Abstract
Enzymatic transfer of the AMP portion of ATP to substrate proteins has recently been described as an essential mechanism of bacterial infection for several pathogens. The first AMPylator to be discovered, VopS from Vibrio parahaemolyticus, catalyzes the transfer of AMP on to the host GTPases Cdc42 and Rac1. Modification of these proteins disrupts downstream signaling events, contributing to cell rounding and apoptosis, and recent studies have suggested that blocking AMPylation may be an effective route to stop infection. To date, however, no small molecule inhibitors have been discovered for any of the AMPylators. Therefore, we developed a fluorescence-polarization based high-throughput-screening assay and used it to discover the first inhibitors of protein AMPylation. Herein we report the discovery of the first small molecule VopS inhibitors (e.g. calmidazolium, GW7647 and MK886) with Kis ranging from 6–50 µM and upwards of 30-fold selectivity versus HYPE, the only known human AMPylator.
INTRODUCTION
Vibrio infections, a main cause of food poisoning, increased 115% between 1998 and 2010.1, 2 While Vibrio gastroenteritis is typically self-limiting, antibiotic intervention is required in at-risk patients with an underlying medical condition. Vibrio parahaemolyticus, the main bacterial cause of this sickness, uses a Type III secretion system to inject host cells with effector proteins including VopS. As recently identified by Orth and colleagues,3 VopS catalyzes the transfer of the AMP portion of ATP to threonine residues present in the host GTPases Cdc42 and Rac1. As a result, this posttranslational modification (PTM) disrupts downstream signaling events eventually leading to cell rounding and apoptosis.3
Enzymatic transfer of the AMP portion of ATP to substrate proteins, coined AMPylation, has since been shown to be an essential mechanism for infections caused by V. parahaemolticus, H. somni4 and Legionella pneumophila,5 suggesting that AMPylators are potential therapeutic targets. Supporting this notion is the recent demonstration that antibodies targeting the Fic domain of IbpA from H. somni block AMPylation and prevent infection.6, 7 The fact that several bacterial pathogens including Klebsiella pneumoniae, Salmonella enterica, Shigella dysenteriae, and Mycobacterium tuberculosis all harbor genes encoding AMPylators further suggests that inhibitors targeting these enzymes may possess broad therapeutic utility.3–5, 8–17 Additionally, AMPylation is important for eukaryotic cell signaling because deletion of the Fic-domain containing protein in Drosophila leads to blindness.18 To date, however, no small molecule AMPylator inhibitors have been discovered. Therefore, we set out to develop a high throughput screen (HTS) to identify inhibitors of protein AMPylation.
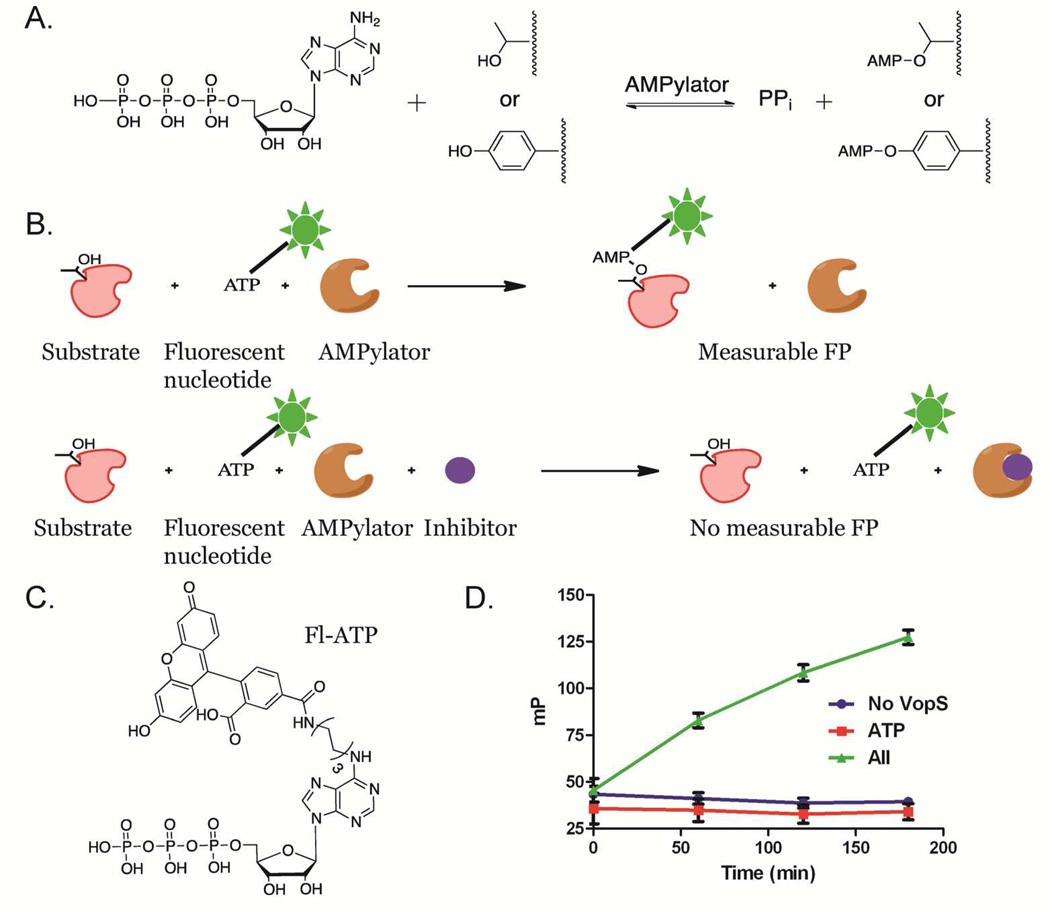
The basis of our assay platform is the enzyme catalyzed transfer of a fluorophore to a protein substrate, which results in an increase in fluorescence polarization (FP) (Figure 1). In the presence of an inhibitor, however, fluorophore transfer is blocked and there is no increase in FP.19 We recently identified Fl-ATP as a fluorescently-tagged ATP analogue that is compatible with this type of HTS assay (Figure 1C). Briefly, the kcat/KM of Fl-ATP is only 10-fold lower than ATP (1.0 × 104 versus 1.2 × 105 M−1s−1 for Fl-ATP and ATP, respectively), and we verified that this fluorescent nucleotide is transferred to VopS substrates.20 Given the high catalytic efficiency of Fl-ATP, we used this analog as the fluorescence source for our FP-based HTS assay, which monitors the VopS catalyzed transfer of Fl-AMP to Cdc42. Using this assay, we identified the first inhibitors of protein AMPylation.
Figure 1.
VopS reaction and assay design. a) AMPylators transfer AMP to side chain threonines or tyrosines. b) FP assay design. A time dependent increase in FP signal occurs as VopS transfers Fl-AMP to Cdc42. In the presence of an inhibitor, however, no Fl-AMP is transferred and the FP signal is diminished. c) Structure of Fl-ATP. d) Substrates (Cdc42 and Fl-ATP) were added to 16 replicates of VopS, VopS with 0.4 mM ATP or VopS Screening Buffer in a 384-well plate. FP was measured as a function of time.
