Abstract
Background
While divided attention tasks are recognized as predictors of falls in older adults, a comprehensive examination of this association is lacking.
Objective
We examined the validity of a ‘walking while talking (WWT)’ task for predicting falls.
Methods
We studied the associations of eight selected gait markers measured during WWT (individually as well as domains derived by factor analysis) with incident falls in 646 adults (mean age 79.9, 61% women) enrolled in an aging study who received quantitative gait assessments. Cox regressions adjusted for multiple potential confounders and normal pace walking were used to examine the associations.
Results
Over a mean follow-up of 2.6 years, 337 participants (52%) fell. Step length was the only individual WWT parameter that predicted falls (HR 0.98, p=0.034). Factor analysis identified three gait domains, of which only the pace factor predicted falls (HR 1.31, p=0.002). Results remained robust after adjusting for multiple potential confounders and accounting for normal pace walking.
Conclusions
WWT performance was a significant predictor of falls. Gait domains in WWT should be further studied to improve current fall risk assessments and to develop new interventions.
Keywords: Falls, walking, talking, divided attention, older adults, epidemiology, cognition, cohort
Introduction
Lundin-Olsson and colleagues [1] observed that nursing home residents who stopped walking when talking were at higher risk of falls. This seminal observation has since spurred exploration of dual-task methods such as the ‘walking while talking (WWT)’ test to understand fall risk by studying individuals as they walk while simultaneously conducting a cognitively demanding task. The WWT paradigm affords the opportunity to manipulate attention demands and measure the effect of taxing the attention system on gait performance. The decrement in gait performance during WWT is considered a measure of dual-task cost that arises from the two tasks interfering with each other and competing for the same brain resources [2, 3].
A systematic review by Beauchet and colleagues [4] concluded that dual-tasks were strong predictors of falls. However, four of the 15 studies reviewed had negative results [4]. Methodological limitations of previous studies identified in this review included small samples, limited follow-up, and lack of standardization for dual-task procedures [4, 5]. Moreover, most studies only report changes in gait velocity, though other gait variables might predict falls. For instance, we reported that stride length variability during normal pace walking was a stronger predictor of falls than velocity [6]. Beauchet and colleagues stressed the need for well-designed, prospective population based studies with large sample sizes to improve the predictive validity of current dual-task based fall assessments tests.[4]
To address some of the limitations of previous dual-task based fall assessment studies, we examined the validity of a WWT-task for predicting falls in 646 community-dwelling older adults. Establishing the role of WWT in falls has potential to improve risk assessments and provide mechanistic insights to guide development of new fall interventions.
Methods
Study population
The Einstein Aging Study (EAS) is a prospective cohort study of community-dwelling residents of Bronx County. The primary aim of the EAS was to identify risk factors for dementia [7]. Study design has been reported [8, 9]. Potential participants (age 70+) identified from population lists of Bronx County were contacted first by letter, then telephone explaining the purpose and nature of the study. Telephone interviews included verbal consent, medical history and cognitive screeners. Exclusion criteria included severe auditory or visual loss, inability to ambulate, and institutionalization. Participants returned annually for clinical, cognitive, and mobility assessments, and were contacted by telephone every 2-3 months to assess function and falls. Informed consent was obtained at enrollment according to protocols approved by the Einstein Institutional Review Board.
Gait assessment
Quantitative gait studies were conducted at baseline using a computerized walkway (180×35.5×0.25 inches) with embedded pressure sensors (GAITRite; CIR Systems, PA) in a quiet well-let hallway. Participants walked on the mat at their normal pace while computer software recorded gait variables as the mean of two trials. To account for initial acceleration and terminal deceleration, data collection started and stopped 3 feet from either end of the walkway edge. The GAITRite system is widely used and has excellent reliability [5, 6, 10].
