Abstract
Peptide loading of class II MHC molecules in endosomal compartments is regulated by HLA-DM. HLA-DO modulates HLA-DM function, with consequences for the spectrum of MHC-bound epitopes presented at the cell surface for interaction with T cells. Here, we summarize and discuss recent progress in investigating the molecular mechanisms of action of HLA-DM and HLA-DO and in understanding their roles in immune responses. Key findings are the long-awaited structures of HLA-DM in complex with its class II substrate and with HLA-DO, and observation of a novel phenotype - autoimmunity combined with immunodeficiency - in mice lacking HLA-DO. We also highlight several areas where gaps persist in our knowledge about this pair of proteins and their molecular biology and immunobiology.
Introduction
HLA-DM and HLA-DO and their murine counterparts H-2M and H-2O (generically referred to here as DM and DO) are non-peptide binding class II major histocompatibility (MHC-II) homologs. Unlike the large family of class I MHC homologs, which have varied roles in many cell types as endocytic receptors, NK ligands, T cell decoys, and presenters of peptides, lipids, and vitamin derivatives [1], for the non-classical MHC-II proteins DM and DO known roles are only in antigen-presenting cells, where they regulate loading of peptides derived from self and foreign antigens. DM functions as a peptide exchange factor required for efficient loading of endosomal peptides onto MHC-II molecules. DO functions as a modulator of DM. The molecular mechanism by which DM promotes peptide exchange and the roles of DM and DO in the overall immune response are outstanding fundamental questions in MHC biology. In the period covered by this review, significant progress has been made towards understanding the structural basis for DM interaction with MHC-II, and new work strengthens the conclusion that DM plays a key role in immunodominance. However, important mechanistic questions about DM action still remain unanswered, and this constrains our ability to integrate these advances into deeper understanding of how DM functions in development, maintenance, and activation of the CD4+ T cell response. For DO, the mechanism of action has been established: DO acts as substrate mimic to competitively inhibit HLA-DM-mediated catalysis of MHC-II peptide exchange. A key role for DO in regulating autoimmunity has been established through studies of H-2O knockout mice. However, the relationship of the molecular mechanism of DO action to its biological role still is not clear.
Insight into DM function from crystal structures of DM-DO and DM-DR
Two crystal structures of trapped DM-MHC complexes provided long-awaited insight into how DM engages MHC-II to promote peptide exchange [2, 3]. DM acts as an enzyme to catalyze peptide exchange [4, 5], and like other enzymes it binds only transiently to its substrate(s) before inducing conversion and releasing product(s). Thus, DM does not bind stably to MHC-peptide complexes [6, 7]. DM does not appear to bind to recombinant peptide-free empty MHC molecules [7], although DM binding to apparently empty MHC molecules produced in their normal cellular context has been reported [8, 9]. The discrepancy may be due to differences between metastable “peptide-receptive” species generated during peptide dissociation [9–11] and stable “peptide-averse” species produced in the absence of peptide [11, 12]. Previously, a few mutated HLA-DR-peptide complexes with weakened MHC-peptide interaction have been shown to bind to DM sufficiently tightly to be observed biochemically [6, 7, 13], but until recently all of these have resisted crystallization and detailed structural analysis. In one of the recent structure reports, Pos et al crystallized a DM-MHCII complex after covalent attachment of DM to HLA-DR1 via sortase-A mediated coupling of the DM beta-subunit C-terminus to the HLA-DR1 beta-subunit C-terminus, with the HLA-DR1 carrying a truncated peptide attached via a disulfide bond engineered into the P6 pocket [2]. The peptide was designed to bind only to the C-terminal side of the binding site, leaving the N-terminal side empty; usually such peptides bind weakly if at all, but here the interaction was stabilized through covalent bonding to the MHC. Crucially, leaving the N-terminal side of the site open allows MHC conformational alteration and stable interaction with DM. In the second of the crystal structure reports, Guce et al crystallized DM with HLA-DO [3]. In the complex, DO adopts an overall conformaton highly similar to classical MHCII proteins with an open groove, but with conformational alterations at the N-terminal side. The DO structure provides insight into the nature of αβ chain association in the MHCII family and constrains possible functional roles for DO in antigen presentation. DO was shown through enzymatic and mutagenesis studies to act as a substrate mimic, binding tightly to DM and competitively inhibiting the interaction with MHC-peptide. In the crystal structure DO was observed to bind to the same lateral face of DM as does DR, with essentially all interface residues conserved.
The DM-MHC interaction in the two structures is virtually identical (Figure 1), alleviating concerns that the protein engineering necessary to trap DM with HLA-DR might have induced a non-physiologically relevant conformation, or that DO’s mimicry of an MHC-peptide complex might not extend to structural details of its interaction with DM. A FRET study revealed a similar side-by-side arrangement for DM bound to DO in solution [14], further supporting the physiological relevance of the complex visually by X-ray crystallography.
Figure 1.

DM engages DR1 and DO similarly. A. Structure of DM bound to HLA-DR1 carrying a covalently attached peptide fragment to mimic a reaction intermediate, from PDB:4FZQ described in Pos et al [2]. B. DM bound to DO, a tight-binding competitive inhibitor that acts as a substrate mimic, from PDB:4IOP described in Guce et al [3].
