Abstract
The kidney possesses the capacity to repair after an acute insult, even one that causes complete organ failure. This regenerative response is characterized by robust proliferation of epithelial cells, principally those located in the proximal tubule. Because defining the origin of these reparative cells has important consequences for stem cell and regenerative approaches to treat kidney injury, this area has been the subject of intense investigation and debate. While progress has been made in narrowing the possible origin of these cells to an intratubular source, there has been no consensus between the possibility of a preexisting intratubular stem or progenitor cell versus the possibility that fully differentiated epithelial cells re-enter the cell cycle after injury and generate new proximal tubule cells through self-duplication. This review will summarize the evidence on both sides of this active controversy and provide support for the notion that no preexisting proximal tubule stem cell population exists, but rather all differentiated proximal tubule epithelia have the capacity to proliferate during repair by a mechanism of dedifferentiation and self-duplication.
Keywords: Acute kidney injury, Bone marrow derived cells, dedifferentiation, stem cell
Introduction
Acute kidney injury (AKI) is defined as the rapid worsening of kidney function within several hours to weeks [1]. For many years, it was believed if a patient survived an episode of AKI that their kidneys made a full recovery. However, recent publications have conclusively shown that patients who survive an episode of AKI are at much greater risk of future development of chronic kidney disease (CKD) [2, 3]. One theory holds that this risk reflects failed, or maladaptive, epithelial repair which eventually leads to interstitial fibrosis and CKD [2–4]. In order to develop targeted therapies that promote kidney repair after an acute injury, and prevent maladaptive repair and the risk of future CKD, it is critical to understand the origins of kidney cells responsible for repair after injury.
The outer medulla is injured most severely in ischemic insults, both in human and rodent, because this region is exposed to severe and persistent hypoxia during ischemia [5, 6]. The outer medulla contains the S3 segment of the proximal tubule, the medullary ascending limb and medullary collecting ducts [6, 7]. Both proximal and distal nephron segments are injured in AKI, and indeed in human AKI both a proximal biomarker (Kidney Injury Molecule-1, KIM-1) and a distal tubule marker (neutrophil gelatinase-associated lipocalin, NGAL) are elevated soon after injury. In rodent AKI models, the predominant injured part of nephron is S3 segment of proximal tubule and expands towards the outer parts of nephron according to the duration of ischemia [7, 8].
While mouse IRI is the most utilized model of human AKI in use today, differences between rodent and human AKI are notable. In human AKI, tubular necrosis is patchy and focal, not frankly necrotic as is seen in mouse. In addition, there is more distal tubule injury in human AKI compared to the mouse kidney IRI model. Despite these differences, mouse kidney IRI is a critical tool to study AKI pathophysiology which has been described as “imperfect but indispensable” [9]. A full discussion of these differences is beyond the scope of this review but the topic has been reviewed [7].
During homeostasis, the rate of renal tubular epithelial cell turnover is very low [10]. However, after an ischemic or toxic insult, there is a rapid (within 24 hours) burst of proliferation among proximal tubule epithelial cells, particularly those in the S3 segment, where most kidney damage occurs. The proliferative index for proximal tubule cells in this segment can increase from 1 in 200 before injury to 1 in 2 cells in cell cycle 24–48 hours after injury [11]. Based on observations that the proliferating cells express certain mesenchymal cell markers like vimentin, and developmental cell markers like Pax2, it has long been felt that these proliferating proximal tubule cells originated by dedifferentiation of epithelial cells to a more primitive developmental state, followed by proliferation [12]. However, during the last decade, a number of reports challenged this model, suggesting that proliferating epithelia arise from cells outside the kidney, such as hematopoietic stem cells (HSCs) or mesenchymal stem cells (MSCs), or alternatively from adult resident kidney stem or progenitor cells, either within the interstitium or within the tubule itself [13].
