Abstract
Objectives
To determine the associations between statin use and major adverse cardiovascular and cerebrovascular events (MACCE) and amputation-free survival in critical limb ischemia (CLI) patients.
Background
CLI is an advanced form of peripheral arterial disease (PAD) associated with nonhealing arterial ulcers and high rates of MACCE and major amputation. While statin medications are recommended for secondary prevention in PAD, their effectiveness in CLI is uncertain.
Methods
We reviewed 380 CLI patients who underwent diagnostic angiography or therapeutic endovascular intervention from 2006–2012. Propensity scores and inverse probability of treatment weighting were used to adjust for baseline differences between patients taking and not taking statins.
Results
246 (65%) patients were prescribed statins. The mean serum low-density lipoprotein (LDL) level was lower in patients prescribed statins (75±28 vs. 96±40 mg/dL, P<0.001). Patients prescribed statins had more baseline comorbidities including diabetes, coronary artery disease, and hypertension, as well as more extensive lower extremity disease (all P <0.05). After propensity weighting, statin therapy was associated with lower one-year rates of MACCE (stroke, myocardial infarction, or death; hazard ratio [HR] 0.53, 95% CI 0.28–0.99), mortality (HR 0.49, 95% CI 0.24–0.97), and major amputation or death (HR 0.53, 95% CI 0.35–0.98). Statin use was also associated with improved lesion patency among patients undergoing infrapopliteal angioplasty. Patients with LDL levels above 130mg/dL had increased hazards of MACCE and mortality compared to patients with lower levels of LDL.
Conclusions
Statins are associated with lower mortality and MACCE and increased amputation-free survival in CLI patients.
Keywords: Peripheral artery disease, statin therapy, major adverse cardiac event, lipids and lipoproteins, secondary prevention
INTRODUCTION
Peripheral arterial disease (PAD) affects four to eight million people in the United States.(1–3) Patients with PAD have significantly increased rates of myocardial infarction (MI), cardiovascular mortality, and stroke.(4) Critical limb ischemia (CLI), the most advanced form of PAD, is characterized by ischemic rest pain, non-healing ischemic ulcers, and gangrene. Patients with CLI have a major amputation rate of up to 40% at six months and a mortality rate of 20–25% in the first year after presentation.(5, 6) While CLI represents only a subset of the total PAD population, the high cardiovascular event and amputation rates in these patients result in a large overall healthcare burden.(7, 8)
The benefits of HMG-CoA reductase inhibitors (statins) on morbidity and mortality have been established in patients with or at high risk for ischemic heart disease.(9–12) There is also evidence for the utility of statins in patients with PAD; a revision of the Adult Treatment Panel III (ATP III) guidelines designates PAD a ‘coronary heart disease risk equivalent’ and consensus guidelines recommend statin therapy to target a low-density lipoprotein (LDL) level of ≤100mg/dL (2.59 mmol/L) for “very high risk” patients.(4, 13) However, these recommendations are based predominantly on data from patients with claudication or population screening ankle brachial indices. Thus, the value of statin therapy for patients with CLI is uncertain.
We hypothesized that statin therapy would be associated with a reduced rate of major adverse cardiovascular and cerebrovascular events (MACCE) and a reduced rate of major amputation in patients with CLI. We tested this hypothesis in a large cohort of patients with CLI who were treated longitudinally at a multidisciplinary vascular center.
METHODS
Design
The PAD-UCD Registry comprises all patients with a clinical diagnosis of PAD who underwent diagnostic angiography and/or therapeutic endovascular intervention at the UC Davis Medical Center from 2006 to 2012. During this interval, three vascular surgeons and one interventional cardiologist performed all of the procedures. At the time of data analysis, the registry included 975 patients and 1,490 procedures. The study protocol was approved by the Institutional Review Board at the University of California, Davis Medical Center.
Data Collection and Definitions
We identified patients who had at least one presentation during the study period for CLI, defined as Rutherford class 4–6 disease (rest pain, non-healing ulceration due to arterial insufficiency, or gangrene).(14) We retrospectively analyzed these patients’ data based on review of electronic medical record documentation. We used pre- and post-procedure hospital and clinic records to identify patient demographics, baseline health status and medical management, clinical presentation, vascular procedures, post-procedure management, and outcomes. All records were reviewed by trained chart abstractors and verified by a board-certified cardiologist. Patients were categorized into the statin group if either their hospitalization data or the most recent pre-procedure clinic visit indicated current statin use. Other medication prescriptions were determined based on the most recent pre-procedure clinic visit. Baseline serum LDL levels were determined using the most recent value within six months pre-procedure.
Routine practice at our institution during this period was to schedule follow-up visits within one month after angiographic procedures, then every three months for the first year and every 6–12 months thereafter. At these visits, patients were assessed for clinical improvement and those who had interventions were evaluated with interval ankle-brachial pressure index (ABI) measurements and duplex ultrasonography (DUS).
Outcomes
The primary endpoint was a composite measure of MACCE, defined as any death, MI, or stroke within one year post-procedure. MI was defined as symptoms of chest pressure and elevation of troponin with evidence of infarct by stress imaging or coronary angiography and ventriculography. Stroke was defined as focal neurologic deficit lasting longer than 24 hours with computed tomography or magnetic resonance imaging evidence of cerebral ischemic infarct or intracerebral hemorrhage.
Secondary outcomes, all at one year post-procedure, included death, MI, stroke, subsequent ipsilateral lower extremity bypass grafting, and ipsilateral major amputation, defined as any amputation above the level of the ankle joint. In order to account for the competing hazard of death among patients at high risk of needing amputation, we also evaluated amputation-free survival as a composite endpoint.
