compact structure than previously reported “m”-shaped TLR dimers [4••] (Figure 1A). Engagement of small chemical agonists triggers rearrangement of the dimeric configuration, resulting in more extensive TLR8:TLR8 interface and closer proximity of their C-termini. The TLR8 ligand-binding pocket is largely conserved in TLR7, in agreement with their similar specificity for ligands. Purified TLR8 was cleaved at a long “Z-loop” insertion between its LRR14 and LRR15. This coincides with previously identified cleavage sites at TLR7 and TLR9 [5-7]. Cleavage at this Z-loop did not result in dissociation of the N- and C-terminal fragments because of the β sheet formation at the concave surface and other interactions among the LRRs. It is not clear if this cleavage is important for small ligand engagement by TLR8, as the Z-loop is not in the immediate vicinity of the bound agonists. However, it remains possible that binding of larger ssRNA ligand may involve the Z-loop residues.
Figure 1.
Ligand-binding and oligomerization of pattern recognition receptors TLR8 (A), NLRC4 (B), RIG-I (C), and AIM2 (D). The signaling, oligomerization, and ligand-binding domains are colored green, cyan and magenta except for the TLR8 ECD, which is in rainbow color from its N- to C-termini. Binding of small molecular agonist CL097 (in van der Waals spheres) induces close proximity of the TLR8 ECD C-termini and the cytoplasmic TIR domains (green) as shown in (A). Flagellin molecules are represented as orange ovals and the NAIP CARDs as green spheres in (B). The ADP/ATP molecules are shown as van der Waals spheres in (B-C). The NLRC4 inflammasome, RIG-I filament, and AIM2 inflammasome are represented schematically on the right side panels of (B-D).
Growing structural and biochemical evidence suggests that the TLRs may be divided into two distinct subtypes based on their ligand specificity, ECD horseshoe architecture, and oligomerization states in the apo-forms. Type I includes TLR1, TLR2, TLR4 and TLR6 that recognize ligands containing hydrophobic moieties. These TLR ECDs show a twist of their horseshoe structure, which partially results from the atypical central LRR motifs that lack the conserved asparagines [8-11]. Interestingly, these sites of distortion are also near the binding pockets for the hydrophobic epitopes of the ligands. By contrast, such structural irregularities are generally absent for type II TLRs such as TLR3 [12,13], TLR5 [14••] and TLR8 [4••] and may also include TLR7 and TLR9. These TLRs recognize nucleic acid or protein ligands with largely hydrophilic surface. These two types of TLRs also demonstrate distinct oligomerization states in the absence of ligand binding: recombinantly expressed type I TLRs are monomers that require ligand engagement to assemble stable dimeric structures [8-11], while most of the type II TLRs such as TLR5, TLR8 and TLR9 adopt pre-formed dimers that are rearranged upon ligand engagement to facilitate signaling [3••,4••,15]. The only exception in type II TLRs is TLR3 that requires dsRNA binding to form stable homodimers [16,17]. There is a caveat to this distinction of preformed TLR dimers: many studies focused on the ECDs of TLRs that contain LRRCT from hagfish VLRs, therefore whether and how the native LRRCT and the transmembrane domains might modulate the TLR oligomerization states remain to be explored. Despite the differences in architecture, modes of ligand recognition and oligomerization states, ligand engagement by all TLR ECDs results in dimeric horseshoe formation that bring their cytoplasmic TIR domains into close proximity to recruit TIR domain-containing adapter molecules. Future studies will need to focus on mechanisms of adapter recruitment and TIR domain signaling complex assembly to fully understand TLR function in the context of the full-length receptors.
