Abstract
Background
We aimed to determine with this randomized, triple-masked, placebo-controlled study if benefits are afforded by adding a multiple-day, ambulatory, continuous ropivacaine paravertebral nerve block to a single-injection ropivacaine paravertebral block following mastectomy.
Methods
Preoperatively, 60 subjects undergoing unilateral (n = 24) or bilateral (n = 36) mastectomy received either unilateral or bilateral paravertebral perineural catheter(s), respectively, inserted between the third and fourth thoracic transverse process(es). All subjects received an initial bolus of ropivacaine 0.5% (15 mL) via the catheter(s). Subjects were randomized to receive either perineural ropivacaine 0.4% or normal saline using portable infusion pump(s) [5 mL/h basal; 300 mL reservoir(s)]. Subjects remained hospitalized for at least 1 night and were subsequently discharged home where the catheter(s) were removed on postoperative day 3. Subjects were contacted by telephone on postoperative days 1, 4, 8, and 28. The primary end point was average pain (scale: 0–10) queried on postoperative day (POD) 1.
Results
Average pain queried on POD 1 for subjects receiving perineural ropivacaine (n=30) was a median (interquartile) of 2 (0–3), compared with 4 (1–5) for subjects receiving saline (n = 30; 95% CI difference in medians, −4.0 – −0.3; P = 0.021]. During this same time period, subjects receiving ropivacaine experienced a lower severity of breakthrough pain (5 [3–6] vs 7 [5–8]; P = 0.046) as well. As a result, subjects receiving perineural ropivacaine experienced less pain-induced physical and emotional dysfunction, as measured with the Brief Pain Inventory (lower score = less dysfunction): 14 (4–37) vs 57 (8–67) for subjects receiving perineural saline (P = 0.012). For the subscale that measures the degree of interference of pain on 7 domains, such as general activity and relationships, subjects receiving perineural saline reported a median score 10 times higher than those receiving ropivacaine (3 [0–24] versus 33 [0–44]; P = 0.035). In contrast, following infusion discontinuation there were no statistically significant differences detected between treatment groups.
Conclusions
Following mastectomy, adding a multiple-day, ambulatory, continuous ropivacaine infusion to a single-injection ropivacaine paravertebral nerve block results in improved analgesia and less functional deficit during the infusion. However, no benefits were identified following infusion discontinuation.
INTRODUCTION
Within the United States alone, more than 3 million women are living with a history of breast cancer;1 approximately 100,000 new cases are diagnosed annually;1,2 and 30% to 40% of women with this diagnosis undergo mastectomy.2 Pain following mastectomy is often severe, with breakthrough pain as measured with a Numeric Rating Scale (NRS; 0–10, 0 = no pain, 10 = worst imaginable)3 a median (interquartile) of 7 (4–8); and 25% of women describing a “continuous aching pain” the day following surgery.4 Analgesia may be provided with a single-injection paravertebral nerve block, which involves the placement of long-acting local anesthetic adjacent to the peripheral nerves that innervate the breast.4 Unfortunately, the longest-acting local anesthetics clinically available usually have a duration of less than 24 hours.5
In contrast, local anesthetic delivery may be continued indefinitely via a percutaneously-inserted perineural catheter—termed a continuous peripheral nerve block—which has been demonstrated to reduce appreciably postoperative pain and opioid use in multiple other anatomic locations.6 Therefore, the primary aim of this randomized, triple-masked (subjects, investigators and all clinical staff, statisticians), placebo-controlled study was to determine if postoperative benefits are afforded following mastectomy by adding a multiple-day, ambulatory, continuous ropivacaine infusion to a single-injection ropivacaine paravertebral nerve block. The primary end point was average postoperative pain level queried the day following surgery.
METHODS
Enrollment
The local Institutional Review Board (University California San Diego, San Diego, CA) approved all study procedures, and all study subjects provided written, informed consent. The trial was prospectively registered at clinicaltrials.gov (NCT01231204). Patients offered enrollment included women 18 years or older undergoing unilateral or bilateral mastectomy with or without axillary lymph node dissection, and desiring a single-injection paravertebral nerve block(s) for postoperative analgesia. Exclusion criteria included morbid obesity (body mass index > 40 kg/m2); renal insufficiency (creatinine > 1.5 mg/dL), current chronic analgesic therapy (daily use > 4 weeks), a history of opioid dependence, pregnancy, incarceration, an inability to communicate with the investigators or hospital staff, or comorbidity that resulted in moderate or severe functional limitation (American Society of Anesthesiologists physical status classification > 2).
Catheter insertion
All subjects had a peripheral intravenous catheter inserted, standard noninvasive monitors applied, supplemental oxygen administered via a face mask; and were placed in a seated position with their forehead resting on the forearms which were crossed and resting on a pillow atop a medical table. Intravenous midazolam and fentanyl were titrated for patient comfort, while ensuring that patients remained responsive to verbal cues. The catheter insertion site(s) was cleansed with chlorhexidine gluconate and isopropyl alcohol, and a clear, sterile, fenestrated drape applied. For all subjects, each target paravertebral space was located using ultrasound guidance. With a low-frequency (5–2 MHz) curved array transducer (C60x MicroMaxx; SonoSite Inc, Bothell, Washington) in a sterile sleeve, the paravertebral space between the third and fourth thoracic vertebrae was identified in a parasagittal view approximately 3 cm lateral to midline on the side of surgery. A local anesthetic skin wheal was raised caudal to the ultrasound transducer. An 8.9 cm, 17-gauge, Tuohy-tip needle (FlexTip Plus; Arrow International, Reading, Pennsylvania) was inserted through the skin wheal in-plane beneath the ultrasound transducer and directed to the paravertebral space. Normal saline (5 mL) was injected via the needle to help open the paravertebral space and observe the pleura being displaced anteriorly. A perineural catheter was inserted 2 to 4 cm beyond the needle tip, the needle withdrawn over the catheter, the catheter affixed with an occlusive sterile dressing, (paper) taped directly caudal and then lateral above the iliac crest, and the injection port subsequently affixed with an anchoring device lateral to the navel on the ipsilateral side.