The crystal structures reveal an extensive interface between DM and MHC-II involving both membrane-proximal immunoglobulin-like domains and membrane-distal helices-atop-sheet domains, generally corresponding to the lateral surfaces previously identified by mutagenesis [15, 16]. The interaction is surprisingly asymmetric, given the structural homology between DM and both HLA-DR and HLA-DO, and involves surfaces not previously observed to be involved in interactions of MHC proteins with other binding partners. Compared to crystal structures of DM alone [17–19], DM does not undergo any appreciable conformational alteration upon DR/DO binding, but HLA-DR undergoes a dramatic change in residues 35–57 of the alpha subunit (boxed in Figure 2A). This region comprises the last two strands (s3-s4) of the beta-sheet “floor” of the peptide-binding site, the short 3–10 helix at the N-terminal end of the site, and the extended strand region in the vicinity of the P1 pocket. The structure of free HLA-DO has not been determined, so whether or not it undergoes conformational change upon binding DM is not known, but HLA-DO in complex with DM assumes a conformation that is similar to the altered MHC-II-peptide structure in the DM/DR complex (Figure 2A). The conformation includes a flipped-out orientation of Trpα43, previously identified as a crucial for the DM/DR interaction [6], and occlusion of the N-terminal side of the peptide-binding site (Figure 2B). The extended strand rearrangement places a phenylalanine in the P1 pocket (Pheα51 for HLA-DR, Pheα54 for HLA-DO) and disrupts hydrogen-bonding interactions between the extended strand and the peptide main-chain atoms. Both the P1 pocket and the main-chain hydrogen bonds in this region are known to be important for stabilizing MHC-peptide interaction. Thus the structures provide a straightforward molecular mechanism for DM-catalyzed peptide exchange, in which DM binding destabilizes the MHC-peptide complex by interfering with key peptide main chain and side chain binding interactions.
Figure 2.
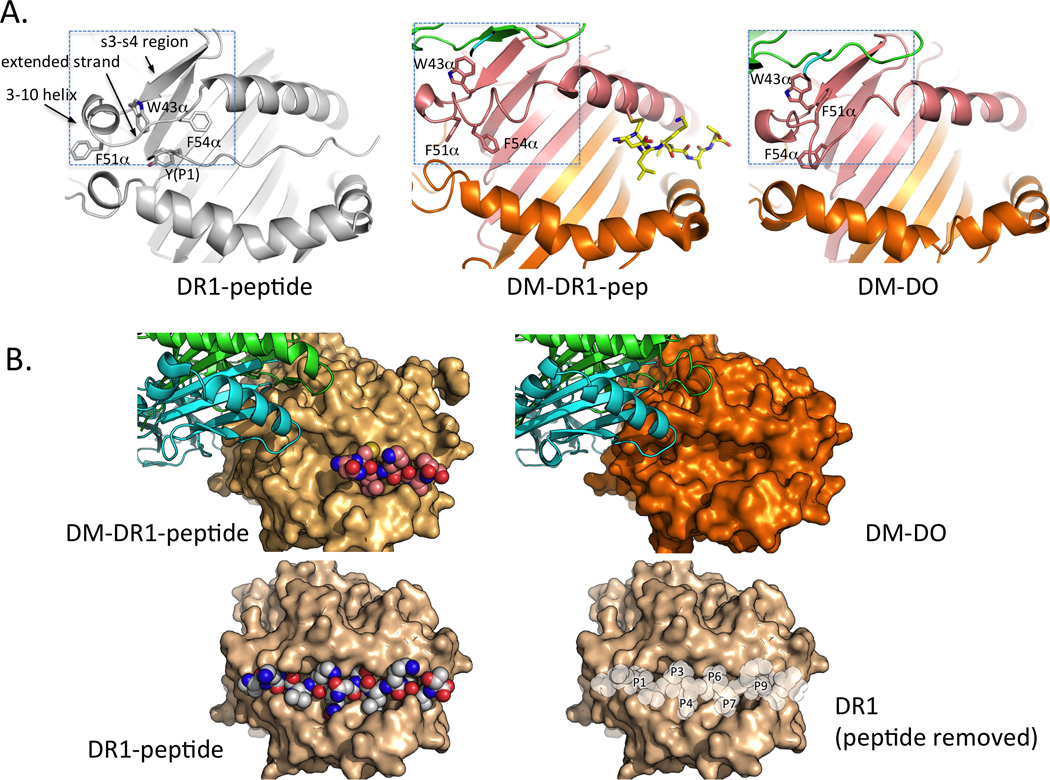
Conformational alteration in DM-DR1-peptide and DM-DO occludes the P1 regions of the MHCII peptide-binding site. A. Major structural alterations are restricted to residues 35–57 in the MHCII alpha subunit (boxed), involving strands 3 and 4 of the beta sheet platform (s3-s4 region), the MHCII 3–10 helix adjacent to the peptide binding site, and the extended strand region alongside the P1 pocket. B. These alterations sterically occlude the N-terminal side of the peptide-binding site. Locations of the peptide side-chain binding pockets are indicated after notional removal of the peptide from the structure of DR1 bound to an influenza-derived peptide.