Genetic lineage analysis using a Six2-Cre driver in which all epithelial cells within the nephron were labeled followed by a cycle of injury and repair, revealed no contribution from (unlabeled) extratubular cells [14]. There is good agreement on this point today. While MSC have known therapeutic effects in AKI, most investigators agree that these arise through paracrine effects [15–18]. On the other hand, there is little agreement about whether an intratubular stem or progenitor might exist, since the original lineage analysis we preformed using Six2-Cre would have labeled both differentiated proximal tubule cells, as well as any putative progenitors. Both sides of this controversy are summarized below.
Evidence for intratubular stem cells
By definition, stem cells possess two qualities: they self-renew and they also have the capacity to differentiate into other cell types by asymmetric cell division [19]. By contrast, progenitor cells are more differentiated, possess a limited proliferative capacity and may be unipotent [20]. The critical question is whether such cells exist prior to injury in adult kidney. A variety of reports have characterized potential markers for stem / progenitor cell populations within the tubule. Maeshima et al. identified a slowly cycling cell population in the normal rat kidney [21, 22]. These tubular “label retaining cells (LRC)” retained BrdU labeling after a BrdU pulse was administered by osmotic pump followed by 2-week chase period. Tubular LRC were scattered among nephron segments. These LRC actively proliferated during the repair process, suggesting specific contribution to repair from label-retaining cells. On the other hand, there was little else besides label retention that distinguished these cells from their neighbors, raising the possibility that they simply happened to divide during the BrdU pulse, since there is a low, basal rate of cell proliferation during kidney homeostasis.
Oliver et al. utilized a different BrdU pulse-chase protocol (a pulse after birth followed by a long chase) and identified LRCs in the renal papilla, both the interstitium and in tubules, proposing this location as a kidney stem cell niche [23]. These cells are quiescent in normal condition, but after the transient ischemia, they entered the cell cycle, indicating active proliferation. In other experiments from the same group, they showed that dye-labeled papillary cells migrated towards the upper papilla and contributed to the renal repair after the transient ischemia [24]. By contrast, we could not detect cell cycle reentry or migration of these LRC after ischemia-reperfusion injury [25].
Kitamura et al. microdissected different segments of the nephron and isolated the cells with robust proliferative capacity from the S3 segment of the proximal tubule. In culture, these cells possessed an immature tubule phenotype including Pax-2 expression, and they had the capacity to differentiate into tubular cells of various nephron segments; proximal tubule, loop of Henle and distal tubules [26]. These findings indicate that a cell population within the S3 segment of the proximal tubule exhibits a multipotent phenotype during in vitro culture. Whether these cells possess the same plasticity in vivo remains a matter of debate.
Using a Cre driver under control of the nuclear factor of activated T-cells, cytoplasmic 1 (NFATc1), Langworthy et al. proposed that NFATc1 expressing proximal tubular cells were an intratubular progenitor subpopulation after injury [27]. After crossing this transgenic mouse line against the LacZ reporter mice, they induced the acute kidney injury by HgCl2 injection. An increase in LacZ-positive tubular cells was observed 5 days after the insult, which further increased by day 10. These NFATc1-positive cells were resistant to apoptosis and proliferated after injury, suggesting they may represent an intratubular progenitor population. On the other, hand, if NFATc1 is a marker of epithelial dedifferentiation or injury, these results could alternatively be interpreted to support a self-duplication model and not a stem cell model – particularly given the fact that NFATc1 expression is not observed in uninjured tubule.
The most intriguing recent developments in this area concern a distinct population of single cells scattered throughout human proximal tubule characterized by co-expression of two stem cell markers; CD133 and CD24 [28]. Ex vivo, these CD133+ CD24+ cells are multipotent and can differentiate into various renal cells, osteocytes, adipocytes, endothelial cells and stromal cells in defined culture conditions [28, 29]. Renal CD133+ CD24 cells can be divided into three subsets according to their marker pattern and their nephron localization [30–32]. CD133+ CD24+ podocalyxin (PDX)+ and CD106+ cells localize at Bowman’s capsule, close to the vascular pole, and apparently differentiate into podocytes [30, 31]. CD133+ CD24+ CD106+ PDX− cells localized at urinary pole of Bowman’s capsule and the proximal tubule just emerging from glomerulus, can differentiate into both podocytes and tubular epithelial cells [31, 32]. CD133+ CD24+ CD106− PDX− cells are scattered among the proximal and distal tubules and can differentiated only into tubular epithelial cell, suggesting they may be unipotent tubular progenitors[31, 32].