Fifteen milliliters of 0.5% ropivacaine with epinephrine, 5 μg/mL, was slowly injected via the catheter with gentle aspiration every 3 mL. Catheter placement was considered successful if, within 30 minutes, the patient experienced any decreased sensation to cold temperature with an alcohol pad over the approximate level of the ipsilateral third thoracic dermatome. Misplaced catheters were replaced successfully, or the patient excluded from further study participation. For subjects undergoing bilateral mastectomy, a catheter using the same protocol was subsequently inserted on the contralateral side.
Randomization
Treatment allocation occurred following confirmation of successful insertion of the perineural catheter(s). Subjects were randomized to 1 of 2 groups: Placebo (normal saline) or ropivacaine (0.4%). Randomization was carried out by investigational pharmacists using computer-generated lists. Pharmacists provided portable, elastomeric infusion pumps (LV5 Infusor; Baxter International Inc, Deerfield, Illinois) with a fixed rate of 5 mL/h and 300 mL reservoir, filled with study solution as determined by these lists. Subjects were assigned to a treatment in sequential order, and randomization was stratified both by procedure (unilateral or bilateral mastectomy) as well as lymph node dissection (with or without). Subjects having a bilateral mastectomy received two separate infusion pumps, each affixed to a separate catheter, and always containing the identical solution (all subjects received only one treatment: ropivacaine or normal saline). Subjects, investigators, observers, statisticians, and all clinical staff were masked to treatment-group assignment.
Perineural infusion was initiated within the operating room. Intraoperatively, all subjects received a general anesthetic using inhaled anesthetic and oxygen. Intravenous fentanyl was administered for cardiovascular responsiveness to noxious stimuli at the discretion of the anesthesia provider.
For postoperative analgesia, all subjects received the single-injection ropivacaine paravertebral block (initiated via the catheter), and oral acetaminophen (975 mg 4 times daily). Administration of rescue analgesics for breakthrough pain was determined by pain severity using the NRS: oxycodone 5 mg (NRS < 4) or 10 mg (NRS ≥ 4). While hospitalized, pain was reassessed 30 minutes later and intravenous morphine (2–4 mg) was repeated every 30 minutes until the NRS < 4.
Subjects remained hospitalized at least 1 night, and were subsequently discharged home with their perineural catheter(s) in situ. Subjects and their caretakers were provided with verbal and written catheter/pump instructions, the telephone and pager numbers of an investigator available at all times, and prescriptions for their outpatient oral medications that did not differ from the oral analgesics provided in the hospital. Subjects were telephoned in the afternoons of postoperative day (POD) 1 and 2, and the morning of POD 3, at which time subjects’ caretakers removed the perineural catheter(s) with physician instructions provided by telephone.
Outcome measurements (end points)
Data was collected in person preoperatively, extracted from computerized records during hospitalization (recovery room and from 00:00 – 12:00 on POD 1), and recorded by telephone on POD 1, 4, 8, and 28. Staff masked to treatment group assignment performed all assessments. We selected outcome measures that have established reliability and validity, with minimal inter-rater discordance, and are recommended by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) consensus statement.7 The primary instrument was the Brief Pain Inventory (short form) administered on POD 1, 4, 8, and 28.8,9 This instrument assesses pain and its interference with physical and emotional functioning within the previous 24 hours. There are 3 domains: (1) pain, with four questions involving “worst,” “average,” “least,” and “current” pain levels using the NRS; (2) percentage of relief provided by pain treatments with 1 question; and, (3) interference with physical and emotional functioning using a 0–10 Likert scale (0 = no interference; 10 = complete interference). The 7 interference questions involve general activity, mood, walking ability, normal work activities inside and outside of the home, relationships, sleep, and enjoyment of life.10,11 The 4 pain scores were combined to produce a pain subscale (0–40), and the 7 functioning questions combined to produce an interference subscale (0–70). Values for the entire Brief Pain Inventory (0 = optimal; 120 = worst possible) consist of both of these subscales combined with the question involving relief provided by pain treatments (percentage relief divided by 10 and then subtracted from 10: 0 = complete relief, 10 = no relief). The use of both single items (eg, average pain) and the composite scores is supported by the IMMPACT recommendations for assessing pain in clinical trials.12–14
Additional information was gathered following the Brief Pain Inventory, including the incidence and intensity (measured on the NRS) of phantom breast pain, defined as painful sensations perceived within breast tissue following surgical resection.15 Also assessed was any difficulty sleeping because of pain (binary variable: yes or no), the perceived number of awakenings due to pain, and nausea using a 0–10 Likert scale (0 = no nausea; 10 = vomiting).16
Statistical analysis
Sample size calculations were centered around the hypothesis that a multiple-day ambulatory continuous paravertebral block decreases the incidence and severity of postmastectomy pain in the week following surgery. To this end, the primary end point was the difference between the 2 treatment groups in average NRS (as administered as part of the Brief Pain Inventory) queried on the day following surgery. Given an n = 60 with subjects equally distributed between the 2 treatment groups, assuming a standard deviation of 2 for the primary end point (based on unpublished data), and a 2-sided type I error protection of 5%, we had 80% power to detect a difference between means of 1.5 (StatMate; GraphPad Software, San Diego, California). No adjustments were made for multiple comparisons, and significant findings in secondary outcomes should be viewed as suggestive, requiring confirmation in a future trial before considering them as definitive.