Lesion-specific secondary outcomes included primary, primary assisted, and secondary patency of all lesions treated with endovascular intervention. Loss of primary patency was defined as a velocity ratio of≥2.0 as assessed by DUS or endovascular or surgical re-intervention to the target vessel. Primary assisted patency was defined as patency after treatment for restenosis, and secondary patency was defined as overall patency after restenosis or occlusion.
All outcomes were adjudicated from physician documentation in the electronic medical record. In order to ensure that deaths outside our institution were captured, patient vital status was also verified using the Social Security Death Index.
Data Analysis
Mean values with standard deviations were used to describe continuous variables and frequencies and percentages were used for categorical variables. Continuous variables were compared using the Wilcoxon rank sum test or analyses of variance, and categorical values using chi squared or Fisher’s exact tests. All analyses were performed using STATA Version 11.2 (STATA Corporation, College Station, Texas).
We developed propensity scores to adjust for confounding in statin use, defined as the conditional probability of being treated with a statin given a patient’s measured demographic and clinical characteristics.(15) To calculate the propensity score for statin treatment, we developed a logistic model for statin treatment using stepwise logistic regression analysis. Baseline covariates in the model included age, gender, and race; history of diabetes, coronary artery disease (CAD), MI, hypertension, heart failure, stroke, carotid artery disease, or chronic obstructive pulmonary disease; smoking status; left ventricular ejection fraction (in 5% increments from ≤10% to ≥65%); prescription of concomitant medications including angiotensin converting enzyme inhibitors or angiotensin receptor blockers, aspirin, and clopidogrel; and year of procedure.
Multiple methodologies were used to validate the propensity model. The c-statistic of the model was 0.79. To assess covariate balance across the distribution of propensity scores, we visually compared propensity score overlap with kernel density plots (Supplementary Figure 1). We also calculated the odds of treatment with a statin for each of the covariates within a given quintile and then used Mantel-Haenszel estimates of common odds ratios to calculate summary odds ratios across all quintiles before and after propensity adjustment.(16) Standardized mean differences were also calculated for each covariate and were verified to be balanced for each covariate after adjustment (Supplemental Table 1).(17) To determine the best estimate with observational data of the treatment effect of statin use, proportional hazards marginal structural models were then developed via weighted regression with inverse probability of treatment weighting (IPTW) using the propensity score.(18, 19) As a sensitivity analysis, propensity modeling was also performed using nearest-neighbor matching, and qualitatively similar results were obtained to that of the IPTW model. In order to account for the possibility that censoring patients at the first event could obscure effects on other competing risks, we also conducted a competing risks analysis using the method of Fine and Gray.(20)
To analyze lesion-level outcomes, we stratified interventions according to anatomic level of disease (femoropopliteal vs. infrapopliteal). We then used Cox proportional hazard models to estimate hazard ratios for the effect of statin medications on restenosis and target vessel revascularization. We included lesion length, proximal vessel reference diameter, diabetes, gender, smoking status, estimated glomerular filtration rate, and, for femoropopliteal lesions, stent placement as multivariable predictors of restenosis.
To study the relationship of baseline LDL level to outcomes, patients were stratified into four LDL subgroups based on ATP III guidelines: <70 mg/dL, 70–100 mg/dL, 100–130 mg/dL, and >130 mg/dL (<1.81 mmol/L, 1.81–2.59 mmol/L, 2.59–3.36 mmol/L, and >3.36 mmol/L).(13) To minimize bias in the outcomes due to missing LDL data, multiple imputation with 10 imputations was used. MACCE and mortality were then analyzed using Cox proportional hazard modeling that included LDL level as an additional covariate in a model that also adjusted for other baseline demographic factors among patients treated with statin medications.
RESULTS
Study Population
380 patients presented with critical limb ischemia and underwent peripheral angiography during the study period (Table 1). Median follow-up time derived from Kaplan-Meier estimates was 409 days. With respect to the primary outcome of MACCE, 4 patients (3.0%) in the no statin group and 9 patients (3.7%) in the statin group were lost to follow-up (P=0.96). Overall, 65% of the patients were taking a statin at baseline, a percentage that ranged from 58% for patients entering the study in 2006 to 71% in 2012 (P=0.07 for trend; Figure 1). Over 40% of the patients were female; we have previously reported differences in the presentation and outcomes for this cohort based on gender, including a higher rate of MACCE for women.(21) Simvastatin and atorvastatin were the two most frequently prescribed statins and together accounted for 73% of the prescribed statins. Additional lipid-lowering medications were prescribed in less than 15% of patients. Among patients not taking a statin, only 6 (4%) had a documented contraindication.
Table 1.
Baseline Characteristics of Patients with Critical Limb Ischemia.