Electron microscopy has proven that these CD24+ cells possess a unique morphology compared to fully differentiated proximal tubule epithelia. They have less cytoplasm, fewer mitochondria and a complete absence of brush border [33]. Isolated CD133+ CD24+ cells have a greater capacity for proliferation than CD133− CD24− cells and are resistant to cell death [31]. When CD133+ CD24+ cells are injected into the kidney of severe combined immunodeficient (SCID) mice with acute kidney injury, they ameliorate injury and appear to incorporate into tubular structures [28, 31]. These studies have not been performed using genetic lineage analysis, however, which is the gold standard for tracking cell fate in this type of experiment, so these results await confirmation.
Despite these findings, published by several independent groups, it is still unclear whether these cells are a preexisting progenitor population or alternatively transiently dedifferentiated cells due to local tubular injury or homeostatic self-renewal. In support of this latter possibility, Smeets et al. recently showed that CD133+ CD24+ cells also co-express Kidney injury molecule-1 (KIM-1) and the mesenchymal marker vimentin [33]. Lindgren et al. also showed that CD133+ cells co-stained with vimentin [34]. Since KIM-1 and vimentin are markers of cell injury, these results argue that these cells are not preexisting progenitors but rather individual injured epithelial cells that arose from fully differentiated cells.
Complicating these studies, CD24 and CD133 protein cannot be measured in mouse kidney. The cell surface protein CD133 was originally identified on CD34+ hematopoietic stem cells [35] and is also expressed in various cancer stem cells [36–38]. Several monoclonal antibodies have been developed, but the most commonly used antibodies, AC133 (CD133/1) and 293C/AC141 (CD133/2), recognize a distinct N-linked glycosylated epitope of the CD133 protein [39]. Kemper et al. proposed that the cancer stem cells contain highly glycosylated CD133, whereas the glycosylation reduced according to the differentiation [40]. These two antibodies were also used in the previous reports concerning renal progenitors [28, 33, 34], but they cannot be used on rodent tissues [41, 42].
It should be noted that CD133+ CD24+ cells can be found not only in human but also in pig and chimpanzee, but not in rodent kidney [43]. This raises the possibility a differences in fundamental mechanisms of tubular repair between mammals. It has been speculated that this difference between species might be attributed to the body size and longevity and that smaller animals like rodents do not require the progenitor population for maintaining the homeostasis under normal conditions [42].
Dedifferentiation of fully differentiated tubular epithelial cells after injury
The traditional concept for kidney repair after injury is that surviving tubular epithelial cells dedifferentiate, proliferate, and eventually replace the neighboring cells that were lost by the acute insult. [1, 11, 12]. Vogetseder et al. showed that the bulk of proximal tubular cells in S3 segment are in the G1-phase of the cell cycle, and a strong mitotic stimulation accelerated the re-entry into the cell cycle, contributing to renal repair [44]. Importantly, they showed that these cells in G1 are fully differentiated epithelia – not a minority population that do not express markers of terminal differentiation. As alluded above, we previously demonstrated that surviving tubular epithelial cells are responsible for kidney regeneration after injury using a genetic fate-mapping techniques using Six2-GFPCre transgenic mice. The Six2 gene expression is observed only in metanephric mesenchymal cells that are fate to become renal epithelia, not interstitial stromal cells [45]. Using Six2-GFPCre transgenic mice, more than 90% of tubular cells, not interstitial cells, were genetically labeled. After a cycle of injury and repair, there was no dilution of labeling within the tubule [14]. Importantly, there was no re-expression of Six2-GFPCre either, as assessed by PCR and immunohistochemistry for endogenous Six2 and GFP, since this could have labeled previously unmarked cells, confounding the analysis. Since no interstitial cells were labeled with this strategy even after the injury and repair [14], this finding excluded the possibility for extra tubular stem / progenitor population that could contribute to renal repair.
