Abstract
The structurally simple glycero- and sphingo-phospholipids, lysophosphatidic acid (LPA) and sphingosine-1-phosphate (S1P), serve as important receptor-active mediators that influence blood and vascular cell function and are positioned to influence the events that contribute to the progression and complications of atherosclerosis. Growing evidence from preclinical, animal models has implicated LPA, LPA receptors, and key enzymes involved in LPA metabolism in pathophysiologic events that may underlie atherosclerotic vascular disease. These observations are supported by genetic analysis in humans implicating a lipid phosphate phosphatase as a novel risk factor for coronary artery disease. In this review we summarize current understanding of LPA production, metabolism and signaling as may be relevant for atherosclerotic and other vascular disease.
Keywords: Lysophosphatidic acid, autotaxin, lipid phosphate phosphatase, atherosclerosis
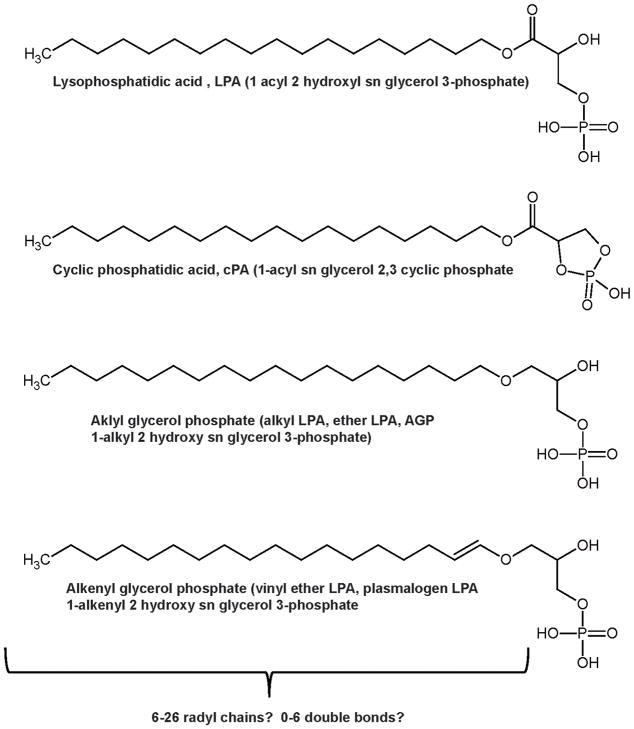
Lysophosphatidic acid (LPA), sphingosine-1-phosphate (S1P), and several other related molecules constitute a family of bioactive lipid phosphoric acids that function as receptor-active mediators to play important roles in cell growth, differentiation, apoptosis and development.1, 2 LPA and S1P are structurally simple glycero- and sphingo-phospholipids. LPA is a common intracellular intermediate in the synthesis of triglycerides and glycerophospholipids. The process by which these “housekeeping” lipids acquired functions as extracellular mediators remains a matter for debate but as discussed in further detail below, at least in the case of LPA separation of the synthesis, actions and inactivation of signaling LPA from the role of this lipid in intracellular metabolism, is accomplished by physical and functional compartmentalization of the key enzymes and receptors. The molecular species diversity of LPA is considerably more complex than S1P because of variations in the chain length, saturation, and linkage of the radyl hydrocarbon substituent (Figure 1). In particular, LPA species containing an ether substituted hydrocarbon chain, which are more properly referred to as alkyl glycerol phosphates, have potent effects at particular LPA receptors suggesting that structural diversity in this class of molecules may have important biological consequences. Plasmalogen LPA species and cyclic phosphatidic acid have also been reported in mammalian systems. The latter molecule, which may be formed by intramolecular transphosphatidylation catalyzed by phospholipase D, is particularly intriguing but relatively understudied.
Figure 1. Structure of LPA and related molecules.
LPA or 1-acyl 2 hydroxy sn glycerol 3-phosphate (top) has potent signaling effects imparted by cell surface receptors and is also a key intermediate in phospholipid and triglyceride synthesis. The length and degree of saturation of the acyl chains in LPA can vary. The broad LPA “family” also includes lysoglycerophospholipids that differ in the nature of the linkage between the acyl chains and the glycerol phosphate backbone. Both alkyl glycerol phosphate or alkyl LPA (middle) and alkenyl glycerol phosphate or plasmalogen LPA (bottom), contain an ether bond between the acyl chain and the glycerol phosphate backbone. LPA subclasses with -acyl-, alkenyl- and alkyl linkages and differing acyl chains exhibit distinctive pharmacological properties and can show different selectivity G-protein coupled receptors.
Pathways for synthesis, signaling, and degradation of LPA
Physiologically relevant levels of LPA can be detected in a variety of biological fluids including the blood, most convincingly using HPLC and tandem mass spectrometry methods which have been reported by a number of groups.3 LPA in blood plasma is predominantly bound to serum albumin4 and can also be detected in lipoprotein fractions, in contrast to S1P, which is predominantly associated with lipoproteins and may be carried specifically by HDL associated apolipoprotein M.5 Studies using stable or radioactive isotope-labeled tracers reveal that intravenously administered LPA is rapidly eliminated from the circulation,6, 7 which at least in mice, can be accounted for by a process involving rapid uptake in the liver.8 This observation, coupled with observations that plasma LPA levels decline with similar rapidity after pharmacological inhibition of plasma LPA synthesis, have led to the conclusion that LPA undergoes rapid turn-over in the circulation.
The secreted lysophospholipase D (lysoPLD) autotaxin (ATX) generates extracellular LPA by hydrolysis of circulating or locally produced lysophosphatidylcholine (lysoPC).9, 10 ATX is a widely expressed gene that is essential for early development of the vasculature.11, 12 Consequently, because efforts to date to generate mice with tissue-specific inactivation of this gene have been inconclusive, the relative contributions of different cell types as sources for circulating ATX are presently unclear. A particular interesting possibility is adipocytes are a major source of circulating ATX. Cultured adipocytes express and release ATX and circulating LPA and ATX levels in humans are positively correlated with body mass index.13–15 Furthermore, mice lacking ATX in adipose tissue have reduced plasma ATX and LPA levels.16 Conversely, ATX in fat and ATX and LPA in plasma increases in mice following high fat diet feeding. (16 and unpublished observations by FY and SSS). Lysophospholipids, most prominently lysoPC, are abundant in plasma and associated with plasma lipoproteins. Although it can be generated as a byproduct of cholesterol esterification in the liver, lysoPC substrate for ATX is also formed by the action of A-type phospholipases (PLA), which are present in plasma and released during platelet activation.17, 18 We and others have shown that ATX binds blood and vascular cell integrins and that the interaction may be important for localized generation of LPA and/or targeting the enzyme to the surface of cells to produce cell specific responses.19–21 Thus, in addition to being present in plasma, LPA can also be generated dynamically in the vicinity of blood and vascular cells through regulated mechanisms involving cell surface recruitment of ATX.
