Abstract
Regulatory myeloid cells (RMC) are emerging as novel targets for immunosuppressive (IS) agents and hold considerable promise as cellular therapeutic agents. Herein, we discuss the ability of regulatory macrophages (Mreg), regulatory dendritic cells (DCreg) and myeloid-derived suppressor cells (MDSC) to regulate alloimmunity, their potential as cellular therapeutic agents and the IS agents that target their function. We consider protocols for the generation of RMC and the selection of donor- or recipient-derived cells for adoptive cell therapy. Additionally, the issues of cell trafficking and antigen (Ag) specificity following RMC transfer are discussed. Improved understanding of the immunobiology of these cells has increased the possibility of moving RMC into the clinic to reduce the burden of current IS agents and promote Ag-specific tolerance. In the second half of this review, we discuss the influence of established and experimental IS agents on myeloid cell populations. IS agents believed historically to act primarily on T cell activation and proliferation are emerging as important regulators of RMC function. Better insights into the influence of IS agents on RMC will enhance our ability to develop cell therapy protocols to promote the function of these cells. Moreover, novel IS agents may be designed to target RMC in situ to promote Ag-specific immune regulation in transplantation and usher in a new era of immune modulation exploiting cells of myeloid origin.
Keywords: immune regulation, myeloid cells, transplantation
INTRODUCTION
Despite excellent short-term outcomes due to the prevention and successful treatment of acute rejection, late graft failure remains an important problem in organ transplantation (1). Moreover, current non-specific suppression of the immune system using anti-rejection drugs carries significant risks, including infection, malignancy and drug toxicity (2). Currently, there is increasing interest in the potential of regulatory innate or adaptive immune cells to control allograft rejection (3). Targeting myeloid cells with the goal of minimizing dependency on immunosuppressive (IS) drugs and promoting donor-specific tolerance represents a promising approach.
Herein, we discuss strategies to target regulatory myeloid cells (RMC) in situ and prospects for cell therapy in transplantation using RMC. Three RMC populations,- regulatory macrophages (Mreg), regulatory dendritic cells (DCreg) and myeloid-derived suppressor cells (MDSC) will be the focus of this review. Mreg will be discussed in the context of studies on peripheral blood mononuclear cell (PBMC)-derived cells differentiated in macrophage colony-stimulating factor (M-CSF) and then stimulated with interferon (IFN)-γ, since most work on Mreg in the field of transplantation has been focused on this population (4, 5). Dendritic cells (DC) are innate professional antigen (Ag)- presenting cells (APC) that serve as critical initiators and regulators of innate and adaptive immunity (6–8). For in-depth analysis of DC ontogeny and the mechanisms that underlie their immune regulatory capacity, please see recent comprehensive reviews (8–12). MDSC are a heterogeneous population of immature myeloid cells and myeloid progenitors that regulate anti-tumor immunity and share the ability to suppress effector T cell responses. The origin and suppressive mechanisms of MDSC have been reviewed in detail (13, 14).
RMC AS CELLULAR IMMUNOTHERAPEUTIC AGENTS
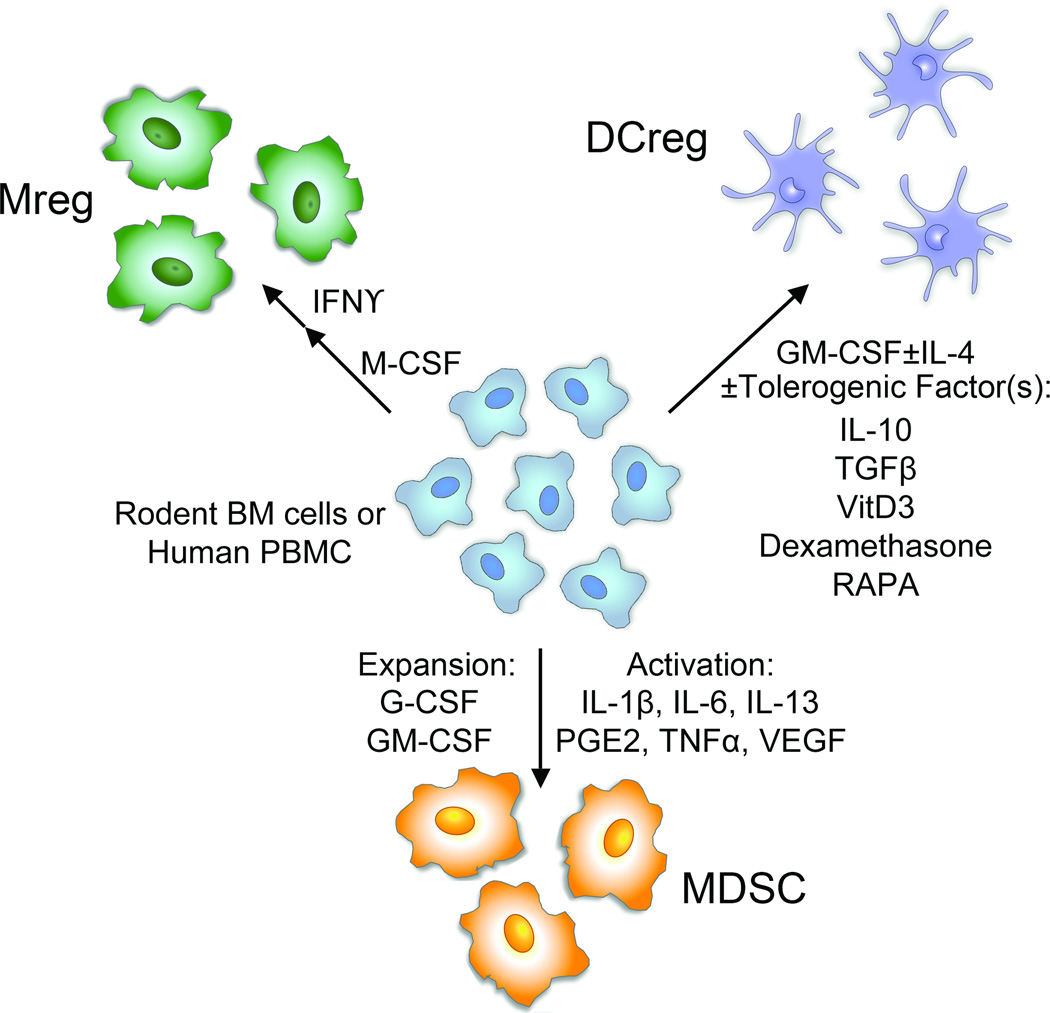
In Vitro Generation of RMC
RMC generated in vitro for therapeutic evaluation are propagated typically from rodent BM (BM) cells or human PBMC (Figure 1). Although differentiation procedures between species are similar, distinct starting cell populations make the translation of findings from rodents to humans difficult (15). Moreover, RMC therapy lacks standard differentiation protocols since the optimal immune regulatory properties of each RMC population are unknown (16). Although MDSC have not been evaluated for immune regulatory function in humans, protocols for the propagation and administration of Mreg and DCreg have been described in human renal transplantation and in healthy volunteers or type 1-diabetics, respectively (Table 1). Importantly, no adverse effects of RMC therapy have been reported in these limited clinical studies to date.
Figure 1.
Generation of RMC in vitro from rodent BM cells or human PBMC. Mreg, DCreg and MDSC can be generated in vitro from precursors in rodent BM or human PBMC exposed to specific growth factors. In some cases, RMC (Mreg and MDSC) are also activated in vitro by the addition of inflammatory cytokines or other soluble factors. DCreg are often generated in the presence of anti-inflammatory cytokines or agents that suppress their activation into stimulatory DC.
Table 1.
Influence of RMC administration in humans.
| RMC | Source | Study Name |
Clinical | Protocol | Outcome | Reference |
|---|---|---|---|---|---|---|
| Immature DC | Autologous blood monocytes cultured in GM-CSF and IL-4 pulsed with Ag | N/A | N/A | 2×106 s.c. | Ag-specific inhibition of CD8+ effector T cell function and generation of CD8+ Treg | (59, 60) |
| Immature or tolDC | Autologous unmanipulated DC (n=3) or DC treated with antisense oligonucleotides for CD40, CD80 and CD86 (n=7) | N/A | Type-1 diabetes (Phase I safety study) | 107 cells intradermally every 2 weeks for 4 doses | 10/10 no adverse events; Significant increase in peripheral B220+CD11c− B cell frequency | (161) |
| Mreg (TAIC) | Donor splenic mononuclear cells cultured in M-CSF and stimulated with IFN-γ | TAIC-I | Deceased donor renal transplantation | 1.0–7.52×106 cells/kg by central venous infusion d5 (Patient receiving 0.55×106 cells/kg excluded); tacrolimus, sirolimus and glucocorticoid triple therapy for first month then weaned to tacrolimus monotherapy with progressive tacrolimus weaning | 8/10 weaned from steroids; 6/10 weaned to low dose tacrolimus monotherapy | (162) |
| Mreg (TAIC) | Donor PBMC cultured in M-CSF and stimulated with IFN-γ then co-cultured with recipient PBMC prior to infusion of all cells | TAIC-II | Living donor renal transplantation | 1.74–10.39×107 co-cultured cells/kg by central venous infusion d-5; ATG (d0, 1 and 2), prednisolone and tacrolimus started at 8–12 ng/ml trough levels and weaned to 5–8 ng/ml; prednisolone stopped by 10 weeks if graft function stable | 5/5 no adverse events; 3/5 on low-dose tacrolimus monotherapy; 1/5 withdrawn from all immunosuppression for 8 months | (53) |
| Mreg (TAIC) | Donor PBMC cultured in M-CSF and stimulated with IFN-γ then co-cultured with recipient PBMC prior to infusion of all cells | Case Study (TAIC-II) | Presensitized living-related renal transplantation | 6.9×107 co-cultured cells/kg by central venous infusion d-17; ATG (d0, 1 and 2), prednisolone and tacrolimus started at 8–12 ng/ml trough levels and weaned to 4–8 ng/ml at week 35 | No acute rejection episodes up to 27 months; donor-specific hyporesponsiveness and loss of donor-specific Ab | (37) |
| Mreg | Donor PBMC cultured in M-CSF and stimulated with IFN-γ | N/A | Living-donor renal transplantation | 2 mg/kg/d AZA beginning 3d prior to central venous infusion of Mreg (7.1 or 8×106 cells/kg) until 8 week post-Tx; Mreg given 6–7d before Tx; tacrolimus and prednisolone begun day of Tx and prednisolone weaned by 10 weeks resulting in tacrolimus monotherapy (4–8 ng/ml trough) | n=2 patients maintained on low dose tacrolimus monotherapy with excellent graft function and no rejection episodes >3 year post-Tx | (17) |
Abbreviations: Ab, antibody; ATG, anti-thymocyte globulin; AZA, azathioprine; GM-CSF, granulocyte macrophage colony stimulating factor; IFN, interferon; IL, interleukin; N/A, not applicable; PBMC, peripheral blood mononuclear cells; RMC, regulatory myeloid cell; TAIC, transplant acceptance-inducing cell; Tx, transplant
Human Mreg are differentiated from donor PBMC acquired by leukapheresis, in recombinant human M-CSF for 6 days, followed by 24h stimulation with IFN-γ (17). Human DCreg are typically differentiated from PBMC or purified monocytes in the presence of granulocyte-macrophage (GM)-CSF and interleukin (IL)-4, with the addition of one or more factors that promote their tolerogenicity (reviewed in (11, 18)). DCreg are typically immature myeloid DC and maturation-resistant, or ‘alternatively-activated’ (e.g. exposed to IL-10 and transforming growth factor β [TGFβ] during propagation, then stimulated with LPS), so that they maintain expression of major histocompatibility complex (MHC) molecules, but display low levels of co-stimulatory molecules and pro-inflammatory cytokines. Vitamin D3 (vitD3) and dexamethasone promote DCreg (19, 20). Thus, activation of human DC cultured in vitD3/dexamethasone with lipopolysaccharide (LPS) results in stable, ‘alternatively-activated,’ semi-mature DC (21). Addition of IL-10 (‘DC-10’) (22) or the mechanistic target of rapamycin (mTOR) inhibitor, rapamycin (RAPA) (23) to human monocyte cultures also produces DCreg. Non-human primate (NHP) monocyte-derived DCreg can be generated using vitD3 and IL-10 (24, 25). DCreg are also made using low dose GM-CSF in the absence of IL-4 (26). Thus, although Mreg differentiation is comparatively well-defined, there is significant variability in methods to generate DCreg. Importantly, generation of recipient-derived RMC for clinical use must be validated with PBMC from patients with pre-existing disease (27). In this regard, DCreg generated from patients with rheumatoid arthritis (28) or relapsing-remitting multiple sclerosis (29) exhibit a similar phenotype and function to DCreg generated from healthy controls.