Walking While Talking
Subjects walked on the mat, as described above, while reciting alternate letters of the alphabet. They were instructed to pay equal attention to walking and talking [3]. To reduce learning effects subjects were randomly assigned to start with the letter “A” or “B.” The number of errors and correctly recited alternate letters were recorded. Testers intervened only if subject safety was an issue. Based on our and other locomotion and falls studies [6, 11, 12] we selected the following eight gait variables: velocity (cm/s), cadence (step/min), step length (cm), swing (percent), stance (percent), double support (percent), step time variability (standard deviation (SD)), and swing time variability (SD).
We have reported high reliability of our current WWT protocol [3] and its association with cognitive processes [13, 14] relevant to dual-tasking. In a preliminary study of WWT, using a previous version of the protocol without quantitative gait assessments, we reported that velocity during WWT predicted falls [9].
Falls
Falls were defined as unintentionally coming down to the floor or a lower level not due to a major intrinsic or extrinsic event [15]. Participants were interviewed about occurrence of falls at baseline, and by telephone every 2-3 months [6]. They were also asked about falls in the prior year during annual clinic visits. Consistency between participant report of falls on the phone and in-person interviews was reported to be highly reliable in our previous study; all participants who reported a fall at six months on the phone recalled the fall at the subsequent in-person interview [9].
Covariates
Presence or absence of depression, diabetes, heart failure, hypertension, angina, myocardial infarction, strokes, Parkinson's disease, chronic obstructive lung disease, and arthritis was used to calculate a summary illness index [6]. Activities of daily living were assessed using a disability scale for community-based cohorts [16]. General cognitive status was assessed by the Blessed Information-Memory-Concentration test [17]. Depression was assessed using the 15-item Geriatric Depression Scale (GDS) [18]. Visual acuity was measured with Snellen's chart (<1/200 low acuity). Clinical gait abnormalities were diagnosed by visual inspection of walking patterns by study clinicians, and have been reported to predict falls in our cohort [8, 19]. Balance and lower extremity strength was assessed by time taken to rise from a chair five times unassisted [20]. Unipedal stance time was recorded as the time balanced on one foot without support [21].
Statistical Analysis
Baseline characteristics of fallers and non-fallers were compared with descriptive statistics. Cox proportional hazards models were used to compute hazard ratios (HR) with 95% confidence intervals (CI) to predict incident falls based on baseline WWT performance on the eight selected parameters. Quantitative gait variables are highly correlated and independent effects are difficult to discern. Hence, the principal component method was used to conduct an orthogonal varimax rotated factor analysis of the eight quantitative gait variables to derive statistically independent gait domains [6, 11, 12, 22]. The time scale was follow-up time (days) to incident fall or final contact. All models were adjusted for age, sex, and education. Further adjustments included illnesses index, prescription medication count, Blessed score, GDS score, chair rise time, falls in the year prior to enrollment, and presence of gait abnormalities. Variables included in the models were identified as fall risk markers in bivariate analysis and in our prior study [6].
We reported that quantitative gait variables during normal pace walking condition predict falls [6]. Hence, to examine the incremental validity of WWT for falls we conducted sensitivity analyses accounting for normal walking velocity and clinical gait status in the fully adjusted model. Additionally, we adjusted for normal walking stride length variability as it has previously been reported to be the best predictor of falls [6].
Proportional hazards assumptions of all models were tested graphically and analytically, and were adequately met. All analyses were conducted using SPSS version 20 (SPSS Inc., Chicago, IL).
Results
Study population
This study began in September 2004 when we started systematically ascertaining falls in our cohort, and ended November 2012. Of the 972 EAS participants seen during this period, 131 had no fall assessments, 141 had no WWT assessments (tester or equipment unavailability, refusals, or inability to ambulate) and 8 had incomplete WWT. Of the 692 participants with baseline WWT and fall follow-up, we excluded 46 with significant cognitive impairment (Blessed scores>6) to minimize recall bias [23]. The 646 participants completed one or more follow-up telephone interviews (100% compliance). Over a mean study follow-up period of 638.3 days (range 17-2,604), 337 (52%) participants reported an incident fall. Mean time to fall was 479.4 days (range 17-2,192).