Normality of distribution was determined using the Shapiro-Wilk test.17 For normally distributed and nonparametric data, single comparisons were made using a 2-sample t-test or Mann-Whitney U test, respectively. Categorical data were analyzed using the Chi square test with Yates continuity correction, or Fisher exact test if expected cell counts were below 5. P < 0.05 was considered significant. Count data (eg, incidence of phantom pain, number of awakenings due to surgical pain, incidence of wound pain, and opioids dose) were analyzed with a Negative Binomial generalized linear model, with group as a categorical covariate. Models were fit by the glm.nb () command from the R package MASS.18 If the model is found to be inappropriate, then a zero-inflated Negative Binomial model is considered as an alternative, with a binomial generalized linear model with group as a covariate for the zero-inflation. Zero-inflated Negative Binomial models are fit by the zeroinfl () command from the R package pscl.† Vuong’s nonnested hypothesis test was used for selecting between Negative Binomial and Zero inflated Negative Binomial models, with a = 0.05 used as the decision criterion.19 These analyses were executed using R version 2.12 (2010),†† and performed according to the intention-to-treat principle.20
RESULTS
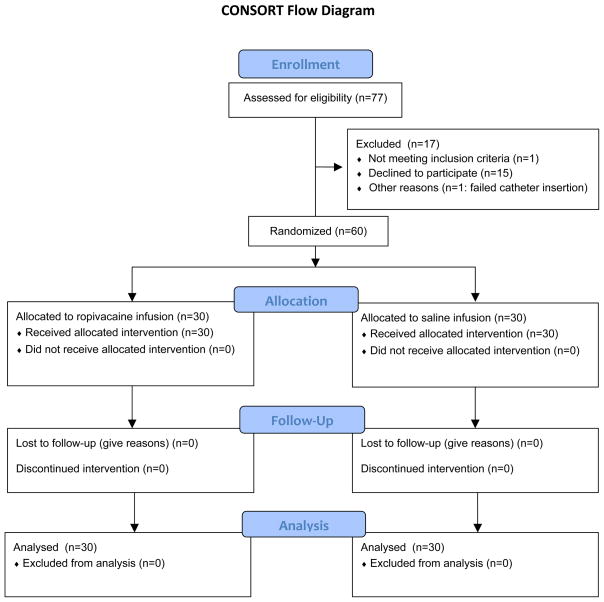
During a 24-month period beginning in December 2010, 61 subjects enrolled, and all but one (98%) had unilateral (n = 24) or bilateral (n = 36) catheter(s) inserted successfully per protocol (Fig. 1; Table 1). Of the 60 remaining subjects, 24 received unilateral and 36 bilateral catheter(s).
Figure 1.
CONSORT Flowchart.
Table 1.
Population data and procedural information
| Perineural Infusion: | Ropivacaine (n=30) | Placebo (n=30) |
|---|---|---|
| Age (yr) | 48 (40–54) | 49 (40–57) |
| Height (cm) | 165 (161–170) | 166 (163–170) |
| Weight (kg) | 62 (56–72) | 61 (54–69) |
| Body mass index (kg/m2) | 23 (20–26) | 24 (20–26) |
| Unilateral mastectomy | ||
| With lymph node dissection | 8 | 10 |
| Without lymph node dissection | 3 | 3 |
| Bilateral mastectomy | ||
| With lymph node dissection | 14 | 12 |
| Without lymph node dissection | 5 | 5 |
| Surgical duration (min) | 190 (125–205) | 184 (132–229) |
| Worst pain during catheter insertion (NRS) | 5.0 (2.5–6.0) | 2.5 (1.3–4.5) |
| Midazolam for catheter insertion(s) (mg) | 2 (2–3) | 2 (2–4) |
| Fentanyl for catheter insertion(s) (μg) | 100 (50–100) | 100 (75–100) |
Values are reported as median (interquartile) or number of subjects, as indicated
NRS: Numeric Rating Scale
Primary End Point
Average pain queried on POD 1 for subjects receiving ropivacaine (n=30) was a median (interquartile) of 2 (0–3), versus 4 (1–5) for subjects receiving saline (n=30; 95% CI difference in medians −4.0 – −0.3; P = 0.021).
Secondary End Points (Table 2)
Table 2.
Secondary end points. Values represent the previous 24 h from time queried, unless otherwise indicated.
| Perineural Infusion: | Ropivacaine (n=30) | Placebo (n=30) | P-value |
|---|---|---|---|
| Pain (NRS POD 1 00:00–12:00)§ | 3.6 (2.0–4.0) | 3.7 (2.5–4.6) | 0.183 |
| Morphine equivalents (mg)§ | |||
| Intraoperative§ | 3.0 (2.5–4.5) | 2.5 (2.5–5.0) | 0.752 |
| Recovery room§ | 1.0 (0.0–2.8) | 2.4 (1.0–5.5) | 0.013 |
| POD 1 (00:00–12:00)§ | 1.5 (0.0–8.5) | 3.3 (1.4–10.3) | 0.402 |
| Oral opioid (oxycodone, mg) | |||
| POD 4 | 10 (0–23) | 10 (0–20) | 0.376 |
| POD 8 | 1 (0–14) | 0 (0–8) | 0.840 |
| Nausea (0–10, 10=vomiting) | |||
| POD 1§ | 0 (0-0) | 0 (0–6) | 0.053 |
| POD 4 | 0 (0-0) | 0 (0-0) | 0.277 |
| POD 8 | 0 (0-0) | 0 (0-0) | 0.294 |
| Difficulty sleeping (# subjects) | |||
| POD 1§ | 9 | 16 | 0.116 |
| POD 4 | 8 | 8 | 1.000 |
| POD 8 | 10 | 8 | 0.848 |
| POD 28 | 6 | 6 | >0.999 |
| Awakenings due to pain (#/subject) | |||
| POD 1§ | 0 (0–1) | 1 (0–3) | 0.306 |
| POD 4 | 0 (0–1) | 0 (0–1) | 0.828 |
| POD 8 | 0 (0–2) | 0 (0-0) | 0.127 |
| POD 28 | 0 (0-0) | 0 (0-0) | 0.445 |
Values are reported as median (interquartile) or number of subjects, as indicated
During perineural infusion
NRS: Numeric Rating Scale
POD: postoperative day
Oral opioid and nausea queried exclusively on POD 4/8 and 1/4/8, respectively
Similarly, during the infusion period on POD 1, subjects receiving ropivacaine experienced a lower severity of breakthrough pain (5 [3–6] vs 7 [5–8]; P = 0.046); and, reported less pain-related interference with physical and emotional functioning (Table 3). For example, for the interference subscale that measures the degree of interference of pain on physical and emotional functioning, subjects receiving perineural saline reported a median score 10 times higher than those receiving ropivacaine (3 [0–24] vs 33 [0–44]; P = 0.035). In contrast, following infusion discontinuation there were no statistically significant differences detected between treatment groups (Tables 4 and 5).