NOD-like receptors
The NLRs have tripartite domain structures containing N-terminal signaling domains such as CARDs or PYDs, central nucleotide-binding domains (NBDs) that bind ATP and mediate oligomerization along with associated domains, and C-terminal leucine-rich repeats (LRRs) that may participate in ligand binding and/or autoinhibition of the receptors. Several NLR family members such as NLRP1, NLRP3, NLRC4, NLRP6, NLRP7 and NLRP12 are known to assemble oligomeric signaling complexes named inflammasomes [18,19]. Inflammasomes are composed of the activated receptor, the adapter ASC, and effector procaspase-1. Formation of the inflammasomes leads to activation of caspase-1 and maturation and secretion of proinflammatory cytokines such as IL-1β and IL-18.
An exciting recent development is the determination of the structure for the NLRC4 core region. NLRC4 is a cytosolic receptor that recognizes bacterial flagellin or components of the type III secretion system in complex with NAIP proteins [20••,21••,22-23]. The structure of NLRC4 is composed of the nucleotide binding domain (NBD), winged helix domain (WHD), helical domain 1 (HD1), HD2, and a C-terminal leucine-rich repeat (LRR) [24••] (Figure 1B). The interaction between NBD and WHD is mediated by ADP and a critical His433 residue in WHD, and is important to maintain the receptor in a monomeric resting state. Mutation of His433 resulted in constitutively active and oligomeric NLRC4. Similarly, the interaction between NBD and HD2/LRR also suppresses NLRC4 activation, as evidenced by activated NLRC4 upon disruption of NBD and HD2/LRR interactions. This autoinhibition mechanism is reminiscent of that adopted by Apaf-1, in which the WD40 domain functions as both the ligand binding site and a repressor domain. While details of the NLRC4 activation mechanism remain to be explored, it is hypothesized that upon association of the NLRC4 LRR with bacterial flagellin or NAIP:flagellin complex, a large conformational change ensues, which releases the autoinhibition state imposed by the WHD, HD2 and LRR domains to facilitate oligomerization and signaling by its NBD and CARD domains, respectively.
RIG-I-like receptors
Three RLRs, RIG-I, MDA5, and LGP2 [25-27], are cytoplasmic viral RNA sensors that mount type I interferon response through MAVS/IPS-1/VISA/Cardif-dependent signaling pathways [28-31]. The RLRs are DExD/H box proteins that belong to the superfamily 2 (SF2) helicases. They contain the RecA-like globular domains (Hel1 and Hel2), a unique helicase insertion domain (Hel2i), and C-terminal regulatory domain (CTD) (Figure 1C). RIG-I and MDA, but not LGP2, contain two N-terminal tandem CARDs believed to mediate downstream signaling [25-27,32]. The Hel1 and Hel2 domains harbor conserved motifs for ATP binding, ATP hydrolysis, and RNA binding [33••,34••,35••]. The Hel2i domain contains an overlapping binding surface for CARD2 in the apo-conformation and for RNA in the ligand-bound form, suggesting that the Hel2i:CARD2 interaction play a role in retaining the apo-receptor in an autoinhibited state [34••]. Binding of dsRNA blunt ends by the RIG-I CTD and ATP by the helicase domains lead to reorientation of the helicase domains to associate with dsRNA, ATP hydrolysis by the helicase domains, and exposure of the two CARDs. Ubiquitination of RIG-I by TRIM25 leads to receptor oligomerization and interaction with MAVS at the mitochondria [36•]. The RIG-I:MAVS interaction induces conformational changes in the latter to form prion-like aggregates to amplify IRF-mediated signaling for interferon induction [37••].