Determinants for DM action
Much recent effort in the field has been devoted to identifying features of the MHCII-peptide complex associated with susceptibility to editing by DM. Resistance to DM editing is thought to be a key aspect of CD4+ T cell epitope selection. Yin et al. validated this idea in the context of the long-term memory response to smallpox vaccination, adding to the existing literature of studies in mice [20]. Various features of the MHC-peptide interaction were measured for HLA-DR1 and a series of peptides from the vaccinia A10L major core protein, and the dissociation lifetime in the presence of DM was found to be the best predictor of immunogenicity. Chaves et al. reviewed currently available algorithms for predicting CD4+ T cell epitopes and concluded that, while these algorithms are useful for many aspects of epitope discovery, their prediction efficiency remains poor [21]. It is likely that approaches that take into account the action of DM would have greatly improved prediction efficiency. However, the peptide sequence determinants for DM editing are not yet clear. One line of evidence implicates P1 pocket occupancy as a key feature determining DM susceptibility. For HLA-DR (and its murine counterpart I-E), the P1 pocket is the largest and most important energetically of the MHCII peptide side-chain binding pockets. The P1 pocket becomes sterically blocked in the DM-MHCII peptide complexes as a result of the DM-induced conformational change (Figure 2). Anders et al found that HLA-DR molecules carrying truncated peptides that left the P1 pocket vacant bound tightly to DM [6]. As the P1 pocket is the major determinant of peptide binding affinity, the truncated peptides bound only very weakly to DR, but could be trapped in the site by engineered disulfide bonds. In a subsequent study, Schulze et al found that that truncated peptides that retained P1 pocket occupancy were resistant to DM [22]. These considerations led Pos et al to a model whereby the ability of a peptide to make strong interactions in the HLA-DR P1 pocket is the crucial factor for resistance to DM editing [23]. For HLA-DQ (and I-A) proteins, there is not as much information on binding specificity and DM susceptibility, but the P1 pocket is smaller and less consequential energetically [24], and so factors in addition to P1 interactions might play a greater role for these proteins.
A different line of evidence implicates MHC conformational change a key feature determining DM susceptibility. Painter et al found that substitution of DRα F54 resulted in MHC-peptide complexes highly sensitive to DM editing and able to form relatively stable DM complexes even with a large anchor residue in the P1 pocket [7]. Structural analysis of the mutant protein revealed conformational changes in the alpha subunit 3–10 helix and extended strand region, smaller but in the same regions as the MHCII conformational changes observed in the DM-MHCII crystal structures. Hou et al investigated HLA-DQ2, an allele that is relatively resistant to DM editing, and found that insertion of a glycine residue at position DQα 53 in the extended strand recovered DM susceptibility [25]. HLA-DQ2 has a deletion at this position relative to most other MHCII proteins, but the deletion does not dramatically change the MHC conformation in this region [26]. How insertion of glycine at DQα 53 increases DM susceptibility is not known, but increasing conformational flexibility would appear to be a likely explanation. Using a gel mobility assay and a series of DR1-binding peptides, Ferrante et al observed two different MHC-peptide conformers populated according to peptide binding affinity, only one of which was a substrate for DM editing [27]. The peptides all shared a tyrosine at the P1 position, but differed at other, non-anchor positions. These considerations lead to a model whereby the ability of a MHC-peptide complex to adopt a new conformation is the key feature determining DM susceptibility.
Most naturally occurring MHC-peptide complexes will occupy the P1 pocket, and indeed usually the entire P-2 to P10 region. Whether the relative DM susceptibility of these complexes is related to how frequently they vacate the P1 pocket, or to how efficiently they populate a DM conformer susceptible to editing, and whether these properties are related in any straightforward way to peptide sequence, remain topics for further investigation. At present conformational properties have been investigated only for a very few HLA-DR complexes, and DM susceptibilities investigated for a somewhat larger set of peptides but almost exclusively for a very few HLA-DR alleles (primarily DRB1*0101). Thus at present there is not enough empirical data is available to resolve these important questions about the sequence and structural determinants for DM susceptibility.
Outstanding questions about the mechanism of DM action
Several important questions remain about the molecular mechanism of DM action. Despite the overall congruence of DM-DR and DM-DO structures, the actual conformation of the crucial region MHC-II α35–57 region is quite different in two structures, with either αF51 or αF54 occupying the P1 pocket (Figure 2A). These phenylalanine residues are conserved, but not invariant, among MHC-II sequences, and understanding their relative roles might bear on the relative DM susceptibilities of MHC alleles with differences at these positions. The relationship of these trapped complexes to an actual reaction intermediate is not clear, and the very basic mechanistic question of whether the DM-bound MHC-II intermediate contains one (destabilized) peptide [2, 6], two (partially bound) peptides [28, 29], or no peptide at all [3, 7], remains to be established. Besides its mechanistic importance, this issue relates to question of peptide competition for MHC-II binding, important both for understanding the factors that regulate the spectrum of peptides displayed normally by antigen presenting cells and also for guiding efforts to alter this spectrum therapeutically. Finally, none of the mechanistic studies to date have addressed the relationship between DM action and those of small molecule [30–32] and short peptide [33, 34] modulators of MHC-II peptide dissociation, which have been described as having a DM-like mechanism of action. A possible mechanism that could reconcile all these ways to promote MHC-peptide dissociation would include an aspect of facilitated dissociation by subsite occupancy [35], as recently proposed for IgE-Fcε receptor inhibitors [36]. In this mechanism, dissociation of a ligand with multiple attachment points is facilitated by blocking only one of the sites of interaction. It is possible that small molecules that occupy the a side-chain binding pocket [30–32], short peptides that occupy only part of the peptide-binding site [33, 34], and DM-induced conformational changes that block P1 pocket and/or hydrogen-bonding interactions [2, 3, 6, 7], all induce peptide dissociation by this mechanism.