Table 3.
Brief Pain Inventory during the perineural infusion.
| Postoperative Day: |
1
|
||
|---|---|---|---|
| Ropivacaine | Placebo | P-value | |
| Pain (0–10 Numeric Rating Scale) | |||
| Worst | 5 (3–6) | 7 (5–8) | 0.046 |
| Average | 2 (0–3) | 4 (1–5) | 0.021 |
| Least | 0 (0–2) | 2 (0–3) | 0.053 |
| Current | 1 (0–4) | 4 (0–5) | 0.050 |
| Pain Subscale Total (0–40) | 9 (4–13) | 16 (7–21) | 0.021 |
| Relief provided by analgesics (%) | 90 (60–100) | 50 (30–100) | 0.060 |
| Interference with (0–10; 0=none): | |||
| General activity | 1 (0–5) | 6 (0–8) | 0.052 |
| Mood | 0 (0–2) | 3 (0–6) | 0.038 |
| Walking | 0 (0–2) | 3 (0–5) | 0.046 |
| Work (inside/outside of home) | 0 (0–4) | 5 (0–8) | 0.017 |
| Relationships | 0 (0–1) | 3 (0–6) | 0.017 |
| Sleep | 0 (0–2) | 3 (0–8) | 0.034 |
| Enjoyment of life | 0 (0–5) | 5 (0–8) | 0.049 |
| Interference Subscale Total (0–70) | 3 (0–24) | 33 (0–44) | 0.035 |
| Brief Pain Inventory Total (0–120) | 14 (4–37) | 57 (8–67) | 0.012 |
Values are reported as median (interquartile) and represent the time between the surgical procedure and the time queried.
Values for the Brief Pain Inventory total score (0 = optimal; 120 = worst possible) consist of the two subscales combined with the question involving relief provided by pain treatments (percentage relief divided by 10 and then subtracted from 10: 0 = complete relief, 10 = no relief).
Table 4.
Brief Pain Inventory following the perineural infusion. There were no statistically significant differences between treatment groups.
| Postoperative Day: |
4
|
8
|
28
|
|||
|---|---|---|---|---|---|---|
| Ropivacaine | Placebo | Ropivacaine | Placebo | Ropivacaine | Placebo | |
| Pain (0–10 Numeric Rating Scale) | ||||||
| Worst | 4 (1–6) | 5 (2–6) | 3 (1–5) | 3 (1–5) | 1 (0–4) | 2 (0–3) |
| Average | 3 (0–3) | 2 (0–4) | 2 (0–3) | 2 (0–3) | 0 (0–2) | 0 (0–1) |
| Least | 0 (0–3) | 0 (0–2) | 0 (0–2) | 0 (0–2) | 0 (0-0) | 0 (0-0) |
| Current | 2 (0–3) | 2 (0–4) | 1 (0–3) | 1 (0–3) | 0 (0–1) | 0 (0-0) |
| Pain Subscale Total (0–40) | 10 (2–13) | 8 (3–15) | 6 (1–12) | 7 (2–12) | 1 (0–9) | 3 (0–5) |
| Relief provided by analgesics (%) | 80 (50–100) | 70 (50–100) | 90 (55–100) | 85 (60–100) | 100 (90–100) | 100 (85–100) |
| Interference with (0–10)… | ||||||
| General activity | 3 (0–6) | 3 (0–7) | 1 (0–4) | 3 (0–6) | 0 (0–2) | 0 (0–3) |
| Mood | 1 (0–3) | 0 (0–4) | 0 (0–3) | 0 (0–4) | 0 (0–1) | 0 (0–1) |
| Walking | 0 (0–2) | 0 (0–3) | 0 (0–1) | 0 (0–2) | 0 (0-0) | 0 (0-0) |
| Work (inside/outside of home) | 3 (0–7) | 1 (0–6) | 1 (0–4) | 0 (0–4) | 0 (0–2) | 0 (0–3) |
| Relationships | 0 (0–2) | 0 (0–3) | 0 (0–2) | 0 (0–2) | 0 (0–1) | 0 (0-0) |
| Sleep | 0 (0–4) | 0 (0–5) | 0 (0–5) | 0 (0–4) | 0 (0–2) | 0 (0–1) |
| Enjoyment of life | 3 (0–5) | 0 (0–5) | 0 (0–3) | 0 (0–5) | 0 (0–1) | 0 (0–2) |
| Subscale Total (0–70) | 14 (0–26) | 12 (0–32) | 6 (0–22) | 3 (0–22) | 0 (0–12) | 0 (0–11) |
| Brief Pain Inventory Total (0–120) | 31 (2–40) | 24 (4–53) | 15 (2–40) | 20 (3–43) | 0 (0–11) | 3 (0–12) |
Values are reported as median (interquartile) and represent the previous 24 h from time queried
Values for the Brief Pain Inventory total score (0 = optimal; 120 = worst possible) consist of both subscales combined with the question relief provided by analgesics calculated as follows: percentage relief divided by 10 and then subtracted from 10 (0 = complete relief, 10 = no relief).
Table 5.