In contrast to the capping of dsRNA ends by the RIG-I CTD, the MDA5 CTD and helicase domains associate with the stem of the dsRNA duplex [38••]. This allows the CTD and helicase domains to mediate the formation of head-to-tail MDA5 filaments through cooperative binding of the dsRNA staircase, which leads to the formation of the MDA5 CARD clusters at the peripheral of such filaments [38••]. The MDA5 CARD clusters along the long dsRNA molecules in turn activate the adapter MAVS. Such filamentous architecture on a dsRNA platform was also recently reported for RIG-I [39•,40•], in which the formation of the RIG-I filament was dependent on translocation of RIG-I from the dsRNA blunt ends to the stem. Even though the RIG-I filament formation was independent of the K63-linked polyubiquitin chains, the filament-based and polyubiquitin-based mechanisms could synergize to boost signaling to MAVS. Major differences between the RIG-I and MDA5 filaments are that the former is less cooperative, requires ATP hydrolysis, and initiates from the end of dsRNA, whereas the latter is highly cooperative but disassembles upon ATP hydrolysis [39]. Nonetheless, both filaments provide multivalent ligand-based platforms that are capable of extremely high signaling output by virtue of their large oligomeric assembly. It will be an important future direction to investigate the mechanistic details of the RLR filaments in association with the MAVS prion-like aggregates.
The RLR signaling pathways are regulated by multiple post-translational modifications [41], including polyubiquitination as mentioned above. Recently, phosphorylation of the RIG-I and MDA5 CARDs have also emerged as an important regulatory mechanism [42,43••]. Interestingly, the sites of phosphorylation, S8 in RIG-I and S88 in MDA5, are at or near the α1 helix of the death domain fold from CARD. This region was reported to mediate the type I death domain complex formation by members of the superfamily [44]. It is possible that phosphorylation near the type I interface may influence the charge-based CARD:CARD association, thus regulate downstream signaling. Alternatively, modification of the RIGI-I surface charge may diminish its association with TRIM25 and the resulting ubiquitination [45•].
As an example of cross-regulation among different PRR families, the NLRX1 at the mitochondria was shown to interact with MAVS to suppress RLR signaling [46]. It was also shown to associate with mitochondrial translation elongation factor TUFM to promote autophagy [47•]. A C-terminal fragment containing the LRR of NLRX1 was crystallized as a hexameric complex [48•]. This hexamer complex was shown to bind RNA and regulate ROS production. Whether the NLRX1:RNA association is important for modulating RLR function remains to be explored.
The PYHIN protein family
The PYHIN family is a group of interferon-inducible proteins. Among the four family members in human, AIM2 and IFI16 have been shown to directly bind dsDNA through their C-terminal HIN domains and induce innate immune responses. The N-terminal PYD is a member of the death domain superfamily implicated in the assembly of immune and apoptotic signaling pathways. DNA-stimulation of AIM2 and IFI16 results in formation of the AIM2 inflammasome and induction of interferon expression, respectively. The murine PYHIN family member p202 does not contain a PYD and functions as a suppressor of the AIM2 activation. The crystal structures of the HIN domains from AIM2, IFI16 and p202 revealed the structural basis of sequence-independent dsDNA recognition through electrostatic interactions [49••,50•,51••] (Figure 1D). The AIM2 HIN domain and IFI16 HINb domain bind the dsDNA sugar phosphate backbone through a highly positively charged concave surface spanning its oligonucleotide/oligosaccharide-binding (OB) 1, linker, and OB2 folds. Such nonspecific DNA association suggests that dsDNA may serve as an oligomerization platform that recruits multiple receptor molecules, which in turn assemble adapters and effector enzymes into large signaling complexes such as the inflammasomes. This mode of DNA-mediated receptor complex assembly bears striking similarity to those adopted by the MDA5 and RIG-I receptors in their filament formation along the dsRNA ligand [38••,39•,40•]. In addition to DNA binding, the AIM2 HIN domain was shown to associate with its PYD in the absence of DNA [52•]. This intramolecular interaction may retain the receptor in an autoinhibited resting state, which can be released by dsDNA engagement that promotes the AIM2 PYD:ASC PYD interaction.
In contrast to the AIM2 and IFI16 HIN domains, the DNA-binding surface of the p202 HINa domain is located on the opposite side of the linker, with OB1 and OB2 contributing a larger interface and higher binding affinity [50•,51••]. In addition, the HINb domain of p202 was shown to mediate tetramerization and directly bind the AIM2 HIN domain, which may underlie the specific inhibition of AIM2 by p202 [51••]. This specific inhibition may be further enhanced by the higher DNA-binding affinity by the p202 HINa domain. In agreement, p202 was shown to prevent ASC cluster formation upon AIM2 activation, thus preventing the AIM2 inflammasome formation and production of inflammatory cytokines [51••].