The effects of extracellular LPA are largely mediated by members of a family of G-protein-coupled receptors with at least six bona fide members (LPA1–6 receptors).1, 2, 22 Additionally, LPA binds with high affinity to the immunoglobulin family receptor for advanced glycan endproducts (RAGE), which may also contribute to cell-surface receptor-mediated LPA signaling.23 Recombinant LPA receptors exhibit different selectivity for LPA species; how the properties of LPA influence biologic responses is not well understood. As mentioned above, LPA is also an obligatory intermediate in intracellular phospholipid synthesis and intracellular LPA generated by the glycerol-3-phosphate pathway, or perhaps cyclic phosphatidic acid formed by phospholipase D may signal via nuclear peroxisome proliferator receptor γ (PPARγ).24, 25
Almost every subtype of primary or cultured blood and vascular cells exhibits some type of response to LPA and/or ATX. For example, LPA triggers vascular smooth muscular cell (SMC) de-differentiation and (because LPA is present in serum at high concentrations) may be a key factor in the serum response for phenotypic modulation of vascular SMCs.2*8 LPA, a potent trigger for Rho activation, promotes endothelial cell migration and has been variably associated with increasing or decreasing endothelial barrier function, with most of the reports supporting the latter response.29–31 ATX expression in endothelial cells can be elicited by VEGF32–35, and exposure to ATX increases angiogenesis into Matrigel implants.32 LPA downregulates endothelial CD36 and may thereby blunt the inhibitory effects of thrombospondin on angiogenesis. In addition to promoting inflammatory responses on endothelial cells and leukocytes (reviewed in),36–38 LPA triggers neutral lipid accumulation in monocytes.39 LPA is a weak platelet activator40 but potently stimulates fibronectin-matrix assembly on platelets,41 a process that may be important for enhancing thrombus formation.
A role for ATX in vascular development has been established in zebrafish and mice. In mice, inherited deficiency of Enpp2 encoding ATX results in embryonic lethality, in part due to failure of formation of yolk sac and embryonic vasculature.11, 12 The Zebrafish genome contains ATX paralogs with high homology to the mammalian protein and lysoPLD activity capable of generating LPA in vitro.42 Zebrafish embryos injected with antisense morpholino oligonucleotides that target expression of ATX display vascular defects, in particular incomplete formation of the intersegmental vessels that sprout from the dorsal aorta.42 The phenotype was recapitulated in morphant embryos targeting both LPA receptors 1 and 4, suggesting that these receptors are mediators of LPA-dependent effects of ATX that are required for proper vascular endothelial cell migration during vascular development.
The phenotype of mice lacking LPA receptors is not as striking as that observed in Enpp2−/− animals1, 43 A subset of Lpar4−/− mice on a C57Bl/6 background exhibit embryonic lethality in association with edema and hemorrhage.44 Angiogenesis in Matrigel implants is impaired in adult Lpar4−/− mice, suggesting that LPA4 receptor may account for at least a portion of the LPA/ATX signaling necessary for post-development blood vessel formation in these animals. Some Lpar1−/− mice develop frontal hematomas but this phenotype that is not exacerbated by combined deficiency of both Lpar1 and Lpar2.45
Local infusion of LPA and, more markedly, alkylglycerolphosphate in the ligated carotid arteries of rats and mice, elicits the development of neointimal hyperplasia.46 In mice, this response is attenuated by genetic deficiency of Pparγ−/− but not of LPA receptors 1 and 2, which is paradoxical because other genetic and pharmacological data identify a normally protective role for PPARγ in models of vascular injury and atherosclerosis.47, 48 We and others have reported that mice, lacking combinations of LPA receptors 1 – 3, display alterations in the response to vascular injury and subsequent vessel remodeling.49, 50 In our hands, the lack of LPA1 receptor exaggerated the positive remodeling response in injured femoral arteries. Interestingly a similar phenotype of excessive vascular remodeling is observed in mice lacking Gα12/Gα13 or their effector, the RhoGEF protein LARG.51 Taken together, these observations underscore the complex interactions between receptors mediating LPA-dependent signaling responses in the vasculature where a combination of apparent redundancy (and perhaps compensatory changes in response to genetic deficiency of one or more of these receptors), continues to make it challenging to ascribe vascular signaling responses to particular LPA receptor classes or subtypes.
The signaling actions of LPA can be terminated by removal of the phosphate group, which generates the corresponding alcohols that are no longer agonists for LPA receptors. Lipid phosphate phosphatases (LPPs) constitute a family of three enzymes that dephosphorylate a broad range of lipid phosphates, including LPA and S1P and these enzymes are members of a larger family of integral membrane enzymes with intra and extracellular roles in lipid metabolism and signaling.52–55 LPPs are encoded by the PPAP2 genes: LPP1 by the PPAP2A gene, LPP2 by the PPAP2C gene, and LPP3 by the PPAP2B gene.56 LPPs localize to both the plasma membrane and intracellular membrane organelles, in particular the endoplasmic reticulum and Golgi apparatus.54, 56, 57 They are predicted to have a core of six transmembrane spanning helical regions with the N- and C-termini in the cytoplasmic side of the membrane. Based on this predicted topology and biochemical analysis, the active site of these enzymes is oriented on the extracellular (or luminal) face of the membrane.