Phantom Breast Pain
| Perineural Infusion: | Ropivacaine (n=30) | Placebo (n=30) | P-value |
|---|---|---|---|
| Prevalence (number of subjects reporting pain) | |||
| POD 1 | 0 | 1 | >0.999 |
| POD 4 | 1 | 2 | >0.999 |
| POD 8 | 3 | 3 | >0.999 |
| POD 28 | 5 | 5 | >0.999 |
| Incidence (times per day for each subject) | |||
| POD 1 | 0 (0-0) | 0 (0-0) | § |
| POD 4 | 0 (0-0) | 0 (0-0) | 0.560 |
| POD 8 | 0 (0–2) | 0 (0-0) | 0.632 |
| POD 28 | 0 (0–2) | 0 (0–2) | 0.875 |
| Severity (Worst NRS previous 24 h) | |||
| POD 1 | 0 (0-0) | 0 (0-0) | § |
| POD 4 | 0 (0-0) | 0 (0-0) | 0.543 |
| POD 8 | 0 (0–1) | 0 (0-0) | 0.674 |
| POD 28 | 0 (0–4) | 0 (0-0) | 0.855 |
| Severity (Average NRS previous 24 h) | |||
| POD 1 | 0 (0-0) | 0 (0-0) | § |
| POD 4 | 0 (0-0) | 0 (0-0) | 0.543 |
| POD 8 | 0 (0–1) | 0 (0-0) | 0.611 |
| POD 28 | 0 (0–1) | 0 (0–1) | 0.650 |
Values for pain severity are reported as median (10th–90th percentiles)
POD: postoperative day
A p-value could not be calculated due to only one non-zero datum
NRS: Numeric Rating Scale
Protocol deviations and adverse events
One subject receiving perineural ropivacaine via bilateral catheters requested removal of one of the catheters that “pinched” at the skin the evening of POD 0; and, subsequently requested removal of the contralateral catheter and study withdrawal the following afternoon for the same reason. The discomfort resolved immediately upon catheter withdrawal and did not recur. For purposes of analysis, this subject was retained within her treatment group until the time of study withdrawal, per both the intention-to-treat principle and United States ethical guidelines.20,21 Of note, there were no unintended catheter dislocations. Another subject had a catheter inserted on the incorrect side for a unilateral mastectomy prior to randomization, and this catheter was withdrawn and a new catheter inserted on the correct side.
One subject with bilateral catheters infusing normal saline experienced contact dermatitis in skin areas with adhesive exposure (occlusive dressing, paper tape, and anchoring device), which resolved following catheter removal on POD 3, per protocol. Another subject exhibited a bilateral block from the first through tenth thoracic dermatome following the initial ropivacaine injection, suggesting epidural spread. She did not exhibit hypotension and elected to remain in the study. All signs of the regional block had resolved within 12 hours, and following study group assignment unmasking, it was revealed that she had received perineural normal saline.
DISCUSSION
This randomized, triple-masked, placebo-controlled study provides evidence that, following unilateral or bilateral mastectomy, adding a multiple-day, ambulatory, continuous ropivacaine infusion to a single-injection ropivacaine paravertebral nerve block results in improved analgesia and decreased functional deficit the day following surgery. In contrast, following the end of the infusion there were no benefits detected from the additional continuous paravertebral block. Given the tens-of-thousands of patients undergoing mastectomy within the United States alone,1,2 these results have broad clinical implications, not only for immediate postoperative analgesia, but potentially persistent postoperative pain as well.
Benefits of continuous peripheral nerve blocks have been documented for various anatomic catheter locations,6 so while it might seem self-evident that paravertebral perineural infusions would provide similar benefits following mastectomy, there are good reasons to not infer this causal relationship from studies involving other anatomic sites. Importantly, mastectomy involves many thoracic levels, and the often-accompanying axillary dissection extends involvement to the lower cervical levels. There is robust, prospectively collected evidence that the vertical spread of local anesthetic resulting from a single injection is greatly limited: a mean (SD) of 3.0 (1.2) dermatomes with a single bolus injection (0.26 mL/kg of local anesthetic plus 0.1 mL/kg of radiopaque dye) compared with 6.5 (2.0) dermatomes with 4 individual bolus injections (each with 25% of the volume) at 4 consecutive thoracic levels.22 Furthermore, with the relatively low volume (5–10 mL/h), dose (20–40 mg/h), and injection pressure of a perineural infusion compared with a single-injection bolus via a Tuohy needle, it is doubtful that a continuous paravertebral block affects more levels than a single-injection bolus.
Previously published literature
There is an abundance of published evidence that single-injection paravertebral blocks improve postmastectomy analgesia.4,23–25 In addition, multiple studies have documented improved analgesia and/or decreased supplemental opioid requirements associated with a continuous paravertebral block.23,26–30 However, all but 1 of these latter investigations included a long-acting single-injection paravertebral block in addition to the perineural infusion, while the control group received neither a single-injection nor perineural infusion.23,26–29 Since the analgesic effects of a single-injection paravertebral block have been documented as long as 72 hours,5 it remained unknown if the benefits cited in the perineural infusion studies were due exclusively to the single-injection block, or perineural infusion as well.23,26–29 And, in fact, the only published study including a control group with an active single-injection block failed to detect any benefits from adding a 72-hour ropivacaine perineural infusion.30 In this respect, the current study is the first randomized, controlled trial demonstrating that postmastectomy patients benefit from a paravertebral local anesthetic infusion.6 There are multiple potential reasons why the current results differed from this previously published investigation,30 including differences in technique of the initial paravertebral block and catheter-insertion, ropivacaine infusion concentration/rate, and inclusion of a nonsteroidal anti-inflammatory drug (naproxen) beginning the day after surgery in the previous study.
As with all medical interventions, perineural local anesthetic infusion has intrinsic risks that must be balanced against the potential benefits. This study’s results suggest there are benefits in providing a continuous paravertebral nerve block following mastectomy, but the infusion-related adverse events must not be overlooked or minimized. In our study, subjects experienced contact dermatitis from adhesive exposure, catheter “pinching” at the skin, bilateral block suggesting possible epidural spread, and catheter insertion on the incorrect side. In addition, both in-hospital and ambulatory perineural infusion require a system for management that may not be available at every practice.