MDSC exhibit considerable phenotypic heterogeneity and are subdivided into those that resemble monocytes or are similar phenotypically to neutrophils (30). They require factors to induce their activation, in addition to their expansion (13). Thus, mouse monocytic MDSC are generated from BM cells in G-CSF, GM-CSF or both, and activated with IL-6 or IL-13 (31, 32). Table 2 outlines adoptive MDSC therapies that have been evaluated in mouse models of skin or pancreatic islet cell transplantation, graft-versus-host disease (GVHD) and type 1-diabetes. Human MDSC generated from PBMC with GM-CSF+IL-6 appear to exert the most potent suppressive capacity, but GM-CSF+IL-1β, prostaglandin (PG)E2, tumor necrosis factor α (TNFα) or vascular endothelial growth factor (VEGF) also induce suppressive MDSC (33). Similarly, GM-CSF and IL-6 can be used to generate suppressive human BM-derived MDSC (31). Addition of PGE2 to GM-CSF and IL-4-stimulated human PBMC cultures blocks DC differentiation and promotes MDSC generation (34).
Table 2.
MDSC transfer for immune modulation or cell therapy of allograft rejection.
| Condition | Source of MDSC | Cell Dose and Route |
Model | Mechanism and Outcome | Reference |
|---|---|---|---|---|---|
| (i) No transplant | Tumor-bearing mouse splenocytes | 3–5×106i.v. 2–3d after T cell transfer | Ag-specific transgenic CD8+ T cell | Ag-specific CD8+ T cell tolerance but T cells remained responsive to non-specific αCD3 stimulation | (50) |
| Tumor-bearing mouse BM | 5×106 i.v. + 5µg/mouse peptide Ag d1 after T cell transfer | Transgenic T cell induction of diabetes | 75% diabetes-free at d30 (Ag-specific) with T cell anergy and induction of Treg | (64) | |
| Tumor-bearing mouse BM | 2×107 i.v. with T cells | NOD/SCID with transfer of diabetogenic T cells | 60% diabetes-free at d100 with reduced lymphocyte infiltration and insulitis | (64) | |
| (ii) Skin transplant | Transplant recipient splenocytes | 2×105 i.v. on d-1 and d3 | MHC class II-mismatched skin allograft | 50% long-term survival when transplant-activated MDSC transferred from ILT2 (HLA-G receptor) transgenic mice but not wild-type mice | (75) |
| Splenocytes from LPS-treated mice | 5×106 i.v. on d-1 | Male to female or MHC class II-mismatched skin allograft | Prolonged allograft survival dependent on heme oxygenase-1 | (80) | |
| (iii) Islet cell transplant | BALB/c BM cultures with GM-CSF+IL-6 or GM-CSF+G-CSF | 107 i.v. on d0, 7, 14 and 21 | Islet allograft (B6 to BALB/c) | Long-term survival in ~75% (GM-CSF+IL-6 MDSC) or ~40% (GM-CSF+G-CSF MDSC) without generalized immune suppression | (31) |
| B6 BM cultures with GM-CSF with liver stellate cells (B6, BALB/c or C3H) | 2.5×106 mixed with islets | Islet allograft (BALB/c to B6) | ~45–65% long-term survival, B7-H1-dependent increase in Treg that mediate T cell hyporesponsiveness | (163) | |
| (iv) GVHD | B6 BM cultures with GM-CSF+G-CSF+IL-13 | 2 or 6×106 i.v. with donor cells | GVHD (B6 to BALB/c) | Cell dose- and arginase-1-dependent improved survival with inhibition of CD4+ and CD8+ T cell responses and maintained graft-versus-leukemia effect | (32) |
| 129SvEv embryonic stem cell line cultured with KL, VEGF, Flt3L, TPO and M-CSF | 2×106 i.v. with donor cells, d4 and d10 | GVHD (129SvEv to BALB/c) | 82% long-term survival | (79) |
Abbreviations: G-CSF, granulocyte colony stimulating factor; GM-CSF, granulocyte macrophage colony stimulating factor; ILT, immunoglobulin-like transcript; KL, c-Kit ligand; M-CSF, macrophage colony stimulating factor; NOD/SCID, non-obese diabetic/severe combined immune deficiency; VEGF, vascular endothelial growth factor; Flt3L, fms-like tyrosine kinase 3 ligand; TPO, thrombopoietin
RMC therapies need to be designed in conjunction with current IS protocols due to the success of the latter in achieving high short-term organ allograft survival rates (15). Thus, experimental RMC therapy needs to be undertaken with an appropriate IS agent(s) that maintains their tolerogenic properties. In rodent organ transplant models, Mreg (35) and DCreg (11) synergize with pharmacologic agents, anti-lymphocyte serum or co-stimulation blockade, but the impact of IS agents on MDSC is largely unknown.
Selection of Donor or Recipient RMC for Therapy
Mouse Mreg prolong allograft survival only when donor-derived (35). Although there is a potential risk of sensitizing the recipient to donor, this has not been observed in the human renal transplant recipients given Mreg to date (36, 37). The risk is mitigated by infusing the cells one week before transplantation (to avoid surgically-induced inflammation) and choosing IS agents that are likely to maintain the tolerogenic properties of RMC in the face of inflammation.
Both donor- and recipient-derived DCreg have been investigated extensively in rodent transplant models (11, 15, 18, 38). While allogeneic DC trafficking from rodent organ grafts may survive in lymphoid tissue for several days in unmanipulated hosts or even weeks in immunosuppressed recipients (39, 40), these donor DC may be also killed by host natural killer (NK) cells (41) and reprocessed by endogenous DC able to present donor alloAg (42). Donor Mreg survive in humans for at least 30 hours in the spleen, liver and BM (17) and 2 weeks in mice in the lung (35). Although DCreg can be generated from the graft recipient at any time, the optimal method of loading donor alloAg (donor cell lysate, exosomes, apoptotic cells) has not been established (18). One group has used unpulsed autologous DCreg to promote long-term rodent allograft survival, thus maturation-resistant DCreg are given in the peri-transplant period and acquire donor alloAg in situ (43–45).
Similar events could accompany cell therapy with MDSC, since these cells are also able to process and present Ag (46, 47). As precursors of myeloid cells, MDSC can differentiate into DC and macrophages (31, 48–50), but MDSC have not been found to potentiate immunity following their adoptive transfer (Table 2) and retain immune regulatory function, even if they do differentiate (31, 50). On the other hand, cyclooxygenase (COX)2 activation by inflammatory mediators such as IL-1β and IFN-γ prevents the differentiation of MDSC into DC (51), while IFN-γ is an important stimulator of MDSC suppressive function (52). These properties resemble those of Mreg that are activated by IFN-γ (36) and provide the advantage that inflammatory conditions such as occur in organ transplantation may reinforce the suppressive activity of MDSC. Thus, selection of donor or recipient RMC presents its own distinct challenges, such as circumventing allosensitization, and the need for/nature of Ag pulsing.
Ag Specificity
The ability of RMC to regulate immune responses in an Ag-specific manner is an important consideration to avoid global immunosuppression. Mouse (35) and human (17) Mreg suppress mitogen-activated CD4+ and CD8+ T cell proliferation, and mouse Mreg delete alloreactive T cells specifically in vitro (35). Moreover, donor-, but not recipient- or third party-derived Mreg, prolong mouse cardiac allograft survival (35), suggesting that Mreg can regulate alloAg-specific immunity in vivo. Administration of transplant acceptance-inducing cells (TAIC), i.e. unpurified Mreg, to human renal transplant recipients has been reported to promote donor-specific hyporesponsiveness, even in a pre-sensitized recipient (37, 53).
Donor- and host-derived DCreg promote long-term allograft survival or donor-specific tolerance in rodent transplant models when combined with anti-lymphocyte serum (ALS), anti-CD40L (CD154) mAb or cytotoxic T lymphocyte Ag (CTLA)4-Ig (54–58). Importantly, local administration of immature autologous DC to healthy human volunteers results in inhibition of Ag-specific CD8+ T cell effector function (59) and generation of regulatory CD8+ T cells (60). These latter findings provide proof-of-principle that DC have the capacity to regulate Ag-specific responses in humans. Recently, donor-derived DCreg have been shown to prolong organ allograft survival in a robust pre-clinical NHP renal transplant model accompanied by reduction in donor-reactive Tmemory cell responses (25).
The Ag specificity of MDSC suppressive function depends on the model, microenvironment and activation of target lymphocytes (61). MDSC can inhibit both CD4+ and CD8+ T cell reactivity (46, 52, 62–64). They can suppress Ag-specific CD8+ T cell responses (46), but it is not known whether they are capable of Ag-specific CD4+ T cell suppression (13), especially in view of their low or absent MHC class II expression (65). Importantly, MDSC generated in vitro can promote Ag-specific CD8+ T cell hyporesponsiveness (31). In a mouse model of cardiac allograft tolerance induced by donor-specific transfusion (DST) and anti-CD40L mAb, suppression of T cells by graft-infiltrating MDSC was non-specific, and BM and splenic monocytes did not suppress (66). Taken together, DCreg and Mreg have Ag-specific regulatory capacity in transplantation, but the conditions under which MDSC suppress alloimmunity in an Ag-specific manner need to be better understood in order to harness these cells for therapeutic application.