RESULTS AND DISCUSSION
Assay design
We hypothesized that Fl-ATP could be used in a FP-based HTS assay, wherein the VopS catalyzed transfer of Fl-ATP to Cdc42 leads to a time-dependent increase in FP (Figure 1). The principle behind this assay is that when plane polarized light is passed through a sample, Fl-ATP will minimally emit polarized light (because the probe rotates rapidly in solution). When transferred to a protein substrate, the protein-fluorophore complex rotates more slowly, resulting in an observable increase in the emission of polarized light, i.e. FP. Key advantages of this assay format are that the read out is homogenous and requires no washing. Additionally, the assay directly measures inhibitory potency, and the same probes can be used to validate the inhibitors in a gel-based screen.
As opposed to a direct competition assay, as occurs in FP-ABPP assays,21–23 an additional advantage of our HTS format comes from the fact that since it is an enzymatic assay, it will identify compounds that inhibit enzyme activity regardless of whether they are ATP competitive, prevent protein substrate binding, or are allosteric inhibitors. Note that while the binding of Fl-ATP to the AMPylator itself could also increase the FP signal, preliminary studies indicate that this scenario does not occur with VopS, likely due to the fact that the KM of VopS for ATP is relatively high (~50 µM).13, 20 Also note that even if Fl-ATP does bind tightly to the enzyme, we are simply biasing the results of the screen towards compounds that bind to the ATP binding pocket.
Initial assay optimization
To provide the initial proof of concept for this assay, we identified the conditions that would yield a robust time dependent increase in FP (Figure 1D). Initially, the nonionic surfactant pluronic acid (0.01%) was added to our traditional VopS AMPylation buffer to eliminate any false positives that may be due to non-specific aggregation.24 We then optimized the concentrations of VopS and substrates to achieve a linear response over several hours, as well as provide suitable signal to baseline (S/B) and Z’ factors, a measure of assay variability, and be amenable to 384-well plate processing. Both Fl-ATP and Cdc42 concentrations are sub KM (0.005× and 0.02× respectively) ensuring our ability to identify inhibitors capable of binding at either site. From these experiments (Figure 1D), we identified the ideal concentrations of VopS (0.02 µM), Fl-ATP (0.25 µM) and Cdc42 (5 µM) that provide both a linear response and suitable S/B (~3) and Z’ values (~0.8) at 3 h. Note that the 3 h time point was chosen to both maximize S/B and Z’ and minimize the number of concurrent robotic handling steps.
Plate-to-plate and day-to-day variability
These optimized conditions were then used to examine the reproducibility of the assay on full 384-well plates using both a DMSO source plate and a control plate. Both the control plate and source plate were pin-transferred (20 nL) into a black 384-well microtiter plate that already contained VopS or no enzyme controls. After pre-incubating for 20 min, both Cdc42 and Fl-ATP were added. A pre-incubation time of 20 min was chosen to allow for the discovery of slow-tight binding inhibitors during the actual screen. ATP was also tested as a low control because this is the normal substrate for the enzyme and effectively acts as an inhibitor by outcompeting binding by Fl-ATP. A random well plot (Figure S1A) shows the clear separation of the high and both low controls. Since ATP and the no VopS columns provided suitable results, the no VopS columns were used for all future Z’ calculations. Also, as can be seen in the well scatter plot, we identified zero false positives. A correlation plot (Figure S1B) shows that there was strong repeatability and clustering of the controls and the DMSO sample field. Z’ factors were excellent, ~0.8 for each plate (Figure S1C), and the S/B was near 3, indicating this assay is highly reproducible and shows very little deviation in the controls.