The 337 subjects who fell were older (p=0.002) and had higher baseline prevalence of previous falls (p<0.001) compared to the 309 non-fallers (Table 1). Fallers and non-fallers did not differ significantly in other descriptive variables.
Table 1. Comparison of Baseline Variables in Subjects with and without Fallsa.
| Variable | Non-Fallers (n=309) | Fallers (n=337) | P-value |
|---|---|---|---|
| Age (years) | 79.2±5.5 | 80.5±5.4 | 0.002* |
| Female, n(%) | 176(57%) | 219(65%) | 0.054 |
| Education (years) | 14.1±3.1 | 14.4±3.2 | 0.282 |
| Illness Index (range 0-10) | 1.17±1.1 | 1.16±95 | 0.980 |
| Medication Number | 3.3±2.8 | 3.3±2.6 | 0.536^ |
| Fall in Past Year, n(%) | 40(13) | 138(40) | <0.001* |
| Disability score (0-14) | 0.81±1.3 | 0.85±1.2 | 0.333^ |
| GDS score (range 0-15) | 2.1±2.3 | 2.2±2.1 | 0.176^ |
| Blessed Score (range 0-32) | 1.7±1.6 | 1.7±1.5 | 0.860^ |
| Visual acuity <1/200, n(%) | 50(18) | 69(21) | 0.378 |
| Clinical gait abnormality, n(%) | 118(39) | 131(39) | 0.947 |
| Repeat chair (s) | 13.8±4.8 | 13.7±4.4 | 0.838 |
| Unipedal Stance (s) | 8.7±8.8 | 7.9±7.8 | 0.406^ |
| Normal walking velocity (cm/s) | 95.8±22.6 | 94.3±22.7 | 0.382 |
Values are mean±SD unless otherwise specified
Mann-Whitney U Test
Significant p-values
Individual Gait Variables
Step length was the only WWT parameter that predicted falls (Table 2) with fallers having significantly shorter steps than non-fallers (mean difference=1.89 cm, p=.042). The number of letters recited (HR 1.010, p=0.318) and errors during WWT (HR 1.003, p=0.879) did not predict falls
Table 2. Individual WWT Gait Variables and Fall Risk.
| WWT Gait Variables | HRa (95% CI) | P-value |
|---|---|---|
| Velocity (cm/s) | 0.997(0.99-1.00) | 0.162 |
| Cadence (step/min) | 0.999(0.99-1.00) | 0.711 |
| Step Length (cm) | 0.989(0.98-0.99) | 0.034 |
| Swing (%) | 1.003(0.98-1.03) | 0.763 |
| Stance (%) | 0.997(0.98-1.02) | 0.776 |
| Double Support (%) | 0.994(0.98-1.01) | 0.344 |
| Step Time SD (s) | 0.879(0.57-1.35) | 0.554 |
| Swing Time SD (s) | 1.049(0.60-1.83) | 0.867 |
Values adjusted for Gender, Age, Education
WWT Gait Domains
Factor analysis of eight WWT gait variables resulted in three independent factors accounting for 86.7% of variance (Table 3). The first factor, denoted “Rhythm,” loaded heavily in swing (percent), double support (percent), and stance (percent), and accounted for the largest variance (34.9%). The “Variability” factor accounted for 31.3% of variance with swing time SD, and step time SD loading highest. The “Pace” factor accounted for 20.6% of variance and loaded highest in velocity and step length. The factors were similar to those reported for normal pace walking by us and other investigators [6, 11].