Although the three LPP enzymes demonstrate similar catalytic activities and substrate preferences in vitro, the phenotypes of mice with targeted inactivation of the Ppap2 genes imply that they have non-redundant functions both during development and after birth. Mice with deficiency of Ppap2c58 or harboring an exon trap inactivated allele of Ppap2a that results in mosaic hypomorphism for LPP1 appear phenotypically normal although the latter animals were reported to have higher plasma LPA levels.7 In contrast, Ppap2b−/− embryos are not viable and display defects in extra-embryonic vascular development.59 Media from Ppap2b-null mouse embryonic fibroblasts contains 2.6 fold higher levels of LPA than is found in media from wild-type cells. Using mice with tissue-specific inactivation of Ppap2b, we observed that LPP3 functions as an intrinsic negative regulator of SMC function.60 Studies of cultured aortic SMCs indicates that increased LPA accumulation and signaling may be account, at least in part, for differences in Ppap2b−/− deficient cells. Results in endothelial-specific knockdown mice also support a role for LPP3 in maintaining vascular integrity both during development and postnatally. The ability of ATX inhibitors and LPA receptor antagonists to blunt the phenotype observed in Ppap2b-null mice indicates that enhanced LPA signaling may partially account enhanced permeability.
LPA accumulates in atherosclerotic plaque and may promote experimental atherosclerosis
Human atheroma contains higher levels of LPA than are found in normal healthy blood vessels and LPA accumulates predominantly in the lipid rich core.61 Multiple LPA species can be detected by mass spectrometry based analysis of lipid extracts from human and induced murine atheromas and atheroma associated LPA was suggested to play a role in platelet activation after plaque rupture.62 Consistent with these observations, plaque-associated LPA was visualized in the core of atheromas by time of flight, single ion mass spectrometry in carotid arteries from Ldlr−/− mice following Western diet feeding and collar placement.63 In advanced lesions in Ldlr−/− mice, LPA levels increase ~20 fold with a particularly prominent accumulation of unsaturated long-chain acyl-LPA species. The changes in LPA levels in plaque are paralleled by alterations in enzymes that generate LPA and in LPA receptors.64 However, at present the precise pathway(s) for LPA generation in atherosclerotic lesions (in particular the relative contributions of LPA generation in situ versus accumulation of LPA from circulating sources) is not known. In support of the concept that LPA present in plaques may have pro-atherosclerotic, pro-inflammatory, and pro-thrombotic effects, exogenous administration of LPA heightens atherosclerotic plaque burden in Apoe−/− mice in an LPA1- and LPA3-receptor dependent manner.65 Additionally, perivascular administration of LPA promotes the recruitment of inflammatory cells, in particular mast cells, to atherosclerotic plaques in carotid arteries and resulted in intraplaque hemorrhage.63
Sampling of blood from coronary arteries at the time of acute myocardial infarction reveals higher local levels of LPA, which correlate with markers of platelet activation such as sCD40L. 66 Hyperlipidemia may also increase steady state levels of LPA in plasma and/or enhance the capacity for LPA synthesis. Studies in rabbits suggest that systemic LPA levels may be influenced by cholesterol feeding which elevates plasma LPC levels and heightens the generation of LPA in serum.67 Recent work suggests a link between levels of LPA in small intestine and experimental atherosclerosis. Feeding mice a diet supplemented with unsaturated LPA mimicked the inflammatory effects of Western diet.68 Interestingly, the effects of the LPA diet could be blocked by an apoA-I mimetic peptide 6F, which binds with high affinity to LPA and potently reduces atherosclerosis in Ldlr−/− mice.69–71 Together, these findings support the contention that LPA is positioned to play a central role in atherothrombotic disease.71 However, generation of definitive data on the role of LPA in experimental models of atherosclerosis have been hampered by redundancy among LPA receptors, an obligatory roles for genes encoding enzymes that generate and inactivate LPA in early development, and a lack of potent selective small molecules targeting these receptors and enzymes. Several promising experimental therapeutics that target the ATX/LPA nexus have recently been reported and should provide tools to establish a role in atherosclerosis in both animals and potentially in humans.
Association of a common polymorphism in PPAP2B with human coronary artery disease
The evidence described in the preceding paragraphs strongly supports a role for LPA and its G-protein-coupled receptors in the development of experimental atherosclerosis in animal models. Investigation of the genetics of coronary artery disease in humans suggests that the findings in mice may be translatable. Approximately 60% of the inter-individual variability in cardiovascular disease risk in humans is estimated to be heritable. Genome-wide association studies (GWAS)72 and a GWAS meta-analysis, identified heritable single nucleotide polymorphisms in the PPAP2B gene encoding LPP3 as a novel loci associated with coronary artery disease (CAD) susceptibility.73 In the meta-analysis of more than 86,000 individuals, the PPAP2B locus independently predicted CAD (odds ratio 1.17; P = 3.81 X 10−19) and lacked association with traditional risk factors such as hypertension, cholesterol, diabetes, obesity or smoking. The major risk allele, rs17114036A allele, is located in the final intron of the six exon PPAP2B gene. A least seven SNPs are in robust linkage disequilibrium (r2>0.9) with rs17114036.74 Although data for rs17114036 are not available in publicly accessible data sets, query of the SNPExpress database for the proxy SNP rs9970807 (r2 of 0.901 with rs17114036) predicts that the major risk-associated allele is associated with lower leukocyte mRNA expression for most of the PPAP2B exons, and two other proxy SNPs show similar patterns. Overall, these results suggest that the major allele of rs9970807 and therefore rs17114036 might be associated with decreased expression of LPP3 protein. To characterize the role of candidate genes in atherosclerosis, Erbilqin et al., performed expression quantitative locus (eQTL) mapping in endothelial cells and observed an upregulation of Ppap2b expression in aortic endothelial cells isolated from four-week-old Apoe−/− mice and in atherosclerotic lesions obtained from 24–week-old Apoe−/− mice.75 Taken together, these observations support the testable hypothesis that LPP3 serves to suppress atherosclerosis and/or stabilize atherosclerotic plaques and that individuals with diminished LPP3 expression are at heightened risk for the development and complications of atherosclerosis.