Assay reproducibility
To further gauge the sensitivity and reproducibility of the assay, we determined the IC50 for the natural substrate, ATP, which inhibits the increase in FP by out competing Fl-ATP. For these studies, 7 replicates of 1/3 dilutions of ATP were pin transferred into the wells and the FP monitored at 0, 30, 60, 120, and 180 min, using the optimized conditions described above (0.02 µM VopS, 0.250 µM Fl-ATP and 5 µM Cdc42) (Figure S2). All replicates showed strong correlations and we obtained good agreement in the IC50 values obtained for ATP (IC50(ATP) = 7.0 ± 1 µM) at 3 h. The IC50 values obtained at the different time points were also identical within error (6.8 – 7.0 ± 1 µM at 1, 2, and 3 h for ATP), further demonstrating that the 3 h time point provides an acceptable point at which to measure changes in FP.
LOPAC Screen
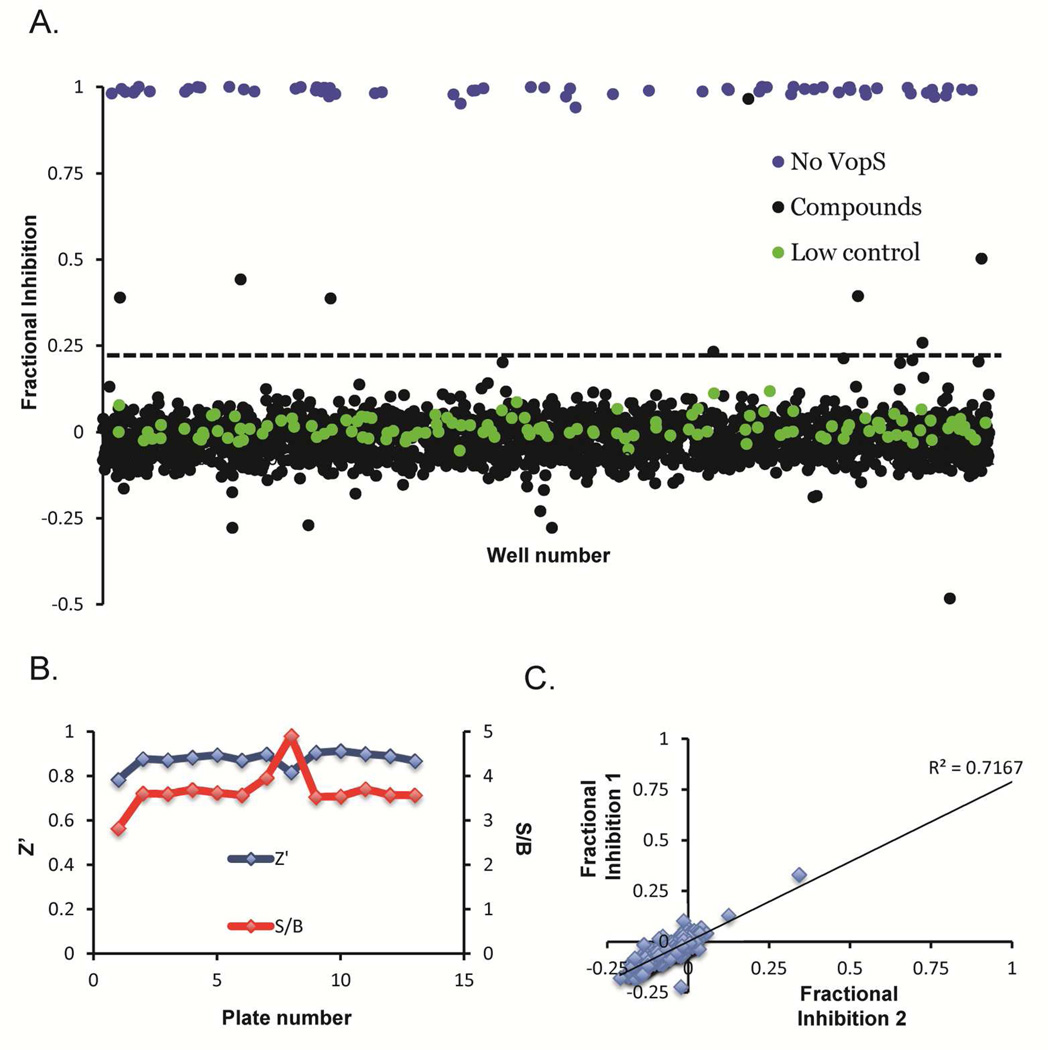
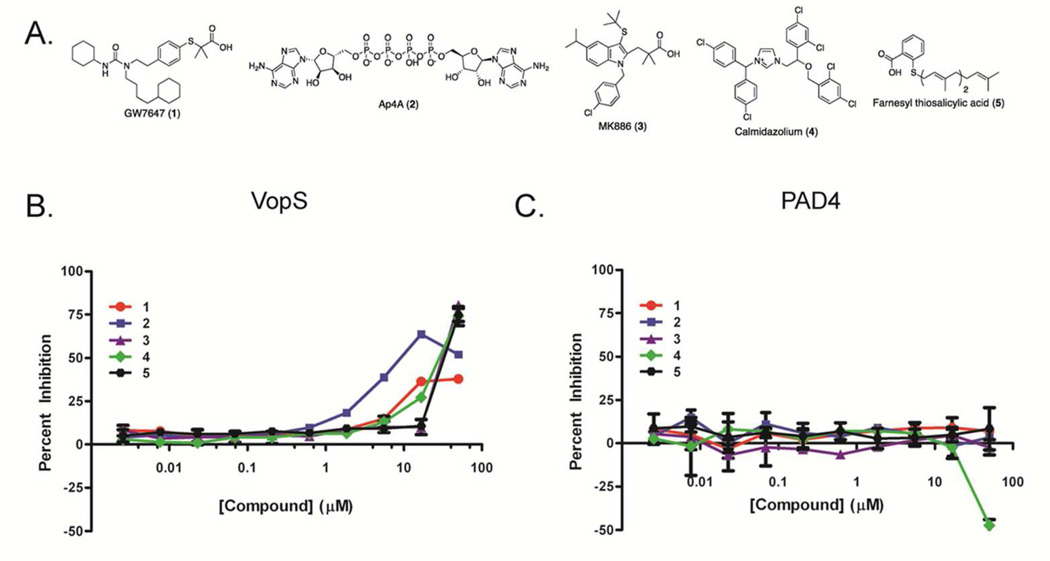
With an optimized and validated assay in hand, we screened the 1,280-compound LOPAC library (Sigma-Aldrich Library Of Pharmacologically Active Compounds) at 11 µM using the conditions and controls outlined above. A randomized-well activity scatter plot (Figure 2A) of the compounds shows a large assay window, i.e. separation between the controls (Figure 2B: average Z’ of 0.89 for the whole assay) and several potential inhibitors in-between. Using a typical assay cut-off,25 the hit rate was calculated to be 0.4%. While a small subset of the wells did show negative inhibition, this phenomenon was not reproducible. Therefore, these wells were considered artifacts. Comparing two replicates of the same LOPAC source plate (Figure 2C) demonstrates the reproducibility of the assay (R2 = 0.72). The structures of the top 5 hits are depicted in Figure 3A.
Figure 2.
LOPAC screen. The LOPAC library was screened in triplicate. The compound activity can be seen in a) Random well scatter of the 3 h normalized FP values. Assay quality is shown by b) Z’ and S/B plots for each of the 13 plates, and c) Well correlation between 2 of the LOPAC plates.
Figure 3.
Hit Validation. a) Structures of the most efficacious 5 hits from the LOPAC screen. Concentration response curves (CRC) for the same compounds with b) VopS and c) PAD4. The CRC for PAD4 were generated using the PAD4 targeted FP-ABPP assay.
Counterscreen
We next used our PAD4 FP-ABPP HTS assay as a counterscreen,22 reasoning that since PAD4 and VopS operate by drastically different mechanisms any compounds that show inhibition are false positives that decrease FP by fluorescence quenching, aggregation or some other non-specific means.26 Here, we used fluorescein-conjugated F-amidine (FFA),27 a PAD targeted ABPP that modifies a cysteine residue present in the PAD4 active site, and confirmed that it leads to a time-dependent increase in FP upon incubation with PAD4 (Figure S3). We observed a robust Z’ factor (~0.7) and S/B (~6) when using the no enzyme wells as the high control. Also, as can be seen in the well-scatter plot, the level of inhibition by Cl-amidine is an excellent indicator that the assay behaves identically to our previous screen,22 which used a rhodamine-based fluorophore.
Since none of the top 5 compounds inhibit PAD4 in the counterscreen, we reasoned that they were likely bona fide VopS inhibitors (Figure 3C). Concentration response curves (CRCs) were then generated to confirm that this is the case (Figure 3B). In all cases, dose dependent inhibition of the top hits was observed with IC50 values ranging from 11–60 µM. While the ATP analogue Ap4A, is most likely an alternative substrate, the other inhibitors have unique non-ATP like structures, which indicates that it is possible to screen large collections of compounds to identify unique chemotypes for this class of enzyme.