Conclusions
Recent structural studies have yielded extraordinary insights into the molecular mechanisms of ligand recognition and downstream signaling by several receptor families. New paradigms are emerging from these structure-function studies. First, autoinhibition is a mechanism that retains the receptors in the resting states to prevent spurious activation of damaging proinflammatory responses. This has been demonstrated for RIG-I, AIM2, and NLRC4 in which the ligand-binding domains serve the double duty of sequestering the signaling domains. This important safeguarding mechanism is likely applicable to other PRRs. Second, ligand- or protein domain-mediated oligomerization platforms such as the TLR dimer/oligomer, MDA5/RIG-I filaments, NLRC4 inflammasome and AIM2 inflammasome are highly efficient signaling mechanisms that can promote cooperative responses to minimal presence of PAMPs. Because some of the activating ligands such as DNA and RNA are universal genetic materials, the inflammatory responses endowed by such oligomeric signaling platforms can also induce or perpetuate autoimmune and autoinflammatory disorders. Lastly, post-translational modifications have been shown to play important roles in regulating PRR functions. Examples are phosphorylation of TLR4 [53], TLR3 [54], RIG-I [43••], MDA5 [43••], and NLRC4 [55•], ubiquitination of RIG-I [36•,56], and proteolysis of TLR7 and 9 [5-7]. Many of these post-translational modifications directly affect receptor:ligand interaction, receptor oligomerization or association with signaling proteins. Future studies on the autoinhibition, oligomerization and post-translational modification of the PRRs will undoubtedly further our understandings of their roles in antimicrobial immune defense and autoimmune and autoinflammatory disorders.
Highlights.
Autoinhibition is a common mechanism for retaining PRRs in the resting states
Receptor oligomerization enhances sensitivity and signaling output
Post-translational modifications can regulate ligand binding and signaling
Acknowledgements
We thank members of the Xiao lab for critical reading of the manuscript. We apologize for omission of references due to space constraints. T.S.X is supported by the Division of Intramural Research, NIAID, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Janeway CA, Medzhitov R. Innate immune recognition. Annu. Rev. Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 2.Kang JY, Lee J-O. Structural biology of the Toll-like receptor family. Annu. Rev. Biochem. 2011;80:917–941. doi: 10.1146/annurev-biochem-052909-141507. [DOI] [PubMed] [Google Scholar]
- 3 ••.Yoon S-I, Kurnasov O, Natarajan V, Hong M, Gudkov AV, Osterman AL, Wilson IA. Structural basis of TLR5-flagellin recognition and signaling. Science. 2012;335:859–864. doi: 10.1126/science.1215584. The authors report the first structure of TLR5 in complex with its protein ligand flagellin, which demonstrates a novel 2:2 hetero-tetrameric complex.
- 4 ••.Tanji H, Ohto U, Shibata T, Miyake K, Shimizu T. Structural Reorganization of the Toll-Like Receptor 8 Dimer Induced by Agonistic Ligands. Science. 2013;339:1426–1429. doi: 10.1126/science.1229159. Here the structures of the native TLR8 extracellular domain are reported, which illustrate a surprising TLR8 dimer rearrangement upon association with small chemical agonists. TLR8 is also cleaved at the “Z-loop”, the cleavage of which in TLR7 and TLR9 was shown to be essential for activation.