Ongoing and published studies support a role for LPP3 as a negative regulator of LPA signaling, likely by counteracting localized LPA synthesis or decreasing extracellular LPA levels. Evidence for effects of LPPs on both cell-specific and cell-autonomous signaling responses comes from studies in Drosophila development in which expression of LPPs encoded by the two wunen genes in both somatic and germ cells, is necessary for proper germ-cell guidance and survival in the developing embryo.76 Because wunen mutations can be complemented by mammalian LPP3 it seems likely that LPP3 expression in mammalian systems will also result in a combination of cell-specific and cell-autonomous effects on LPA production and signaling. For example, in the complex setting of atherosclerosis, cardiovascular disease risk-associated alterations in expression of LPP3 in vascular endothelial cells, vascular smooth muscle cells and monocytes might result in heightened cell-specific signaling responses to LPA during lesion formation, as well as cell-type independent increases in accumulation of LPA in the developing atheroma. If the primary mechanism accounting for alterations in expression of LPP3 on human cardiovascular disease risk stems from a role of this enzyme in attenuating cell-type specific LPA signaling responses or accumulation of LPA in vascular lesions, then pharmacologic approaches that inhibit ATX activity or block the signaling responses mediated by LPA receptors, would also be expected to reduce atherosclerosis in humans. These ideas also support the view that adipose-derived ATX could serve as a novel mediator of accelerated atherosclerosis that is associated with obesity (Figure 2). However, it is important to note that additional non-LPA dependent mechanisms could contribute to a role for LPP3 in limiting atherosclerosis. For example, LPP3 also hydrolyses S1P and changes in S1P signaling could impact vessel responses to injury. LPP3 may77 also have roles in regulating intracellular lipid metabolism and signaling that are not directly related to effects on cell-surface receptor-mediated LPA responses. LPP3 has also been suggested to have non-enzymatic functions that may be mediated by integrin binding or catenin signaling.59, 78, 79 Notwithstanding these caveats, determining the mechanism by which the risk-associated allele of PPAP2B predicts CAD risk is now an important goal to both validate this new genetic marker as a biomarker of disease risk and to provide insights into the basic mechanisms of CAD that may be used to improve therapeutic strategies to prevent its development and complications.
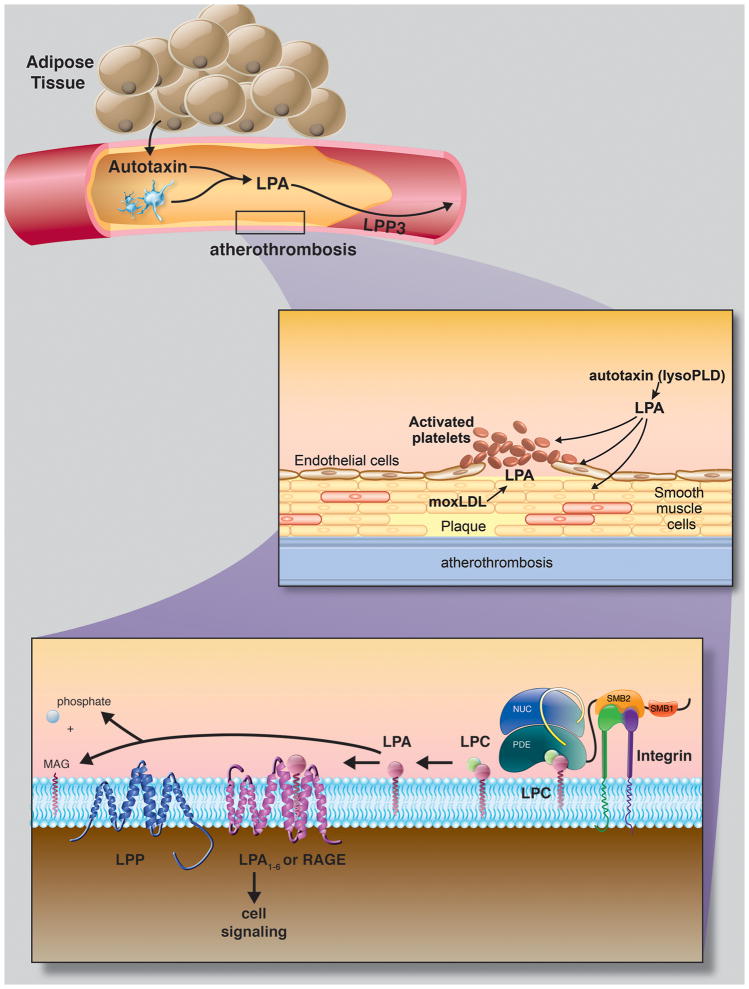
Figure 2. Proposed model for autotaxin – LPA- LPP3 nexus in atherosclerosis and it complications.
Top: At least a portion of circulating autotaxin may derive from adipose tissue. Middle: Autotaxin-derived LPA has potent effects on a number of blood and vascular cells that could potentially contribute to biologic events that underlie atherosclerosis. Additionally, atherosclerotic plaque is enriched in LPA. Bottom: circulating and/or secreted autotaxin may interact with integrins and through non-integrin mediated mechanisms to generate a local gradient of LPA. LPA signaling through G-protein couple receptors LPA1–6 and possible RAGE may be terminated by enzymatic dephosphorylation catalyzed by LPPs. Whether autotaxin promotes and LPP3 suppresses atherosclerosis remains to be established. Illustration by Matt Hazzard, University of Kentucky, Information Technology.
Significance.
A significant proportion of the risk of coronary artery disease is inherited. Efforts to understand the genetic basis of CAD have revealed a potential role for lysopholipid signaling in the development and/or complications of atherosclerosis. Growing evidence from experiment model systems supports a role for the autotaxin - lysophosphatidic acid (LPA) – lipid phosphate phosphate 3 system in regulating molecular events that may control blood and vascular cell involvement in atherosclerosis.
Acknowledgments
This material is based on work supported in part by resources at the Lexington VA Medical Center. This work was also supported in part by grants from the Heart Lung and Blood Institute (R01HL078663), the National Center for Research Resources (P20RR021954), and by an IDeA award from the National Institute of General Medical Sciences (P20GM103527), the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, ( UL1TR000117 and TL1TR000115). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- ATX
Autotaxin
- CAD
Coronary artery disease
- Eqtl
Expression quantitative locus
- GWAS
Genome-wide association studies
- HDL
High density lipoprotein
- HPLC
High-performance liquid chromatography
- LARG
Leukemia-associated RhoGEF protein
- LPA
Lysophosphatidic acid
- LPP3
Lipid phosphate phosphase 3
- LysoPC
Lysophosphatidyl choline
- LysoPLD
Lysophospholipase D
- PPARγ
Peroxisome proliferator receptor γ
- RAGE
Receptor for advanced glycan endproducts
- SIP
Sphingosine-1-phosphate
- SMC
Smooth muscle cell
- SNP
Single Nucleotide Polymorphism
Footnotes
Disclosures:
The authors have no conflicts of interest to report.