| Variable | Statin (N= 246) | No Statin (N= 134) | P value |
|---|---|---|---|
| Age, years | 69.0 ± 13.3 | 68.0 ± 13.9 | 0.4 |
| Male (%) | 145 (59) | 68 (51) | 0.1 |
| Race/Ethnicity (%) | 0.7 | ||
| Caucasian | 179 (73) | 98 (73) | |
| Hispanic | 29 (12) | 20 (15) | |
| African American | 31 (13) | 13 (10) | |
| Asian | 7 (3) | 3 (2) | |
| BMI, kg/m2 | 27.4 ± 6.2 | 26.6 ± 6.5 | 0.2 |
| Tobacco, former or current (%) | 168 (69) | 87 (66) | 0.5 |
| CHF (%) | 79 (33) | 36 (27) | 0.3 |
| DM (%) | 167 (68) | 65 (50) | <0.001 |
| GFR, ml/min | 59.8 ± 39.4 | 61.2 ± 40.8 | 0.8 |
| HTN (%) | 221 (90) | 103 (77) | <0.001 |
| SBP (mmHg) | 134 ± 23 | 132 ± 23 | 0.99 |
| CAD (%) | 144 (59) | 40 (31) | <0.001 |
| History of MI (%) | 72 (29) | 12 (9) | <0.001 |
| Ejection Fraction | 53.1 ± 17.3 | 52.6 ± 18.5 | 0.9 |
| History of Stroke/ TIA (%) | 58 (24) | 17 (13) | 0.01 |
| History of Malignancy (%) | 30 (12) | 14 (10) | 0.6 |
| COPD (%) | 40 (16) | 16 (12) | 0.2 |
| History of AAA (%) | 10 (4) | 4 (3) | 0.6 |
| History of Carotid Stenosis (%) | 42 (18) | 5 (4) | <0.001 |
| History of Contralateral Amputation (%) | 14 (10) | 37 (15) | 0.2 |
| Total Cholesterol, mg/dl | 142 ± 41 | 167 ± 60 | 0.001 |
| LDL, mg/dl | 75 ± 28 | 96 ± 40 | <0.001 |
| HDL, mg/dl | 39 ± 18 | 35 ± 17 | 0.3 |
| TG, mg/dl | 130 ± 65 | 145 ± 84 | 0.2 |
| HbA1c, % | 7.9 ± 2.2 | 7.8 ± 2.1 | 0.9 |
| ACE inhibitor or ARB (%) | 162 (66) | 57 (43) | <0.001 |
| Beta blocker (%) | 154 (63) | 47 (35) | <0.001 |
| Aspirin (%) | 225 (91) | 117 (87) | 0.2 |
| Clopidogrel (%) | 168 (68) | 77 (57) | 0.04 |
| Other Lipid Medication* | 31 (13) | 12 (9) | 0.6 |
| Rutherford score | 0.8 | ||
| 4 | 38 (15) | 22 (16) | |
| 5 | 177 (73) | 95 (70) | |
| 6 | 29 (12) | 18 (13) | |
| ABI† | 0.48 ± 0.24 | 0.48 ± 0.27 | 0.9 |
| TBI | 0.19 ± 0.17 | 0.18 ± 0.20 | 0.7 |
Abbreviations: AAA, abdominal aortic aneurysm; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; GFR, glomerular filtration rate; HBA1c, hemoglobin A1c; HDL, high density lipoprotein; HTN, hypertension; LDL, low density lipoprotein; TG, triglyceride; TIA, transient ischemic attack; TBI, toe brachial index
Other lipid medications included fibrate, fish oil, niacin, and ezetimibe.
Excluding subjects with ABI>1.4, for whom TBI was also measured
Figure 1. Rate of Statin Prescription Over Time.
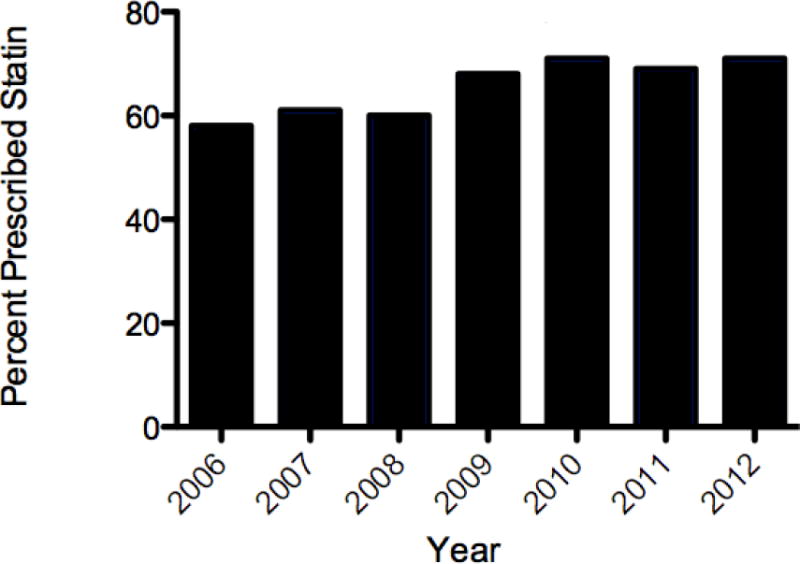
The percentage of patients with critical limb ischemia prescribed a statin medication by year, 2006–2012 (P=0.07 for trend).
Patients prescribed statin medications had higher rates of baseline medical comorbidities, including higher prevalence of diabetes mellitus, hypertension, CAD, and carotid artery stenosis (Table 1). Patients prescribed a statin were also more likely to have history of prior MI or stroke/transient ischemic attack. Other baseline differences between the two groups included a higher rate of concomitant medical therapies among patients receiving statins including beta-blockers, angiotensin converting enzyme inhibitors or angiotensin receptor blockers, and clopidogrel. Patients taking a statin medication also had lower baseline levels of total cholesterol and LDL (Figure 2). Rates of baseline comorbidities including history of diabetes and CAD did not show significant trends by year (P=0.21 and P=0.73, respectively). The angiographic characteristics of the two groups were similar, with the exception of a higher rate of multilevel stenosis (defined as at least one >50% stenosis in both a femoro-popliteal and an infrapopliteal vessel) in the statin group (Table 2).
Figure 2. Total Cholesterol and LDL Levels by Statin Use.

Baseline total cholesterol (A, P<0.01) and LDL (B, P<0.01) levels were lower in the group prescribed statin medications.