Trafficking and Migration of RMC under Inflammatory Conditions and Following their Adoptive Transfer
There is evidence that human Mreg administered via central venous access migrate to the lungs and then distribute to the liver, spleen and BM within 30h of their infusion (17). Murine Mreg demonstrate a similar distribution pattern following intravenous (i.v.) injection and notably do not migrate to lymph nodes (35). Little is known about chemokine receptor expression on Mreg and the location(s) where they exert their regulatory function in vivo is not known (4).
Expression of CCR7 by DC directs them to secondary lymphoid organs where they interact with T cells. Adoptively-transferred, IL-10-expressing DC require CCR7 to prolong mouse cardiac allograft survival (67), suggesting that DCreg, and likely Mreg, must traffick to secondary lymphoid for their regulatory function. Notably, IL-10 reduces DC CCR7 expression and lymph node homing ability (68). Upregulation of CCR7 following activation of DCreg by Toll-like receptor (TLR) ligation in vitro may be required to improve the migratory function of these cells (69). Following i.v. injection, rodent host-derived DCreg migrate rapidly to the spleen (70, 71), while RAPA-conditioned DC migrate to the lymph nodes following intramuscular injection (72). The route of DCreg administration may be critical to optimize their function in vivo (69). While i.v. DCreg injection prolongs cardiac allograft survival in mice, subcutaneous injection of the same DCreg does not affect graft survival (73). Similarly, in a NHP model, i.v. administration of DCreg results in immune regulation (24), whereas intradermal injection may boost the immune response (15). In human cancer patients, intradermal injection increases the migration of immature DC to the draining lymph nodes compared to subcutaneous administration (74); however, subcutaneous administration of immature DC has been shown to regulate CD8+ T cell responses to model Ags in humans (59, 60). Together, these studies suggest that optimization of delivery route is critical to DCreg function and that directing their migration to secondary lymphoid organs is important.
MDSC express chemokine receptors, such as CX3CR1 (75) or CCR2 (46, 76), that direct them towards sites of inflammation, but they can also be directed towards secondary lymphoid organs by expression of CD62L (32, 46) and CCR7 (32). It is unknown whether MDSC migration to the allograft, secondary lymphoid organs or both is preferable following their adoptive transfer; however, MDSC are required to migrate to the graft and not lymph nodes for experimental transplant tolerance induced by donor-specific infusion and anti-CD154 mAb (66). The complement component C5a participates in the recruitment of MDSC to tumors and peripheral lymphoid organs in mice (77). Thus, it will be of interest to determine whether C5a plays a similar role in transplant rejection, since C5 is integral to Ab-mediated rejection (78). In vitro-generated MDSC traffic to peripheral lymphoid tissue and sites of inflammation in GVHD, including the liver and spleen (79) or spleen and lymph nodes (32). MDSC expanded in vivo in response to LPS that inhibited alloimmunity migrated to the spleen when transferred to skin transplant recipients, but their migration to the graft was not assessed (80). MDSC accumulate within tumors (50) and at sites of inflammation in murine experimental allergic encephalomyelitis (EAE) (52) and chronic contact eczema (63). They also accumulate within the spleen (50, 63, 81) and lymph nodes (50, 63) in inflammatory disease and cancer. Following transplantation, rodent MDSC are found in the allograft and peripheral blood (66, 82, 83) as the result of migration from the BM (66). Although human MDSC were reported to be elevated in the peripheral blood of renal transplant recipients, they were not assessed in biopsy tissue (84).
In summary, RMC therapies have demonstrated promising immune regulatory capacity. However, it will be necessary to rationally design protocols in transplantation that optimize in vitro generation of RMC whose in vivo migration (to the appropriate sites) and function are supported by the IS regimen. Further pre-clinical studies are warranted to optimize each parameter in increasingly stringent models from rodent to NHP, while also continuing to progress RMC therapy in human transplant recipients.
TARGETING RMC WITH THERAPEUTIC AGENTS
This section summarizes reports concerning the influence of IS drugs, specific therapeutic Abs and novel immunoregulatory strategies on DC, macrophages and MDSC (Table 3).
Table 3.
Influence of immunosuppressive drugs, biologic agents and novel immunoregulatory agents on myeloid DC, macrophages and MDSC in vivo.
| Therapeutic Agent | Type of cell | Species/Model | Effect/s on cells | Reference |
|---|---|---|---|---|
| GCs, Dexamethasone | mDCs & Mϕ | Delayed-type hypersensitivity mice | Depletion of mDC and pDC, and Mϕ enrichment | (89) |
| MDSC | Trauma model mice | Expansion of MDSC | (90) | |
| 1α,25(OH)2D3 (VitD3) | DCs | - | Modulation of phenotype and function towards tolerogenic DC | (164, 165) |
| MDSC | Tumor-bearing mice | Diminished presence of MDSC within regional lymph nodes, spleens and tumors, restoration of their Ag-presenting ability and differentiation towards a DC phenotype | (166–168) | |
| Cyclosporine A | DC | D-type hypersensitivity mice | Defective Ag acquisition and MHC-restricted Ag presentation | (169) |
| DC & Mϕ | Rat | Reduced numbers in thymus | (96–98) | |
| Mϕ | Cardiac Tx in NHP (combined with CCR5 blockade) | Generation of alternatively-activated Mϕ | (100) | |
| DC | Heart Tx patients | Increase in circulating mDC percentage | (102) | |
| DC & Mϕ | Mouse kidney Tx model and humans | Reduction in bacterial phagocytosis | (170) | |
| Tacrolimus | DC & Mϕ | LPS-induced inflammatory response (mouse) | Decreased responsiveness to LPS, and blocking of MHC-restricted Ag presentation | (94, 95) |
| Mϕ | Brain-injured rats | Reduction in the number recruited to the inflammatory site, and their proliferative activity | (171) | |
| DC | Atopic dermatitis patients | Decrease in IgE receptors | (172) | |
| Rapamycin | DC | - | Impairment of DC costimulatory molecule up-regulation, production of pro-inflammatory cytokines, and T cell allostimulatory capacity, and induction of apoptosis | (113, 114) |
| Kidney transplant patients | Increased immuno-stimulatory potential | (115) | ||
| Mycophenolate Mofetil(MMF) | DC | Contact hypersensitivity (mice) | Impaired Ag-presenting capacity | (109) |
| Mϕ | Renal Tx rats | Inhibition of Mϕ infiltration | (173) | |
| HDAC inhibitors | DC | Graft-versus-host-disease mice, and humans | Reduced costimulatory molecule expression, pro-inflammatory cytokine release, and T cell allostimulatory activity | (120, 121) |
| Proteasome inhibitors | DC | Mice | Impairment of DC maturation and cytokine production, as well as DC-mediated T cell stimulation | (130) |
| NFκB inhibition: | ||||
| - Azithromycin | DC | Murine histo-incompatibility model | Inhibition of DC maturation | (174) |
| - Liposomes containing NFκB decoy oligodeoxynucleotides (ODN) | Mϕ | Kidney transplantation (rats) | Reduction of periarterial Mϕ infiltration | (175) |
| PGE2 receptor (EP4) agonist | Mϕ | Cardiac Tx mice | Suppression of Mϕ activation | (138) |
| Polyclonal antithymocyte globulin (ATG) Ab | mDC | Allogeneic stem cell Tx patients | Reduction of circulating mDCs | (176) |
| Anti-CD52 mAb | mDC | Kidney transplant patients | Strong and sustained reduction in the total number of peripheral DC and a significant shift from myeloid to plasmacytoid DC subsets | (143) |
| CTLA4Ig | DC | Cardiac Tx rats | Secretion of inhibitory products that suppress alloAg-induced T cell proliferative responses | (151) |
| Anti-CD154 | DC | Cardiac Tx mice | Potentiation of DC tolerogenicity | (156) |
| Anti-CD28 mAb | MDSC | Kidney Tx rat | MDSC accumulation in the blood and allograft | (82) |
| siRNA gene silencing of MyD88 and TRIF | DC | Cardiac Tx mice | Reduction of DC maturation | (158) |
| Recombinant G-CSF (Neupogen) | MDSC (Gr-1+CD11b+) | Skin Tx mice | Induction of a high frequency of MDSC in the peripheral lymphoid compartments | (159) |
| Human ILT2 | MDSC | Skin Tx mice | Increased MDSC (CD11b+Gr-1+) and enhanced long-term survival of allografts | (75) |
Abbreviations: DC, dendritic cell; GCs, glucocorticoids; HDAC, histone deacetylase; ILT2, immunoglobulin-like transcript 2; mDC, myeloid dendritic cell; Mϕ, macrophage; MyD88, myeloid differentiation primary response gene 88; NHP, non-human primate; TRIF, Toll-IL-1 receptor domain containing adaptor-inducing interferon-β; tx, transplant; vitD3, vitamin D3
Conventional IS Drugs
Transplant recipients receive pharmacologic and biological agents to control graft rejection, and although the principal mechanism of action of these agents is inhibition of T cell responses, they also modulate RMC. The influence of anti-inflammatory agents, IS drugs and biologic IS, on DC function in vivo has been reviewed in detail elsewhere (10, 85, 86). Studies of their influence on Mreg and MDSC are limited.
The most extensively-studied IS drugs that target DC in vivo are glucocorticoids (GC), calcineurin inhibitors (CNI), RAPA (sirolimus) and mycophenolate mofetil (MMF) (10). The in vivo effects of GC on DC have been reviewed by van Kooten et al (87). Specifically, GC reduce peripheral DC numbers and inhibit their maturation and production of pro-inflammatory cytokines, while enriching for Mreg (88, 89). Endogenous GC promote the expansion of MDSC in a murine model of trauma (90), and exposure of monocytes to GC induces CD11b+Gr-1+CD124+Ly6Cmed MDSC (91). Administration of dexamethasone to glioblastoma patients increases circulating CD14+HLA-DRlo/negCD80− immunosuppressive cells, that resemble MDSC (92).
CNI, i.e. cyclosporine A (CsA) and tacrolimus (FK506), are front-line anti-rejection agents used in combination with an anti-proliferative agent, in particular MMF. CsA and tacrolimus, but not RAPA, inhibit MHC-restricted Ag presentation by DC in vitro (93) and in vivo (94). Tacrolimus treatment of mice reduces responsiveness of macrophages and DC to LPS (95). Numbers of thymic DC and macrophages are decreased in rats during CsA treatment (96–98); however, their function appears to be unaffected (96). On the other hand, increased numbers of DC have been reported in NHP with long-surviving renal allografts treated with both tacrolimus and sirolimus (99). CsA combined with CCR5 blockade increases cardiac graft survival in NHP, an effect that is associated with generation of alternatively-activated macrophages through activation of the peroxisome proliferator-activated receptor (PPAR)γ (100). Additionally, CsA inhibits the phenotypic maturation, endocytic activity and allostimulatory function of human peripheral blood DC (101). CsA or tacrolimus increases the incidence of mDC in peripheral blood of human heart transplant recipients, but no difference in expression of the DC maturation marker CD83 is observed (102). To our knowledge, direct effects of CNI on MDSC have not been studied; however, expression of the immunophilin FK506 binding protein 51 is increased in monocytic and granulocytic MDSC isolated from tumor-bearing mice and regulates their suppressive function (103). Additionally, calcineurin and nuclear factor of activated T cells (NFAT) signaling are negative regulators of myelopoiesis, and CsA augments numbers of differentiated DC in vitro (104). Therefore it appears likely that CNI impact MDSC.