To address the possibility that a preexisting, intratubular stem or progenitor cell might account for repair after injury [42, 46], we next performed lineage analysis of tubular epithelial cell proliferation by the sequential pulsing of distinct thymidine analogs [25]. If an intratubular stem cell is responsible for repair, then this rare population will become activated to divide after injury, producing a population of “transit-amplifying cells” – which arise from stem cells and divide rapidly for a finite number of times until they differentiate into proximal tubule epithelia. This experiment revealed that different populations of epithelial cells were proliferating at 24 vs. 48 hours after injury – which is not consistent with a rapidly proliferating, stem cell derived transit amplifying population. Instead, it suggests that any surviving cell after injury is capable of cell division and that proximal tubule repairs by self-duplication. It remains formally possible that preexisting intratubular progenitors, if they exist, could be resistant to injury and preferentially survive compared with normal tubular epithelial cells [31]. In this case, it may not be necessary for these progenitors to proliferate rapidly after injury, because they might constitute the bulk of surviving cells [42]. To formally address this possibility, we recently performed lineage analysis of fully differentiated proximal tubule epithelial cells to ask whether they are capable of proliferating after injury.
Direct evidence that terminally differentiated tubular epithelial cells contribute to repair
A critical piece of evidence missing from the current debate is a lineage analysis of terminally differentiated proximal tubule cells after injury. The dueling hypotheses on renal repair offer very distinct predictions concerning the fate of such cells after acute injury. The stem cell hypothesis holds that these differentiated cells would die, and would not proliferate after injury; therefore the number of genetically labeled cells would decrease after injury and repair, since the CD24+/CD133+ cells would not have been labeled, and would preferentially proliferate after injury. By contrast, the self-duplication hypothesis would predict that genetically labeled differentiated epithelial cells would proliferate after injury, and the fraction of genetic label within the tubule would remain constant (Figure 1). To perform such an experiment, we generated a knockin mouse with CreERT2 under the control of SLC34a1 locus [47] (Figure 2A). SLC34a1, the sodium-phosphorus co-transporter, is expressed on the apical side of terminally differentiated proximal tubular epithelial cells, especially in the S1 and S2 segments [48].
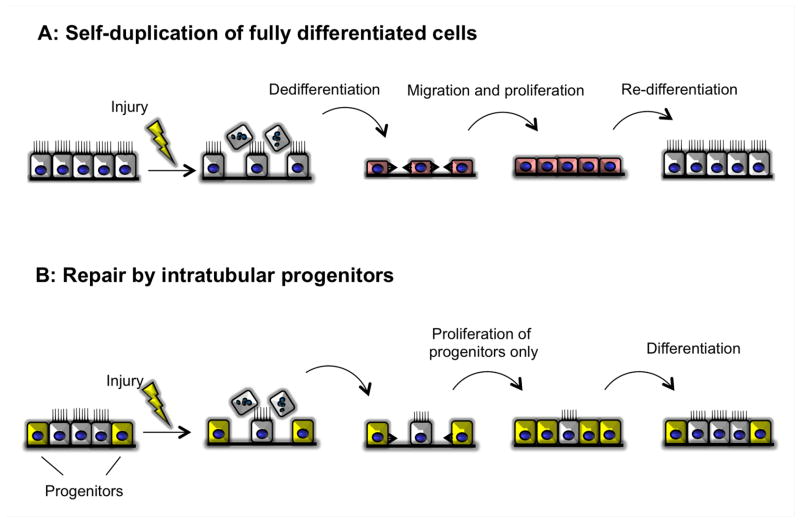
Figure 1. Concepts for the cellular origin of tubular regeneration after injury.
(A) After the injury, terminally differentiated cells dedifferentiated and flattened, then proliferate and migrate to cover the denuded tubular basement membrane, and eventually re-differentiated to the terminally differentiated cells.
(B) After the injury, the cells from outside migrate through the tubular basement membrane, thereafter differentiated to the site specific tubular epithelial cells.