Table 2.
Lower Extremity Angiographic Characteristics of Patients With and Without Statin Use.
| Statin (N = 246) |
No Statin (N = 134) |
P Value | |
|---|---|---|---|
| Level of Disease* | |||
| Aorto - Iliac | 54 (29) | 33 (31) | 0.7 |
| Femoropopliteal | 188 (82) | 93 (73) | 0.06 |
| Infrapopliteal | 205 (91) | 105 (86) | 0.2 |
| Multilevel Stenosis† | 161 (68) | 75 (57) | 0.03 |
| Chronic Total Occlusion | |||
| Aorto-Iliac | 22 (12) | 19 (18) | 0.2 |
| Femoropopliteal | 114 (50) | 60 (47) | 0.7 |
| Infrapopliteal | 205 (91) | 105 (86) | 0.2 |
| Lower Extremity Runoff | 0.3 | ||
| 0 | 32 (15) | 19 (16) | |
| 1–2 | 159 (73) | 77 (66) | |
| 3 | 26 (12) | 20 (17) |
Level of disease was defined as >50% stenosis within a given segment. The totals are greater than 100%, since a given patient could have stenoses at multiple levels.
Defined as at least one stenosis >50% in both a femoro-popliteal and an infrapopliteal vessel.
Outcomes by Statin Use
The event rates and hazard ratios for all clinical outcomes are summarized in Table 3. Patients prescribed statin therapy had lower absolute event rates for all outcomes despite greater baseline comorbidities. The propensity model showed good balance in adjusted variables, with no significant difference in the pooled odds of statin prescribing after propensity weighting for all measured covariates (Figure 3). After adjustment, MACCE (HR 0.53, 95% CI 0.28–0.99; Figure 4A) and mortality (HR 0.49, 95% CI 0.24–0.97; Figure 4B) were lower in the statin group. Rates of MI (HR 0.48, 95% CI 0.16–1.44), stroke (HR 0.18, 95% CI 0.02–1.78), and amputation (HR 0.68, 95% CI 0.32–1.39) did not differ significantly but all trended in a direction favoring statin use. The risk of death or major amputation was also significantly decreased in those prescribed statins (HR 0.53, 95% CI 0.35–0.98; Figure 4C). There was no association between statin use and rates of lower extremity bypass, which were 18% at one year in both groups. Outcomes using a competing risks analysis showed similar point estimates for the relationships between statin use and MI, stroke, bypass, and major amputation. (Supplemental Table 2).
Table 3.
One Year Outcome Rates and Unadjusted and Adjusted Hazard Ratios
| Variables | Outcomes Rates*
|
Unadjusted
|
IPTW Adjusted
|
|||
|---|---|---|---|---|---|---|
| No Statin (N=134) |
Statin (N=246) |
HR (95% CI) |
P | HR (95% CI) |
P | |
| Cardiac Events | ||||||
| Death | 27(21%) | 35(15%) | 0.69 (0.42–1.14) | 0.1 | 0.49 (0.24–0.97) | 0.04 |
| MI | 7(6%) | 9 (5%) | 0.69 (0.26–1.85) | 0.4 | 0.48 (0.16–1.44) | 0.2 |
| Stroke | 3 (3%) | 1 (1%) | 0.18 (0.02–1.72) | 0.2 | 0.18 (0.02–1.78) | 0.1 |
| MACCE | 31 (23%) | 43 (18%) | 0.73 (0.46–1.16) | 0.2 | 0.53 (0.28–0.99) | 0.048 |
| Lower Extremity Bypass | 22 (18%) | 43 (18%) | 0.96 (0.57–1.62) | 0.9 | 1.17 (0.61–2.24) | 0.6 |
| Amputation | 19 (18%) | 25 (12%) | 0.70 (0.39–1.27) | 0.2 | 0.68 (0.32–1.39) | 0.3 |
| Death/Amputation | 41 (35%) | 56 (27%) | 0.73 (0.49–1.09) | 0.1 | 0.59 (0.35–0.98) | 0.04 |
Raw number of events (Kaplan-Meier estimated percentage)
Abbreviations: CI, confidence interval; HR, hazard ratio; IPTW, inverse probability of treatment weighting; MACCE, major adverse cardiovascular and cerebrovascular events (MI, stroke, or death); MI, myocardial infarction
Figure 3. Odds Ratios of Statin Use by Baseline Demographics.
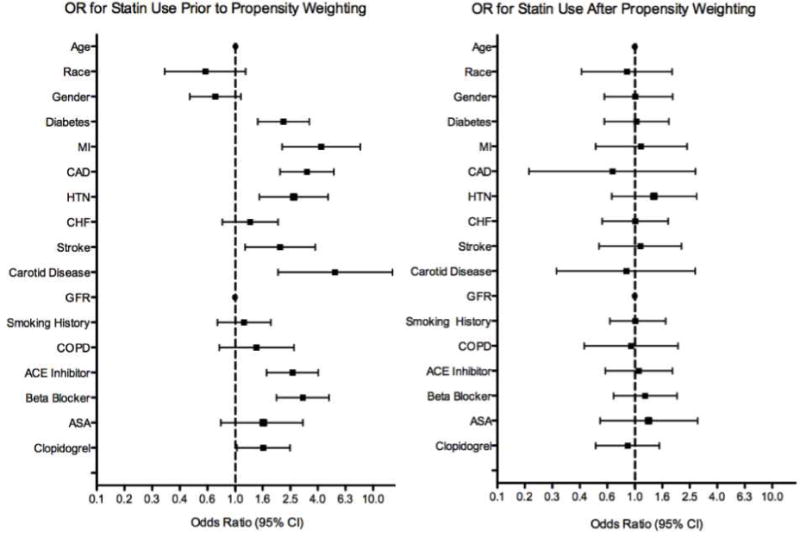
Prior to propensity weighting, significant differences in baseline patient characteristics were associated with statin use. (A) After adjustment, 95% confidence intervals of the odds ratios for all measured covariates cross 1.0. (B)
Figure 4. Statin Use and MACCE, Mortality, and Amputation-Free Survival.