Progress in elucidating the elusive role of DO in immunity
In an important paper, Gu et al report immunodeficiency and autoimmunity in H2-O knockout mice [37]. Previous biochemical and cellular work on H2-O had identified potential roles for DO in regulating antigen presentation [38–42] and in shaping the spectrum of MHC-II-bound peptides [41, 42], but only very limited immune changes were identified in knockout mice [38, 39]. Gu et al show that H2-O−/− mice spontaneously develop high titers of anti-nuclear antibodies. Despite the production of autoantibodies, T-dependent IgG antibody responses to model antigens are delayed, perhaps due to the reduced frequency of marginal zone B cells in these mice. Thus DO decreases immunity to self-antigens while increasing immunity to (at least some) foreign antigens. CD4+ T cells that developed in H2-O−/− mice were required for autoantibody production and higher ANA titers were observed when B cells also lacked H2-0. T cell receptor repertoire was altered, as reflected by greater homeostatic proliferation of H2-0−/− T cells when they were transferred into H2-0−/− (compared to H2-0+/+) hosts. The observations from these mice argue that DO’s regulation of self-peptide presentation is important in restraining peripheral activation of autoreactive CD4+ T cells, consistent with Yi et al [43]. In a separate avenue of research, Kremer et al showed that DO is required for efficient MHC-II presentation of particular human self-antigens [44]. Similarly, DO expression is generally required for high expression of class II invariant chain peptides (CLIP) presentation at the cell surface; new work from the Pezeshki et al shows that this increases binding of particular superantigens, SEA and TSST-1 [45]. The finding that HLA-DO is a competitive inhibitor of DM action would appear to indicate that DO acts simply by attenuating DM, and not, for example, by modulating DM specificity [3], although Poluektov et al have proposed an additional role for HLA-DO in direct interaction with MHC-II [46].
Gu et al suggest that if DO is acting by inhibiting DM, that inhibition should be spatially or temporally regulated to result in a qualitatively different spectrum of peptides presented on MHC-II [37], as has been reported in class II peptide elution studies of B cells with and without DO [40]. DO is relatively unstable in the absence of DM and free DO does not traffic beyond the ER [47], so most or all DO in an antigen-presenting cell is associated with DM. However, there appear to be mechanisms that result in differential steady state distribution of DM/HLA-DO complexes and free DM within the endosomal pathway in B cells, with DM/DO enriched in early endocytic compartments and free DM enriched in late endosomal/lysosomal compartments [48]. Within the latter, evidence suggests that there is differential distribution of DM and DM/DO [49]. Consistent with this prior finding, Xiu et al used B cell and HeLa cell transfectants to show that DM redistributes to the limiting membrane from the internal vesicles of lysosomal multivesicular bodies though its interaction with DO, in a process mediated by the sorting motif present in the HLA-DO beta subunit cytoplasmic tail [50]. Jahnke et al report evidence supporting ubiquitination as a direct regulator of DO intracellular localization, with additional indirect effects on endocytic machinery [51]. Thus, intracellular sorting and localization might provide the spatial regulation hypothesized by Gu et al to generate a different spectrum of self and foreign peptide antigens bound to MHC-II in the presence or absence of DO.
Role of DM in autoimmunity
Several papers have reported on a requirement for DM activity in development of type 1 diabetes. Hyperexpression of DO, with consequent reduced DM function, inhibited T1D in NOD mice [43]. Direct ablation of DM function in NOD mice, achieved by gene targeting of NOD ES cells, also blocked diabetes [52]. In the second model, reduced numbers of pathogenic CD4 T cells were observed. The expression on antigen-presenting cells of the NOD class II molecule I-Ag7 is lower in both DO-overexpressing and DM-deficient strains as compared to wild type NOD, reflecting dependence of I-Ag7 on DM chaperoning. In these models, the role of DM in autoimmunity is hypothesized to be related to peripheral presentation of DM-dependent disease-initiating peptide(s), with reduced DM activity resulting in reduced presentation and reduced disease pathology. In other studies of the role of DM in the NOD model of type 1 diabetes, In other studies of the role of DM in the NOD model of type 1 diabetes, evidence has been presented supporting a critical role in disease pathology for CD4+ T cells that recognize DM-susceptible complexes of I-A/insulin peptide, generated by capture of extracellular peptide at the cell surface or in re-cycling vesicles, locations where DM activity is low. DM is hypothesized to edit out disease-related peptide(s) during tolerance development, with reduced DM activity resulting in increased tolerance to DM-susceptible self-antigens [53]. Further, evidence has been presented supporting a critical role in disease pathology for insulin-reactive CD4+ T cells recognizing a DM-independent/susceptible epitope [54]. An integrated picture would suggest multiple roles for DM in T1D pathogenesis. Notably, the DM-resistant DQ2 allele is a well-established high risk allele for human TID, and a recent bioinformatic analysis based on GWAS data indicates a DMB polymorphism with consequences for risk for several autoimmune diseases, including T1D [55].