- 5.Ewald SE, Lee BL, Lau L, Wickliffe KE, Shi G-P, Chapman HA, Barton GM. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature. 2008;456:658–662. doi: 10.1038/nature07405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park B, Brinkmann MM, Spooner E, Lee CC, Kim Y-M, Ploegh HL. Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-like receptor 9. Nat Immunol. 2008;9:1407–1414. doi: 10.1038/ni.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sepulveda FE, Maschalidi S, Colisson R, Heslop L, Ghirelli C, Sakka E, Lennon-DumEnil A-M, Amigorena S, Cabanie L, Manoury B. Critical Role for Asparagine Endopeptidase in Endocytic Toll-like Receptor Signaling in Dendritic Cells. Immunity. 2009;31:737–748. doi: 10.1016/j.immuni.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, Paik S-G, Lee H, Lee J-O. Crystal Structure of the TLR1-TLR2 Heterodimer Induced by Binding of a Tri-Acylated Lipopeptide. Cell. 2007;130:1071–1082. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Kim HM, Park BS, Kim J-I, Kim SE, Lee J, Oh SC, Enkhbayar P, Matsushima N, Lee H, Yoo OJ, et al. Crystal Structure of the TLR4-MD-2 Complex with Bound Endotoxin Antagonist Eritoran. Cell. 2007;130:906–917. doi: 10.1016/j.cell.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Kang JY, Nan X, Jin MS, Youn S-J, Ryu YH, Mah S, Han SH, Lee H, Paik S-G, Lee J-O. Recognition of Lipopeptide Patterns by Toll-like Receptor 2-Toll-like Receptor 6 Heterodimer. Immunity. 2009;31:873–884. doi: 10.1016/j.immuni.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Park BS, Song DH, Kim HM, Choi B-S, Lee H, Lee J-O. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 12.Choe J, Kelker MS, Wilson IA. Crystal structure of human toll-like receptor 3 (TLR3) ectodomain. Science. 2005;309:581–585. doi: 10.1126/science.1115253. [DOI] [PubMed] [Google Scholar]
- 13.Bell JK, Botos I, Hall PR, Askins J, Shiloach J, Segal DM, Davies DR. The molecular structure of the Toll-like receptor 3 ligand-binding domain. Proc. Natl. Acad. Sci. U.S.A. 2005;102:10976–10980. doi: 10.1073/pnas.0505077102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14 ••.Zhou K, Kanai R, Lee P, Wang H-W, Modis Y. Toll-like receptor 5 forms asymmetric dimers in the absence of flagellin. Journal of Structural Biology. 2012;177:402–409. doi: 10.1016/j.jsb.2011.12.002. The authors report electron microscopy studies of the full-length TLR5 and docking of a homology model into the EM density. This study provides the first evidence that the full-length TLR5 receptor forms asymmetric dimers in the absence of ligand binding.
- 15.Latz E, Verma A, Visintin A, Gong M, Sirois CM, Klein DCG, Monks BG, McKnight CJ, Lamphier MS, Duprex WP, et al. Ligand-induced conformational changes allosterically activate Toll-like receptor 9. Nat Immunol. 2007;8:772–779. doi: 10.1038/ni1479. [DOI] [PubMed] [Google Scholar]
- 16.Leonard JN, Ghirlando R, Askins J, Bell JK, Margulies DH, Davies DR, Segal DM. The TLR3 signaling complex forms by cooperative receptor dimerization. Proceedings of the National Academy of Sciences. 2008;105:258–263. doi: 10.1073/pnas.0710779105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu L, Botos I, Wang Y, Leonard JN, Shiloach J, Segal DM, Davies DR. Structural basis of toll-like receptor 3 signaling with double-stranded RNA. Science. 2008;320:379–381. doi: 10.1126/science.1155406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Molecular Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 19.Atianand MK, Rathinam VA, Fitzgerald KA. SnapShot: Inflammasomes. Cell. 2013;153:272–272.e1. doi: 10.1016/j.cell.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 20 ••.Kofoed EM, Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 2011;477:592–595. doi: 10.1038/nature10394. This and the following study below report the identification of the NAIP proteins as the co-receptor for recognition of flagellin and type III secretion system by NLRC4.
- 21 ••.Zhao Y, Yang J, Shi J, Gong Y-N, Lu Q, Xu H, Liu L, Shao F. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477:596–600. doi: 10.1038/nature10510. Same as Ref. [20].