Secondary screens and inhibitor validation
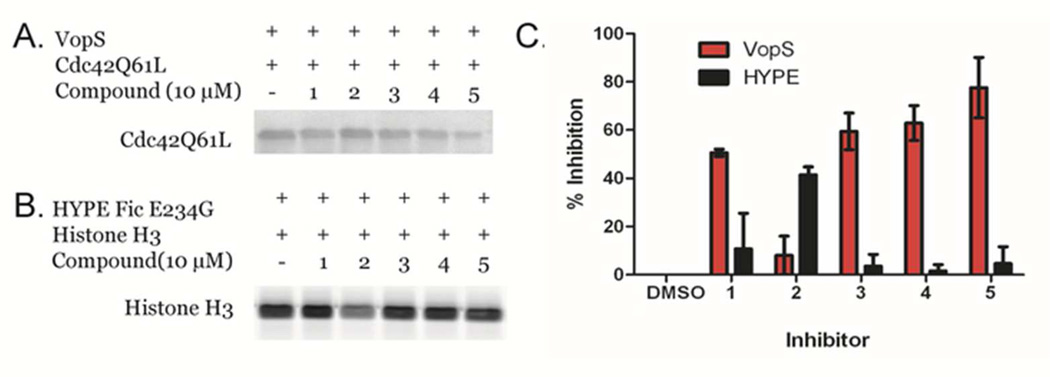
To further confirm that the top hits block Fl-AMP transfer, as opposed to a non-specific effect on the FP signal, we developed a gel-based version of our primary screen. In this screen, if the inhibitors merely interfere with FP and do not block transfer, fluorescently-labeled Cdc42 will still be detected since the inhibitor is removed during the electrophoresis stage of the assay; changes in fluorescence are directly monitored in gel. Similar secondary screens have been described.21, 28 An additional benefit of this assay comes from the fact that it is possible to quantify changes in fluorescence intensity, and thereby provide a semi-quantitative read-out of inhibitor potency. Here, VopS was treated with 10 µM of inhibitors 1–5 before adding sub KM concentrations of Fl-ATP and Cdc42. After electrophoresis, strong labeling was seen in the control DMSO lane, but there was a decrease in labeling for compounds 1, 3, 4, and 5 confirming that they do in fact inhibit Fl-AMP transfer to Cdc42 (Figure 4A, Table 1). In contrast, compound 2 appears to be a false positive as it shows only 3% inhibition in this assay.
Figure 4.
Gel-based counterscreen. The top five hits from the LOPAC CRC were analyzed using a gel-based assay with a) VopS or b) HYPE Fic E234G. c) Normalized percent inhibition from duplicate data relative to the DMSO control from the gel based screen for both VopS and HYPE Fic E234G.
Table 1.
Inhibition values for the HTS
| Compound | Primary screen inhibitiona |
CRC IC50 VopS (µM) |
CRC IC50 PAD4 (µM) |
Gel based % inhibition VopSb |
Gel based % inhibition HYPEb |
Fold selective |
|---|---|---|---|---|---|---|
| 1 | 61% ± 38 | >59 | >59 | 55% | 10% | 6 |
| 2 | 47% ± 4 | 11 | >59 | 3% | 41% | 0 |
| 3 | 41% ± 3 | 50 | >59 | 22% | 4% | 6 |
| 4 | 33% ± 1 | 40 | >59 | 63% | 2% | 32 |
| 5 | 25% ± 1 | 52 | >59 | 79% | 5% | 16 |
Average of three replicates;
10 µM compound;
To gauge selectivity versus other Fic domain containing proteins, we adapted this gel-based assay for HYPE, the only human Fic domain containing protein. Identifying inhibitors that preferentially inhibit VopS over HYPE is important for limiting the potential toxicity of novel VopS targeted therapeutics. For these studies, our HYPE Fic E234G construct was treated analogously to VopS but instead of using Cdc42 as a substrate, we used histone H3 (Figure 4B, Table 1). The HYPE Fic E234G mutant is constitutively active8 and we recently identified H3 as a HYPE substrate (Figure 4B). Based on this assay, compound 4 shows the highest selectivity (32-fold selective) (Figure 4C), indicating that our screen can discover inhibitors that are specific for bacterial versus human Fic domain containing proteins. The selectivity for VopS over HYPE further indicates that inhibition is not due to a non-specific mechanism.
Inhibitor kinetics
After confirming that four of the top five compounds inhibit VopS in both the primary HTS assay and the gel-based secondary screen, we used our continuous pyrophosphate detection assay to determine modes of inhibition for 1, 3, and 5. Inhibitor 4 interfered with the downstream enzymes in the continuous assay, so a radioactive α33P-ATP assay was used for this compound. Based on the Lineweaver-Burk plots and an analysis of the errors, inhibitors 1 and 3 are non-competitive with respect to both Cdc42 and ATP with Kii values of 20 ± 1 and 50 ± 6 µM respectively (Table 1). Inhibitor 5 is competitive with respect to Cdc42 (Ki = 410 ± 110 µM) and has a mixed pattern of inhibition with respect to ATP (Kis = 66 ± 19 µM). Compound 4 is the most potent inhibitor, and is competitive with respect to Cdc42 (Ki = 6 ± 1 µM) and non-competitive with respect to ATP. As expected, the Ki values of 1, 3 and 4 correspond to the IC50 values calculated from the CRCs since the concentrations of both substrates are well below KM in the 384-well assay. Inhibitor 5, however, showed poor inhibition in this continuous assay, possibly due to solubility issues caused by the farnesyl chain, as such this compound was not used in any further experiments.
Cellular Efficacy
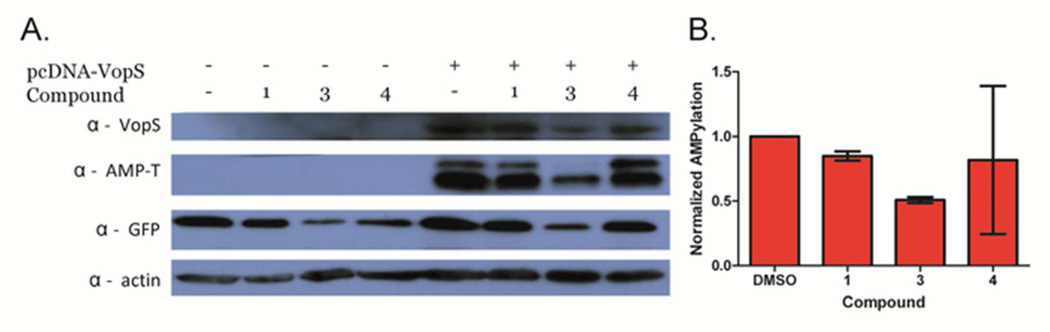
To explore their cellular efficacy, we examined whether compounds 1–4 inhibited cellular AMPylation and cell rounding in VopS transfected cells.3 Compounds 1 and 3 were evaluated at 10 µM and compound 4 was tested at 5 µM, due to its cellular toxicity.29 As depicted in Figure 5, compounds 1 and 3 decreased cellular protein AMPylation, as measured by Western Blotting, however, compound 4 did not show a significant decrease. While compound 4 was the most potent inhibitor identified from our screen, its high cellular toxicity likely limits its utility as a VopS inhibitor. Furthermore, while compounds 1 and 3 do decrease the amount of protein AMPylation in cells, they were unable to reverse the cell rounding phenotype induced by VopS overexpression (Figure S4).3 The inability to prevent the cell rounding phenotype is likely due to the fact that the VopS catalyzed AMPylation reaction is thought to be irreversible in human cells. Thus, without more potent inhibitors, cellular rescue may not be possible.
Figure 5.
In cellulo efficacy of validated VopS inhibitors. a) Western blot of the lysates from GFP or VopS/GFP transfected HeLa cells in the presence or absence of compounds 1 (10 µM), 3 (10 µM), or 4 (5 µM). b) Quantified band intensities of the AMP-Threonine blots normalized against VopS.