(C) The progenitor population scatters among the tubule at the normal condition. After injury, only these cells can proliferate and migrate cover the denuded tubular basement membrane, and eventually some of these differentiate to the terminally differentiated cells and the other remains as the progenitor population.
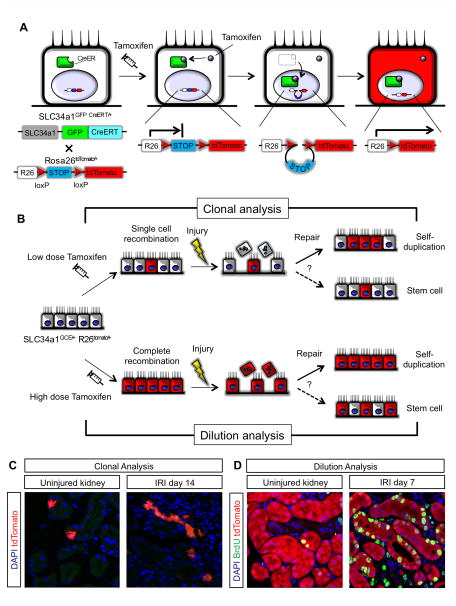
Figure 2. Clonal and label dilution analysis using SLC34a1 GFPCreER (SLC34a1GCE/+), R26tomato/+ mice [49].
(A) Temporal labeling the terminally differentiated cells using SLC34a1GCE/+ mice. Cre recombinase allele to which the ligand binding domain of estrogen receptor (ER) is fused (CreER). CreER normally located in the cytoplasmic, but once exogenous tamoxifen bind to the ER, CreER translocates into the nucleus. CreER delete the floxed STOP sequence and reporter gene (tdTomato) transcription occurs. Eventually, terminally differentiated tubular cells express tdTomato and this genetic labeling is permanent, even if the cell will lose the phenotype of terminally differentiation.
(B) Rationale for the clonal and dilution analysis. Recombination by tamoxifen in SLC34a1GCE/+ mice was induced only in “terminally differentiated” proximal tubular epithelial cells, “not in progenitor population”. For clonal analysis, we first induce the single cell labeling in the terminally differentiated proximal tubular epithelial cells by low dose tamoxifen and then evaluate the clone size after injury. If terminally differentiated cells contributed to the renal repair, the clone size will expand (Solid arrow in the repair process). For dilution analysis, we first induce the complete cell labeling in the terminally differentiated proximal tubular epithelial cells by high dose tamoxifen and then evaluate the frequency of labeled cells after injury. If only terminally differentiated cells contributed to the renal repair, the labeling will remain compete (Solid arrow in the repair).
(C) Clonal analysis of SLC34a1GCE/+, R26tomato/+ mice. In uninjured contralateral kidney, cells were labeled solely by low dose tamoxifen (left panel). In IRI kidney, the clone size of labeled cells expanded after repair (right panel).
(D) Dilution analysis of SLC34a1GCE/+, R26tomato/+ mice. In uninjured kidney, proximal tubule was complete labeled. After injury and repair, the complete labeling still remained. In addition, after injury, genetically labeled tubular epithelial cells co-stained BrdU for labeling the cell division during repair, indicating proliferated cells came from labeled terminally differentiated cells.
After sparse labeling of terminally differentiated tubular epithelial cells by low dose tamoxifen injection (Figure 2B), if terminally differentiated cells participate in the repair process after injury, the clone size of labeled cells will expand. But if (unlabeled) progenitor cells contribute renal repair, clone size will remain single. 14 days after 35min IRI, the clone size of labeled cells expanded in both inner and outer cortex, and some clones had more than 5 cells (Figure 2D). This result indicates that terminally differentiated cells actively proliferate and directly contribute tubular repair after injury [47]. To investigate whether an undifferentiated progenitor population could contribute to proximal tubular repair at all, complete recombination of terminally differentiated cells was induced by high dose tamoxifen injection (Figure 2C). If an unlabeled progenitor population participates in tubular regeneration after injury, proximal tubule label will be diluted after repair. If terminally differentiated tubular epithelial cells contribute to renal repair, then label will remain complete. 7days after 35min IRI, the fraction of labeled proximal tubular epithelial cells did not change, indicating that only terminally differentiated cells contributed to renal repair (Figure 2D). Labeled cells also stained positively with vimentin and Pax2 after injury, whereas no positive staining of these molecules in uninjured tubules was observed, indicating that terminally differentiated cells undergo dedifferentiation during the repair.