MMF is an anti-proliferative pro-drug of mycophenolic acid (MPA) that inhibits B and T cell proliferation (105). MPA also suppresses DC maturation and reduces Ag presentation to T lymphocytes (106–109). As MPA has been reported to suppress granulopoiesis, it is possible that it also affects MDSC.
RAPA inhibits the serine threonine kinase mechanistic target of rapamycin (mTOR) (110). Its administration to mice impairs DC costimulatory molecule up-regulation, production of proinflammatory cytokines, and T cell allostimulatory function (111–113). Moreover, RAPA induces apoptosis in DC, but not in monocytes or macrophages (114). Haidinger et al (115) found that DC in kidney transplant patients treated with RAPA displayed increased immunostimulatory potential compared with those in patients treated with CNI and in healthy controls. Interestingly, RAPA prevents the anti-inflammatory effects of GC on human monocytes as well as myeloid DC (116). Moreover, RAPA conditioning augments IL-12 production by mouse BM-derived DC or human monocyte-derived DC stimulated with LPS or pro-inflammatory cytokines, respectively (117, 118). Thus, under different circumstances RAPA can exert pro- or anti-inflammatory effects on DC. mTOR is required for DC development, so it will be interesting to determine whether RAPA affects MDSC due to its ability to inhibit myelopoiesis (119).
Thus, in addition to the ability of conventional IS agents to inhibit B and T cell activation, these drugs exert profound, but variable, effects on macrophage and DC differentiation and function.
Experimental IS Agents
Histone deacetylase (HDAC) inhibitors (including suberoylanilide hydroxamic acid, trichostatin A and valproic acid) are anti-tumor agents that also have anti-inflammatory properties. HDAC inhibitors reduce TLR-induced costimulatory molecule expression and pro-inflammatory cytokine release by DC and their T cell allostimulatory activity in vitro and in vivo (120–122). HDAC inhibition blocks GM-CSF-dependent function in macrophages and their differentiation to DC (123), but there are contradictory reports regarding its influence on cytokine secretion (124, 125), that may reflect the specific HDAC inhibitor or dose used. We have demonstrated recently (126) that HDAC inhibitors augment GM-CSF-mediated murine MDSC expansion in vitro and in vivo, and that these MDSC exhibit similar suppressive potency to control MDSC.
Proteasome inhibitors, such as bortezomib, are believed to block the activation and nuclear translocation of NF-κB, a transcription factor central to DC maturation and inflammatory responses (127). In experimental hematopoietic stem cell transplantation, bortezomib attenuates GVHD, yet preserves graft-versus-leukemia activity (128, 129). Administration of bortezomib to mice results in a more immature DC phenotype (130). Bortezomib reduces the phagocytic capacity of human monocyte-derived DC, skews their phenotypic maturation and reduces their cytokine production and immunostimulatory capacity. It also reduces their chemokine secretion and migration (131), while promoting their apoptosis and reducing the yield of viable DC (131), preferentially targeting immature DC (127).
There are also anti-inflammatory agents that modulate RMC function. NF-κB inhibitors block DC maturation and can induce tolerance in murine cardiac transplantation (132–134). Interestingly, NF-κB is implicated as a critical regulator of MDSC suppressive function (135). Furthermore, COX-2 inhibitors prevent production of PGE2 and reduce numbers of MDSC (136), and can prolong murine cardiac allograft survival (137). There is also evidence that a PGE2 receptor (EP4) agonist suppresses the activation of macrophages and prolongs mouse cardiac allograft survival (138).
Thus, various experimental IS agents currently under investigation are capable of modifying RMC function. Typically, they reduce DC maturation, but appear to have varying effects on MDSC expansion and function.
In Vivo RMC Targeting with Abs and Other Novel Approaches
T cell-depleting Abs also target RMC. Thus, polyclonal anti-thymocyte globulin (ATG) inhibits human DC Ag uptake and maturation, induces complement-mediated lysis of DC, and decreases the capacity of DC to stimulate allogeneic T cells in vitro (139). Additionally, ATG polarizes DC towards expression of indoleamine dioxygenase (IDO) (140) that inhibits T cell proliferation. Anti-CD52 mAb (Alemtuzumab; Campath-1H) depletes peripheral blood DC, but not tissue DC, due to differential expression of CD52 on DC in these sites (141, 142). It causes a sustained reduction of total peripheral DC in kidney transplant recipients (143). In addition to T cells, human DC express CD25 after stimulation (144, 145), making them a potential target for anti-CD25 (IL-2 receptor α subunit) mAb. Furthermore, anti-CD25 mAb treatment diminishes the ability of human DC to stimulate T helper cells (144), but does not affect HLA-DR or costimulatory molecule expression by the DC after LPS stimulation (145). Recent work using daclizumab (humanized anti-CD25 mAb) has shown that it potently inhibits Ag-specific T cell activation by human mature DC in vitro (146). Interestingly, anti-CD25 mAb combined with IL-12 depletes MDSC in a mouse model of colon carcinoma (147).
Co-stimulation blockade is an emerging strategy to promote graft survival by interfering with T cell activation, in which APC play an important role. Development of co-stimulation blockers has focused mainly on targeting T cell surface co-stimulatory molecules, although some also target APC (148, 149). Notably, anti-CD28 mAb induces tolerance to rat kidney allografts in association with accumulation of circulating and graft-infiltrating MDSC that suppress effector T cell expansion (82). Belatacept (CTLA4-Ig) is the first costimulation blocker approved for renal transplantation. There is evidence that CTLA4-Ig binding to CD80/CD86 molecules provides a reverse signal to DC that results in the induction of indoleamine dioxygenase (150), and that enhanced secretion of inhibitory products by CTLA4Ig-exposed DC promotes alloantigen-specific transplant tolerance (151). However, it has been reported recently that CTLA4-Ig immunosuppressive activity may not depend on a DCreg phenotype, but on its presence during DC/T cell interaction (152). Interestingly, Ab blockade of CTLA-4 reduces the suppressive potential of MDSC in tumor-bearing mice (153). Anti-CD40 mAbs prolong renal and islet allograft survival in NHP (154, 155), while mouse mDC under CD40 blockade have a tolerogenic profile in vivo (156) and are responsible for inducing peripheral Treg and delaying cardiac allograft rejection (157).
Gene silencing of TLR adaptors, namely myeloid differentiation primary response gene (MyD) 88 and TIR-domain-containing adapter-inducing interferon-β (TRIF), using siRNA reduces DC maturation and prolongs murine cardiac allograft survival (158). Administration of recombinant G-CSF (Neupogen) prolongs skin transplant survival in mice and induces MDSC in peripheral lymphoid compartments (159). Suppressive granulocytic and monocytic MDSC are expanded in human stem cell donors during G-CSF-mobilization protocols for allogeneic hematopoietic stem cell transplantation (160). Furthermore, human inhibitory receptor ILT2, expressed on activated T cells and engaged by HLA-G on DC, has been shown to amplify MDSC and to promote long-term allograft survival (75).
Thus, although previously thought to act primarily on T cells, T cell-depleting inhibitory Abs also profoundly affect DC function, and novel approaches using costimulation blockade, siRNA or recombinant growth factors can promote MDSC.
CONCLUSION
RMC constitute an important, heterogeneous innate immune cell population with considerable promise for cell therapy. The influence of IS agents on these cells is becoming increasingly apparent. While the use of RMC as cellular therapeutics is beginning to advance from pre-clinical models to patients with inflammatory diseases, further insights into the differentiation and function of Mreg, DCreg and MDSC are required in order to maximize the utility of these cells. In addition to conventional IS drugs, novel therapeutic agents can promote the regulatory function of RMC, while preventing their immunostimulatory potential. These agents are likely to prove of considerable importance in exploiting the properties of RMC to promote transplant tolerance.
Acknowledgments
The authors’ work is supported by National Institutes of Health grants R01AI67541, U01AI51698 and P01AI81678 (AWT) and R00HL097155 (HRT). BRR is the recipient of a NIH T32 pre-doctoral fellowship (T32AI07449) and an American Heart Association pre-doctoral fellowship (11PRE7070020). DR-R is in receipt of an ESOT/AST Exchange Grant. We thank Ms. Miriam Freeman for excellent administrative support.
Abbreviations
- Ag
antigen
- ALS
anti-lymphocyte serum
- APC
antigen-presenting cell
- BM
bone marrow
- CNI
calcineurin inhibitor
- COX
cyclooxygenase
- CsA
cyclosporine A
- CTLA4
cytotoxic T lymphocyte Ag 4
- DC
dendritic cell
- DCreg
regulatory dendritic cell
- DST
donor-specific transfusion
- EAE
experimental allergic encephalomyelitis
- GC
glucocorticoids
- GM-CSF
granulocyte macrophage colony stimulating factor
- GMP
good manufacturing practice
- GVHD
graft-versus-host disease
- HDAC
histone deacetylase
- IFN
interferon
- IL
interleukin
- ILT2
inhibitory receptor Ig-like transcript 2
- IS
immunosuppressant/immunosuppressive
- i.v.
intravenous
- LPS
lipopolysaccharide
- MDSC
myeloid-derived suppressor cell
- M-CSF
macrophage colony stimulating factor
- MHC
major histocompatibility complex
- MMF
mycophenolate mofetil
- Mϕ
macrophage
- MPA
mycophenolic acid
- Mreg
regulatory macrophage
- mTOR
mechanistic target of rapamycin
- MyD88
myeloid differentiation primary response gene 88
- NFAT
nuclear factor of activated T cells
- NHP
non-human primate
- NK
natural killer
- PBMC
peripheral blood mononuclear cell
- PG
prostaglandin
- RAPA
rapamycin
- RMC
regulatory myeloid cell
- TAIC
transplant acceptance-inducing cell
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
- Tx
transplantation
- vitD3
vitamin D3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BRR, DR-R, HRT and AWT each contributed to the writing and editing of the manuscript.
Disclosure
One of the authors (AWT) is co-inventor of US patents for generation of regulatory dendritic cells to promote transplant tolerance.