Table 3. Factor Analysis of Eight WWT Gait Variablesa.
| Gait Variables | Rhythm Factor | Variability Factor | Pace Factor |
|---|---|---|---|
| Swing (%) | .967 | .-.020 | .195 |
| Double Support (%) | -.800 | .182 | -.328 |
| Stance (%) | -.967 | .020 | -.194 |
| Swing Time SD (s) | .131 | .913 | -.078 |
| Step Time SD (s) | -.131 | .925 | -.023 |
| Cadence (step/min) | .248 | -.714 | .334 |
| Velocity (cm/s) | .312 | -.516 | .742 |
| Step Length (cm) | .306 | -.029 | .888 |
| Variance Explained (%) | 34.90 | 31.25 | 20.58 |
Highest loading variables in bold
“Pace” was the only factor that predicted incident falls in both models (Table 4). For each one SD decrement in the “Pace” factor, there was a 31% increased hazard of falling in Model 2 (HR 1.312, p=0.002). Exclusion of participants with diagnosis of Parkinson's disease (n=3) and depression (GDS>10) (n=5) did not materially change the association of the Pace factor with falls (HR 1.30, p = 0.002).
Table 4. WWT Gait Domains and Fall Risk.
| Factors | Model 1a | Model 2b | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Rhythm Factor | 0.917(0.81-1.04) | 0.161 | 0.960(0.84-1.09) | 0.540 |
|
| ||||
| Variability Factor | 1.041(0.92-1.18) | 0.539 | 1.044(0.91-1.19) | 0.537 |
|
| ||||
| Pace Factor | 1.174(1.04-1.33) | 0.011 | 1.312(1.11-1.55) | 0.002 |
Adjusted for Gender, Age, Education
Adjusted for Gender, Age, Education, Illness Index, Prescription Medicines, GDS, Blessed Score, Chair Rise test, Clinical gait abnormalities, Falls last year, Normal velocity (cm/s), and Normal stride length variability (cm)
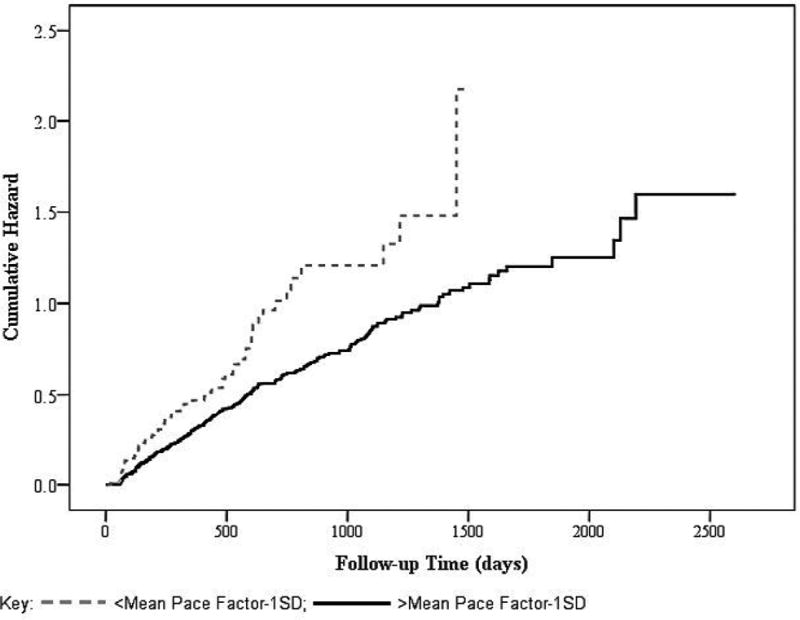
Kaplan-Meier curves for risk of falls by WWT “Pace” factor scores are presented in Figure 1. We dichotomized the “Pace” factor to compare the fall risk of slowest 14% with the rest of the sample. The category with poorer gait performance in the WWT “Pace” domain shows higher risk of falls. In addition, we examined fall risk of the fastest 14% in the WWT “Pace” domain. Results indicated significantly less fall risk (HR .685 95% CI .489-.960, p=.028) for this group in comparison to the rest of the sample.
Figure 1. Kaplan-Meier Plots of Falls.