Concluding remarks
The work described here provided long-sought answers to questions in the class II antigen presentation field: What is the conformation of a class II transition state stabilized by DM? What is the mechanism of DO inhibition of DM? And what are (at least some of) the immunological consequences of DO deficiency? However, key issues remain. The molecular basis for the difference in stability of transient DM/DR complexes as compared to tight and essentially irreversible binding of DM/DO complexes is not clear from current structural and mutational analysis. The peptide sequence determinants of DM susceptibility are not known, and the DM susceptibility of HLA-DQ/peptide and HLA-DP/peptide complexes has been investigated in few if any cases. The contribution of DO in central tolerance has not been directly addressed. The compartments and subdomains where DM and DO localize in primary antigen presenting cells are not known, nor is the function (if any) of DM expressed at the cell surface. How do DO deficient mice handle infection? These and other questions will shape the next efforts in the field.
Highlights.
Two recent crystal structures show how DM interacts with MHCII proteins
Studies of H2-O knockout mice reveal a role for DO in regulating autoimmunity
Key questions about HLA-DM and HLA-DO function remain unanswered
Acknowledgements
Supported by NIH grants AI38996 (LJS), AI48833 (LJS) and AI095813 (EDM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, and have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Adams EJ, Luoma AM. The adaptable major histocompatibility complex (MHC) fold: structure and function of nonclassical and MHC class I-like molecules. Annu Rev Immunol. 2013;31:529–561. doi: 10.1146/annurev-immunol-032712-095912. [DOI] [PubMed] [Google Scholar]
- 2. Pos W, Sethi DK, Call MJ, Schulze MS, Anders AK, Pyrdol J, Wucherpfennig KW. Crystal structure of the HLA-DM-HLA-DR1 complex defines mechanisms for rapid peptide selection. Cell. 2012;151:1557–1568. doi: 10.1016/j.cell.2012.11.025.. Using a HLA-DR1 molecule engineered to allow stable peptide-binding to the C-terminal end of the peptide binding groove and an unoccupied P1 pocket, these authors produced a long-awaited structure of an HLA- DM/HLA-DR1 complex. The authors propose a mechanism of peptide editing by DM based the rearrangements observed in the P1 pocket of peptide binding groove.
- 3. Guce AI, Mortimer SE, Yoon T, Painter CA, Jiang W, Mellins ED, Stern LJ. HLA-DO acts as a substrate mimic to inhibit HLA-DM by a competitive mechanism. Nat Struct Mol Biol. 2013;20:90–98. doi: 10.1038/nsmb.2460.. Enzymatic studies, mutational analysis, and X-ray crystallography all show that DO binds to the same site on DM as do MHC-peptide complexes, and thus that DO acts as a competitive inhibitor. DO adopts a conformation highly similar to classical MHCII proteins with an open groove but with conformational alterations at the N-terminal side. The DO-DM complex is highly similar to that of DO-DR.
- 4.Sloan VS, Cameron P, Porter G, Gammon M, Amaya M, Mellins E, Zaller DM. Mediation by HLA-DM of dissociation of peptides from HLA-DR. Nature. 1995;375:802–806. doi: 10.1038/375802a0. [DOI] [PubMed] [Google Scholar]
- 5.Vogt AB, Kropshofer H, Moldenhauer G, Hammerling GJ. Kinetic analysis of peptide loading onto HLA-DR molecules mediated by HLA-DM. Proc Natl Acad Sci U S A. 1996;93:9724–9729. doi: 10.1073/pnas.93.18.9724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anders AK, Call MJ, Schulze MS, Fowler KD, Schubert DA, Seth NP, Sundberg EJ, Wucherpfennig KW. HLA-DM captures partially empty HLA-DR molecules for catalyzed removal of peptide. Nat Immunol. 2011;12:54–61. doi: 10.1038/ni.1967.. Using surface plasmon resonance and recombinant molecules, the authors present evidence for a long-lived complex of peptide-free HLA-DR and HLA-DM that is induced to dissociate by the binding to DR of high affinity peptide. Peptide truncation such that N-terminal hydrogen bonds and P1 anchor interactions were destroyed increased binding to DM.
- 7. Painter CA, Negroni MP, Kellersberger KA, Zavala-Ruiz Z, Evans JE, Stern LJ. Conformational lability in the class II MHC 310 helix and adjacent extended strand dictate HLA-DM susceptibility and peptide exchange. Proc Natl Acad Sci U S A. 2011;108:19329–19334. doi: 10.1073/pnas.1108074108.. The αF54C of HLA-DR1 results in a protein with increased DM affinity, weakened MHC-peptide hydrogen bonding as measured by a novel hydrogen-deuterium exchange assay, and altered conformation in the 3–10 helix and extended strand region adjacent to the P1 pocket. The authors propose a mechanism for DM editing in which DM induces conformational alterations at the N-terminal side of the binding site that promote peptide exchange.