REFERENCES
- 1.Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4(3):378. doi: 10.1111/j.1600-6143.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 2.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351(26):2715. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 3.Wood KJ, Bushell A, Hester J. Regulatory immune cells in transplantation. Nat Rev Immunol. 2012;12(6):417. doi: 10.1038/nri3227. [DOI] [PubMed] [Google Scholar]
- 4.Hutchinson JA, Riquelme P, Geissler EK. Human regulatory macrophages as a cell-based medicinal product. Curr Opin Organ Transplant. 2012;17(1):48. doi: 10.1097/MOT.0b013e32834ee64a. [DOI] [PubMed] [Google Scholar]
- 5.Broichhausen C, Riquelme P, Geissler EK, Hutchinson JA. Regulatory macrophages as therapeutic targets and therapeutic agents in solid organ transplantation. Curr Opin Organ Transplant. 2012;17(4):332. doi: 10.1097/MOT.0b013e328355a979. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 7.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 8.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 9.Belz GT, Nutt SL. Transcriptional programming of the dendritic cell network. Nat Rev Immunol. 2012;12(2):101. doi: 10.1038/nri3149. [DOI] [PubMed] [Google Scholar]
- 10.Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol. 2004;4(1):24. doi: 10.1038/nri1256. [DOI] [PubMed] [Google Scholar]
- 11.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7(8):610. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 12.Stenger EO, Turnquist HR, Mapara MY, Thomson AW. Dendritic cells and regulation of graft-versus-host disease and graft-versus-leukemia activity. Blood. 2012;119(22):5088. doi: 10.1182/blood-2011-11-364091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Kooten C, Lombardi G, K.A. G, et al. Dendritic cells as a tool to induce transplantation tolerance: obstacles and opportunities. Transplantation. 2011;91:2. doi: 10.1097/tp.0b013e31820263b3. [DOI] [PubMed] [Google Scholar]
- 16.Kalantari T, Kamali-Sarvestani E, Ciric B, et al. Generation of immunogenic and tolerogenic clinical-grade dendritic cells. Immunol Res. 2011;51(2–3):153. doi: 10.1007/s12026-011-8255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutchinson JA, Riquelme P, Sawitzki B, et al. Cutting Edge: Immunological consequences and trafficking of human regulatory macrophages administered to renal transplant recipients. J Immunol. 2011;187(5):2072. doi: 10.4049/jimmunol.1100762. [DOI] [PubMed] [Google Scholar]
- 18.Ezzelarab M, Thomson AW. Tolerogenic dendritic cells and their role in transplantation. Seminars in Immunology. 2011;23(4):252. doi: 10.1016/j.smim.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piemonti L, Monti P, Sironi M, et al. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2000;164(9):4443. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- 20.Pedersen AE, Gad M, Walter MR, Claesson MH. Induction of regulatory dendritic cells by dexamethasone and 1alpha,25-Dihydroxyvitamin D(3) Immunol Lett. 2004;91(1):63. doi: 10.1016/j.imlet.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Anderson AE, Sayers BL, Haniffa MA, et al. Differential regulation of naive and memory CD4+ T cells by alternatively activated dendritic cells. J Leukoc Biol. 2008;84(1):124. doi: 10.1189/jlb.1107744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregori S, Tomasoni D, Pacciani V, et al. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood. 2010;116(6):935. doi: 10.1182/blood-2009-07-234872. [DOI] [PubMed] [Google Scholar]
- 23.Macedo C, Turquist H, Metes D, Thomson AW. Immunoregulatory properties of rapamycin-conditioned monocyte-derived dendritic cells and their role in transplantation. Transplant Res. 2012;1(1):16. doi: 10.1186/2047-1440-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zahorchak AF, Kean LS, Tokita D, et al. Infusion of stably immature monocyte-derived dendritic cells plus CTLA4Ig modulates alloimmune reactivity in rhesus macaques. Transplantation. 2007;84(2):196. doi: 10.1097/01.tp.0000268582.21168.f6. [DOI] [PubMed] [Google Scholar]
- 25.Ezzelarab M, Zahorchak AF, Lu L, et al. Regulatory dendritic cell infusion prolongs kidney allograft survival in non-human primates. Am J Transplant. doi: 10.1111/ajt.12310. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreau A, Varey E, Bouchet-Delbos L, Cuturi MC. Cell therapy using tolerogenic dendritic cells in transplantation. Transplant Res. 2012;1(1):13. doi: 10.1186/2047-1440-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solari MG, Thomson AW. Human dendritic cells and transplant outcome. Transplantation. 2008;85(11):1513. doi: 10.1097/TP.0b013e318173a768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harry RA, Anderson AE, Isaacs JD, Hilkens CM. Generation and characterisation of therapeutic tolerogenic dendritic cells for rheumatoid arthritis. Ann Rheum Dis. 2010;69(11):2042. doi: 10.1136/ard.2009.126383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raich-Regue D, Grau-Lopez L, Naranjo-Gomez M, et al. Stable antigen-specific T-cell hyporesponsiveness induced by tolerogenic dendritic cells from multiple sclerosis patients. Eur J Immunol. 2012;42(3):771. doi: 10.1002/eji.201141835. [DOI] [PubMed] [Google Scholar]
- 30.Boros P, Ochando JC, Chen SH, Bromberg JS. Myeloid-derived suppressor cells: natural regulators for transplant tolerance. Hum Immunol. 2010;71(11):1061. doi: 10.1016/j.humimm.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marigo I, Bosio E, Solito S, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32(6):790. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Highfill SL, Rodriguez PC, Zhou Q, et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood. 2010;116(25):5738. doi: 10.1182/blood-2010-06-287839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol. 2010;185(4):2273. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Obermajer N, Kalinski P. Generation of myeloid-derived suppressor cells using prostaglandin E2. Transplant Res. 2012;1(1):15. doi: 10.1186/2047-1440-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riquelme P, Tomiuk S, Kammler A, et al. IFN-gamma-induced iNOS expression in mouse regulatory macrophages prolongs allograft survival in fully immunocompetent recipients. Mol Ther. 2013;21(2):409. doi: 10.1038/mt.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riquelme P, Geissler EK, Hutchinson JA. Alternative approaches to myeloid suppressor cell therapy in transplantation: comparing regulatory macrophages to tolerogenic DCs and MDSCs. Transplant Res. 2012;1(1):17. doi: 10.1186/2047-1440-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hutchinson JA, Roelen D, Riquelme P, et al. Preoperative treatment of a presensitized kidney transplant recipient with donor-derived transplant acceptance-inducing cells. Transpl Int. 2008;21(8):808. doi: 10.1111/j.1432-2277.2008.00712.x. [DOI] [PubMed] [Google Scholar]
- 38.Beriou G, Moreau A, Cuturi MC. Tolerogenic dendritic cells: applications for solid organ transplantation. Curr Opin Organ Transplant. 2012;17(1):42. doi: 10.1097/MOT.0b013e32834ee662. [DOI] [PubMed] [Google Scholar]
- 39.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel pathway for initiation of rejection. J Exp Med. 1990;171(1):307. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Demetris AJ, Murase N, Starzl TE. Donor dendritic cells after liver and heart allotransplantation under short-term immunosuppression. Lancet. 1992;339(8809):1610. doi: 10.1016/0140-6736(92)91875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu G, Xu X, Vu MD, Kilpatrick ED, Li XC. NK cells promote transplant tolerance by killing donor antigen-presenting cells. J Exp Med. 2006;203(8):1851. doi: 10.1084/jem.20060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Divito SJ, Wang Z, Shufesky WJ, et al. Endogenous dendritic cells mediate the effects of intravenously injected therapeutic immunosuppressive dendritic cells in transplantation. Blood. 2010;116(15):2694. doi: 10.1182/blood-2009-10-251058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beriou G, Peche H, Guillonneau C, Merieau E, Cuturi MC. Donor-specific allograft tolerance by administration of recipient-derived immature dendritic cells and suboptimal immunosuppression. Transplantation. 2005;79(8):969. doi: 10.1097/01.tp.0000158277.50073.35. [DOI] [PubMed] [Google Scholar]
- 44.Moreau A, Hill M, Thebault P, et al. Tolerogenic dendritic cells actively inhibit T cells through heme oxygenase-1 in rodents and in nonhuman primates. FASEB Journal. 2009;23(9):3070. doi: 10.1096/fj.08-128173. [DOI] [PubMed] [Google Scholar]
- 45.Hill M, Thebault P, Segovia M, et al. Cell therapy with autologous tolerogenic dendritic cells induces allograft tolerance through interferon-gamma and epstein-barr virus-induced gene 3. Am J Transplant. 2011;11(10):2036. doi: 10.1111/j.1600-6143.2011.03651.x. [DOI] [PubMed] [Google Scholar]
- 46.Movahedi K, Guilliams M, Van den Bossche J, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111(8):4233. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 47.Nagaraj S, Gupta K, Pisarev V, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13(7):828. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caquard M, Ferret-Bernard S, Haurogne K, et al. Diabetes acceleration by cyclophosphamide in the non-obese diabetic mouse is associated with differentiation of immunosuppressive monocytes into immunostimulatory cells. Immunol Lett. 2010;129(2):85. doi: 10.1016/j.imlet.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 49.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181(8):5791. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kusmartsev S, Nagaraj S, Gabrilovich DI. Tumor-associated CD8+ T cell tolerance induced by bone marrow-derived immature myeloid cells. J Immunol. 2005;175(7):4583. doi: 10.4049/jimmunol.175.7.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Obermajer N, Muthuswamy R, Lesnock J, Edwards RP, Kalinski P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood. 2011;118(20):5498. doi: 10.1182/blood-2011-07-365825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu B, Bando Y, Xiao S, et al. CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J Immunol. 2007;179(8):5228. doi: 10.4049/jimmunol.179.8.5228. [DOI] [PubMed] [Google Scholar]
- 53.Hutchinson JA, Brem-Exner BG, Riquelme P, et al. A cell-based approach to the minimization of immunosuppression in renal transplantation. Transpl Int. 2008;21(8):742. doi: 10.1111/j.1432-2277.2008.00692.x. [DOI] [PubMed] [Google Scholar]