Summary
A better understanding of the mechanisms of tubular repair after injury is required to develop therapeutic strategies for AKI. This remains a contentious field, but recent findings suggest that proliferation of fully differentiated proximal tubule cells after injury represents the predominant mechanism of repair. Since these dedifferentiated cells appear to have special properties ex vivo, including resistance to cell death and multi potency, an important future challenge is to understand how epithelial dedifferentiation might ‘reprogram’ these cells to confer regenerative properties.
References
- 1.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. The Journal of clinical investigation. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012;82:516–524. doi: 10.1038/ki.2012.208. [DOI] [PubMed] [Google Scholar]
- 4.Humphreys BD, Xu F, Sabbisetti V, Grgic I, Naini SM, Wang N, Chen G, Xiao S, Patel D, Henderson JM, Ichimura T, Mou S, Soeung S, McMahon AP, Kuchroo VK, Bonventre JV. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J Clin Invest. 2013;123:4023–4035. doi: 10.1172/JCI45361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brezis M, Rosen S. Hypoxia of the renal medulla--its implications for disease. The New England journal of medicine. 1995;332:647–655. doi: 10.1056/NEJM199503093321006. [DOI] [PubMed] [Google Scholar]
- 6.Lieberthal W, Nigam SK. Acute renal failure. I. Relative importance of proximal vs. distal tubular injury. Am J Physiol. 1998;275:F623–631. doi: 10.1152/ajprenal.1998.275.5.F623. [DOI] [PubMed] [Google Scholar]
- 7.Heyman SN, Rosenberger C, Rosen S. Experimental ischemia-reperfusion: biases and myths-the proximal vs. distal hypoxic tubular injury debate revisited. Kidney Int. 2010;77:9–16. doi: 10.1038/ki.2009.347. [DOI] [PubMed] [Google Scholar]
- 8.Wei Q, Dong Z. Mouse model of ischemic acute kidney injury: technical notes and tricks. American journal of physiology Renal physiology. 2012;303:F1487–1494. doi: 10.1152/ajprenal.00352.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lieberthal W, Nigam SK. Acute renal failure. II. Experimental models of acute renal failure: imperfect but indispensable. Am J Physiol Renal Physiol. 2000;278:F1–F12. doi: 10.1152/ajprenal.2000.278.1.F1. [DOI] [PubMed] [Google Scholar]
- 10.Carlson BM. Some principles of regeneration in mammalian systems. Anatomical record Part B, New anatomist. 2005;287:4–13. doi: 10.1002/ar.b.20079. [DOI] [PubMed] [Google Scholar]
- 11.Baddour JA, Sousounis K, Tsonis PA. Organ repair and regeneration: an overview. Birth defects research Part C, Embryo today : reviews. 2012;96:1–29. doi: 10.1002/bdrc.21006. [DOI] [PubMed] [Google Scholar]
- 12.Bonventre JV. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol. 2003;14(Suppl 1):S55–61. doi: 10.1097/01.asn.0000067652.51441.21. [DOI] [PubMed] [Google Scholar]
- 13.Little MH. Regrow or repair: potential regenerative therapies for the kidney. J Am Soc Nephrol. 2006;17:2390–2401. doi: 10.1681/ASN.2006030218. [DOI] [PubMed] [Google Scholar]
- 14.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Imberti B, Morigi M, Tomasoni S, Rota C, Corna D, Longaretti L, Rottoli D, Valsecchi F, Benigni A, Wang J, Abbate M, Zoja C, Remuzzi G. Insulin-like growth factor-1 sustains stem cell mediated renal repair. J Am Soc Nephrol. 2007;18:2921–2928. doi: 10.1681/ASN.2006121318. [DOI] [PubMed] [Google Scholar]
- 16.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 17.Togel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. American journal of physiology Renal physiology. 2007;292:F1626–1635. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- 18.Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG. Stromal Cells Protect against Acute Tubular Injury via an Endocrine Effect. J Am Soc Nephrol. 2007;18:2486–2496. doi: 10.1681/ASN.2007020140. [DOI] [PubMed] [Google Scholar]
