Abstract
Background
The importance and specific role(s) of eosinophils in modulating the immune/inflammatory phenotype of allergic pulmonary disease remain to be defined. Established animals models assessing the role(s) of eosinophils as contributors and/or causative agents of disease have relied on congenitally deficient mice where the developmental consequences of eosinophil depletion are unknown.
Methods
We developed a novel conditional eosinophil-deficient strain of mice (iPHIL) through a gene knock-in strategy inserting the human diphtheria toxin (DT) receptor (DTR) into the endogenous eosinophil peroxidase genomic locus.
Results
Expression of DTR rendered resistant mouse eosinophil progenitors sensitive to DT without affecting any other cell types. The presence of eosinophils was shown to be unnecessary during the sensitization phase of either ovalbumin (OVA) or house dust mite (HDM) acute asthma models. However, eosinophil ablation during airway challenge led to a predominantly neutrophilic phenotype (>15% neutrophils) accompanied by allergen-induced histopathologies and airway hyperresponsiveness in response to methacholine indistinguishable from eosinophilic wild type mice. Moreover, the iPHIL neutrophilic airway phenotype was shown to be a steroid-resistant allergic respiratory variant that was reversible upon restoration of peripheral eosinophils.
Conclusions
Eosinophil contributions to allergic immune/inflammatory responses appear to be limited to the airway challenge and not the sensitization phase of allergen provocation models. The reversible steroid-resistant character of the iPHIL neutrophilic airway variant suggests underappreciated mechanisms by which eosinophils shape the character of allergic respiratory responses.
Keywords: Asthma, Eosinophil-deficient, Neutrophil, Steroid-resistant
INTRODUCTION
The inability to specifically define the roles and importance of eosinophils in allergic respiratory responses has contributed to the complexity of disease management approaches for patients (reviewed in (1, 2). This complexity, in part, occurs as a consequence of the apparently multi-faceted roles eosinophils have in allergic inflammatory responses in both animal models of disease and human asthma patients (reviewed in (3)). In particular, various transgenic and knockout strains of mice with exaggerated or depleted peripheral eosinophil levels have demonstrated that these granulocytes are likely to have several contributory roles in the modulation of immune and inflammatory responses occurring in mice following allergen challenge. Studies using either of the available congenitally eosinophil-deficient strains of mice, PHIL (4) and ΔdblGATA (5), suggested that pulmonary eosinophils modulate lung inflammatory T cell recruitment ((6, 7)) and polarize the immune response by suppression of Th1/Th17 pathways (8). Unfortunately, these strains of mice also displayed significant differences regarding the roles of eosinophils in allergic pulmonary responses that have been attributed to background strain variability (e.g., C57BL/6J (6) vs. BALB/cJ (7), use of different allergens (OVA (4, 5) vs. HDM (9)), the consequences of additional environmental cues (10, 11), and developmental effects on other immune cells (12, 13) or tissues (14, 15) as result of the life-long loss of eosinophils associated with congenitally eosinophil-deficient mice.
We developed a novel inducible eosinophil-deficient strain of mice (iPHIL) with the objective of demonstrating that the “on-demand” targeting of eosinophils provides a model system to define the specific role of eosinophils in allergic pulmonary inflammation without the inescapable caveats associated with congenital eosinophil-deficiency. Moreover, the availability of this model now will permit the definition of anti-inflammatory effector functions involved in disease resolution and, in turn, the definition of eosinophil activities in studies investigating the efficacy of therapies targeting eosinophils in established disease. To these ends, the selective depletion of eosinophils during allergen sensitization, airway challenge, and later during memory challenge provocations were performed to define the roles of eosinophils during each stage of the immune response to allergen exposure. These data showed that eosinophils are not required for polarization or progression of the immune response during sensitization to antigen in acute OVA or HDM allergen provocation models. Conversely, the loss of eosinophils during the allergen challenge phase of each allergen protocol resulted in a lung inflammatory phenotype that displayed a neutrophilic airway infiltrate with allergen-dependent airway histopathologies and induced airway hyperresponsiveness (AHR) virtually identical to those observed in allergen provoked eosinophil-sufficient wild type mice. Significantly, secondary allergen challenges (memory recall assessments) of iPHIL mice after restoration of their peripheral eosinophils levels reversed the induced lung phenotype from airway neutrophilic inflammation to an inflammatory response dominated by eosinophils. More importantly, the neutrophilic inflammation linked with allergen provocation of iPHIL mice was found to be steroid-resistant. Thus, our studies using iPHIL mice suggest that eosinophils contribute to the character of allergen-induced pathologies and the underlying immune responses and highlight that allergen provocation of eosinophil-deficient mice may replicate some of the complexities of the inflammatory phenotypes displayed by asthma patient subgroups (e.g., steroid refractory neutrophilic subjects (16)).