Literature
- 1.Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN, Chun J. Lpa receptors: Subtypes and biological actions. Annu Rev Pharmacol Toxicol. 2010;50:157–186. doi: 10.1146/annurev.pharmtox.010909.105753. [DOI] [PubMed] [Google Scholar]
- 2.Blaho VA, Hla T. Regulation of mammalian physiology, development, and disease by the sphingosine 1-phosphate and lysophosphatidic acid receptors. Chem Rev. 2011;111:6299–6320. doi: 10.1021/cr200273u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker DL, Umstot ES, Desiderio DM, Tigyi GJ. Quantitative analysis of lysophosphatidic acid in human blood fractions. Ann N Y Acad Sci. 2000;905:267–269. doi: 10.1111/j.1749-6632.2000.tb06557.x. [DOI] [PubMed] [Google Scholar]
- 4.Minnear FL, Patil S, Bell D, Gainor JP, Morton CA. Platelet lipid(s) bound to albumin increases endothelial electrical resistance: Mimicked by lpa. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1337–1344. doi: 10.1152/ajplung.2001.281.6.L1337. [DOI] [PubMed] [Google Scholar]
- 5.Christoffersen C, Obinata H, Kumaraswamy SB, Galvani S, Ahnstrom J, Sevvana M, Egerer-Sieber C, Muller YA, Hla T, Nielsen LB, Dahlback B. Endothelium-protective sphingosine-1-phosphate provided by hdl-associated apolipoprotein m. Proc Natl Acad Sci U S A. 2011;108:9613–9618. doi: 10.1073/pnas.1103187108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albers HM, Dong A, van Meeteren LA, Egan DA, Sunkara M, van Tilburg EW, Schuurman K, van Tellingen O, Morris AJ, Smyth SS, Moolenaar WH, Ovaa H. Boronic acid-based inhibitor of autotaxin reveals rapid turnover of lpa in the circulation. Proc Natl Acad Sci U S A. 2010;107:7257–7262. doi: 10.1073/pnas.1001529107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomsig JL, Snyder AH, Berdyshev EV, Skobeleva A, Mataya C, Natarajan V, Brindley DN, Lynch KR. Lipid phosphate phosphohydrolase type 1 (lpp1) degrades extracellular lysophosphatidic acid in vivo. Biochem J. 2009;419:611–618. doi: 10.1042/BJ20081888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salous AK, Panchatcharam M, Sunkara M, Mueller P, Dong A, Wang Y, Graf GA, Smyth SS, Morris AJ. Mechanism of rapid elimination of lysophosphatidic acid and related lipids from the circulation of mice. J Lipid Res. 2013;54:2775–2784. doi: 10.1194/jlr.M039685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moolenaar WH, Perrakis A. Insights into autotaxin: How to produce and present a lipid mediator. Nature reviews Molecular cell biology. 2011;12:674–679. doi: 10.1038/nrm3188. [DOI] [PubMed] [Google Scholar]
- 10.Hausmann J, Kamtekar S, Christodoulou E, et al. Structural basis of substrate discrimination and integrin binding by autotaxin. Nat Struct Mol Biol. 2011;18:198–204. doi: 10.1038/nsmb.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka M, Okudaira S, Kishi Y, Ohkawa R, Iseki S, Ota M, Noji S, Yatomi Y, Aoki J, Arai H. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem. 2006;281:25822–25830. doi: 10.1074/jbc.M605142200. [DOI] [PubMed] [Google Scholar]
- 12.van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, van Rooijen MA, Pradere JP, Pettit TR, Wakelam MJ, Saulnier-Blache JS, Mummery CL, Moolenaar WH, Jonkers J. Autotaxin, a secreted lysophospholipase d, is essential for blood vessel formation during development. Mol Cell Biol. 2006;26:5015–5022. doi: 10.1128/MCB.02419-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boucher J, Quilliot D, Praderes JP, Simon MF, Gres S, Guigne C, Prevot D, Ferry G, Boutin JA, Carpene C, Valet P, Saulnier-Blache JS. Potential involvement of adipocyte insulin resistance in obesity-associated up-regulation of adipocyte lysophospholipase d/autotaxin expression. Diabetologia. 2005;48:569–577. doi: 10.1007/s00125-004-1660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferry G, Tellier E, Try A, et al. Autotaxin is released from adipocytes, catalyzes lysophosphatidic acid synthesis, and activates preadipocyte proliferation. Up-regulated expression with adipocyte differentiation and obesity. J Biol Chem. 2003;278:18162–18169. doi: 10.1074/jbc.M301158200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simon MF, Daviaud D, Pradere JP, Gres S, Guigne C, Wabitsch M, Chun J, Valet P, Saulnier-Blache JS. Lysophosphatidic acid inhibits adipocyte differentiation via lysophosphatidic acid 1 receptor-dependent down-regulation of peroxisome proliferator-activated receptor gamma2. J Biol Chem. 2005;280:14656–14662. doi: 10.1074/jbc.M412585200. [DOI] [PubMed] [Google Scholar]
- 16.Dusaulcy R, Rancoule C, Gres S, Wanecq E, Colom A, Guigne C, van Meeteren LA, Moolenaar WH, Valet P, Saulnier-Blache JS. Adipose-specific disruption of autotaxin enhances nutritional fattening and reduces plasma lysophosphatidic acid. J Lipid Res. 2011;52:1247–1255. doi: 10.1194/jlr.M014985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolen AL, Naren AP, Yarlagadda S, Beranova-Giorgianni S, Chen L, Norman D, Baker DL, Rowland MM, Best MD, Sano T, Tsukahara T, Liliom K, Igarashi Y, Tigyi G. The phospholipase a1 activity of lysophospholipase a-i links platelet activation to lpa production during blood coagulation. J Lipid Res. 2011;52:958–970. doi: 10.1194/jlr.M013326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aoki J, Taira A, Takanezawa Y, Kishi Y, Hama K, Kishimoto T, Mizuno K, Saku K, Taguchi R, Arai H. Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J Biol Chem. 2002;277:48737–48744. doi: 10.1074/jbc.M206812200. [DOI] [PubMed] [Google Scholar]