- 8.Kropshofer H, Arndt SO, Moldenhauer G, Hammerling GJ, Vogt AB. HLA-DM acts as a molecular chaperone and rescues empty HLA-DR molecules at lysosomal pH. Immunity. 1997;6:293–302. doi: 10.1016/s1074-7613(00)80332-5. [DOI] [PubMed] [Google Scholar]
- 9.Denzin LK, Hammond C, Cresswell P. HLA-DM interactions with intermediates in HLA-DR maturation and a role for HLA-DM in stabilizing empty HLA-DR molecules. J Exp Med. 1996;184:2153–2165. doi: 10.1084/jem.184.6.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabinowitz JD, Vrljic M, Kasson PM, Liang MN, Busch R, Boniface JJ, Davis MM, McConnell HM. Formation of a highly peptide-receptive state of class II MHC. Immunity. 1998;9:699–709. doi: 10.1016/s1074-7613(00)80667-6. [DOI] [PubMed] [Google Scholar]
- 11.Joshi RV, Zarutskie JA, Stern LJ. A three-step kinetic mechanism for peptide binding to MHC class II proteins. Biochemistry. 2000;39:3751–3762. doi: 10.1021/bi9923656. [DOI] [PubMed] [Google Scholar]
- 12.Frayser M, Sato AK, Xu L, Stern LJ. Empty and peptide-loaded class II major histocompatibility complex proteins produced by expression in Escherichia coli and folding in vitro. Protein Expr Purif. 1999;15:105–114. doi: 10.1006/prep.1998.0987. [DOI] [PubMed] [Google Scholar]
- 13.Chou CL, Sadegh-Nasseri S. HLA-DM recognizes the flexible conformation of major histocompatibility complex class II. J Exp Med. 2000;192:1697–1706. doi: 10.1084/jem.192.12.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yoon T, Macmillan H, Mortimer SE, Jiang W, Rinderknecht CH, Stern LJ, Mellins ED. Mapping the HLA-DO/HLA-DM complex by FRET and mutagenesis. Proc Natl Acad Sci U S A. 2012;109:11276–11281. doi: 10.1073/pnas.1113966109.. An intermolecular FRET assay and a set of interface mutants are used to investigate the relative orientation of DO and DM in the bound complex, and DO is found to inhibit DM over a wide pH range.
- 15.Pashine A, Busch R, Belmares MP, Munning JN, Doebele RC, Buckingham M, Nolan GP, Mellins ED. Interaction of HLA-DR with an acidic face of HLA-DM disrupts sequence-dependent interactions with peptides. Immunity. 2003;19:183–192. doi: 10.1016/s1074-7613(03)00200-0. [DOI] [PubMed] [Google Scholar]
- 16.Doebele RC, Busch R, Scott HM, Pashine A, Mellins ED. Determination of the HLA-DM interaction site on HLA-DR molecules. Immunity. 2000;13:517–527. doi: 10.1016/s1074-7613(00)00051-0. [DOI] [PubMed] [Google Scholar]
- 17.Mosyak L, Zaller DM, Wiley DC. The structure of HLA-DM, the peptide exchange catalyst that loads antigen onto class II MHC molecules during antigen presentation. Immunity. 1998;9:377–383. doi: 10.1016/s1074-7613(00)80620-2. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson MJ, Moradi B, Seth NP, Xing X, Cuny GD, Stein RL, Wucherpfennig KW. Small molecules that enhance the catalytic efficiency of HLA-DM. J Immunol. 2006;176:4208–4220. doi: 10.4049/jimmunol.176.7.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fremont DH, Crawford F, Marrack P, Hendrickson WA, Kappler J. Crystal structure of mouse H2-M. Immunity. 1998;9:385–393. doi: 10.1016/s1074-7613(00)80621-4. [DOI] [PubMed] [Google Scholar]
- 20.Yin L, Calvo-Calle JM, Dominguez-Amorocho O, Stern LJ. HLA-DM constrains epitope selection in the human CD4 T cell response to vaccinia virus by favoring the presentation of peptides with longer HLA-DM-mediated half-lives. J Immunol. 2012;189:3983–3994. doi: 10.4049/jimmunol.1200626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaves FA, Lee AH, Nayak JL, Richards KA, Sant AJ. The utility and limitations of current Web-available algorithms to predict peptides recognized by CD4 T cells in response to pathogen infection. J Immunol. 2012;188:4235–4248. doi: 10.4049/jimmunol.1103640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulze MS, Anders AK, Sethi DK, Call MJ. Disruption of hydrogen bonds between major histocompatibility complex class II and the peptide N-terminus is not sufficient to form a human leukocyte antigen-DM receptive state of major histocompatibility complex class II. PLoS One. 2013;8:e69228. doi: 10.1371/journal.pone.0069228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pos W, Sethi DK, Wucherpfennig KW. Mechanisms of peptide repertoire selection by HLA-DM. Trends Immunol. 2013;34:495–501. doi: 10.1016/j.it.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sidney J, Steen A, Moore C, Ngo S, Chung J, Peters B, Sette A. Divergent motifs but overlapping binding repertoires of six HLA-DQ molecules frequently expressed in the worldwide human population. J Immunol. 2010;185:4189–4198. doi: 10.4049/jimmunol.1001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou T, Macmillan H, Chen Z, Keech CL, Jin X, Sidney J, Strohman M, Yoon T, Mellins ED. An insertion mutant in DQA1*0501 restores susceptibility to HLA-DM: implications for disease associations. J Immunol. 2011;187:2442–2452. doi: 10.4049/jimmunol.1100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim CY, Quarsten H, Bergseng E, Khosla C, Sollid LM. Structural basis for HLA-DQ2-mediated presentation of gluten epitopes in celiac disease. Proc Natl Acad Sci U S A. 2004;101:4175–4179. doi: 10.1073/pnas.0306885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ferrante A, Gorski J. A Peptide/MHCII conformer generated in the presence of exchange peptide is substrate for HLA-DM editing. Sci Rep. 2012;2:386. doi: 10.1038/srep00386.. A gel mobility assay is used to show that individual DR-peptide complexes can adopt two conformers, of which only one interacts with DM. The authors proposal that peptides induce a conformational state of the MHCII-peptide complex permissive for DM editing, with ability to form this state proportional to peptide Kd.