Influence of Normal Pace Walking
Dual-task costs measured as change in gait velocity between dual-task and single-task conditions (HR 1.00, p=0.412) did not predict falls. The “Pace” factor remained a significant predictor of falls even after adjusting for normal walking velocity (HR 1.297, p=0.002) and stride length variability (HR 1.312, p=0.002).
Discussion
We investigated the association between WWT and falls in a prospective cohort of 646 community-dwelling older adults. To obtain a comprehensive picture of the complex relationship between WWT and falls we report both individual gait measures and gait domains. In contrast to previous studies, we identified step length, but not velocity or other variables, as the sole gait marker to predict falls in our community residing elderly cohort. Our results also indicate that the WWT “Pace” factor significantly predicted falls, and remained a robust predictor after adjusting for multiple potential confounders [24] as well as baseline normal walking velocity and stride length variability. Step length was the only independent gait variable that predicted falls; however, the “Pace” domain, which was primarily comprised of velocity and step length, strengthened the effect of the association. Moreover, supplemental analyses comparing groups in the fastest and slowest 14% of the WWT “Pace” domain to the rest of the sample illustrated additional support for the predictive validity of slow gait, derived from factor analysis, for predicting falls in this cohort.
To our knowledge, this is the largest study of the predictive validity of WWT as a predictor of fall that has been conducted to date in a prospective cohort. Moreover, as recommended in a recent review article by Beauchet and colleagues[4], assessments were conducted using a standardized and previously validated dual-task test[9] and results accounted for multiple confounding variables including cognition and health status as well as single-task walking variables to improve the predictive validity of WWT.[4]
Unlike our study, most previous dual-task and falls studies were conducted in smaller or select samples, had limited follow-up, lacked standardized protocols, or did not account for single-task walking [4]. Aside from a study by Kressig and colleagues which reported that higher stride time variability measured during a dual-task increased fall risk for elderly inpatients [5], exploration of dual-task gait variables other than speed to predict falls is limited. Velocity was only significant in our study as a fall predictor as a contributor to the “Pace” domain, supporting the incremental contribution of the factor analysis approach. Our study also indicated that dual-task costs, measured by gait slowing between WWT and normal pace walking, did not predict falls. These methodological differences may help explain discrepant results in some studies that reported gait slowing as the only measure of WWT [4]. Two recent publications by Lord et al.,[11] and Holloman et al.,[12] that were influenced by our novel application of factor analysis to studying gait[6, 13] support the utility of this statistical approach to capture the multidimensional characteristics of gait. All variables included in the factor analysis contribute to the final factors that are derived; but the magnitude of their contribution to the different factors will vary. Identifying domains of gait through the factor analysis is a more powerful and sensitive approach for understanding potential underlying motor, cognitive, or behavioral disturbances. Hence, it is appropriate to study the factors separately from the individual variables.
Our findings in the current and previous studies of the same cohort [6, 9] highlight differences between single and dual-task conditions with respect to predictive validity of individual gait variables and domains for falls. For example, step length was the only individual WWT gait variable that predicted falls, while, velocity, stride length, double support percent, stride length variability, and swing time variability during normal walking were reported to predict falls in the same cohort [6]. The “Pace” factor accounts for the greatest variance in normal pace walking [11, 22] but the least in the dual-task condition. Studies of single-task normal walking in the same cohort [6] showed that “Variability” and “Rhythm” factors rather than “Pace” predicted falls. Moreover, after accounting for baseline normal walking velocity and stride length variability “Pace” remained a robust predictor of falls.