- 19.Pamuklar Z, Federico L, Liu S, et al. Autotaxin/lysopholipase d and lysophosphatidic acid regulate murine hemostasis and thrombosis. J Biol Chem. 2009;284:7385–7394. doi: 10.1074/jbc.M807820200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fulkerson Z, Wu T, Sunkara M, Kooi CV, Morris AJ, Smyth SS. Binding of autotaxin to integrins localizes lysophosphatidic acid production to platelets and mammalian cells. J Biol Chem. 2011;286:34654–34663. doi: 10.1074/jbc.M111.276725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanda H, Newton R, Klein R, Morita Y, Gunn MD, Rosen SD. Autotaxin, an ectoenzyme that produces lysophosphatidic acid, promotes the entry of lymphocytes into secondary lymphoid organs. Nat Immunol. 2008;9:415–423. doi: 10.1038/ni1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moolenaar WH, Hla T. Snapshot: Bioactive lysophospholipids. Cell. 2012;148:378–378. e372. doi: 10.1016/j.cell.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rai V, Toure F, Chitayat S, Pei R, Song F, Li Q, Zhang J, Rosario R, Ramasamy R, Chazin WJ, Schmidt AM. Lysophosphatidic acid targets vascular and oncogenic pathways via rage signaling. J Exp Med. 2012;209:2339–2350. doi: 10.1084/jem.20120873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McIntyre TM, Pontsler AV, Silva AR, St Hilaire A, Xu Y, Hinshaw JC, Zimmerman GA, Hama K, Aoki J, Arai H, Prestwich GD. Identification of an intracellular receptor for lysophosphatidic acid (lpa): Lpa is a transcellular ppargamma agonist. Proc Natl Acad Sci U S A. 2003;100:131–136. doi: 10.1073/pnas.0135855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stapleton CM, Mashek DG, Wang S, Nagle CA, Cline GW, Thuillier P, Leesnitzer LM, Li LO, Stimmel JB, Shulman GI, Coleman RA. Lysophosphatidic acid activates peroxisome proliferator activated receptor-gamma in cho cells that over-express glycerol 3-phosphate acyltransferase-1. PLoS One. 2011;6:e18932. doi: 10.1371/journal.pone.0018932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ai S, Kuzuya M, Koike T, Asai T, Kanda S, Maeda K, Shibata T, Iguchi A. Rho-rho kinase is involved in smooth muscle cell migration through myosin light chain phosphorylation-dependent and independent pathways. Atherosclerosis. 2001;155:321–327. doi: 10.1016/s0021-9150(00)00585-2. [DOI] [PubMed] [Google Scholar]
- 27.Dulin NO, Orlov SN, Kitchen CM, Voyno-Yasenetskaya TA, Miano JM. G-protein-coupled-receptor activation of the smooth muscle calponin gene. Biochem J. 2001;357:587–592. doi: 10.1042/0264-6021:3570587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayashi K, Takahashi M, Nishida W, Yoshida K, Ohkawa Y, Kitabatake A, Aoki J, Arai H, Sobue K. Phenotypic modulation of vascular smooth muscle cells induced by unsaturated lysophosphatidic acids. Circ Res. 2001;89:251–258. doi: 10.1161/hh1501.094265. [DOI] [PubMed] [Google Scholar]
- 29.Schulze C, Smales C, Rubin LL, Staddon JM. Lysophosphatidic acid increases tight junction permeability in cultured brain endothelial cells. J Neurochem. 1997;68:991–1000. doi: 10.1046/j.1471-4159.1997.68030991.x. [DOI] [PubMed] [Google Scholar]
- 30.Neidlinger NA, Larkin SK, Bhagat A, Victorino GP, Kuypers FA. Hydrolysis of phosphatidylserine-exposing red blood cells by secretory phospholipase a2 generates lysophosphatidic acid and results in vascular dysfunction. J Biol Chem. 2006;281:775–781. doi: 10.1074/jbc.M505790200. [DOI] [PubMed] [Google Scholar]
- 31.Tager AM, LaCamera P, Shea BS, et al. The lysophosphatidic acid receptor lpa1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 32.Nam JH, Shin DH, Min JE, Ye SK, Jeon JH, Kim SJ. Ca2+ signaling induced by sphingosine 1-phosphate and lysophosphatidic acid in mouse b cells. Mol Cells. 2010;29:85–91. doi: 10.1007/s10059-010-0020-4. [DOI] [PubMed] [Google Scholar]
- 33.Ptaszynska MM, Pendrak ML, Bandle RW, Stracke ML, Roberts DD. Positive feedback between vascular endothelial growth factor-a and autotaxin in ovarian cancer cells. Mol Cancer Res. 2008;6:352–363. doi: 10.1158/1541-7786.MCR-07-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ptaszynska MM, Pendrak ML, Stracke ML, Roberts DD. Autotaxin signaling via lysophosphatidic acid receptors contributes to vascular endothelial growth factor-induced endothelial cell migration. Mol Cancer Res. 2010;8:309–321. doi: 10.1158/1541-7786.MCR-09-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Im E, Motiejunaite R, Aranda J, Park EY, Federico L, Kim TI, Clair T, Stracke ML, Smyth S, Kazlauskas A. Phospholipase cgamma activation drives increased production of autotaxin in endothelial cells and lysophosphatidic acid-dependent regression. Mol Cell Biol. 2010;30:2401–2410. doi: 10.1128/MCB.01275-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smyth SS, Cheng HY, Miriyala S, Panchatcharam M, Morris AJ. Roles of lysophosphatidic acid in cardiovascular physiology and disease. Biochim Biophys Acta. 2008;1781:563–570. doi: 10.1016/j.bbalip.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris AJ, Panchatcharam M, Cheng HY, Federico L, Fulkerson Z, Selim S, Miriyala S, Escalante-Alcalde D, Smyth SS. Regulation of blood and vascular cell function by bioactive lysophospholipids. J Thromb Haemost. 2009;7 (Suppl 1):38–43. doi: 10.1111/j.1538-7836.2009.03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morris AJ, Selim S, Salous A, Smyth SS. Blood relatives: Dynamic regulation of bioactive lysophosphatidic acid and sphingosine-1-phosphate metabolism in the circulation. Trends Cardiovasc Med. 2009;19:135–140. doi: 10.1016/j.tcm.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci U S A. 2004;101:11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haseruck N, Erl W, Pandey D, Tigyi G, Ohlmann P, Ravanat C, Gachet C, Siess W. The plaque lipid lysophosphatidic acid stimulates platelet activation and platelet-monocyte aggregate formation in whole blood: Involvement of p2y1 and p2y12 receptors. Blood. 2004;103:2585–2592. doi: 10.1182/blood-2003-04-1127. [DOI] [PubMed] [Google Scholar]