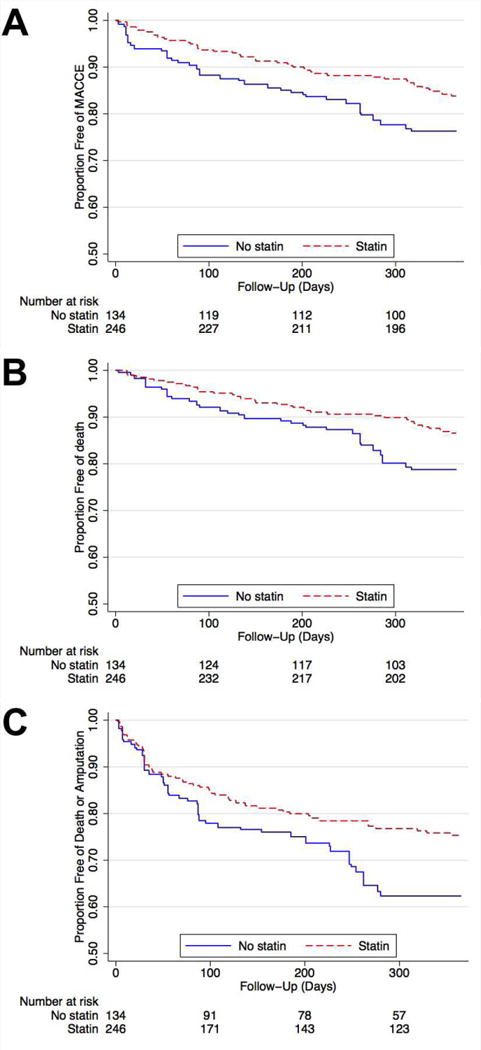
Kaplan-Meier curves to one year post-procedure showing the proportion free of (A) MACCE (MI, stroke, or death; log rank P<0.05), (B) death (P<0.05), and (C) death or amputation (P<0.05). All curves are after propensity weighted adjustment.
Among the overall cohort, 295 (78%) patients underwent endovascular intervention for limb salvage at the time of baseline angiography, while 23 patients (6%) underwent major amputation and 38 patients (10%) underwent lower extremity bypass grafting as initial management. Overall procedural success rates for endovascular intervention were similar in both groups. The mean gain in ABI post procedure was 0.45±0.26 in the statin group and 0.39 ± 0.19 in the no statin group (P=0.2). Among infrapopliteal lesions, statin use was associated with improved patency at one year (Figure 5). Using Cox proportional modeling, statin use was associated with a non-significant improvement in primary patency and significantly improved primary assisted patency and secondary patency of infrapopliteal lesions (Table 4). These results remained significant after multivariable adjustment. There was no significant effect of statins on one-year patency of femoropopliteal lesions. These results suggest that the lower amputation rates among patients treated with statins may be due in part to improved infrapopliteal vessel patency after endovascular intervention.
Figure 5. Statin Use and Patency of Infrapopliteal Lesions.
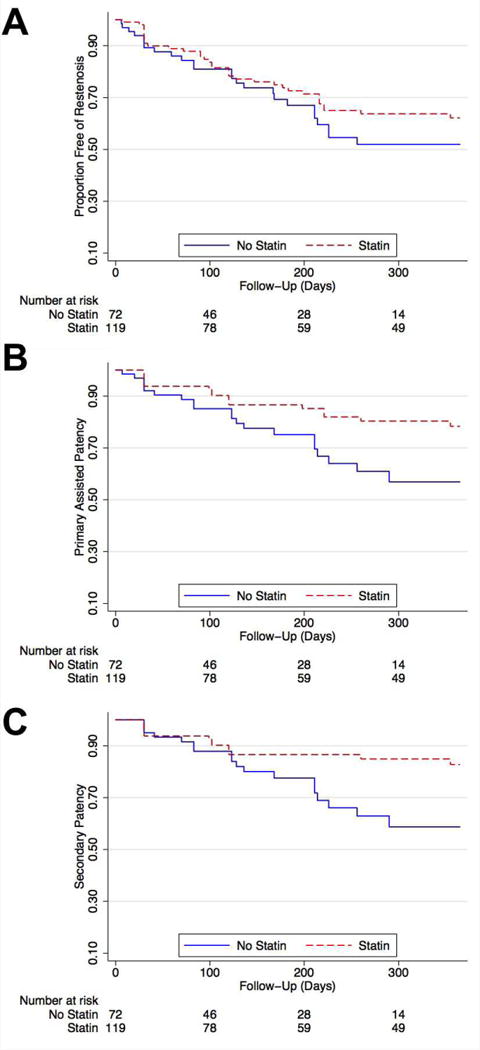
Kaplan-Meier curves to one year post-angiography showing proportion free of loss of (A) primary patency (log rank P=0.5); (B) primary assisted patency (P=0.04); and (C) secondary patency (P=0.02).
Table 4.