- 54.Lu L, Li W, Fu F, et al. Blockade of the CD40-CD40 ligand pathway potentiates the capacity of donor-derived dendritic cell progenitors to induce long-term cardiac allograft survival. Transplantation. 1997;64(12):1808. doi: 10.1097/00007890-199712270-00031. [DOI] [PubMed] [Google Scholar]
- 55.DePaz HA, Oluwole OO, Adeyeri AO, et al. Immature rat myeloid dendritic cells generated in low-dose granulocyte macrophage-colony stimulating factor prolong donor-specific rat cardiac allograft survival. Transplantation. 2003;75(4):521. doi: 10.1097/01.TP.0000048380.84355.4A. [DOI] [PubMed] [Google Scholar]
- 56.Wang Q, Zhang M, Ding G, et al. Anti-ICAM-1 antibody and CTLA-4Ig synergistically enhance immature dendritic cells to induce donor-specific immune tolerance in vivo. Immunol Lett. 2003;90(1):33. doi: 10.1016/s0165-2478(03)00160-3. [DOI] [PubMed] [Google Scholar]
- 57.Garrovillo M, Ali A, Depaz HA, et al. Induction of transplant tolerance with immunodominant allopeptide-pulsed host lymphoid and myeloid dendritic cells. Am J Transplant. 2001;1(2):129. [PubMed] [Google Scholar]
- 58.Mirenda V, Berton I, Read J, et al. Modified dendritic cells coexpressing self and allogeneic major histocompatibility complex molecules: an efficient way to induce indirect pathway regulation. J Am Soc Nephrol. 2004;15(4):987. doi: 10.1097/01.asn.0000119575.98696.1d. [DOI] [PubMed] [Google Scholar]
- 59.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193(2):233. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dhodapkar MV, Steinman RM. Antigen-bearing immature dendritic cells induce peptide-specific CD8(+) regulatory T cells in vivo in humans. Blood. 2002;100(1):174. doi: 10.1182/blood.v100.1.174. [DOI] [PubMed] [Google Scholar]
- 61.Solito S, Bronte V, Mandruzzato S. Antigen specificity of immune suppression by myeloid-derived suppressor cells. J Leukoc Biol. 2011;90(1):31. doi: 10.1189/jlb.0111021. [DOI] [PubMed] [Google Scholar]
- 62.Kerr EC, Raveney BJ, Copland DA, Dick AD, Nicholson LB. Analysis of retinal cellular infiltrate in experimental autoimmune uveoretinitis reveals multiple regulatory cell populations. J Autoimmun. 2008;31(4):354. doi: 10.1016/j.jaut.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 63.Marhaba R, Vitacolonna M, Hildebrand D, Baniyash M, Freyschmidt-Paul P, Zoller M. The importance of myeloid-derived suppressor cells in the regulation of autoimmune effector cells by a chronic contact eczema. J Immunol. 2007;179(8):5071. doi: 10.4049/jimmunol.179.8.5071. [DOI] [PubMed] [Google Scholar]
- 64.Yin B, Ma G, Yen CY, et al. Myeloid-derived suppressor cells prevent type 1 diabetes in murine models. J Immunol. 2010;185(10):5828. doi: 10.4049/jimmunol.0903636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lees JR, Azimzadeh AM, Bromberg JS. Myeloid derived suppressor cells in transplantation. Curr Opin Immunol. 2011;23(5):692. doi: 10.1016/j.coi.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 66.Garcia MR, Ledgerwood L, Yang Y, et al. Monocytic suppressive cells mediate cardiovascular transplantation tolerance in mice. J Clin Invest. 2010;120(7):2486. doi: 10.1172/JCI41628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garrod KR, Chang CK, Liu FC, Brennan TV, Foster RD, Kang SM. Targeted lymphoid homing of dendritic cells is required for prolongation of allograft survival. J Immunol. 2006;177(2):863. doi: 10.4049/jimmunol.177.2.863. [DOI] [PubMed] [Google Scholar]
- 68.Takayama T, Morelli AE, Onai N, et al. Mammalian and viral IL-10 enhance C-C chemokine receptor 5 but down-regulate C-C chemokine receptor 7 expression by myeloid dendritic cells: impact on chemotactic responses and in vivo homing ability. J Immunol. 2001;166(12):7136. doi: 10.4049/jimmunol.166.12.7136. [DOI] [PubMed] [Google Scholar]
- 69.Leishman AJ, Silk KM, Fairchild PJ. Pharmacological manipulation of dendritic cells in the pursuit of transplantation tolerance. Curr Opin Organ Transplant. 2011;16(4):372. doi: 10.1097/MOT.0b013e3283484b42. [DOI] [PubMed] [Google Scholar]
- 70.Taner T, Hackstein H, Wang Z, Morelli AE, Thomson AW. Rapamycin-treated, alloantigen-pulsed host dendritic cells induce Ag-specific T cell regulation and prolong graft survival. Am J Transplant. 2005;5(2):228. doi: 10.1046/j.1600-6143.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- 71.Peche H, Trinite B, Martinet B, Cuturi MC. Prolongation of heart allograft survival by immature dendritic cells generated from recipient type bone marrow progenitors. Am J Transplant. 2005;5(2):255. doi: 10.1111/j.1600-6143.2004.00683.x. [DOI] [PubMed] [Google Scholar]
- 72.Reichardt W, Durr C, von Elverfeldt D, et al. Impact of mammalian target of rapamycin inhibition on lymphoid homing and tolerogenic function of nanoparticle-labeled dendritic cells following allogeneic hematopoietic cell transplantation. J Immunol. 2008;181(7):4770. doi: 10.4049/jimmunol.181.7.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Emmer PM, van der Vlag J, Adema GJ, Hilbrands LB. Dendritic cells activated by lipopolysaccharide after dexamethasone treatment induce donor-specific allograft hyporesponsiveness. Transplantation. 2006;81(10):1451. doi: 10.1097/01.tp.0000208801.51222.bd. [DOI] [PubMed] [Google Scholar]
- 74.Ridolfi R, Riccobon A, Galassi R, et al. Evaluation of in vivo labelled dendritic cell migration in cancer patients. J Transl Med. 2004;2(1):27. doi: 10.1186/1479-5876-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang W, Liang S, Wu J, Horuzsko A. Human inhibitory receptor immunoglobulin-like transcript 2 amplifies CD11b+Gr1+ myeloid-derived suppressor cells that promote long-term survival of allografts. Transplantation. 2008;86(8):1125. doi: 10.1097/TP.0b013e318186fccd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang B, Lei Z, Zhao J, et al. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett. 2007;252(1):86. doi: 10.1016/j.canlet.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 77.Markiewski MM, DeAngelis RA, Benencia F, et al. Modulation of the antitumor immune response by complement. Nat Immunol. 2008;9(11):1225. doi: 10.1038/ni.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang H, Arp J, Liu W, et al. Inhibition of terminal complement components in presensitized transplant recipients prevents antibody-mediated rejection leading to long-term graft survival and accommodation. J Immunol. 2007;179(7):4451. doi: 10.4049/jimmunol.179.7.4451. [DOI] [PubMed] [Google Scholar]
- 79.Zhou Z, French DL, Ma G, et al. Development and function of myeloid-derived suppressor cells generated from mouse embryonic and hematopoietic stem cells. Stem Cells. 2010;28(3):620. doi: 10.1002/stem.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Wilde V, Van Rompaey N, Hill M, et al. Endotoxin-induced myeloid-derived suppressor cells inhibit alloimmune responses via heme oxygenase-1. Am J Transplant. 2009;9(9):2034. doi: 10.1111/j.1600-6143.2009.02757.x. [DOI] [PubMed] [Google Scholar]
- 81.Ezernitchi AV, Vaknin I, Cohen-Daniel L, et al. TCR zeta down-regulation under chronic inflammation is mediated by myeloid suppressor cells differentially distributed between various lymphatic organs. J Immunol. 2006;177(7):4763. doi: 10.4049/jimmunol.177.7.4763. [DOI] [PubMed] [Google Scholar]
- 82.Dugast AS, Haudebourg T, Coulon F, et al. Myeloid-derived suppressor cells accumulate in kidney allograft tolerance and specifically suppress effector T cell expansion. J Immunol. 2008;180(12):7898. [Google Scholar]
- 83.Dilek N, Poirier N, Usal C, Martinet B, Blancho G, Vanhove B. Control of transplant tolerance and intragraft regulatory T cell localization by myeloid-derived suppressor cells and CCL5. J Immunol. 2012;188(9):4209. doi: 10.4049/jimmunol.1101512. [DOI] [PubMed] [Google Scholar]
- 84.Hock BD, Mackenzie KA, Cross NB, et al. Renal transplant recipients have elevated frequencies of circulating myeloid-derived suppressor cells. Nephrol Dial Transplant. 2012;27(1):402. doi: 10.1093/ndt/gfr264. [DOI] [PubMed] [Google Scholar]
- 85.Abe M, Thomson AW. Influence of immunosuppressive drugs on dendritic cells. Transpl Immunol. 2003;11(3–4):357. doi: 10.1016/S0966-3274(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 86.Svajger U, Obermajer N, Jeras M. Novel findings in drug-induced dendritic cell tolerogenicity. Int Rev Immunol. 2010;29(6):574. doi: 10.3109/08830185.2010.522280. [DOI] [PubMed] [Google Scholar]
- 87.Kooten C, Stax AS, Woltman AM, Gelderman KA. In: Handbook of Experimental Pharmacology "Dendritic Cells". Lombardi G, Riffo-Vasquez Y, editors. Berlin, Heidelberg: Springer Berlin Heidelberg; 2013. [Google Scholar]
- 88.Piemonti L, Monti P, Allavena P, et al. Glucocorticoids affect human dendritic cell differentiation and maturation. J Immunol. 1999;162(11):6473. [PubMed] [Google Scholar]
- 89.Zheng G, Zhong S, Geng Y, et al. Dexamethasone promotes tolerance in vivo by enriching CD11clo CD40lo tolerogenic macrophages. Eur J Immunol. 2013;43(1):219. doi: 10.1002/eji.201242468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang K, Bai X, Li R, et al. Endogenous glucocorticoids promote the expansion of myeloid-derived suppressor cells in a murine model of trauma. Int J Mol Med. 2012;30(2):277. doi: 10.3892/ijmm.2012.1014. [DOI] [PubMed] [Google Scholar]
- 91.Varga G, Ehrchen J, Tsianakas A, et al. Glucocorticoids induce an activated, anti-inflammatory monocyte subset in mice that resembles myeloid-derived suppressor cells. J Leukoc Biol. 2008;84(3):644. doi: 10.1189/jlb.1107768. [DOI] [PubMed] [Google Scholar]
- 92.Gustafson MP, Lin Y, New KC, et al. Systemic immune suppression in glioblastoma: the interplay between CD14+HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro Oncol. 2010;12(7):631. doi: 10.1093/neuonc/noq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee YR, Yang IH, Lee YH, et al. Cyclosporin A and tacrolimus, but not rapamycin, inhibit MHC-restricted antigen presentation pathways in dendritic cells. Blood. 2005;105(10):3951. doi: 10.1182/blood-2004-10-3927. [DOI] [PubMed] [Google Scholar]
- 94.Lee YH, Lee YR, Im SA, et al. Calcineurin inhibitors block MHC-restricted antigen presentation in vivo. J Immunol. 2007;179(9):5711. doi: 10.4049/jimmunol.179.9.5711. [DOI] [PubMed] [Google Scholar]
- 95.Jennings C, Kusler B, Jones PP. Calcineurin inactivation leads to decreased responsiveness to LPS in macrophages and dendritic cells and protects against LPS-induced toxicity in vivo. Innate Immun. 2009;15(2):109. doi: 10.1177/1753425908100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Damoiseaux JG, Beijleveld LJ, van Breda Vriesman PJ. Quantification and phenotypic characterization of the rat thymic dendritic cell population upon in vivo cyclosporine administration. Transplant Proc. 1993;25(5):2814. [PubMed] [Google Scholar]