- 22.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 23.Franchi L, Amer A, Body-Malapel M, Kanneganti T-D, Özören N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 24 ••.Hu Z, Yan C, Liu P, Huang Z, RuiMa, Zhang C, Wang R, Zhang Y, FabioMartinon DHJJ, Chai J. Crystal Structure of NLRC4 Reveals Its Autoinhibition Mechanism. Science. 2013;341:172–175. doi: 10.1126/science.1236381. This is the first report on the structure of a NLR family member containing the NBD and LRR domains. The autoinhibition mechanism has been long suspected, and was elegantly demonstrated here.
- 25.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 26.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 27.Yoneyama M, Kikuchi M, Matsumoto K, Imaizumi T, Miyagishi M, Taira K, Foy E, Loo Y-M, Gale M, Akira S, et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol. 2005;175:2851–2858. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- 28.Seth RB, Sun L, Ea C-K, Chen ZJ. Identification and Characterization of MAVS, a Mitochondrial Antiviral Signaling Protein that Activates NF-κB and IRF3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Ishii KJ, Takeuchi O, Akira S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 30.Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, Tschopp J. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature Cell Biology. 2005;437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 31.Xu L-G, Wang Y-Y, Han K-J, Li L-Y, Zhai Z, Shu H-B. VISA Is an Adapter Protein Required for Virus-Triggered IFN-β Signaling. Molecular Cell. 2005;19:727–740. doi: 10.1016/j.molcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 32.Rothenfusser S, Goutagny N, DiPerna G, Gong M, Monks BG, Schoenemeyer A, Yamamoto M, Akira S, Fitzgerald KA. The RNA helicase Lgp2 inhibits TLR-independent sensing of viral replication by retinoic acid-inducible gene-I. J Immunol. 2005;175:5260–5268. doi: 10.4049/jimmunol.175.8.5260. [DOI] [PubMed] [Google Scholar]
- 33 ••.Jiang F, Ramanathan A, Miller MT, Tang G-Q, Gale M, Patel SS, Marcotrigiano J. Structural basis of RNA recognition and activation by innate immune receptor RIG-I. Nature. 2011;479:423–427. doi: 10.1038/nature10537. Reports in [33-35] illustrate structures of RIG-I in complex with dsRNA and in isolation. Structures of multiple fragments of RIG-I from different species demonstrate mechanisms of receptor autoinhibition and dsRNA recognition.
- 34 ••.Kowalinski E, Lunardi T, McCarthy AA, Louber J, Brunel J, Grigorov B, Gerlier D, Cusack S. Structural basis for the activation of innate immune pattern-recognition receptor RIG-I by viral RNA. Cell. 2011;147:423–435. doi: 10.1016/j.cell.2011.09.039. Same as Ref. [33].
- 35 ••.Luo D, Ding SC, Vela A, Kohlway A, Lindenbach BD, Pyle AM. Structural insights into RNA recognition by RIG-I. Cell. 2011;147:409–422. doi: 10.1016/j.cell.2011.09.023. Same as Ref. [33].
- 36 •.Jiang X, Kinch LN, Brautigam CA, Chen X, Du F, Grishin NV, Chen ZJ. Ubiquitin-induced oligomerization of the RNA sensors RIG-I and MDA5 activates antiviral innate immune response. Immunity. 2012;36:959–973. doi: 10.1016/j.immuni.2012.03.022. The authors report that ubiquitination induces oligomerization of RIG-I and MDA5, which is necessary for activation of antiviral signaling pathways.
- 37 ••.Hou F, Sun L, Zheng H, Skaug B, Jiang Q-X, Chen ZJ. MAVS Forms Functional Prion-like Aggregates to Activate and Propagate Antiviral Innate Immune Response. Cell. 2011 doi: 10.1016/j.cell.2011.06.041. doi:10.1016/j.cell.2011.06.041. The authors report that engagement of RIG-I and MAVS leads to conformational changes in MAVS and formation of prion-like aggregates that significantly amplify interferon production in response to viral infection.