The ability to recite alternate letters of the alphabet while walking has been shown to involve similar cortical regions as gait.[25] Specifically, attention, executive function, and memory regions of the prefrontal cortex are involved in completing both tasks; [22] therefore, the dual-task paradigm could reveal early indicators of dysfunctions in particular brain regions.[25, 26] Different brain processes and substrates have been correlated with WWT-like tasks and normal walking [22, 27-29]. Doi and colleagues [27] found an association between dual-task costs in trunk instability and brain atrophy, suggesting trunk instability as a marker of brain volume decline. Change in gait speed between single and dual-tasks was greater in participants with decreased cortical volume of the primary motor cortex in adults with mild cognitive impairment [28]. Cognitively impaired adults with greater subcortical hyperintensity severity have also been shown to perform worse in dual-tasks compared to normal controls [29]. These findings suggest that dual and single-task walking assessments may tap into different fall mechanisms [11, 12]. Intact gait control requires the efficient integration of many neural systems and cognitive processes, such as memory, attention, and executive function.[2, 26, 30] Therefore, gait dysfunction, particularly during the WWT protocol could be a potential early indicator of cognitive impairments.[31]
Our study included community-residing older adults, and may not be generalizable to those who are institutionalized or with cognitive impairments. Older patients with Mild Cognitive Impairment (MCI) syndrome have impaired performance on dual tasks as well as higher risk of falls.[32, 33] Though we lacked a prospective MCI diagnosis procedure, we accounted for this possibility by excluding participants with dementia and significant cognitive impairment as well as adjusting our analyses for baseline Blessed scores. Lack of power analyses has been noted as a limitation of previous dual-task studies [4]. Post-hoc power analysis indicated that we had 82% power to examine WWT and fall associations. While reduced recall of falls is a potential problem [23], we reduced bias by excluding participants with dementia, monitoring falls at short intervals, and through high follow-up interview completion rates.
In conclusion, our findings support the predictive validity of WWT-tasks for falls in older adults, and demonstrate the incremental validity of dual-task over single-task walking assessments. While the WWT individual variables and domains require quantitative methods, they provide valuable insights into improving current risk assessments and developing new therapeutic options. For instance, dual-task performance has been reported to be improved by training even in frail or cognitively impaired older adults [34, 35] and could be studied further as a novel falls prevention strategy.
Acknowledgments
Funding: National Institute on Aging (AG03949, PI: RB Lipton).
Grant support funding for this project was provided by the National Institute on Aging (AG03949, PI: RB Lipton).
Footnotes
Disclosure of conflict of interest: None.
References
- 1.Lundin-Olsson L, Nyberg L, Gustafson Y. “Stops walking when talking” as a predictor of falls in elderly people. The Lancet. 1997;349(9052):617. doi: 10.1016/S0140-6736(97)24009-2. [DOI] [PubMed] [Google Scholar]
- 2.Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc. 2012;60(11):2127–36. doi: 10.1111/j.1532-5415.2012.04209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verghese J, Kuslansky G, Holtzer R, Katz M, Xue XN, Buschke H, et al. Walking while talking: Effect of task prioritization in the elderly. Arch Phys Med Rehabil. 2007 Jan;88(1):50–3. doi: 10.1016/j.apmr.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beauchet O, Annweiler C, Dubost V, Allali G, Kressig R, Bridenbaugh S, et al. Stops walking when talking: a predictor of falls in older adults? European Journal of Neurology. 2009;16(7):786–95. doi: 10.1111/j.1468-1331.2009.02612.x. [DOI] [PubMed] [Google Scholar]