Patient-oriented outcomes
Simple pain scores and recognition of the importance of patient-oriented outcomes (eg, health-related quality of life,31 quality of recovery,32 global outcome measures of function33) have greatly increased over the last decade, both to improve the quality of healthcare and to provide value data (eg, cost-benefit analysis) for external organizations such as government agencies and insurance companies that increasingly require evidence of performance.34 For these reasons, we administered the Brief Pain Inventory—an instrument assessing pain and its interference on physical and emotional functioning—for the present study8,9 and documented dramatically decreased pain-related physical and emotional functional interference during a perineural local anesthetic infusion. To date, few studies involving continuous peripheral nerve blocks have examined quality-of-life measures, and there is a conspicuous lack of evidence in documented improvements of global measures during perineural infusion.6
Persistent postoperative pain
Chronic postsurgical pain is a well-documented risk following mastectomy.35 In 1 study, the addition of a single-injection paravertebral block decreased the intensity of motion-related pain 4 weeks following surgery (P = 0.005).36 In a second investigation, a single-injection paravertebral block followed by 48 hours of infusion decreased the prevalence of pain 10 weeks postoperatively from 80% (control group) to 0% (P = 0.009).26 Of note, the control groups for both of these studies received no regional anesthetic/analgesic, so it remains unknown if the benefits of the latter are related to the perineural infusion or exclusively the initial surgical block.26 In the current study, subjects who received a ropivacaine perineural infusion in addition to a single-injection block reported a maximum (“worst”) NRS at 4 weeks of a median (interquartile) of 0.5 (0.0–3.5) compared with 2.0 (0.0–3.0) for subjects with only a single-injection block (and little difference in opioid use following the infusion). This difference did not reach statistical significance (P = 0.305); and, the average and least pain scores at 4 weeks were exceptionally low for both groups (Table 4), suggesting that the single-injection surgical block is of primary importance in decreasing the risk of persistent postsurgical pain relative to the subsequent perineural infusion.
Reports of the prevalence of postmastectomy phantom breast pain—painful sensations perceived in the breast that is no longer present—range from 14% to 44% in patients provided traditional opioid postoperative analgesics.35 To our knowledge, the association between paravertebral blocks/infusions and this pain phenomenon has not been previously investigated. However, data involving upper- and lower-extremity amputation suggest that continuous peripheral nerve blocks may decrease the incidence and/or severity of phantom limb pain.6,37,38 When a nerve is severed, evidence indicates that the bombardment of the peripheral and central nervous system with nociceptive signals results in changes to the spinal cord, thalamus, and cerebral cortex.39 In an uncontrolled series of 19 patients with phantom limb pain treated with continuous peripheral nerve blocks in the immediate postamputation period, pain intensity decreased by approximately 50% at 1 and 6 months.40 While our study failed to detect any trend of decreased phantom breast pain prevalence or severity in the active treatment group (Table 3), it was also not powered for these secondary end points.
Study limitations
This investigation has several limitations. First, we provided exclusively a basal infusion of 5 mL/h (ropivacaine 0.4%, or 20 mg/h), without patient-controlled bolus doses. The optimal delivery regimen for paravertebral infusions remains unknown; and, therefore, a higher basal infusion rate (>5 mL/h) and/or local anesthetic concentration (>0.4%) than used in this study may yield superior results. In a previous study involving continuous paravertebral blocks providing a basal dose similar to the present investigation (levobupivacaine 0.2% at 8 mL/h, or 16 mg/h) combined with patient-controlled bolus doses (3 mL with 15-minute lockout), subjects triggered their bolus a mean (SD) of 72 (16) times in the 36 hours following mastectomy.41 These results suggest that providing patient-controlled bolus doses in the present study may have improved analgesia and produced even greater differences between the two treatment groups.42 Since the current study evaluated 1 perineural delivery regimen on various post-mastectomy end points, it does not provide data to support any specific recommendations on the optimal basal rate, local anesthetic concentration, inclusion of a bolus dose, bolus dose volume, or optimal lockout duration. Relatedly, nonsteroidal anti-inflammatory agents, gabapentin, and local anesthetic wound infiltration/infusion were not used per the surgeon’s preferred standard analgesic regimen, the addition of which may have decreased the differences found within the 2 treatment groups.43,44
In addition, while the overwhelming number of investigations—including the present study—involving breast surgery and paravertebral infusion included the third thoracic level for catheter insertion,23,26–30,41,45 the optimal level for mastectomy remains unknown. Furthermore, while the current study provides evidence that a continuous paravertebral block provides benefits through the day following surgery, we did not collect data on pain on postoperative days 2 or 3. Therefore, it remains unknown what the optimal duration of infusion is following mastectomy. However, we suspect that this case is much like catheters in most other anatomic locations: the intensity and duration of pain are highly variable, and a subset of patients benefit from a more prolonged infusion while others require no infusion at all.6 Lastly, we did not measure blood levels of nociceptive processing or stress response markers that could have helped elucidate any association between perioperative analgesia, and persistent postoperative pain and cancer recurrence.26,46
In summary, this investigation provides evidence that following mastectomy, adding a multiple-day, ambulatory, continuous ropivacaine infusion to a single-injection ropivacaine paravertebral nerve block results in improved analgesia with less functional deficit during the infusion.
Acknowledgments
Financial Support: Funding for this project provided by the National Institutes of Health grant GM077026 (P.I.: Dr. Ilfeld) from the National Institute of General Medical Sciences (Bethesda, Maryland); the Clinical and Translational Research Institute, University of California, San Diego (San Diego, California), with funding provided by the National Institutes of Health National Center for Research Resources grant UL1RR031980; the Department of Anesthesiology, University of California San Diego (San Diego, California); and Baxter Healthcare International (Deerfield, Illinois). This company also provided the portable infusion pumps used in this investigation, and had no input into any aspect of study conceptualization, design, and implementation; data collection, analysis and interpretation; or manuscript preparation. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the funding entities.
Footnotes
Zeileis A, Kleiber C, Jackman S: Regression models for count data in R. J Stat Software 2008; 27. URL http://www.jstatsoft.org/v27/i08. Accessed February 22, 2013
R Software Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org. Accessed February 19, 2013
Conflict of Interest:
The authors declare no conflict of interest.