- 97.Rezzani R, Rodella L, Corsetti G, Ventura RG. Effects of cyclosporin A on some accessory cells of rat thymus. Int J Exp Pathol. 1995;76(4):247. [PMC free article] [PubMed] [Google Scholar]
- 98.De Waal EJ, Rademakers LH, Schuurman HJ, Vos JG, Van Loveren H. Alterations of dendritic cells in the rat thymus without epithelial cell loss during cyclosporine treatment and recovery. Toxicology. 1996;110(1–3):133. doi: 10.1016/0300-483x(96)03332-x. [DOI] [PubMed] [Google Scholar]
- 99.Ma A, Qi S, Xu D, Daloze P, Chen H. Immunological evaluation of combination therapy with tacrolimus and sirolimus on long-term allograft survival in nonhuman primates. Transplant Proc. 2005;37(1):150. doi: 10.1016/j.transproceed.2004.12.275. [DOI] [PubMed] [Google Scholar]
- 100.Li J, Chen G, Ye P, et al. CCR5 blockade in combination with cyclosporine increased cardiac graft survival and generated alternatively activated macrophages in primates. J Immunol. 2011;186(6):3753. doi: 10.4049/jimmunol.1002143. [DOI] [PubMed] [Google Scholar]
- 101.Tajima K, Amakawa R, Ito T, Miyaji M, Takebayashi M, Fukuhara S. Immunomodulatory effects of cyclosporin A on human peripheral blood dendritic cell subsets. Immunology. 2003;108(3):321. doi: 10.1046/j.1365-2567.2003.01585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barten MJ, Garbade J, Bittner HB, et al. Affects of immunosuppression on circulating dendritic cells: an adjunct to therapeutic drug monitoring after heart transplantation. Int Immunopharmacol. 2006;6(13–14):2011. doi: 10.1016/j.intimp.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 103.Kim YS, Kim YJ, Lee JM, et al. Functional changes in myeloid-derived suppressor cells (MDSCs) during tumor growth: FKBP51 contributes to the regulation of the immunosuppressive function of MDSCs. J Immunol. 2012;188(9):4226. doi: 10.4049/jimmunol.1103040. [DOI] [PubMed] [Google Scholar]
- 104.Fric J, Lim CX, Koh EG, et al. Calcineurin/NFAT signalling inhibits myeloid haematopoiesis. EMBO Mol Med. 2012;4(4):269. doi: 10.1002/emmm.201100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fulton B, Markham A. Mycophenolate mofetil. A review of its pharmacodynamic and pharmacokinetic properties and clinical efficacy in renal transplantation. Drugs. 1996;51(2):278. doi: 10.2165/00003495-199651020-00007. [DOI] [PubMed] [Google Scholar]
- 106.Colic M, Stojic-Vukanic Z, Pavlovic B, Jandric D, Stefanoska I. Mycophenolate mofetil inhibits differentiation, maturation and allostimulatory function of human monocyte-derived dendritic cells. Clin Exp Immunol. 2003;134(1):63. doi: 10.1046/j.1365-2249.2003.02269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cicinnati VR, Hou J, Lindemann M, et al. Mycophenolic acid impedes the antigen presenting and lymph node homing capacities of human blood myeloid dendritic cells. Transplantation. 2009;88(4):504. doi: 10.1097/TP.0b013e3181b0e608. [DOI] [PubMed] [Google Scholar]
- 108.Lagaraine C, Hoarau C, Chabot V, Velge-Roussel F, Lebranchu Y. Mycophenolic acid-treated human dendritic cells have a mature migratory phenotype and inhibit allogeneic responses via direct and indirect pathways. Int Immunol. 2005;17(4):351. doi: 10.1093/intimm/dxh215. [DOI] [PubMed] [Google Scholar]
- 109.Mehling A, Grabbe S, Voskort M, Schwarz T, Luger TA, Beissert S. Mycophenolate mofetil impairs the maturation and function of murine dendritic cells. J Immunol. 2000;165(5):2374. doi: 10.4049/jimmunol.165.5.2374. [DOI] [PubMed] [Google Scholar]
- 110.Augustine JJ, Bodziak KA, Hricik DE. Use of sirolimus in solid organ transplantation. Drugs. 2007;67(3):369. doi: 10.2165/00003495-200767030-00004. [DOI] [PubMed] [Google Scholar]
- 111.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177(12):8338. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 112.Horibe EK, Sacks J, Unadkat J, et al. Rapamycin-conditioned, alloantigen-pulsed dendritic cells promote indefinite survival of vascularized skin allografts in association with T regulatory cell expansion. Transpl Immunol. 2008;18(4):307. doi: 10.1016/j.trim.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 113.Fischer R, Turnquist HR, Taner T, Thomson AW. Use of rapamycin in the induction of tolerogenic dendritic cells. Handb Exp Pharmacol. 2009;(188):215. doi: 10.1007/978-3-540-71029-5_10. [DOI] [PubMed] [Google Scholar]
- 114.Woltman AM, de Fijter JW, Kamerling SW, et al. Rapamycin induces apoptosis in monocyte- and CD34-derived dendritic cells but not in monocytes and macrophages. Blood. 2001;98(1):174. doi: 10.1182/blood.v98.1.174. [DOI] [PubMed] [Google Scholar]
- 115.Haidinger M, Poglitsch M, Geyeregger R, et al. A versatile role of mammalian target of rapamycin in human dendritic cell function and differentiation. J Immunol. 2010;185(7):3919. doi: 10.4049/jimmunol.1000296. [DOI] [PubMed] [Google Scholar]
- 116.Weichhart T, Haidinger M, Katholnig K, et al. Inhibition of mTOR blocks the anti-inflammatory effects of glucocorticoids in myeloid immune cells. Blood. 2011;117(16):4273. doi: 10.1182/blood-2010-09-310888. [DOI] [PubMed] [Google Scholar]
- 117.Turnquist HR, Cardinal J, Macedo C, et al. mTOR and GSK-3 shape the CD4+ T cell stimulatory and differentiation capacity of myeloid DC following exposure to LPS. Blood. 2010;115:4758. doi: 10.1182/blood-2009-10-251488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Macedo C, Turnquist HR, Rosborough BR, Thomson AW, Metes D. Augmented IL-12p70 and IL-27 secretion by mTOR1-inhibited human monocyte-derived DC promotes allogeneic Type-1 polarization regulated by NK cells. Am J Transplant. doi: 10.1111/ajt.12351. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sathaliyawala T, O'Gorman WE, Greter M, et al. Mammalian target of rapamycin controls dendritic cell development downstream of Flt3 ligand signaling. Immunity. 2010;33(4):597. doi: 10.1016/j.immuni.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Reddy P, Sun Y, Toubai T, et al. Histone deacetylase inhibition modulates indoleamine 2,3-dioxygenase-dependent DC functions and regulates experimental graft-versus-host disease in mice. J Clin Invest. 2008;118(7):2562. doi: 10.1172/JCI34712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Song W, Tai YT, Tian Z, et al. HDAC inhibition by LBH589 affects the phenotype and function of human myeloid dendritic cells. Leukemia. 2011;25(1):161. doi: 10.1038/leu.2010.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Frikeche J, Simon T, Brissot E, Gregoire M, Gaugler B, Mohty M. Impact of valproic acid on dendritic cells function. Immunobiology. 2012;217(7):704. doi: 10.1016/j.imbio.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 123.Sebastian C, Serra M, Yeramian A, Serrat N, Lloberas J, Celada A. Deacetylase activity is required for STAT5-dependent GM-CSF functional activity in macrophages and differentiation to dendritic cells. J Immunol. 2008;180(9):5898. doi: 10.4049/jimmunol.180.9.5898. [DOI] [PubMed] [Google Scholar]
- 124.Wang Y, Camirand G, Lin Y, et al. Regulatory T cells require mammalian target of rapamycin signaling to maintain both homeostasis and alloantigen-driven proliferation in lymphocyte-replete mice. J Immunol. 2011;186(5):2809. doi: 10.4049/jimmunol.0903805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu C, Li A, Leng Y, Li Y, Kang J. Histone deacetylase inhibition by sodium valproate regulates polarization of macrophage subsets. DNA Cell Biol. 2012;31(4):592. doi: 10.1089/dna.2011.1401. [DOI] [PubMed] [Google Scholar]
- 126.Rosborough BR, Castellaneta A, Natarajan S, Thomson AW, Turnquist HR. Histone deacetylase inhibition facilitates GM-CSF-mediated expansion of myeloid-derived suppressor cells in vitro and in vivo. J Leukoc Biol. 2012;91(5):701. doi: 10.1189/jlb.0311119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Subklewe M, Sebelin-Wulf K, Beier C, et al. Dendritic cell maturation stage determines susceptibility to the proteasome inhibitor bortezomib. Hum Immunol. 2007;68(3):147. doi: 10.1016/j.humimm.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 128.Vodanovic-Jankovic S, Hari P, Jacobs P, Komorowski R, Drobyski WR. NF-kappaB as a target for the prevention of graft-versus-host disease: comparative efficacy of bortezomib and PS-1145. Blood. 2006;107(2):827. doi: 10.1182/blood-2005-05-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sun K, Welniak LA, Panoskaltsis-Mortari A, et al. Inhibition of acute graft-versus-host disease with retention of graft-versus-tumor effects by the proteasome inhibitor bortezomib. Proc Natl Acad Sci U S A. 2004;101(21):8120. doi: 10.1073/pnas.0401563101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zinser E, Rossner S, Littmann L, Luftenegger D, Schubert U, Steinkasserer A. Inhibition of the proteasome influences murine and human dendritic cell development in vitro and in vivo. Immunobiology. 2009;214(9–10):843. doi: 10.1016/j.imbio.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 131.Nencioni A, Garuti A, Schwarzenberg K, et al. Proteasome inhibitor-induced apoptosis in human monocyte-derived dendritic cells. Eur J Immunol. 2006;36(3):681. doi: 10.1002/eji.200535298. [DOI] [PubMed] [Google Scholar]
- 132.Min WP, Zhou D, Ichim TE, et al. Inhibitory feedback loop between tolerogenic dendritic cells and regulatory T cells in transplant tolerance. J Immunol. 2003;170(3):1304. doi: 10.4049/jimmunol.170.3.1304. [DOI] [PubMed] [Google Scholar]
- 133.Yang J, Bernier SM, Ichim TE, et al. LF15-0195 generates tolerogenic dendritic cells by suppression of NF-kappaB signaling through inhibition of IKK activity. J Leukoc Biol. 2003;74(3):438. doi: 10.1189/jlb.1102582. [DOI] [PubMed] [Google Scholar]
- 134.Zeyda M, Kirsch BM, Geyeregger R, et al. Inhibition of human dendritic cell maturation and function by the novel immunosuppressant FK778. Transplantation. 2005;80(8):1105. doi: 10.1097/01.tp.0000178301.19732.a1. [DOI] [PubMed] [Google Scholar]
- 135.Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32(1):19. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fujita M, Kohanbash G, Fellows-Mayle W, et al. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res. 2011;71(7):2664. doi: 10.1158/0008-5472.CAN-10-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ma N, Szabolcs MJ, Sun J, et al. The effect of selective inhibition of cyclooxygenase (COX)-2 on acute cardiac allograft rejection. Transplantation. 2002;74(11):1528. doi: 10.1097/00007890-200212150-00009. [DOI] [PubMed] [Google Scholar]