- 28.Ferrante A, Anderson MW, Klug CS, Gorski J. HLA-DM mediates epitope selection by a "compare-exchange" mechanism when a potential peptide pool is available. PLoS One. 2008;3:e3722. doi: 10.1371/journal.pone.0003722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tampe R, Clark BR, McConnell HM. Energy transfer between two peptides bound to one MHC class II molecule. Science. 1991;254:87–89. doi: 10.1126/science.1656526. [DOI] [PubMed] [Google Scholar]
- 30.Call MJ, Xing X, Cuny GD, Seth NP, Altmann DM, Fugger L, Krogsgaard M, Stein RL, Wucherpfennig KW. In vivo enhancement of peptide display by MHC class II molecules with small molecule catalysts of peptide exchange. J Immunol. 2009;182:6342–6352. doi: 10.4049/jimmunol.0803464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marin-Esteban V, Falk K, Rotzschke O. "Chemical analogues" of HLA-DM can induce a peptide-receptive state in HLA-DR molecules. J Biol Chem. 2004;279:50684–50690. doi: 10.1074/jbc.M407598200. [DOI] [PubMed] [Google Scholar]
- 32.Hopner S, Dickhaut K, Hofstatter M, Kramer H, Ruckerl D, Soderhall JA, Gupta S, Marin-Esteban V, Kuhne R, Freund C, et al. Small organic compounds enhance antigen loading of class II major histocompatibility complex proteins by targeting the polymorphic P1 pocket. J Biol Chem. 2006;281:38535–38542. doi: 10.1074/jbc.M606437200. [DOI] [PubMed] [Google Scholar]
- 33.Gupta S, Hopner S, Rupp B, Gunther S, Dickhaut K, Agarwal N, Cardoso MC, Kuhne R, Wiesmuller KH, Jung G, et al. Anchor side chains of short peptide fragments trigger ligand-exchange of class II MHC molecules. PLoS One. 2008;3:e1814. doi: 10.1371/journal.pone.0001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chou CL, Mirshahidi S, Su KW, Kim A, Narayan K, Khoruzhenko S, Xu M, Sadegh-Nasseri S. Short peptide sequences mimic HLA-DM functions. Mol Immunol. 2008;45:1935–1943. doi: 10.1016/j.molimm.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 35.Prinz H, Striessnig J. Ligand-induced accelerated dissociation of (+)-cis-diltiazem from L-type Ca2+ channels is simply explained by competition for individual attachment points. J Biol Chem. 1993;268:18580–18585. [PubMed] [Google Scholar]
- 36.Kim B, Eggel A, Tarchevskaya SS, Vogel M, Prinz H, Jardetzky TS. Accelerated disassembly of IgE-receptor complexes by a disruptive macromolecular inhibitor. Nature. 2012;491:613–617. doi: 10.1038/nature11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gu Y, Jensen PE, Chen X. Immunodeficiency and autoimmunity in H2-O-deficient mice. J Immunol. 2013;190:126–137. doi: 10.4049/jimmunol.1200993.. In the first evidence for striking phenotype in HLA-DO deficient mice, older H2-0−/− mice are shown to spontaneously develop high titer anti-nuclear autoantibodies, but delayed generation of antibody repsonses to model antigens. Development of autoimmune phenotype required CD4+ T cells that developed in the H2-0−/− mice.