Structure Activity Relationships
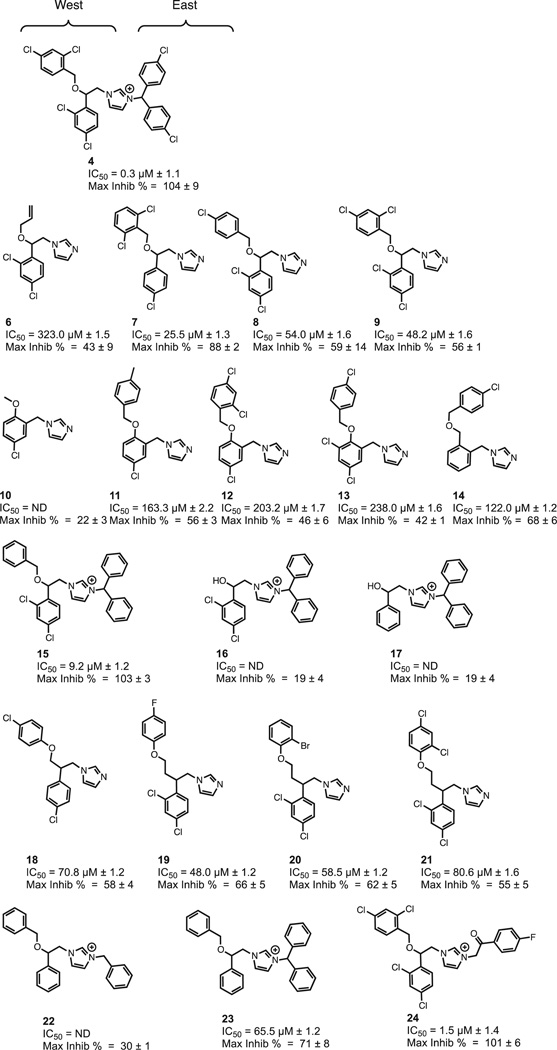
Given the high in vitro potency of 4, we set out to develop a structure activity relationship to identify the critical features that are required for the potent inhibition observed with this compound. Analogues of 4 were either synthesized or obtained from the NCI/DTP Open Chemical Repository30 (Figure 6). Using the HTS assay, we obtained CRCs and IC50 values for 4 and 19 derivatives (Figures 6, S5).
Figure 6.
Calmidazolium derivative characterization. Structures and inhibition values of the calmidazolium derivatives as determined from the CRC screen grouped by their structural differences.
By grouping the analogues according to whether they mimic the eastern or western half of the molecule, it became clear that the eastern biphenyl ring is critical for potency. For example, comparing 9 and 4 shows that the loss of the dichlorophenyl ring decreases potency by more than 100 fold. However, the dichlorophenyl moiety is not absolutely required because some of the potency can be regained with a smaller N-alkylation, as can be seen with 24. This increase in potency is not solely due to the cationic charge acquired from the N,N disubstitution, however, since compounds 16, 17 and 22 are poor inhibitors. Examining the northwestern portion of 4, it is apparent when comparing 6 with 7 – 9 or 10 with 11 – 14 that aryl ethers are much more potent than the aliphatic substituents. The third observation from this series of compounds is that the halogens on the southeastern phenethyl aryl ring are important. For example, the IC50s increase in the 4, 15, 23 series as chlorides are removed from the molecule. The fourth and final feature from this series is the tolerance on the northwestern aryl ring by different halogens. Comparing 18 – 21 shows that regardless of the halogens or substitution pattern on the northwestern ring, there is little change in potency. This is further confirmed by the minimal changes between 7 – 9 and also between 10 and 13. Therefore, our next generation of compounds should all share the imidazoline core with a western di-aryl system but there can be a wide variety of modifications on the eastern side, which can be tuned for specificity.
Differential Hydrogen Deuterium Exchange (HDX) mass spectrometry
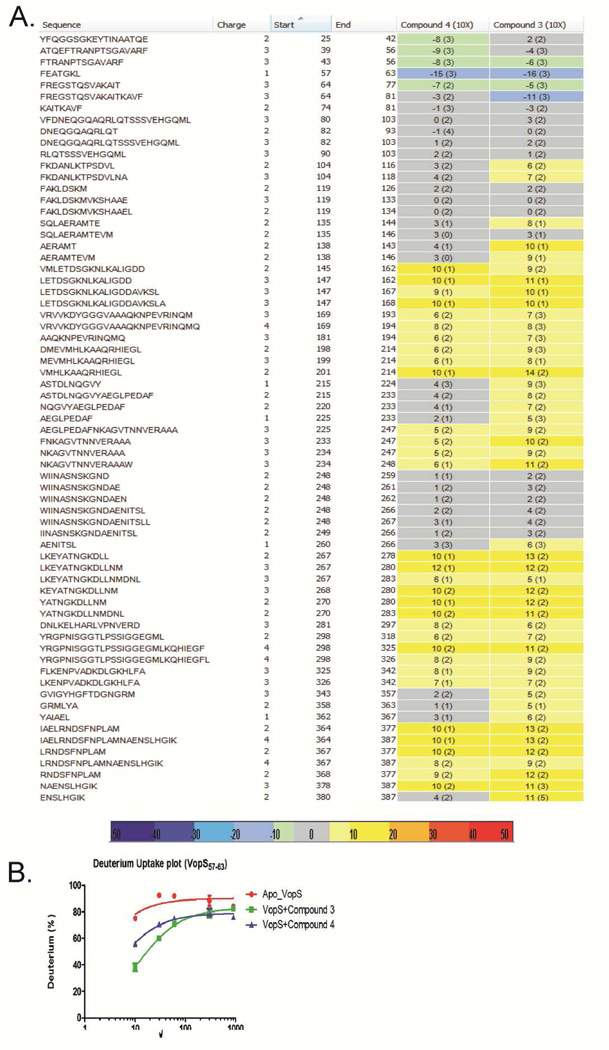
Since none of the compounds were competitive with ATP, we wished to identify where the inhibitors bind to VopS. Since attempts to co-crystallize the compounds with VopS were unsuccessful, we used HDX-MS to map putative binding sites for 3 and 4. Briefly, compounds 3 and 4 were incubated with 10 µM of VopSΔ30 at a molar excess of 10:1 or 3:1 (ligand:protein) for 1 h before H/D exchange, quenching, on-column pepsin digestion and analysis by ESI-MS. When compared to apo-VopS, VopS in the presence of 4 (100 µM) shows decreased hydrogen exchange with solvent deuterium in the N-terminal region (VopS 31–63), with VopS residues 58–63 showing highest protection against HDX (Figure 7A, B). Protection to solvent exchange would suggest increased stabilization of this region of the protein upon ligand binding and or decreased accessibility of the protected amide hydrogens to solvent. The same region also showed protection at 30 µM, a 3-fold excess, of 4 (Figure S6), suggesting that 4 binds to the N-terminal region of VopS (in particular residues 59–63). The decreased protection observed for the 30 µM sample may reflect the moderate Ki value for 4, as it is possible that not all VopS binding sites are saturated at the lower concentration.