Prior Presentation:
Presented, in part, as a scientific abstract at the annual meeting of the American Society of Anesthesiologists in San Francisco, California, October 14, 2013.
The majority of study contributions of Dr. Loland occurred while working at the University of California San Diego, San Diego, California. Dr. Loland subsequently moved to the University of Washington, Seattle, Washington.
References
- 1.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2009. Bethesda, MD: 2012. [Google Scholar]
- 2.Habermann EB, Abbott A, Parsons HM, Virnig BA, Al-Refaie WB, Tuttle TM. Are mastectomy rates really increasing in the United States? J Clin Oncol. 2010;28:3437–3441. doi: 10.1200/JCO.2009.27.6774. [DOI] [PubMed] [Google Scholar]
- 3.Cepeda MS, Africano JM, Polo R, Alcala R, Carr DB. What decline in pain intensity is meaningful to patients with acute pain? Pain. 2003;105:151–157. doi: 10.1016/s0304-3959(03)00176-3. [DOI] [PubMed] [Google Scholar]
- 4.Kairaluoma PM, Bachmann MS, Korpinen AK, Rosenberg PH, Pere PJ. Single-injection paravertebral block before general anesthesia enhances analgesia after breast cancer surgery with and without associated lymph node biopsy. Anesth Analg. 2004;99:1837–1843. doi: 10.1213/01.ANE.0000136775.15566.87. [DOI] [PubMed] [Google Scholar]
- 5.Klein SM, Bergh A, Steele SM, Georgiade GS, Greengrass RA. Thoracic paravertebral block for breast surgery. Anesth Analg. 2000;90:1402–1405. doi: 10.1097/00000539-200006000-00026. [DOI] [PubMed] [Google Scholar]
- 6.Ilfeld BM. Continuous peripheral nerve blocks: a review of the published evidence. Anesth Analg. 2011;113:904–925. doi: 10.1213/ANE.0b013e3182285e01. [DOI] [PubMed] [Google Scholar]
- 7.Dworkin RH, Turk DC, Peirce-Sandner S, et al. Research design considerations for confirmatory chronic pain clinical trials: IMMPACT recommendations. Pain. 2010;149:177–193. doi: 10.1016/j.pain.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 8.Cleeland CS, Ryan KM. Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 9.Mendoza TR, Chen C, Brugger A, Hubbard R, Snabes M, Palmer SN, Zhang Q, Cleeland CS. The utility and validity of the modified brief pain inventory in a multiple-dose postoperative analgesic trial. Clinical J Pain. 2004;20:357–362. doi: 10.1097/00002508-200409000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Broderick JE, Schwartz JE, Vikingstad G, Pribbernow M, Grossman S, Stone AA. The accuracy of pain and fatigue items across different reporting periods. Pain. 2008;139:146–157. doi: 10.1016/j.pain.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zelman DC, Gore M, Dukes E, Tai KS, Brandenburg N. Validation of a modified version of the Brief Pain Inventory for painful diabetic peripheral neuropathy. J Vasc Nurs. 2005;23:97–104. doi: 10.1016/j.jvn.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Dworkin RH, Turk DC, McDermott MP, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain. 2009;146:238–244. doi: 10.1016/j.pain.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 14.Turk DC, Dworkin RH, Burke LB, et al. Developing patient-reported outcome measures for pain clinical trials: IMMPACT recommendations. Pain. 2006;125:208–215. doi: 10.1016/j.pain.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 15.Dijkstra PU, Rietman JS, Geertzen JH. Phantom breast sensations and phantom breast pain: a 2-year prospective study and a methodological analysis of literature. Eur J Pain. 2007;11:99–108. doi: 10.1016/j.ejpain.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Chan A, Low XH, Yap KY. Assessment of the relationship between adherence with antiemetic drug therapy and control of nausea and vomiting in breast cancer patients receiving anthracycline-based chemotherapy. J Managed Care Pharm. 2012;18:385–394. doi: 10.18553/jmcp.2012.18.5.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Razali MR, Wah YB. Power comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lilliefors and Anderson-Darling tests. JOSMA. 2011;2:21–33. [Google Scholar]
- 18.Venables WN, Ripley BD. Modern applied statistics with S. 4. New York: Springer; 2002. [Google Scholar]
- 19.Vuong QH. Likelihood ratio tests for model selection and non-nested hypotheses. Econometrica. 1989;57:307–333. [Google Scholar]
- 20.Todd MM. Clinical research manuscripts in Anesthesiology. Anesthesiology. 2001;95:1051–1053. doi: 10.1097/00000542-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 21.The Common Rule, Title 45 (Public Welfare), Code of Federal Regulations, Part 46 (Protection of Human Subjects), 2001, 1–18.