- 38 ••.Wu B, Peisley A, Richards C, Yao H, Zeng X, Lin C, Chu F, Walz T, Hur S. Structural basis for dsRNA recognition, filament formation, and antiviral signal activation by MDA5. Cell. 2013;152:276–289. doi: 10.1016/j.cell.2012.11.048. The authors report that MDA5 assembles into helical filaments using long pieces of dsRNA as a platform. This receptor filament formation has important implications in cooperative signaling and the dsRNA length requirement for MDA5 activation.
- 39 •.Peisley A, Bin Wu, Yao H, Walz T, Hur S. RIG-I Forms Signaling-Competent Filaments in an ATP-Dependent, Ubiquitin-Independent Manner. Molecular Cell. 2013;51:1–11. doi: 10.1016/j.molcel.2013.07.024. The authors report that RIG-I can also form filaments that are dependent on ATP hydrolysis, even though there is less cooperativity.
- 40 •.Patel JR, Jain A, Chou Y-Y, Baum A, Ha T, García-Sastre A. ATPase-driven oligomerization of RIG-I on RNA allows optimal activation of type-I interferon. EMBO Rep. 2013;14:780–787. doi: 10.1038/embor.2013.102. Similar to [39], the authors demonstrate that RIG-I assembles into helical filaments that are important for activation and signaling.
- 41.Goubau D, Deddouche S, Reis e Sousa C. Cytosolic Sensing of Viruses. Immunity. 2013;38:855–869. doi: 10.1016/j.immuni.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nistal-Villan E, Gack MU, Martinez-Delgado G, Maharaj NP, Inn KS, Yang H, Wang R, Aggarwal AK, Jung JU, Garcia-Sastre A. Negative Role of RIG-I Serine 8 Phosphorylation in the Regulation of Interferon-βProduction. Journal of Biological Chemistry. 2010;285:20252–20261. doi: 10.1074/jbc.M109.089912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43 ••.Wies E, Wang MK, Maharaj NP, Chen K, Zhou S, Finberg RW, Gack MU. Dephosphorylation of the RNA Sensors RIG-I and MDA5 by the Phosphatase PP1 Is Essential for Innate Immune Signaling. Immunity. 2013;38:437–449. doi: 10.1016/j.immuni.2012.11.018. The authors report that phosphorylation is an important regulatory mechanism for RIG-I and MDA5 activities, and identify the phosphatases that dephosphorylate the receptors.
- 44.Kersse K, Verspurten J, Berghe TV, Vandenabeele P. The death-fold superfamily of homotypic interaction motifs. Trends in Biochemical Sciences. 2011;36:541–552. doi: 10.1016/j.tibs.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 45 •.Feng M, Ding Z, Xu L, Kong L, Wang W, Jiao S, Shi Z, Greene MI, Cong Y, Zhou Z. Structural and biochemical studies of RIG-I antiviral signaling. Protein Cell. 2013;4:142–154. doi: 10.1007/s13238-012-2088-4. The authors report that phosphorylation is an important mechanism in regulating RIG-I activation.
- 46.Moore CB, Bergstralh DT, Duncan JA, Lei Y, Morrison TE, Zimmermann AG, Accavitti-Loper MA, Madden VJ, Sun L, Ye Z, et al. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature. 2008;451:573–577. doi: 10.1038/nature06501. [DOI] [PubMed] [Google Scholar]
- 47 •.Lei Y, Wen H, Ting JPY. The NLR protein, NLRX1, and its partner, TUFM, reduce type I interferon, and enhance autophagy. Autophagy. 2013;9:432–433. doi: 10.4161/auto.23026. The authors report that mitochondria proteins NLRX1 and TUFM regulate autophagy and interfeon production.