- 19.Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- 20.Seaberg RM, van der Kooy D. Stem and progenitor cells: the premature desertion of rigorous definitions. Trends in neurosciences. 2003;26:125–131. doi: 10.1016/S0166-2236(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 21.Maeshima A, Yamashita S, Nojima Y. Identification of renal progenitor-like tubular cells that participate in the regeneration processes of the kidney. J Am Soc Nephrol. 2003;14:3138–3146. doi: 10.1097/01.asn.0000098685.43700.28. [DOI] [PubMed] [Google Scholar]
- 22.Maeshima A, Sakurai H, Nigam SK. Adult kidney tubular cell population showing phenotypic plasticity, tubulogenic capacity, and integration capability into developing kidney. J Am Soc Nephrol. 2006;17:188–198. doi: 10.1681/ASN.2005040370. [DOI] [PubMed] [Google Scholar]
- 23.Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q. The renal papilla is a niche for adult kidney stem cells. J Clin Invest. 2004;114:795–804. doi: 10.1172/JCI20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliver JA, Klinakis A, Cheema FH, Friedlander J, Sampogna RV, Martens TP, Liu C, Efstratiadis A, Al-Awqati Q. Proliferation and migration of label-retaining cells of the kidney papilla. J Am Soc Nephrol. 2009;20:2315–2327. doi: 10.1681/ASN.2008111203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Humphreys BD, Czerniak S, Dirocco DP, Hasnain W, Cheema R, Bonventre JV. Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci U S A. 2011;108:9226–9231. doi: 10.1073/pnas.1100629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitamura S, Yamasaki Y, Kinomura M, Sugaya T, Sugiyama H, Maeshima Y, Makino H. Establishment and characterization of renal progenitor like cells from S3 segment of nephron in rat adult kidney. Faseb J. 2005;19:1789–1797. doi: 10.1096/fj.05-3942com. [DOI] [PubMed] [Google Scholar]
- 27.Langworthy M, Zhou B, de Caestecker M, Moeckel G, Baldwin HS. NFATc1 identifies a population of proximal tubule cell progenitors. J Am Soc Nephrol. 2009;20:311–321. doi: 10.1681/ASN.2008010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J Am Soc Nephrol. 2006;17:2443–2456. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- 29.Lazzeri E, Crescioli C, Ronconi E, Mazzinghi B, Sagrinati C, Netti GS, Angelotti ML, Parente E, Ballerini L, Cosmi L, Maggi L, Gesualdo L, Rotondi M, Annunziato F, Maggi E, Lasagni L, Serio M, Romagnani S, Vannelli GB, Romagnani P. Regenerative potential of embryonic renal multipotent progenitors in acute renal failure. J Am Soc Nephrol. 2007;18:3128–3138. doi: 10.1681/ASN.2007020210. [DOI] [PubMed] [Google Scholar]
- 30.Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol. 2009;20:322–332. doi: 10.1681/ASN.2008070709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Angelotti ML, Ronconi E, Ballerini L, Peired A, Mazzinghi B, Sagrinati C, Parente E, Gacci M, Carini M, Rotondi M, Fogo AB, Lazzeri E, Lasagni L, Romagnani P. Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cells. 2012;30:1714–1725. doi: 10.1002/stem.1130. [DOI] [PubMed] [Google Scholar]