Lesion Outcomes Among Patients Undergoing Endovascular Interventions.
| Statin | No Statin | Adjusted HR | |
|---|---|---|---|
| Femoropopliteal Lesions | |||
| Primary Patency | 55% | 53% | 0.87 (0.50–1.50) |
| Primary Assisted Patency | 73% | 67% | 0.62 (0.31–1.30) |
| Secondary Patency | 84% | 69% | 0.50 (0.22–1.30) |
| Infrapopliteal Lesions | |||
| Primary Patency | 62% | 52% | 0.84 (0.50–1.42) |
| Primary Assisted Patency | 78% | 57% | 0.50 (0.26–0.96) |
| Secondary Patency | 83% | 59% | 0.45 (0.22–0.89) |
Abbreviations: HR, hazard ratio. Adjusted HR includes multivariable adjustment for lesion length, proximal vessel reference diameter, diabetes, gender, smoking status, and estimated glomerular filtration rate. Femoropopliteal lesions were also adjusted for stent placement.
Outcomes by LDL Level
We also explored the relationship between LDL levels and MACCE among patients prescribed statin medications. Among patients treated with statins, the group with baseline LDL levels above 130 mg/dL had the highest unadjusted one-year event rate (22%). After multivariable adjustment including age, gender, history of prior myocardial infarction, diabetes, and carotid disease, LDL levels of 100–130 (HR 0.51, 95% CI 0.32–0.80), 70–100 (HR 0.54, 95% CI 0.36–0.83), and <70 (HR 0.71, 95% CI 0.48–1.05) were each associated with a decreased HR of MACCE relative to patients taking statin medications but with baseline LDL values > 130. Similar results were observed for composite death and amputation among patients treated with statins. Among patients not treated with statins, there was also a qualitatively similar relationship between LDL levels and MACCE.
DISCUSSION
This study has three major findings. First, we report the prevalence of statin use in a cohort of CLI patients treated at a tertiary care center, and a trend towards increasing statin use over time. Second, statin use was associated with decreased subsequent hazard of MACCE (primarily due to decreased mortality), increased likelihood of amputation-free survival, and improved patency of infrapopliteal lesions. Third, we found that lower LDL levels were associated with decreased hazards of MACCE and mortality. To our knowledge, this is the first study in CLI patients to investigate the relationships between statin use and MACCE.
Rates of Statin Use in CLI Remain Low
Our data on the rates of statin use in PAD in general and CLI in particular extend prior findings on this subject. Subherwal et al. recently reported on trends in statin use in a general PAD population at the time of diagnosis during the period 2000–2007 using Danish nationwide administrative registries.(22) The overall statin use rate in our population (65%) is higher than the 56% rate they reported for patients without CAD and similar to the rate for patients with both PAD and CAD. Our data show a similar, though not statistically significant, trend towards higher statin use over time. Given the lack of significant increases over time of other indications for statin therapy, this may indicate improving quality of care or increasing awareness of PAD as an indication for statin therapy. Previous reports in the CLI population have indicated even lower rates of statin use, ranging from 23–49%.(23–25) In contrast, estimates of statin use rates in patients with CAD range from approximately 68–78%.(26–28) Further education and awareness of the benefits of statin use among patients with CLI will be necessary to increase rates of medication prescription. Consistent with this goal, statin prescription was recently included as a core performance measure for the treatment of patients with PAD.(29)
Further Support for Statin Therapy in CLI
Our results provide further evidence for statin therapy for patients with CLI. The strongest evidence underlying the American College of Cardiology and American Heart Association (ACC/AHA) recommendations of statin therapy derive from subgroup analysis of the Heart Protection Study, which included primarily patients with low baseline ABI or stable claudication.(12, 30) More recently, a large prospective observational cohort study in the Netherlands demonstrated lower all-cause and cardiac mortality in statin users with PAD.(31, 32) However, these studies were conducted in general PAD populations and included only very small numbers of patients with CLI.
A small number of observational studies of CLI patients have reported outcomes by statin use, with mixed results. Aiello et al recently studied patients undergoing endovascular therapy for CLI and reported 24 month results showing improved vessel patency, limb salvage, and survival with statin use, but the authors did not consider MACCE.(23) Other studies have yielded conflicting results on the effect of statins on graft patency, limb salvage, and mortality among patients with CLI undergoing surgical bypass.(24, 25, 33–35) Our results contribute to this developing body of literature by studying outcomes in a large representative cohort of CLI patients with and without bypass grafts, whether or not they underwent intervention. Unlike previous studies, we report MACCE, capturing important outcomes that might be neglected if only patency or mortality were studied. We also present lesion results divided by anatomic site. The efficacy of statins in promoting patency of infrapopliteal vessels may be related to the smaller caliber of these arteries and may partially explain the effects of statins on reduced amputation rates.
Higher LDL Levels Associated with Increased MACCE in CLI
Our data also begin to explore the relationship between LDL levels and clinical outcomes in CLI patients. The ACC/AHA and ATP III guidelines recommend LDL goals on the basis of the cardiovascular risk associated with PAD and the evidence for LDL treatment goals in CAD. Feringa and colleagues strengthened the evidence for lower LDL treatment targets in the general PAD population by reporting progressively increasing mortality with higher LDL levels.(31) In CLI patients, Aiello et al. reported baseline cholesterol and LDL levels in their statin and control groups, yet total cholesterol and LDL levels did not differ between the groups. Our results provide the first evidence that higher levels of LDL are associated with increased rates of mortality and MACCE in CLI patients, a population with an already elevated baseline risk. Future studies should continue to investigate the optimal target level of LDL among this high-risk group of patients.