- 41.Olorundare OE, Peyruchaud O, Albrecht RM, Mosher DF. Assembly of a fibronectin matrix by adherent platelets stimulated by lysophosphatidic acid and other agonists. Blood. 2001;98:117–124. doi: 10.1182/blood.v98.1.117. [DOI] [PubMed] [Google Scholar]
- 42.Yukiura H, Hama K, Nakanaga K, Tanaka M, Asaoka Y, Okudaira S, Arima N, Inoue A, Hashimoto T, Arai H, Kawahara A, Nishina H, Aoki J. Autotaxin regulates vascular development via multiple lysophosphatidic acid (lpa) receptors in zebrafish. J Biol Chem. 2011;286:43972–43983. doi: 10.1074/jbc.M111.301093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mutoh T, Rivera R, Chun J. Insights into the pharmacological relevance of lysophospholipid receptors. Br J Pharmacol. 2012;165:829–844. doi: 10.1111/j.1476-5381.2011.01622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sumida H, Noguchi K, Kihara Y, et al. Lpa4 regulates blood and lymphatic vessel formation during mouse embryogenesis. Blood. 2010;116:5060–5070. doi: 10.1182/blood-2010-03-272443. [DOI] [PubMed] [Google Scholar]
- 45.Choi JW, Lee CW, Chun J. Biological roles of lysophospholipid receptors revealed by genetic null mice: An update. Biochim Biophys Acta. 2008;1781:531–539. doi: 10.1016/j.bbalip.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida K, Nishida W, Hayashi K, Ohkawa Y, Ogawa A, Aoki J, Arai H, Sobue K. Vascular remodeling induced by naturally occurring unsaturated lysophosphatidic acid in vivo. Circulation. 2003;108:1746–1752. doi: 10.1161/01.CIR.0000089374.35455.F3. [DOI] [PubMed] [Google Scholar]
- 47.Zhang C, Baker DL, Yasuda S, Makarova N, Balazs L, Johnson LR, Marathe GK, McIntyre TM, Xu Y, Prestwich GD, Byun HS, Bittman R, Tigyi G. Lysophosphatidic acid induces neointima formation through ppargamma activation. J Exp Med. 2004;199:763–774. doi: 10.1084/jem.20031619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng Y, Makarova N, Tsukahara R, Guo H, Shuyu E, Farrar P, Balazs L, Zhang C, Tigyi G. Lysophosphatidic acid-induced arterial wall remodeling: Requirement of ppargamma but not lpa1 or lpa2 gpcr. Cell Signal. 2009;21:1874–1884. doi: 10.1016/j.cellsig.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Panchatcharam M, Miriyala S, Yang F, Rojas M, End C, Vallant C, Dong A, Lynch K, Chun J, Morris AJ, Smyth SS. Lysophosphatidic acid receptors 1 and 2 play roles in regulation of vascular injury responses but not blood pressure. Circ Res. 2008;103:662–670. doi: 10.1161/CIRCRESAHA.108.180778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Subramanian P, Karshovska E, Reinhard P, Megens RT, Zhou Z, Akhtar S, Schumann U, Li X, van Zandvoort M, Ludin C, Weber C, Schober A. Lysophosphatidic acid receptors lpa1 and lpa3 promote cxcl12-mediated smooth muscle progenitor cell recruitment in neointima formation. Circ Res. 2010;107:96–105. doi: 10.1161/CIRCRESAHA.109.212647. [DOI] [PubMed] [Google Scholar]
- 51.Althoff TF, Albarran Juarez J, Troidl K, Tang C, Wang S, Wirth A, Takefuji M, Wettschureck N, Offermanns S. Procontractile g protein-mediated signaling pathways antagonistically regulate smooth muscle differentiation in vascular remodeling. J Exp Med. 2012;209:2277–2290. doi: 10.1084/jem.20120350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McDermott MI, Sigal YJ, Crump JS, Morris AJ. Enzymatic analysis of lipid phosphate phosphatases. Methods. 2006;39:169–179. doi: 10.1016/j.ymeth.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 53.Sciorra VA, Morris AJ. Roles for lipid phosphate phosphatases in regulation of cellular signaling. Biochim Biophys Acta. 2002;1582:45–51. doi: 10.1016/s1388-1981(02)00136-1. [DOI] [PubMed] [Google Scholar]
- 54.Pyne S, Long JS, Ktistakis NT, Pyne NJ. Lipid phosphate phosphatases and lipid phosphate signalling. Biochem Soc Trans. 2005;33:1370–1374. doi: 10.1042/BST0331370. [DOI] [PubMed] [Google Scholar]
- 55.Ren H, Panchatcharam M, Mueller P, Escalante-Alcalde D, Morris AJ, Smyth SS. Lipid phosphate phosphatase (lpp3) and vascular development. Biochim Biophys Acta. 2013;1831:126–132. doi: 10.1016/j.bbalip.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sigal YJ, McDermott MI, Morris AJ. Integral membrane lipid phosphatases/phosphotransferases: Common structure and diverse functions. Biochem J. 2005;387:281–293. doi: 10.1042/BJ20041771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brindley DN. Lipid phosphate phosphatases and related proteins: Signaling functions in development, cell division, and cancer. Journal of cellular biochemistry. 2004;92:900–912. doi: 10.1002/jcb.20126. [DOI] [PubMed] [Google Scholar]
- 58.Zhang N, Sundberg JP, Gridley T. Mice mutant for ppap2c, a homolog of the germ cell migration regulator wunen, are viable and fertile. Genesis. 2000;27:137–140. doi: 10.1002/1526-968x(200008)27:4<137::aid-gene10>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 59.Escalante-Alcalde D, Hernandez L, Le Stunff H, Maeda R, Lee HS, Jr, Gang C, Sciorra VA, Daar I, Spiegel S, Morris AJ, Stewart CL. The lipid phosphatase lpp3 regulates extra-embryonic vasculogenesis and axis patterning. Development. 2003;130:4623–4637. doi: 10.1242/dev.00635. [DOI] [PubMed] [Google Scholar]