- 22.Naja ZM, El-Rajab M, Al-Tannir MA, Ziade FM, Tayara K, Younes F, Lonnqvist PA. Thoracic paravertebral block: Influence of the number of injections. Reg Anesth Pain Med. 2006;31:196–201. doi: 10.1016/j.rapm.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Schnabel A, Reichl SU, Kranke P, Pogatzki-Zahn EM, Zahn PK. Efficacy and safety of paravertebral blocks in breast surgery: a meta-analysis of randomized controlled trials. Br J Anaesth. 2010;105:842–852. doi: 10.1093/bja/aeq265. [DOI] [PubMed] [Google Scholar]
- 24.Moller JF, Nikolajsen L, Rodt SA, Ronning H, Carlsson PS. Thoracic paravertebral block for breast cancer surgery: a randomized double-blind study. Anesth Analg. 2007;105:1848–1851. doi: 10.1213/01.ane.0000286135.21333.fd. [DOI] [PubMed] [Google Scholar]
- 25.Boughey JC, Goravanchi F, Parris RN, et al. Prospective randomized trial of paravertebral block for patients undergoing breast cancer surgery. Am J Surg. 2009;198:720–725. doi: 10.1016/j.amjsurg.2008.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iohom G, Abdalla H, O’Brien J, et al. The associations between severity of early postoperative pain, chronic postsurgical pain and plasma concentration of stable nitric oxide products after breast surgery. Anesth Analg. 2006;103:995–1000. doi: 10.1213/01.ANE.0000240415.49180.4A. [DOI] [PubMed] [Google Scholar]
- 27.Buggy DJ, Kerin MJ. Paravertebral analgesia with levobupivacaine increases postoperative flap tissue oxygen tension after immediate latissimus dorsi breast reconstruction compared with intravenous opioid analgesia. Anesthesiology. 2004;100:375–380. doi: 10.1097/00000542-200402000-00029. [DOI] [PubMed] [Google Scholar]
- 28.Burlacu CL, Frizelle HP, Moriarty DC, Buggy DJ. Fentanyl and clonidine as adjunctive analgesics with levobupivacaine in paravertebral analgesia for breast surgery. Anaesthesia. 2006;61:932–937. doi: 10.1111/j.1365-2044.2006.04793.x. [DOI] [PubMed] [Google Scholar]
- 29.Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105:660–664. doi: 10.1097/00000542-200610000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buckenmaier CC, 3rd, Kwon KH, Howard RS, et al. Double-blinded, placebo-controlled, prospective randomized trial evaluating the efficacy of paravertebral block with and without continuous paravertebral block analgesia in outpatient breast cancer surgery. Pain Med. 2010;11:790–799. doi: 10.1111/j.1526-4637.2010.00842.x. [DOI] [PubMed] [Google Scholar]
- 31.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273:59–65. [PubMed] [Google Scholar]
- 32.Wu CL, Rowlingson AJ, Partin AW, et al. Correlation of postoperative pain to quality of recovery in the immediate postoperative period. Reg Anesth Pain Med. 2005;30:516–522. doi: 10.1016/j.rapm.2005.07.190. [DOI] [PubMed] [Google Scholar]
- 33.Wu CL, Raja SN. Optimizing postoperative analgesia: the use of global outcome measures. Anesthesiology. 2002;97:533–534. doi: 10.1097/00000542-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Macario A, Vasanawala A. Improving quality of anesthesia care: opportunities for the new decade. Can J Anaesth. 2001;48:6–11. doi: 10.1007/BF03019807. [DOI] [PubMed] [Google Scholar]
- 35.Jung BF, Ahrendt GM, Oaklander AL, Dworkin RH. Neuropathic pain following breast cancer surgery: proposed classification and research update. Pain. 2003;104:1–13. doi: 10.1016/s0304-3959(03)00241-0. [DOI] [PubMed] [Google Scholar]
- 36.Kairaluoma PM, Bachmann MS, Rosenberg PH, Pere PJ. Preincisional paravertebral block reduces the prevalence of chronic pain after breast surgery. Anesth Analg. 2006;103:703–708. doi: 10.1213/01.ane.0000230603.92574.4e. [DOI] [PubMed] [Google Scholar]
- 37.Ilfeld BM, Moeller-Bertram T, Hanling SR, et al. Treating intractable phantom limb pain with ambulatory continuous peripheral nerve blocks: a pilot study. Pain Med. 2013;14:935–942. doi: 10.1111/pme.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borghi B, D’Addabbo M, White PF, et al. The use of prolonged peripheral neural blockade after lower extremity amputation: the effect on symptoms associated with phantom limb syndrome. Anesth Analg. 2010;111:1308–1315. doi: 10.1213/ANE.0b013e3181f4e848. [DOI] [PubMed] [Google Scholar]
- 39.Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- 40.Schley M, Topfner S, Wiech K, et al. Continuous brachial plexus blockade in combination with the NMDA receptor antagonist memantine prevents phantom pain in acute traumatic upper limb amputees. Eur J Pain. 2007;11:299–308. doi: 10.1016/j.ejpain.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 41.McElwain J, Freir NM, Burlacu CL, Moriarty DC, Sessler DI, Buggy DJ. The feasibility of patient-controlled paravertebral analgesia for major breast cancer surgery: a prospective, randomized, double-blind comparison of two regimens. Anesth Analg. 2008;107:665–668. doi: 10.1213/ane.0b013e31817b7f01. [DOI] [PubMed] [Google Scholar]
- 42.Murata H, Salviz EA, Chen S, Vandepitte C, Hadzic A. Case report: ultrasound-guided continuous thoracic paravertebral block for outpatient acute pain management of multilevel unilateral rib fractures. Anesth Analg. 2013;116:255–257. doi: 10.1213/ANE.0b013e31826f5e25. [DOI] [PubMed] [Google Scholar]
- 43.Vallejo MC, Phelps AL, Sah N, et al. Preemptive analgesia with bupivacaine for segmental mastectomy. Reg Anesth Pain Med. 2006;31:227–232. doi: 10.1016/j.rapm.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Sidiropoulou T, Buonomo O, Fabbi E, et al. A prospective comparison of continuous wound infiltration with ropivacaine versus single-injection paravertebral block after modified radical mastectomy. Anesth Analg. 2008;106:997–1001. doi: 10.1213/ane.0b013e31816152da. [DOI] [PubMed] [Google Scholar]
- 45.Buckenmaier CC, 3rd, Klein SM, Nielsen KC, Steele SM. Continuous paravertebral catheter and outpatient infusion for breast surgery. Anesth Analg. 2003;97:715–717. doi: 10.1213/01.ANE.0000075836.53838.EB. [DOI] [PubMed] [Google Scholar]
- 46.Naesh O, Haljamae H, Hindberg I, Holm J, Jivegard L, Wennmalm A. Epidural anaesthesia prolonged into the postoperative period prevents stress response and platelet hyperaggregability after peripheral vascular surgery. Eur J Vasc Surg. 1994;8:395–400. doi: 10.1016/s0950-821x(05)80956-9. [DOI] [PubMed] [Google Scholar]