Figure 7.
HDX of VopS with 3 or 4. a) Heat map of the change in deuterium exchange of VopS incubated with 100 µM 3 or 4 shows protection in the N-terminal region VopS 57–63. b) Deuterium uptake plot for VopS 57–63 shows a time dependent decrease in HDX in the presence of 100 µM 4 or 100 µM 3.
It should be noted that large areas of destabilization are observed throughout the rest of the protein (residues 147–387) when using 100 µM 4, which are not present at the lower concentration. This destabilization could be caused by a) 4 is insoluble at 100 µM in the VopS buffer and it causes the protein to precipitate/un-fold, resulting in destabilization/increased deuterium uptake. This hypothesis seems less likely because some regions are unchanged (colored grey) or show increased protection (blue/green) at the higher concentration; b) VopS is a multimer in solution and the inhibitor binding leads to monomerization/dimerization and can expose the protein dimerization interfaces to more deuterium exchange; or c) inhibitor binding in the N-terminal subdomain induces a global conformational change in the protein. Although the N-terminal region (i.e., residues 31–63) is distal from the active site (residues 348–356) and a deletion construct lacking the first 75 amino acids is still active, the entire N-terminal subdomain is necessary for full activity.13 Furthermore, previous studies have suggested that a large conformational change occurs upon substrate binding.13 Thus, we favor the latter explanation for the increased HDX in the core of the protein upon inhibitor binding.
Using a similar differential HDX approach, we characterized the binding of 3 to VopS (Figure 7A, C). Compound 3, which is a non-competitive inhibitor of both ATP and Cdc42, also showed increased protection within the N-terminus of VopS (residues 59–75). Furthermore, as we saw with 4, there is substantial destabilization throughout many regions of the rest of the protein. Taken together, these data suggest either that 3 and 4 bind to the N-terminus of VopS or inhibitor binding induces a dramatic conformational change in this region of the enzyme.
Conclusion
In conclusion, we have developed a FP-based HTS compatible assay that relies on the inherent activity of VopS to transfer AMP to Cdc42. We used this assay to screen the 1,280 member LOPAC library and discovered several potential hits. We further characterized these compounds and found 3 of the top 5 are selective for VopS with Kis in the 6–50 µM range. Compounds 1, 3 and 4 display moderate cellular efficacy via their ability to inhibit AMPylation in VopS transfected cells. Structure activity relationships also identified the imadazoline core of calmidazolium, 4, as the key pharmacophore that binds to the N-terminal subdomain of VopS. In total, these studies detail the discovery of the first inhibitors for protein AMPylation and set the stage for the discovery of new and more potent derivatives.
METHODS
VopS HTS Assay Validation
8 µL of VopS Screening Buffer (20 mM HEPES pH 8.0, 100 mM NaCl, 1 mM DTT, 5 mM MgCl2, 0.01% pluronic acid) (column 1) or 8 µL of VopSΔ30 (20 nM final) in Screening Buffer (columns 2–23) were added to a black 384-well microtiter plate (Greiner 784076) using a Beckman Coulter Flying Reagent Dispenser (FRD). Controls (columns 1: DMSO (no VopS, high control); 2: 20 mM ATP (high control); and 23: DMSO (no inhibitors, low control)) and source DMSO (100%) (columns 3–22) were pinned 2× using the 10 nL head on a Beckman Coulter BioMek NXP to achieve a final concentration of 0.4% v/v. After a 20 min incubation, 2 µL of substrates, that is Cdc42Q61L (5 µM final) and Fl-ATP (250 nM final) in Screening Buffer, were added using the FRD. The plates were read at 0, 30, 60, 120, 180 and 240 min using a Perkin-Elmer EnVision plate reader (Filters: Ex: FITC FP 480, Em 1: FITC FP P-pol 535, Em 2: FITC FP S-pol 535).
LOPAC Screen
8 µL of VopS Screening Buffer (column 1) or 8 µL of VopSS30 (20 nM final) in Screening Buffer (columns 2–23) were added to a black 384-well microtiter plate (Greiner 784076) using the FRD. Controls (columns 1: DMSO (no VopS, high control); 2: 20 mM ATP (high control); and 23: DMSO (no inhibitors, low control)) and LOPAC molecules (columns 3–22) were pinned 2× using the 10 nL head on a Beckman Coulter BioMek NXP to achieve a final concentration of 11 µM. After a 20 min incubation, 2 µL of substrates (Cdc42Q61L (5 µM final) and Fl-ATP (250 nM final)) in Screening Buffer were added using the FRD. The plates were read after 3 h as described above.
Compound Response Curves (CRC)
8 µL of VopS Screening Buffer (column 1) or 8 µL of VopSΔ30 (20 nM final) in Screening Buffer (columns 2–23) were added to a black 384-well microtiter plate (Greiner 784076) using the FRD. Controls (columns 1: DMSO (no VopS, high control); 2: 20 mM ATP (high control); and 23: DMSO (no inhibitors, low control)) and CRC molecules (columns 3–22) were pinned using the 100 nL head on a Beckman Coulter BioMek NXP to achieve a final concentration of 2 nM to 50 µM. After a 20 min incubation, 2 µL of substrates (Cdc42Q61L (5 µM final) and Fl-ATP (250 nM final)) in Screening Buffer were added using the FRD. The plates were read after 3 h as described above. For each test compound, percent inhibition was plotted against compound concentration. To calculate the IC50, the data were fit to eq. 1,
| (1) |
where Ymax is the maximum signal, I is the concentration of compound, n is the Hill slope, IC50 is the inflection point concentration and Ymin is the baseline response using Assay Explorer software (Accelrys Inc). In cases where the highest concentration tested (i.e., 58.6 µM) did not result in greater than 50% inhibition, the IC50 was determined manually as greater than 58.6 µM.
Gel-based secondary screens
VopS (20 nM final) was preincubated with inhibitor (10 µM final) in VopS Screening Buffer for 20 min. Cdc42Q61L (5 µM final) and Fl-ATP (1 µM final) were then added. After 15 min, 6× SDS-PAGE loading buffer was added to quench the reaction. The proteins were then separated on a 12% SDS-PAGE gel and imaged to 50 µm on a Typhoon 9410 (GE Healthcare) set at 532 nm. The fluorescent intensities were quantified using Image Quant. Percent inhibition was calculated by normalizing the fluorescence relative to the DMSO control.
HYPE Fic E234G (2.5 µM final) was preincubated with inhibitor (10 µM final) in VopS Screening Buffer for 20 min. Histone H3 (30 µM final) and Fl-ATP (5 µM final) were then added. After 20 min, 6× SDS-PAGE loading buffer was added to quench the reaction. The proteins were then separated on a 12% SDS-PAGE gel and imaged to 50 µm on a Typhoon 9410 (GE Healthcare) set at 532 nm. The fluorescent intensities were quantified using Image Quant. Percent inhibition was calculated by normalizing the fluorescence relative to the DMSO control.