Limitations
This study has several limitations. First, we document a single institution experience at a tertiary care facility; patterns of care and disease may differ at other sites of care. Also, as with all observational studies, the reported associations may not represent underlying causality. Our use of propensity score weighting balanced the cohorts and reduced the effect of measured confounding variables on our outcomes, though it cannot account for unmeasured confounding variables. Statin use may be a marker for quality of or access to care, which could not be assessed with our study design. However, we included numerous measured covariates and demonstrated excellent balance after propensity weighting. We also used multiple methodologies to verify the adequacy of adjustment for differences in baseline covariates as well as sensitivity analyses that were consistent with our overall results.
Similarly, the duration of statin therapy and compliance could not be quantified in this study, and it is possible that an increasing number of patients began taking statin medications during follow-up. We also found an average LDL level of only 96 mg/dL in the group not taking statins, despite low rates of use of other lipid medications, which may indicate that some patients were using statins but discontinued use prior to angiography. “Cross-over” to statin use, ceasing statin use either prior to baseline or during the study, or non-compliance would not negatively affect the validity of our findings, but instead strengthen our reported effect size of statin use on subsequent MACCE. Given the existing support for statin use in claudicants, we believe that evidence for statin therapy in CLI should be interpreted as support for continuous statin therapy beginning at the time of any PAD diagnosis.
While the majority of the endpoints were driven by a decrease in overall mortality, we believe that the composite endpoints of MACCE and death or major amputation are useful because they are common, clinically meaningful endpoints. Not ascertaining the cause of death, along with losing patients to follow-up, limits our analyses. However, prior studies have found that the majority of deaths in patients with CLI are due to cardiovascular causes.(36, 37) That our results, like those of PREVENT III, indicate a difference in mortality but not in sub-lethal vascular events may indicate effective prevention of fatal strokes and MIs. Low overall event rates for amputations and non-fatal MIs and strokes, however, limit our power to detect differences in these secondary outcomes.
Conclusions
In conclusion, this study extends our understanding of the effects of statin therapy in PAD and CLI in particular. The improved rates of one-year MACCE with statin use strengthens the evidence supporting the guideline recommendations of statin therapy for all PAD patients, including those with even the most advanced stages of disease. While our data on statin use rates compares favorably to previously published values, statins remain an under-utilized therapy.(38) Our finding of superior outcomes for patients with lower LDL levels also provides support for the use of LDL as a treatment target in patients with PAD. Future studies should determine the optimal statin type and dose, further explore potential treatment targets including LDL for statins in PAD patients, and investigate barriers to more widespread use of statins among patients with CLI.
Supplementary Material
Acknowledgments
This project was supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR 000002 and linked award TL1 TR 000133.
ABBREVIATIONS
- ABI
ankle brachial index
- ATP III
Adult Treatment Panel III
- CAD
coronary artery disease
- CLI
critical limb ischemia
- DUS
duplex ultrasound
- LDL
low density lipoprotein
- MACCE
major adverse cardiovascular and cerebrovascular events
- MI
myocardial infarction
- PAD
peripheral arterial disease
Footnotes
Dr. Yeo is on the Speakers Bureau for Abbott Vascular. Dr. Laird is a consultant for Boston Scientific, Covidien, Abbott, Bard, and Medtronic. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Criqui MH, Fronek A, Barrett-Connor E, Klauber MR, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation. 1985;71:510–515. doi: 10.1161/01.cir.71.3.510. [DOI] [PubMed] [Google Scholar]
- 2.Allison MA, Ho E, Denenberg JO, et al. Ethnic-Specific Prevalence of Peripheral Arterial Disease in the United States. Am J Prev Med. 2007;32:328–333. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110:738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) Circulation. 2006;113:e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 5.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45:S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 6.Varu VN, Hogg ME, Kibbe MR. Critical limb ischemia. J Vasc Surg. 2010;51:230–241. doi: 10.1016/j.jvs.2009.08.073. [DOI] [PubMed] [Google Scholar]
- 7.Sigvant B, Wiberg-Hedman K, Bergqvist D, et al. A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. J Vasc Surg. 2007;45:1185–1191. doi: 10.1016/j.jvs.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Peacock JM, Keo HH, Duval S, et al. The Incidence and Health Economic Burden of Ischemic Amputation in Minnesota, 2005–2008. Prev Chronic Dis. 2011:8. Available at: /pmc/articles/PMC3221580/?report=abstract. Accessed January 29, 2013. [PMC free article] [PubMed]
- 9.Anon. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 10.Sacks FM, Pfeffer MA, Moye LA, et al. The Effect of Pravastatin on Coronary Events after Myocardial Infarction in Patients with Average Cholesterol Levels. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 11.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 12.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 13.Grundy SM, Cleeman JI, Merz CNB, et al. Implications of Recent Clinical Trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 14.Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: Revised version. J Vasc Surg. 1997;26:517–538. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 15.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 16.Austin PC. The relative ability of different propensity score methods to balance measured covariates between treated and untreated subjects in observational studies. Med Decis Mak Int J Soc Med Decis Mak. 2009;29:661–677. doi: 10.1177/0272989X09341755. [DOI] [PubMed] [Google Scholar]