- 48 •.Hong M, Yoon S-I, Wilson IA. Structure and Functional Characterization of the RNA-Binding Element of the NLRX1 Innate Immune Modulator. Immunity. 2012;36:337–347. doi: 10.1016/j.immuni.2011.12.018. Here a hexameric structure of the NLRX1 C-terminal fragment is reported, which associates with RNA to regulate ROS production.
- 49 ••.Jin T, Perry A, Jiang J, Smith P, Curry JA, Unterholzner L, Jiang Z, Horvath G, Rathinam VA, Johnstone RW, et al. Structures of the HIN domain:DNA complexes reveal ligand binding and activation mechanisms of the AIM2 inflammasome and IFI16 receptor. Immunity. 2012;36:561–571. doi: 10.1016/j.immuni.2012.02.014. The authors report the first structures of the AIM2 and IFI16 HIN domains in complex with dsDNA and demonstrate the structural basis of sequence-independent dsDNA recognition and autoinhibition mechanism for the AIM2 receptor.
- 50 •.Ru H, Ni X, Zhao L, Crowley C, Ding W, Hung L-W, Shaw N, Cheng G, Liu Z-J. Structural basis for termination of AIM2-mediated signaling by p202. Cell Res. 2013 doi: 10.1038/cr.2013.52. doi:10.1038/cr.2013.52. The authors report the structure of the p202 HINa domain in complex with dsDNA that utilizes a different binding surface compared with the AIM2 HIN domain.
- 51 ••.Yin Q, Sester DP, Tian Y, Hsiao Y-S, Lu A, Cridland JA, Sagulenko V, Thygesen SJ, Choubey D, Hornung V, et al. Molecular Mechanism for p202-Mediated Specific Inhibition of AIM2 Inflammasome Activation. Cell Reports. 2013;4:327–339. doi: 10.1016/j.celrep.2013.06.024. The authors report the structure of the p202 HINa domain in complex with dsDNA, and the tetrameric p202 HINb domain that directly associate with AIM2, which explains the specificity of the AIM2 inflammasome suppression by p202.
- 52 •.Jin T, Perry A, Smith P, Jiang J, Xiao TS. Structure of the Absent in Melanoma 2 (AIM2) Pyrin Domain Provides Insights into the Mechanisms of AIM2 Autoinhibition and Inflammasome Assembly. Journal of Biological Chemistry. 2013;288:13225–13235. doi: 10.1074/jbc.M113.468033. Here the authors report structural and biochemical evidence that the AIM2 PYD and HIN domains associate with each other in the absence of DNA binding, which supports the AIM2 autoinhibition model.
- 53.Medvedev AE, Piao W, Shoenfelt J, Rhee SH, Chen H, Basu S, Wahl LM, Fenton MJ, Vogel SN. Role of TLR4 Tyrosine Phosphorylation in Signal Transduction and Endotoxin Tolerance. Journal of Biological Chemistry. 2007;282:16042–16053. doi: 10.1074/jbc.M606781200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee K-G, Xu S, Kang Z-H, Huo J, Huang M, Liu D, Takeuchi O, Akira S, Lam K-P. Bruton’s tyrosine kinase phosphorylates Toll-like receptor 3 to initiate antiviral response. Proceedings of the National Academy of Sciences. 2012;109:5791–5796. doi: 10.1073/pnas.1119238109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55 •.Qu Y, Misaghi S, Izrael-Tomasevic A, Newton K, Gilmour LL, Lamkanfi M, Louie S, Kayagaki N, Liu J, Kömüves L, et al. Phosphorylation of NLRC4 is critical for inflammasome activation. Nature. 2012;490:539–542. doi: 10.1038/nature11429. The authors report that phosphorylation of NLRC4 S533 is an important mechanism to regulate inflammasome activation, and identify the candidate kinase as PKCδ.
- 56.Gack MU, Shin YC, Joo C-H, Urano T, Liang C, Sun L, Takeuchi O, Akira S, Chen Z, Inoue S, et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446:916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]