Cell based inhibition assay
HeLa cells (1.5 × 105 cells/well) were seeded in a 6 well plate and grown overnight in DMEM supplemented with 10% fetal bovine serum (Sigma), 2 mM L-glutamine, 100 µg/mL streptomycin, 100 U/mL penicillin, 0.1 mM non-essential amino acids, and 1 mM sodium pyruvate (Invitrogen) at 37 °C with 5% CO2. The cells were transfected with 100 ng pcDNA3-VopS, 200 ng pGFP-n1 and empty vector pcDNA3 to a total of 2 µg of DNA/well using XtremeGene Hp (Roche). Compounds (5 µM calmidazolium, 10 µM GW7647 or 10 µM MK886) were added to the cells with the transfection reagents. After 6 h, the cells were washed with fresh DMEM and inhibitors were re-added. 18 h post transfection, the cells were fixed using 3.2% paraformaldehyde in 1× PBS for 10 min at room temperature. The fixed cells were imaged using Zeiss LSM 510 confocal microscope. Images were processed using ImageJ and Adobe Photoshop. Alternatively, 18 h after transfection, the cells were harvested in PBS using a cell scraper. The cells were spun down, washed and 50 µL lysis buffer (50 mM Tris, 150 mM NaCl, 1% TX-100, Roche mini protease inhibitor tablet) was added and incubated for 10 min on ice. 5× SDS-PAGE loading buffer was added to the cleared cell lysate, boiled for 10 min and the proteins separated on a 17% SDS-PAGE gel. Proteins were then transferred to PVDF membrane and blocked with 5% milk. Membranes were blotted with rabbit anti-VopS,3 mouse anti-GFP (BD Biosciences), mouse anti-actin (Sigma) and rabbit anti-AMP-threonine.31 Percent inhibition was calculated by normalizing the AMP-T band intensity to the VopS band intensity.
Differential Hydrogen deuterium exchange (HDX) Mass Spectrometry
VopSΔ30 (10 µM) was mixed with a 1:3 or 1:10 (protein:ligand) molar excess of calmidozolium (4), and MK886 (3), and incubated for 1 h at 4 °C for complex formation before subjecting to HDX. For the differential HDX experiments, 5 µL of VopSΔ30 with or without compound were mixed with 20 µL of D2O-containing HDX buffer (20 mM HEPES pH 8.0, 150 mM NaCl, 1 mM EDTA) and incubated at 4 °C for 0, 10, 30, 60, 90 or 3,600 s and analyzed as previously described.32
Supplementary Material
Table 2.
Mechanism of inhibition values for VopS
| Compound | Varied substrate | Mechanism of inhibition |
Kis (µM) |
Kii (µM) |
|---|---|---|---|---|
| 1 | Cdc42 | Non-competitive | 20 ± 1 | |
| ATP | Non-competitive | 41 ± 5 | ||
| 3 | Cdc42 | Non-competitive | 99 ± 16 | |
| ATP | Non-competitive | 50 ± 6 | ||
| 4 | Cdc42 | Competitive | 6 ± 1 | |
| ATP | Non-competitive | 26 ± 4 | ||
| 5 | Cdc42 | Competitive | 410 ± 110 | |
| ATP | Mixed | 66 ± 19 | 310 ± 38 |
ACKNOWLEDGEMENTS
We thank P. Baillargeon and L. DeLuca (Lead Identification, Scripps Florida) for LOPAC screen compound management. K.O. is a Burroughs Welcome Investigator in Pathogenesis of Infectious Disease, a W.W. Caruth, Jr. Biomedical Scholar and has an Earl A. Forsythe Chair in Biomedical Science.
ABBREVIATIONS
- AMP
adenosine monophosphate
- ATP
adenosine triphosphate
- MeCN
acetonitrile
- PPi
inorganic pyrophosphate
- HEPES
N-(2-hydroxyethyl)piperazine-N’-(2-ethanesulfonic acid)
- EDTA
ethylenediamine tetraacetic acid
- CRC
compound response curves
- MALDI
matrix-assisted laser desorption/ionization
- DTT
dithiothreitol
- Fl-ATP
N6-(6-amino)hexyl-ATP-5-carboxyl-fluorescein
- Fl-AMP
N6-(6-amino)hexyl-AMP-5-carboxyl-fluorescein
- FFA
fluorescein tagged F-amidine.
Footnotes
This work is supported by funds provided by TSRI to P.R.T. and NIH grants R01-AI056404 to K.O., GM084041 to P.G. and MH084512 to P.H. K.O. and A.S. are supported by Grant I-1561 from the Welch Research Foundation.
Supporting Information Available: This material is available free of charge via the Internet.
ASSOCIATED CONTENT
Supporting Information
Supplemental Figures 1 to 11 including detailed figure legends as well as full methods for the PAD4 counterscreen, VopS kinetic assays, differential HDX and compound synthesis are in the Supporting Information. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. Foodborne illness acquired in the United States--major pathogens. Emerg Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(Cdc), C. f. D. C. a. P. Vital Signs: Incidence and Trends of Infection with Pathogens Transmitted Commonly Through Food --- Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 1996–2010. MMWR. Morbidity and Mortality Weekly Reports. 2010 Retrieved from http://cdc.gov/mmwr/preview/mmwrhtml/mm6022a6025.htm?s_cid=mm6022a6025w. [PubMed]
- 3.Yarbrough ML, Li Y, Kinch LN, Grishin NV, Ball HL, Orth K. AMPylation of Rho GTPases by Vibrio VopS Disrupts Effector Binding and Downstream Signaling. Science. 2009;323:269–272. doi: 10.1126/science.1166382. [DOI] [PubMed] [Google Scholar]
- 4.Worby CA, Mattoo S, Kruger RP, Corbeil LB, Koller A, Mendez JC, Zekarias B, Lazar C, Dixon JE. The Fic Domain: Regulation of Cell Signaling by Adenylylation. Mol Cell. 2009;34:93–103. doi: 10.1016/j.molcel.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Müller MP, Peters H, Blümer J, Blankenfeldt W, Goody RS, Itzen A. The Legionella effector protein DrrA AMPylates the membrane traffic regulator Rab1b. Science. 2010;329:946–949. doi: 10.1126/science.1192276. [DOI] [PubMed] [Google Scholar]
- 6.Geertsema RS, Worby C, Kruger RP, Tagawa Y, Russo R, Herdman DS, Lo K, Kimball RA, Dixon J, Corbeil LB. Protection of mice against H. somni septicemia by vaccination with recombinant immunoglobulin binding protein subunits. Vaccine. 2008;26:4506–4512. doi: 10.1016/j.vaccine.2008.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geertsema RS, Zekarias B, La Franco Scheuch L, Worby C, Russo R, Gershwin LJ, Herdman DS, Lo K, Corbeil LB. IbpA DR2 subunit immunization protects calves against Histophilus somni pneumonia. Vaccine. 2011;29:4805–4812. doi: 10.1016/j.vaccine.2011.04.075. [DOI] [PubMed] [Google Scholar]