- 5.Kressig RW, Herrmann FR, Grandjean R, Michel JP, Beauchet O. Gait variability while dual-tasking: fall predictor in older inpatients? Aging clinical and experimental research. 2008 Apr;20(2):123–30. doi: 10.1007/BF03324758. [DOI] [PubMed] [Google Scholar]
- 6.Verghese J, Holtzer R, Lipton RB, Wang C. Quantitative gait markers and incident fall risk in older adults. J Gerontol A Biol Sci Med Sci. 2009 Aug;64(8):896–901. doi: 10.1093/gerona/glp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipton RB, Katz MJ, Kuslansky G, Sliwinski MJ, Stewart WF, Verghese J, et al. Screening for dementia by telephone using the memory impairment screen. J Am Geriatr Soc. 2003 Oct;51(10):1382–90. doi: 10.1046/j.1532-5415.2003.51455.x. [DOI] [PubMed] [Google Scholar]
- 8.Verghese J, LeValley A, Hall CB, Katz MJ, Ambrose AF, Lipton RB. Epidemiology of gait disorders in community-residing older adults. J Am Geriatr Soc. 2006 Feb;54(2):255–61. doi: 10.1111/j.1532-5415.2005.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verghese J, Buschke H, Viola L, Katz M, Hall C, Kuslansky G, et al. Validity of divided attention tasks in predicting falls in older individuals: a preliminary study. J Am Geriatr Soc. 2002 Sep;50(9):1572–6. doi: 10.1046/j.1532-5415.2002.50415.x. [DOI] [PubMed] [Google Scholar]
- 10.Menz HB, Latt MD, Tiedemann A, Mun San Kwan M, Lord SR. Reliability of the GAITRite® walkway system for the quantification of temporo-spatial parameters of gait in young and older people. Gait & posture. 2004;20(1):20–5. doi: 10.1016/S0966-6362(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 11.Lord S, Galna B, Verghese J, Coleman S, Burn D, Rochester L. Independent Domains of Gait in Older Adults and Associated Motor and Nonmotor Attributes: Validation of a Factor Analysis Approach. J Gerontol A Biol Sci Med Sci. 2012 Dec 18; doi: 10.1093/gerona/gls255. [DOI] [PubMed] [Google Scholar]
- 12.Hollman JH, McDade EM, Petersen RC. Normative spatiotemporal gait parameters in older adults. Gait & Posture. 2011 May;34(1):111–8. doi: 10.1016/j.gaitpost.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. Journal of neurology, neurosurgery, and psychiatry. 2007;78(9):929–35. doi: 10.1136/jnnp.2006.106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holtzer R, Mahoney JR, Izzetoglu M, Izzetoglu K, Onaral B, Verghese J. fNIRS study of walking and walking while talking in young and old individuals. J Gerontol A Biol Sci Med Sci. 2011 Aug;66(8):879–87. doi: 10.1093/gerona/glr068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tinetti ME, Baker DI, Mcavay G, Claus EB, Garrett P, Gottschalk M, et al. A Multifactorial Intervention to Reduce the Risk of Falling among Elderly People Living in the Community. The New England journal of medicine. 1994 Sep 29;331(13):821–7. doi: 10.1056/NEJM199409293311301. [DOI] [PubMed] [Google Scholar]
- 16.Gill TM, Allore HG, Holford TR, Guo Z. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004 Nov 3;292(17):2115–24. doi: 10.1001/jama.292.17.2115. [DOI] [PubMed] [Google Scholar]
- 17.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Brit J Psychiat. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 18.Yesavage J, Brink T, Rose T. Geriatric depression scale (GDS) Handbook of psychiatric measures. 2000:544–6. [Google Scholar]
- 19.Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H. Abnormality of gait as a predictor of non-Alzheimer's dementia. New Engl J Med. 2002 Nov 28;347(22):1761–8. doi: 10.1056/NEJMoa020441. [DOI] [PubMed] [Google Scholar]
- 20.Schenkman M, Hughes MA, Samsa G, Studenski S. The relative importance of strength and balance in chair rise by functionally impaired older individuals. J Am Geriatr Soc. 1996 Dec;44(12):1441–6. doi: 10.1111/j.1532-5415.1996.tb04068.x. [DOI] [PubMed] [Google Scholar]