- 17.Longmore RB, Yeh RW, Kennedy KF, et al. Clinical referral patterns for carotid artery stenting versus carotid endarterectomy: results from the Carotid Artery Revascularization and Endarterectomy Registry. Circ Cardiovasc Interv. 2011;4:88–94. doi: 10.1161/CIRCINTERVENTIONS.110.958843. [DOI] [PubMed] [Google Scholar]
- 18.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiol Camb Mass. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 21.McCoach CE, Armstrong EJ, Singh S, et al. Gender-related variation in the clinical presentation and outcomes of critical limb ischemia. Vasc Med Lond Engl. 2013;18:19–26. doi: 10.1177/1358863X13475836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Subherwal S, Patel MR, Kober L, et al. Missed Opportunities: Despite Improvement in Use of Cardioprotective Medications Among Patients With Lower-Extremity Peripheral Artery Disease, Underuse Remains. Circulation. 2012;126:1345–1354. doi: 10.1161/CIRCULATIONAHA.112.108787. [DOI] [PubMed] [Google Scholar]
- 23.Aiello FA, Khan AA, Meltzer AJ, Gallagher KA, McKinsey JF, Schneider DB. Statin therapy is associated with superior clinical outcomes after endovascular treatment of critical limb ischemia. J Vasc Surg Off Publ Soc Vasc Surg Int Soc Cardiovasc Surg North Am Chapter. 2012;55:371–380. doi: 10.1016/j.jvs.2011.08.044. [DOI] [PubMed] [Google Scholar]
- 24.Schanzer A, Hevelone N, Owens CD, Beckman JA, Belkin M, Conte MS. Statins are independently associated with reduced mortality in patients undergoing infrainguinal bypass graft surgery for critical limb ischemia. J Vasc Surg Off Publ Soc Vasc Surg Int Soc Cardiovasc Surg North Am Chapter. 2008;47:774–781. doi: 10.1016/j.jvs.2007.11.056. [DOI] [PubMed] [Google Scholar]
- 25.Isma N, Barani J, Mattiasson I, Lindblad B, Gottsäter A. Lipid-lowering therapy is related to inflammatory markers and 3-year mortality in patients with critical limb ischemia. Angiology. 2008;59:542–548. doi: 10.1177/0003319707306144. [DOI] [PubMed] [Google Scholar]
- 26.Cooke CE, Hammerash WJ., Jr Retrospective review of sex differences in the management of dyslipidemia in coronary heart disease: an analysis of patient data from a Maryland-based health maintenance organization. Clin Ther. 2006;28:591–599. doi: 10.1016/j.clinthera.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Arnold SV, Spertus JA, Tang F, et al. Statin use in outpatients with obstructive coronary artery disease. Circulation. 2011;124:2405–2410. doi: 10.1161/CIRCULATIONAHA.111.038265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Connor PJ, Gray RJ, Maciosek MV, et al. Cholesterol levels and statin use in patients with coronary heart disease treated in primary care settings. Prev Chronic Dis. 2005;2:A05. [PMC free article] [PubMed] [Google Scholar]
- 29.Olin JW, Allie DE, Belkin M, et al. ACCF/AHA/ACR/SCAI/SIR/SVM/SVN/SVS 2010 Performance Measures for Adults With Peripheral Artery Disease: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures, the American College of Radiology, the Society for Cardiac Angiography and Interventions, the Society for Interventional Radiology, the Society for Vascular Medicine, the Society for Vascular Nursing, and the Society for Vascular Surgery (Writing Committee to Develop Clinical Performance Measures for Peripheral Artery Disease) Circulation. 2010;122:2583–2618. doi: 10.1161/CIR.0b013e3182031a3c. [DOI] [PubMed] [Google Scholar]
- 30.Heart Protection Study Collaborative Group. Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20,536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg. 2007;45:645–654.e1. doi: 10.1016/j.jvs.2006.12.054. [DOI] [PubMed] [Google Scholar]
- 31.Feringa HHH, Karagiannis SE, van Waning VH, et al. The effect of intensified lipid-lowering therapy on long-term prognosis in patients with peripheral arterial disease. J Vasc Surg. 2007;45:936–943. doi: 10.1016/j.jvs.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 32.Feringa HHH, van Waning VH, Bax JJ, et al. Cardioprotective Medication Is Associated With Improved Survival in Patients With Peripheral Arterial Disease. J Am Coll Cardiol. 2006;47:1182–1187. doi: 10.1016/j.jacc.2005.09.074. [DOI] [PubMed] [Google Scholar]
- 33.Henke PK, Blackburn S, Proctor MC, et al. Patients undergoing infrainguinal bypass to treat atherosclerotic vascular disease are underprescribed cardioprotective medications: effect on graft patency, limb salvage, and mortality. J Vasc Surg. 2004;39:357–365. doi: 10.1016/j.jvs.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 34.Abbruzzese TA, Havens J, Belkin M, et al. Statin therapy is associated with improved patency of autogenous infrainguinal bypass grafts. J Vasc Surg. 2004;39:1178–1185. doi: 10.1016/j.jvs.2003.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandez N, McEnaney R, Marone LK, et al. Predictors of failure and success of tibial interventions for critical limb ischemia. J Vasc Surg. 2010;52:834–842. doi: 10.1016/j.jvs.2010.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faglia E, Clerici G, Clerissi J, et al. Long-term prognosis of diabetic patients with critical limb ischemia: a population-based cohort study. Diabetes Care. 2009;32:822–827. doi: 10.2337/dc08-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jämsén T, Manninen H, Tulla H, Matsi P. The Final Outcome of Primary Infrainguinal Percutaneous Transluminal Angioplasty in 100 Consecutive Patients with Chronic Critical Limb Ischemia. J Vasc Interv Radiol. 2002;13:455–463. doi: 10.1016/s1051-0443(07)61525-5. [DOI] [PubMed] [Google Scholar]
- 38.Pande RL, Perlstein TS, Beckman JA, Creager MA. Secondary Prevention and Mortality in Peripheral Artery Disease: National Health and Nutrition Examination Study, 1999 to 2004. Circulation. 2011;124:17–23. doi: 10.1161/CIRCULATIONAHA.110.003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