- 8.Engel P, Goepfert A, Stanger FV, Harms A, Schmidt A, Schirmer T, Dehio C. Adenylylation control by intra- or intermolecular active-site obstruction in Fic proteins. Nature. 2012;482:107–110. doi: 10.1038/nature10729. [DOI] [PubMed] [Google Scholar]
- 9.Mishra S, Bhagavat R, Chandra N, Vijayarangan N, Rajeswari H, Ajitkumar P. Cloning, expression, purification, and biochemical characterisation of the FIC motif containing protein of Mycobacterium tuberculosis. Protein Expr Purif. 2012;86:58–67. doi: 10.1016/j.pep.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Grammel M, Luong P, Orth K, Hang HC. A chemical reporter for protein AMPylation. J. Am. Chem. Soc. 2011;133:17103–17105. doi: 10.1021/ja205137d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kinch LN, Yarbrough ML, Orth K, Grishin NV. Fido, a novel AMPylation domain common to fic, doc, and AvrB. PLoS ONE. 2009;4:e5818. doi: 10.1371/journal.pone.0005818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Al-Eryani R, Yarbrough ML, Orth K, Ball HL. Characterization of AMPylation on Threonine, Serine, and Tyrosine Using Mass Spectrometry. J Am Soc Mass Spectrom. 2011;22:752–761. doi: 10.1007/s13361-011-0084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luong P, Kinch LN, Brautigam CA, Grishin NV, Tomchick DR, Orth K. Kinetic and structural insights into the mechanism of AMPylation by VopS Fic domain. J Biol Chem. 2010;285:20155–20163. doi: 10.1074/jbc.M110.114884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neunuebel MR, Chen Y, Gaspar AH, Backlund PS, Yergey A, Machner MP. De-AMPylation of the Small GTPase Rab1 by the Pathogen Legionella pneumophila. Science. 2011;333:453–456. doi: 10.1126/science.1207193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan Y, Luo Z-Q. Legionella pneumophila SidD is a deAMPylase that modifies Rab1. Nature. 2011;475:506–509. doi: 10.1038/nature10307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattoo S, Durrant E, Chen MJ, Xiao J, Lazar CS, Manning G, Dixon JE, Worby CA. Comparative analysis of Histophilus somni immunoglobulin-binding protein A (IbpA) with other fic domain-containing enzymes reveals differences in substrate and nucleotide specificities. J Biol Chem. 2011;286:32834–32842. doi: 10.1074/jbc.M111.227603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao J, Worby CA, Mattoo S, Sankaran B, Dixon JE. Structural basis of Fic-mediated adenylylation. Nature Structural & Molecular Biology. 2010;17:1004. doi: 10.1038/nsmb.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahman M, Ham H, Liu X, Sugiura Y, Orth K, Krämer H. Visual neurotransmission in Drosophila requires expression of Fic in glial capitate projections. Nature Neuroscience. 2012;15:871. doi: 10.1038/nn.3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, Mehdi S, Patel I, Kawooya J, Judkins M, Zhang W, Diener K, Lozada A, Dunnington D. An ultra-high throughput screening approach for an adenine transferase using fluorescence polarization. J Biomol Screen. 2000;5:31–38. doi: 10.1177/108705710000500107. [DOI] [PubMed] [Google Scholar]
- 20.Lewallen DM, Steckler CJ, Knuckley B, Chalmers MJ, Thompson PR. Probing adenylation: using a fluorescently labelled ATP probe to directly label and immunoprecipitate VopS substrates. Mol Biosyst. 2012;8:1701–1706. doi: 10.1039/c2mb25053e. [DOI] [PubMed] [Google Scholar]
- 21.Cravatt BF, Wright AT, Kozarich JW. Activity-based protein profiling: from enzyme chemistry to proteomic chemistry. Annu Rev Biochem. 2008;77:383–414. doi: 10.1146/annurev.biochem.75.101304.124125. [DOI] [PubMed] [Google Scholar]
- 22.Knuckley B, Jones JE, Bachovchin DA, Slack J, Causey CP, Brown SJ, Rosen H, Cravatt BF, Thompson PR. A fluopol-ABPP HTS assay to identify PAD inhibitors. Chem Commun (Camb) 2010;46:7175–7177. doi: 10.1039/c0cc02634d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuhl AM, Mohr JT, Bachovchin DA, Niessen S, Hsu KL, Berlin JM, Dochnahl M, Lopez-Alberca MP, Fu GC, Cravatt BF. Competitive Activity-Based Protein Profiling Identifies Aza-beta-Lactams as a Versatile Chemotype for Serine Hydrolase Inhibition. J Am Chem Soc. 2012;134:5068–5071. doi: 10.1021/ja300799t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryan AJ, Gray NM, Lowe PN, Chung CW. Effect of detergent on "promiscuous" inhibitors. J Med Chem. 2003;46:3448–3451. doi: 10.1021/jm0340896. [DOI] [PubMed] [Google Scholar]
- 25.Minond D, Saldanha SA, Spicer T, Qin L, Mercer BA, Roush WR, Hodder P. HTS Assay for Discovery of Novel Metallo-Beta-lactamase (MBL) Inhibitors. 2010 [PubMed] [Google Scholar]
- 26.Luo Y, Knuckley B, Bhatia M, Pellechia PJ, Thompson PR. Activity-based protein profiling reagents for protein arginine deiminase 4 (PAD4): synthesis and in vitro evaluation of a fluorescently labeled probe. J. Am. Chem. Soc. 2006;128:14468–14469. doi: 10.1021/ja0656907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slack JL, Causey CP, Luo Y, Thompson PR. Development and use of clickable activity based protein profiling agents for protein arginine deiminase 4. ACS Chem Biol. 2011;6:466–476. doi: 10.1021/cb1003515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knuckley B, Luo Y, Thompson PR. Profiling Protein Arginine Deiminase 4 (PAD4): a novel screen to identify PAD4 inhibitors. Bioorg Med Chem. 2008;16:739–745. doi: 10.1016/j.bmc.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Database", N. C. f. B. I. P. B. AID = 588440 [Google Scholar]
- 30. http://dtp.cancer.gov.
- 31.Hao YH, Chuang T, Ball HL, Luong P, Li Y, Flores-Saaib RD, Orth K. Characterization of a rabbit polyclonal antibody against threonine-AMPylation. J Biotechnol. 2011;151:251–254. doi: 10.1016/j.jbiotec.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goswami D, Devarakonda S, Chalmers MJ, Pascal BD, Spiegelman BM, Griffin PR. Time window expansion for HDX analysis of an intrinsically disordered protein. J Am Soc Mass Spectrom. 2013;24:1584–1592. doi: 10.1007/s13361-013-0669-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.