- 38.Liljedahl M, Winqvist O, Surh CD, Wong P, Ngo K, Teyton L, Peterson PA, Brunmark A, Rudensky AY, Fung-Leung WP, et al. Altered antigen presentation in mice lacking H2-O. Immunity. 1998;8:233–243. doi: 10.1016/s1074-7613(00)80475-6. [DOI] [PubMed] [Google Scholar]
- 39.Alfonso C, Williams GS, Karlsson L. H2-O influence on antigen presentation in H2-E-expressing mice. Eur J Immunol. 2003;33:2014–2021. doi: 10.1002/eji.200323853. [DOI] [PubMed] [Google Scholar]
- 40.Alfonso C, Williams GS, Han JO, Westberg JA, Winqvist O, Karlsson L. Analysis of H2-O influence on antigen presentation by B cells. J Immunol. 2003;171:2331–2337. doi: 10.4049/jimmunol.171.5.2331. [DOI] [PubMed] [Google Scholar]
- 41.van Ham SM, Tjin EP, Lillemeier BF, Gruneberg U, van Meijgaarden KE, Pastoors L, Verwoerd D, Tulp A, Canas B, Rahman D, et al. HLA-DO is a negative modulator of HLA-DM-mediated MHC class II peptide loading. Curr Biol. 1997;7:950–957. doi: 10.1016/s0960-9822(06)00414-3. [DOI] [PubMed] [Google Scholar]
- 42.Perraudeau M, Taylor PR, Stauss HJ, Lindstedt R, Bygrave AE, Pappin DJ, Ellmerich S, Whitten A, Rahman D, Canas B, et al. Altered major histocompatibility complex class II peptide loading in H2-O-deficient mice. Eur J Immunol. 2000;30:2871–2880. doi: 10.1002/1521-4141(200010)30:10<2871::AID-IMMU2871>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 43.Yi W, Seth NP, Martillotti T, Wucherpfennig KW, Sant'Angelo DB, Denzin LK. Targeted regulation of self-peptide presentation prevents type I diabetes in mice without disrupting general immunocompetence. J Clin Invest. 2010;120:1324–1336. doi: 10.1172/JCI40220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kremer AN, van der Meijden ED, Honders MW, Goeman JJ, Wiertz EJ, Falkenburg JH, Griffioen M. Endogenous HLA class II epitopes that are immunogenic in vivo show distinct behavior toward HLA-DM and its natural inhibitor HLA-DO. Blood. 2012;120:3246–3255. doi: 10.1182/blood-2011-12-399311. [DOI] [PubMed] [Google Scholar]
- 45.Pezeshki AM, Azar GA, Mourad W, Routy JP, Boulassel MR, Denzin LK, Thibodeau J. HLA-DO increases bacterial superantigen binding to human MHC molecules by inhibiting dissociation of class II-associated invariant chain peptides. Hum Immunol. 2013;74:1280–1287. doi: 10.1016/j.humimm.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 46.Poluektov YO, Kim A, Hartman IZ, Sadegh-Nasseri S. HLA-DO as the optimizer of epitope selection for MHC class II antigen presentation. PLoS One. 2013;8:e71228. doi: 10.1371/journal.pone.0071228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deshaies F, Brunet A, Diallo DA, Denzin LK, Samaan A, Thibodeau J. A point mutation in the groove of HLA-DO allows egress from the endoplasmic reticulum independent of HLA-DM. Proc Natl Acad Sci U S A. 2005;102:6443–6448. doi: 10.1073/pnas.0500853102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gondre-Lewis TA, Moquin AE, Drake JR. Prolonged antigen persistence within nonterminal late endocytic compartments of antigen-specific B lymphocytes. J Immunol. 2001;166:6657–6664. doi: 10.4049/jimmunol.166.11.6657. [DOI] [PubMed] [Google Scholar]
- 49.Zwart W, Griekspoor A, Kuijl C, Marsman M, van Rheenen J, Janssen H, Calafat J, van Ham M, Janssen L, van Lith M, et al. Spatial separation of HLA-DM/HLA-DR interactions within MIIC and phagosome-induced immune escape. Immunity. 2005;22:221–233. doi: 10.1016/j.immuni.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 50.Xiu F, Cote MH, Bourgeois-Daigneault MC, Brunet A, Gauvreau ME, Shaw A, Thibodeau J. Cutting edge: HLA-DO impairs the incorporation of HLA-DM into exosomes. J Immunol. 2011;187:1547–1551. doi: 10.4049/jimmunol.1100199. [DOI] [PubMed] [Google Scholar]
- 51.Jahnke M, Trowsdale J, Kelly AP. Ubiquitination of HLA-DO by MARCH family E3 ligases. Eur J Immunol. 2013;43:1153–1161. doi: 10.1002/eji.201243043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Morgan MA, Muller PS, Mould A, Newland SA, Nichols J, Robertson EJ, Cooke A, Bikoff EK. The nonconventional MHC class II molecule DM governs diabetes susceptibility in NOD mice. PLoS One. 2013;8:e56738. doi: 10.1371/journal.pone.0056738.. DM-deficient NOD mice were found to be completely protected against type 1 diabetes. A significantly increased proportion of Treg cells was found, but depletion of these cells did not unmask pathogenic potential in the effector T cell population.
- 53.Mohan JF, Unanue ER. Antigen presentation events in autoimmune diabetes. Nat Rev Immunol. 2012;12:721–728. doi: 10.1038/nri3294. [DOI] [PubMed] [Google Scholar]
- 54.Mohan JF, Levisetti MG, Calderon B, Herzog JW, Petzold SJ, Unanue ER. Unique autoreactive T cells recognize insulin peptides generated within the islets of Langerhans in autoimmune diabetes. Nat Immunol. 2010;11:350–354. doi: 10.1038/ni.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sirota M, Schaub MA, Batzoglou S, Robinson WH, Butte AJ. Autoimmune disease classification by inverse association with SNP alleles. PLoS Genet. 2009;5:e1000792. doi: 10.1371/journal.pgen.1000792. [DOI] [PMC free article] [PubMed] [Google Scholar]