- 32.Romagnani P, Remuzzi G. Renal progenitors in non-diabetic and diabetic nephropathies. Trends in endocrinology and metabolism: TEM. 2013;24:13–20. doi: 10.1016/j.tem.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Smeets B, Boor P, Dijkman H, Sharma SV, Jirak P, Mooren F, Berger K, Bornemann J, Gelman IH, Floege J, van der Vlag J, Wetzels JF, Moeller MJ. Proximal tubular cells contain a phenotypically distinct, scattered cell population involved in tubular regeneration. J Pathol. 2012;229:645–659. doi: 10.1002/path.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindgren D, Bostrom AK, Nilsson K, Hansson J, Sjolund J, Moller C, Jirstrom K, Nilsson E, Landberg G, Axelson H, Johansson ME. Isolation and characterization of progenitor-like cells from human renal proximal tubules. Am J Pathol. 2011;178:828–837. doi: 10.1016/j.ajpath.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT, Bray RA, Waller EK, Buck DW. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997;90:5013–5021. [PubMed] [Google Scholar]
- 36.Monzani E, Facchetti F, Galmozzi E, Corsini E, Benetti A, Cavazzin C, Gritti A, Piccinini A, Porro D, Santinami M, Invernici G, Parati E, Alessandri G, La Porta CA. Melanoma contains CD133 and ABCG2 positive cells with enhanced tumourigenic potential. Eur J Cancer. 2007;43:935–946. doi: 10.1016/j.ejca.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 37.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer research. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 38.Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, Tripodo C, Russo A, Gulotta G, Medema JP, Stassi G. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell stem cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Green CL, Loken M, Buck D, Deeg HJ. Discordant expression of AC133 and AC141 in patients with myelodysplastic syndrome (MDS) and acute myelogeneous leukemia (AML) Leukemia. 2000;14:770–772. doi: 10.1038/sj.leu.2401736. [DOI] [PubMed] [Google Scholar]
- 40.Kemper K, Sprick MR, de Bree M, Scopelliti A, Vermeulen L, Hoek M, Zeilstra J, Pals ST, Mehmet H, Stassi G, Medema JP. The AC133 epitope, but not the CD133 protein, is lost upon cancer stem cell differentiation. Cancer research. 2010;70:719–729. doi: 10.1158/0008-5472.CAN-09-1820. [DOI] [PubMed] [Google Scholar]
- 41.Angelotti ML, Lazzeri E, Lasagni L, Romagnani P. Only anti-CD133 antibodies recognizing the CD133/1 or the CD133/2 epitopes can identify human renal progenitors. Kidney Int. 2010;78:620–621. doi: 10.1038/ki.2010.243. author reply 621. [DOI] [PubMed] [Google Scholar]
- 42.Romagnani P. Of mice and men: the riddle of tubular regeneration. J Pathol. 2012;229:641–644. doi: 10.1002/path.4162. [DOI] [PubMed] [Google Scholar]
- 43.Axelson H, Johansson ME. Renal stem cells and their implications for kidney cancer. Semin Cancer Biol. 2013;23:56–61. doi: 10.1016/j.semcancer.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Vogetseder A, Picard N, Gaspert A, Walch M, Kaissling B, Le Hir M. Proliferation capacity of the renal proximal tubule involves the bulk of differentiated epithelial cells. Am J Physiol Cell Physiol. 2008;294:C22–28. doi: 10.1152/ajpcell.00227.2007. [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008;3:169–181. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benigni A, Morigi M, Remuzzi G. Kidney regeneration. Lancet. 2010;375:1310–1317. doi: 10.1016/S0140-6736(10)60237-1. [DOI] [PubMed] [Google Scholar]
- 47.Kusaba T, Lalli M, Kramann R, Kobayashi A, Humphreys BD. Differentiated kidney epithelial cells repair injured proximal tubule. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1310653110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Madjdpour C, Bacic D, Kaissling B, Murer H, Biber J. Segment-specific expression of sodium-phosphate cotransporters NaPi-IIa and -IIc and interacting proteins in mouse renal proximal tubules. Pflugers Arch. 2004;448:402–410. doi: 10.1007/s00424-004-1253-x. [DOI] [PubMed] [Google Scholar]