- 138.Ogawa M, Suzuki J, Kosuge H, Takayama K, Nagai R, Isobe M. The mechanism of anti-inflammatory effects of prostaglandin E2 receptor 4 activation in murine cardiac transplantation. Transplantation. 2009;87(11):1645. doi: 10.1097/TP.0b013e3181a5c84c. [DOI] [PubMed] [Google Scholar]
- 139.Naujokat C, Berges C, Fuchs D, Sadeghi M, Opelz G, Daniel V. Antithymocyte globulins suppress dendritic cell function by multiple mechanisms. Transplantation. 2007;83(4):485. doi: 10.1097/01.tp.0000251975.81281.22. [DOI] [PubMed] [Google Scholar]
- 140.Gillet-Hladky S, de Carvalho CM, Bernaud J, Bendahou C, Bloy C, Rigal D. Rabbit antithymocyte globulin inhibits monocyte-derived dendritic cells maturation in vitro and polarizes monocyte-derived dendritic cells towards tolerogenic dendritic cells expressing indoleamine 2,3-dioxygenase. Transplantation. 2006;82(7):965. doi: 10.1097/01.tp.0000235549.47976.d0. [DOI] [PubMed] [Google Scholar]
- 141.Buggins AG, Mufti GJ, Salisbury J, et al. Peripheral blood but not tissue dendritic cells express CD52 and are depleted by treatment with alemtuzumab. Blood. 2002;100(5):1715. [PubMed] [Google Scholar]
- 142.Auffermann-Gretzinger S, Eger L, Schetelig J, Bornhauser M, Heidenreich F, Ehninger G. Alemtuzumab depletes dendritic cells more effectively in blood than in skin: a pilot study in patients with chronic lymphocytic leukemia. Transplantation. 2007;83(9):1268. doi: 10.1097/01.tp.0000260433.86776.ec. [DOI] [PubMed] [Google Scholar]
- 143.Kirsch BM, Haidinger M, Zeyda M, et al. Alemtuzumab (Campath-1H) induction therapy and dendritic cells: Impact on peripheral dendritic cell repertoire in renal allograft recipients. Transpl Immunol. 2006;16(3–4):254. doi: 10.1016/j.trim.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 144.Mnasria K, Lagaraine C, Manaa J, Lebranchu Y, Oueslati R. Anti CD25 treatment of human dendritic cells modulates both their cytokine synthesis profiles and their capacity to activate allogeneic CD4 T cells: a potential tolerogenic effect. Int Immunopharmacol. 2008;8(3):414. doi: 10.1016/j.intimp.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 145.Mnasria K, Lagaraine C, Velge-Roussel F, Lebranchu Y, Baron C. Anti-CD25 antibodies decrease the ability of human dendritic cells to prime allogeneic CD4 T cells. Transplant Proc. 2009;41(2):695. doi: 10.1016/j.transproceed.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 146.Wuest SC, Edwan JH, Martin JF, et al. A role for interleukin-2 trans-presentation in dendritic cell-mediated T cell activation in humans, as revealed by daclizumab therapy. Nat Med. 2011;17(5):604. doi: 10.1038/nm.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Medina-Echeverz J, Fioravanti J, Zabala M, Ardaiz N, Prieto J, Berraondo P. Successful colon cancer eradication after chemoimmunotherapy is associated with profound phenotypic change of intratumoral myeloid cells. J Immunol. 2011;186(2):807. doi: 10.4049/jimmunol.1001483. [DOI] [PubMed] [Google Scholar]
- 148.Pilat N, Schwarz C, Wekerle T. Modulating T-cell costimulation as new immunosuppressive concept in organ transplantation. Curr Opin Organ Transplant. 2012;17(4):368. doi: 10.1097/MOT.0b013e328355fc94. [DOI] [PubMed] [Google Scholar]
- 149.Kinnear G, Jones ND, Wood KJ. Costimulation blockade: current perspectives and implications for therapy. Transplantation. 2013;95(4):527. doi: 10.1097/TP.0b013e31826d4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Grohmann U, Orabona C, Fallarino F, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3(11):1097. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 151.Guillot C, Menoret S, Guillonneau C, et al. Active suppression of allogeneic proliferative responses by dendritic cells after induction of long-term allograft survival by CTLA4Ig. Blood. 2003;101(8):3325. doi: 10.1182/blood-2002-07-2076. [DOI] [PubMed] [Google Scholar]
- 152.Mayer E, Holzl M, Ahmadi S, et al. CTLA4-Ig immunosuppressive activity at the level of dendritic cell/T cell crosstalk. Int Immunopharmacol. 2013;15(3):638. doi: 10.1016/j.intimp.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu Y, Yu Y, Yang S, et al. Regulation of arginase I activity and expression by both PD-1 and CTLA-4 on the myeloid-derived suppressor cells. Cancer Immunol Immunother. 2009;58(5):687. doi: 10.1007/s00262-008-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Aoyagi T, Yamashita K, Suzuki T, et al. A human anti-CD40 monoclonal antibody, 4D11, for kidney transplantation in cynomolgus monkeys: induction and maintenance therapy. Am J Transplant. 2009;9(8):1732. doi: 10.1111/j.1600-6143.2009.02693.x. [DOI] [PubMed] [Google Scholar]
- 155.Pearson TC, Trambley J, Odom K, et al. Anti-CD40 therapy extends renal allograft survival in rhesus macaques. Transplantation. 2002;74(7):933. doi: 10.1097/00007890-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 156.Niimi M, Shirasugi N, Ikeda Y, Kan S, Takami H, Hamano K. Operational tolerance induced by pretreatment with donor dendritic cells under blockade of CD40 pathway. Transplantation. 2001;72(9):1556. doi: 10.1097/00007890-200111150-00014. [DOI] [PubMed] [Google Scholar]
- 157.Fu W, Zhu J, Qiu Y, Li W. Induction of CD4+CD25+ T cells and control of cardiac allograft rejection by CD40/CD40L costimulatory pathway blockade in mice. Transplant Proc. 2013;45(2):611. doi: 10.1016/j.transproceed.2012.10.044. [DOI] [PubMed] [Google Scholar]
- 158.Zhang X, Beduhn M, Zheng X, et al. Induction of alloimmune tolerance in heart transplantation through gene silencing of TLR adaptors. Am J Transplant. 2012;12(10):2675. doi: 10.1111/j.1600-6143.2012.04196.x. [DOI] [PubMed] [Google Scholar]
- 159.Adeegbe D, Serafini P, Bronte V, Zoso A, Ricordi C, Inverardi L. In vivo induction of myeloid suppressor cells and CD4(+)Foxp3(+) T regulatory cells prolongs skin allograft survival in mice. Cell Transplant. 2011;20(6):941. doi: 10.3727/096368910X540621. [DOI] [PubMed] [Google Scholar]
- 160.Luyckx A, Schouppe E, Rutgeerts O, et al. G-CSF stem cell mobilization in human donors induces polymorphonuclear and mononuclear myeloid-derived suppressor cells. Clin Immunol. 2012;143(1):83. doi: 10.1016/j.clim.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 161.Giannoukakis N, Phillips B, Finegold D, Harnaha J, Trucco M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care. 2011;34(9):2026. doi: 10.2337/dc11-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hutchinson JA, Riquelme P, Brem-Exner BG, et al. Transplant acceptance-inducing cells as an immune-conditioning therapy in renal transplantation. Transpl Int. 2008;21(8):728. doi: 10.1111/j.1432-2277.2008.00680.x. [DOI] [PubMed] [Google Scholar]
- 163.Chou HS, Hsieh CC, Charles R, et al. Myeloid-derived suppressor cells protect islet transplants by B7-H1 mediated enhancement of T regulatory cells. Transplantation. 2012;93(3):272. doi: 10.1097/TP.0b013e31823ffd39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Adorini L, Penna G. Induction of tolerogenic dendritic cells by vitamin D receptor agonists. Handb Exp Pharmacol. 2009;(188):251. doi: 10.1007/978-3-540-71029-5_12. [DOI] [PubMed] [Google Scholar]
- 165.Pedersen AW, Claesson MH, Zocca MB. Dendritic cells modified by vitamin D: future immunotherapy for autoimmune diseases. Vitam Horm. 2011;86:63. doi: 10.1016/B978-0-12-386960-9.00003-4. [DOI] [PubMed] [Google Scholar]
- 166.Young MR, Lozano Y, Ihm J, Wright MA, Prechel MM. Vitamin D3 treatment of tumor bearers can stimulate immune competence and reduce tumor growth when treatment coincides with a heightened presence of natural suppressor cells. Cancer Lett. 1996;104(2):153. doi: 10.1016/0304-3835(96)04241-3. [DOI] [PubMed] [Google Scholar]
- 167.Prechel MM, Lozano Y, Wright MA, Ihm J, Young MR. Immune modulation by interleukin-12 in tumor-bearing mice receiving vitamin D3 treatments to block induction of immunosuppressive granulocyte/macrophage progenitor cells. Cancer Immunol Immunother. 1996;42(4):213. doi: 10.1007/s002620050273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wiers K, Wright MA, Vellody K, Young MR. Failure of tumor-reactive lymph node cells to kill tumor in the presence of immune-suppressive CD34+ cells can be overcome with vitamin D3 treatment to diminish CD34+ cell levels. Clin Exp Metastasis. 1998;16(3):275. doi: 10.1023/a:1006501110857. [DOI] [PubMed] [Google Scholar]
- 169.Knight SC, Roberts M, Macatonia SE, Edwards AJ. Blocking of acquisition and presentation of antigen by dendritic cells with cyclosporine. Studies with fluorescein isothiocyanate. Transplantation. 1988;46(2 Suppl):48S. doi: 10.1097/00007890-198808001-00010. [DOI] [PubMed] [Google Scholar]
- 170.Tourneur E, Ben Mkaddem S, Chassin C, et al. Cyclosporine A impairs nucleotide binding oligomerization domain (Nod1)-mediated innate antibacterial renal defenses in mice and human transplant recipients. PLoS Pathog. 2013;9(1):e1003152. doi: 10.1371/journal.ppat.1003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Setkowicz Z, Caryk M, Szafraniec M, Zmudzinska A, Janeczko K. Tacrolimus (FK506) and cyclosporin A reduce macrophage recruitment to the rat brain injured at perinatal and early postnatal periods. Neurol Res. 2009;31(10):1060. doi: 10.1179/174313209X383295. [DOI] [PubMed] [Google Scholar]
- 172.Wollenberg A, Sharma S, von Bubnoff D, Geiger E, Haberstok J, Bieber T. Topical tacrolimus (FK506) leads to profound phenotypic and functional alterations of epidermal antigen-presenting dendritic cells in atopic dermatitis. J Allergy Clin Immunol. 2001;107(3):519. doi: 10.1067/mai.2001.112942. [DOI] [PubMed] [Google Scholar]
- 173.Jiang S, Tang Q, Rong R, et al. Mycophenolate mofetil inhibits macrophage infiltration and kidney fibrosis in long-term ischemia-reperfusion injury. Eur J Pharmacol. 2012;688(1–3):56. doi: 10.1016/j.ejphar.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 174.Iwamoto S, Azuma E, Kumamoto T, et al. Efficacy of azithromycin in preventing lethal graft-versus-host disease. Clin Exp Immunol. 2013;171(3):338. doi: 10.1111/cei.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Vos IH, Govers R, Grone HJ, et al. NFkappaB decoy oligodeoxynucleotides reduce monocyte infiltration in renal allografts. FASEB J. 2000;14(5):815. doi: 10.1096/fasebj.14.5.815. [DOI] [PubMed] [Google Scholar]
- 176.Fang L, Fehse B, Engel M, Zander A, Kroger N. Antithymocyte globulin induces ex vivo and in vivo depletion of myeloid and plasmacytoid dendritic cells. Transplantation. 2005;79(3):369. doi: 10.1097/01.tp.0000150210.77543.1b. [DOI] [PubMed] [Google Scholar]