- 60.Panchatcharam M, Miriyala S, Salous A, Wheeler J, Dong A, Mueller P, Sunkara M, Escalante-Alcalde D, Morris AJ, Smyth SS. Lipid phosphate phosphatase 3 negatively regulates smooth muscle cell phenotypic modulation to limit intimal hyperplasia. Arterioscler Thromb Vasc Biol. 2013;33:52–59. doi: 10.1161/ATVBAHA.112.300527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siess W, Zangl KJ, Essler M, Bauer M, Brandl R, Corrinth C, Bittman R, Tigyi G, Aepfelbacher M. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc Natl Acad Sci U S A. 1999;96:6931–6936. doi: 10.1073/pnas.96.12.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rother E, Brandl R, Baker DL, Goyal P, Gebhard H, Tigyi G, Siess W. Subtype-selective antagonists of lysophosphatidic acid receptors inhibit platelet activation triggered by the lipid core of atherosclerotic plaques. Circulation. 2003;108:741–747. doi: 10.1161/01.CIR.0000083715.37658.C4. [DOI] [PubMed] [Google Scholar]
- 63.Bot M, de Jager SC, MacAleese L, Lagraauw HM, van Berkel TJ, Quax PH, Kuiper J, Heeren RM, Biessen EA, Bot I. Lysophosphatidic acid triggers mast cell-driven atherosclerotic plaque destabilization by increasing vascular inflammation. J Lipid Res. 2013;54:1265–1274. doi: 10.1194/jlr.M032862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bot M, Bot I, Lopez-Vales R, van de Lest CH, Saulnier-Blache JS, Helms JB, David S, van Berkel TJ, Biessen EA. Atherosclerotic lesion progression changes lysophosphatidic acid homeostasis to favor its accumulation. Am J Pathol. 2010;176:3073–3084. doi: 10.2353/ajpath.2010.090009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou Z, Subramanian P, Sevilmis G, Globke B, Soehnlein O, Karshovska E, Megens R, Heyll K, Chun J, Saulnier-Blache JS, Reinholz M, van Zandvoort M, Weber C, Schober A. Lipoprotein-derived lysophosphatidic acid promotes atherosclerosis by releasing cxcl1 from the endothelium. Cell Metab. 2011;13:592–600. doi: 10.1016/j.cmet.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 66.Dohi T, Miyauchi K, Ohkawa R, et al. Increased lysophosphatidic acid levels in culprit coronary arteries of patients with acute coronary syndrome. Atherosclerosis. 2013;229:192–197. doi: 10.1016/j.atherosclerosis.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 67.Tokumura A, Kanaya Y, Kitahara M, Miyake M, Yoshioka Y, Fukuzawa K. Increased formation of lysophosphatidic acids by lysophospholipase d in serum of hypercholesterolemic rabbits. J Lipid Res. 2002;43:307–315. [PubMed] [Google Scholar]
- 68.Navab M, Hough G, Buga GM, et al. Transgenic 6f tomatoes act on the small intestine to prevent systemic inflammation and dyslipidemia caused by western diet and intestinally derived lysophosphatidic acid. J Lipid Res. 2013 doi: 10.1194/jlr.M042051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao F, Vasquez SX, Su F, et al. L-5f, an apolipoprotein a-i mimetic, inhibits tumor angiogenesis by suppressing vegf/basic fgf signaling pathways. Integr Biol (Camb) 2011;3:479–489. doi: 10.1039/c0ib00147c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Navab M, Reddy ST, Anantharamaiah GM, Imaizumi S, Hough G, Hama S, Fogelman AM. Intestine may be a major site of action for the apoa-i mimetic peptide 4f whether administered subcutaneously or orally. J Lipid Res. 2011;52:1200–1210. doi: 10.1194/jlr.M013144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Navab M, Reddy ST, Van Lenten BJ, Buga GM, Hough G, Wagner AC, Fogelman AM. High-density lipoprotein and 4f peptide reduce systemic inflammation by modulating intestinal oxidized lipid metabolism: Novel hypotheses and review of literature. Arterioscler Thromb Vasc Biol. 2012;32:2553–2560. doi: 10.1161/ATVBAHA.112.300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.A genome-wide association study in europeans and south Asians identifies five new loci for coronary artery disease. Nat Genet. 2011;43:339–344. doi: 10.1038/ng.782. [DOI] [PubMed] [Google Scholar]
- 73.Schunkert H, Konig IR, Kathiresan S, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43:333–338. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnson AD, Handsaker RE, Pulit SL, Nizzari MM, O'Donnell CJ, de Bakker PI. Snap: A web-based tool for identification and annotation of proxy snps using hapmap. Bioinformatics. 2008;24:2938–2939. doi: 10.1093/bioinformatics/btn564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Erbilgin A, Civelek M, Romanoski CE, Pan C, Hagopian R, Berliner JA, Lusis AJ. Identification of cad candidate genes in gwas loci and their expression in vascular cells. J Lipid Res. 2013;54:1894–1905. doi: 10.1194/jlr.M037085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Renault AD, Sigal YJ, Morris AJ, Lehmann R. Soma-germ line competition for lipid phosphate uptake regulates germ cell migration and survival. Science. 2004;305:1963–1966. doi: 10.1126/science.1102421. [DOI] [PubMed] [Google Scholar]
- 77.Wamhoff BR, Lynch KR, Macdonald TL, Owens GK. Sphingosine-1-phosphate receptor subtypes differentially regulate smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2008;28:1454–1461. doi: 10.1161/ATVBAHA.107.159392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chatterjee I, Humtsoe JO, Kohler EE, Sorio C, Wary KK. Lipid phosphate phosphatase-3 regulates tumor growth via beta-catenin and cyclin-d1 signaling. Mol Cancer. 2011;10:51. doi: 10.1186/1476-4598-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Humtsoe JO, Liu M, Malik AB, Wary KK. Lipid phosphate phosphatase 3 stabilization of beta-catenin induces endothelial cell migration and formation of branching point structures. Mol Cell Biol. 2010;30:1593–1606. doi: 10.1128/MCB.00038-09. [DOI] [PMC free article] [PubMed] [Google Scholar]