- 21.Studenski S, Perera S, Wallace D, Chandler JM, Duncan PW, Rooney E, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003 Mar;51(3):314–22. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 22.Holtzer R, Friedman R, Lipton RB, Katz M, Xue X, Verghese J. The relationship between specific cognitive functions and falls in aging. Neuropsychology. 2007 Sep;21(5):540–8. doi: 10.1037/0894-4105.21.5.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganz DA, Higashi T, Rubenstein LZ. Monitoring falls in cohort studies of community-dwelling older people: effect of the recall interval. J Am Geriatr Soc. 2005 Dec;53(12):2190–4. doi: 10.1111/j.1532-5415.2005.00509.x. [DOI] [PubMed] [Google Scholar]
- 24.Verghese J, Ambrose AF, Lipton RB, Wang C. Neurological gait abnormalities and risk of falls in older adults. J Neurol. 2010 Mar;257(3):392–8. doi: 10.1007/s00415-009-5332-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Movement Disorders. 2008;23(3):329–42. doi: 10.1002/mds.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holtzer R, Verghese J, Xue X, Lipton RB. Cognitive processes related to gait velocity: results from the Einstein Aging Study. Neuropsychology. 2006 Mar;20(2):215–23. doi: 10.1037/0894-4105.20.2.215. [DOI] [PubMed] [Google Scholar]
- 27.Doi T, Makizako H, Shimada H, Yoshida D, Ito K, Kato T, et al. Brain atrophy and trunk stability during dual-task walking among older adults. J Gerontol A Biol Sci Med Sci. 2012 Jun;67(7):790–5. doi: 10.1093/gerona/glr214. [DOI] [PubMed] [Google Scholar]
- 28.Annweiler C, Beauchet O, Bartha R, Wells JL, Borrie MJ, Hachinski V, et al. Motor cortex and gait in mild cognitive impairment: a magnetic resonance spectroscopy and volumetric imaging study. Brain : a journal of neurology. 2013 Mar;136(Pt 3):859–71. doi: 10.1093/brain/aws373. [DOI] [PubMed] [Google Scholar]
- 29.Nadkarni NK, Levine B, McIlroy WE, Black SE. Impact of subcortical hyperintensities on dual-tasking in Alzheimer disease and aging. Alzheimer disease and associated disorders. 2012 Jan;26(1):28–35. doi: 10.1097/WAD.0b013e3182172c58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herman T, Mirelman A, Giladi N, Schweiger A, Hausdorff JM. Executive control deficits as a prodrome to falls in healthy older adults: a prospective study linking thinking, walking, and falling. J Gerontol A Biol Sci Med Sci. 2010 Oct;65(10):1086–92. doi: 10.1093/gerona/glq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verghese J, Robbins M, Holtzer R, Zimmerman M, Wang C, Xue X, et al. Gait dysfunction in mild cognitive impairment syndromes. J Am Geriatr Soc. 2008;56(7):1244–51. doi: 10.1111/j.1532-5415.2008.01758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delbaere K, Kochan NA, Close JC, Menant JC, Sturnieks DL, Brodaty H, et al. Mild cognitive impairment as a predictor of falls in community-dwelling older people. The American Journal of Geriatric Psychiatry. 2012;20(10):845–53. doi: 10.1097/JGP.0b013e31824afbc4. [DOI] [PubMed] [Google Scholar]
- 33.Montero-Odasso M, Muir SW, Speechley M. Dual-task complexity affects gait in people with mild cognitive impairment: the interplay between gait variability, dual tasking, and risk of falls. Archives of physical medicine and rehabilitation. 2012;93(2):293–9. doi: 10.1016/j.apmr.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 34.Andersson AG, Kamwendo K, Seiger A, Appelros P. How to identify potential fallers in a stroke unit: validity indexes of 4 test methods. J Rehabil Med. 2006 May;38(3):186–91. doi: 10.1080/16501970500478023. [DOI] [PubMed] [Google Scholar]
- 35.Verghese J, Mahoney J, Ambrose AF, Wang C, Holtzer R. Effect of cognitive remediation on gait in sedentary seniors. J Gerontol A Biol Sci Med Sci. 2010 Dec;65(12):1338–43. doi: 10.1093/gerona/glq127. [DOI] [PubMed] [Google Scholar]