MATERIALS AND METHODS
Mice
All studies were performed with mice on 6–14 week old animals on a C57BL/6J background. Inducible eosinophil-deficient knock in mice (iPHIL) were generated via a knock in strategy (Ozgene (Bentley DC, WA, Australia)). Briefly, the human HB-EGF (DTR) open reading frame was inserted by homologous recombination in C57BL/6J embryonic stem (ES) cells at the start codon of the eosinophil peroxidase gene (epx) (17) (Supplementary Figure 1 (A)). This engineered locus also included an Internal Ribosomal Entry Site (IRES) upstream of the otherwise intact epx gene, allowing for the expression of a dicistronic mRNA ostensibly encoding both DTR and EPX. Homozygous or heterozygous iPHIL mice were bred to C57BL/6J mice from Jackson Laboratories (Jackson Research Laboratories, Bar Harbor, ME) with wild type littermates as controls to heterozygous iPHIL mice. Mice in these studies were maintained in ventilated micro-isolator cages housed in the specific pathogen-free animal facility at the Mayo Clinic in Arizona under “low barrier” (www.jax.org) conditions (see Supplementary Methods). All protocols and studies involving animals were performed in accordance with National Institutes of Health and Mayo Foundation institutional guidelines.
Hematologic assays: Blood/Bone marrow isolation, Cell counts/Differentials of blood films, Cytospins, and Bone Marrow smears
All assays/assessments were performed as previously described (18, 19). Unless otherwise indicated, cell counts and differentials were performed using ≥300 cells per sample.
Bone Marrow Culture
The ex vivo differentiation/proliferation of eosinophil lineage-committed progenitors was performed using a marrow culture system (20) with modifications that have been previously described (21).
Allergen Sensitization/Airway challenge protocols
The OVA sensitization/airway challenge model was performed as described previously (8). Briefly, two similar protocols were used to assess the role of eosinophils in OVA sensitization vs. airway challenge. Mice were sensitized with (i.p.) injections of 40μg OVA (grade VI; Sigma) emulsify in 2.25mg of Imject™ adjuvant (Thermo Scientific) either on OVA-protocol days 0 and 5 (sensitization studies) or protocol days 0 and 14 (airway provocation studies). Sensitized mice were challenged with aerosolized 1% (w/v) OVA (grade VI, Sigma) solution (control animals received saline alone) for 20 minutes either on OVA-protocol days 13–16 prior to assessment of pulmonary pathologies on protocol day 17 (sensitization studies) or OVA-protocol days 24–26 prior to assessment of pulmonary pathologies on protocol day 28 (allergen challenge studies). In experiments with steroid administration, dexamethasone (DEX) (Sigma) at 5mg/kg mouse weight was (i.p.) injected on day 27 of the allergen protocol (airway provocation studies) and mice were assessed on day 28.
OVA-specific memory recall assessments of inflammation were performed in iPHIL mice that were sensitized (i.p.) with OVA/Imject® adjuvant protocol days 0 and 14. These mice were administered DT ((i.p.) - 15ng/gram body weight) prior to, and during, an initial airway challenge phase on days 20, 21, and 24–27. Mice were assessed at two time points in this protocol: (i) Two days following the last of the initial OVA airway challenges - day 28. (ii) OVA sensitized mice that were previously rendered eosinophil-deficient during the initial airway challenge phase (day 24–27) were allowed to “rest” (i.e., DT administration was discontinued) 12 days to allow restoration of the peripheral eosinophils prior to re-challenge with 1% OVA (control mice received saline alone) on day 39 and assessed on day 41 (OVA Day 41).
Diphtheria toxin (DT) administration to mice
Diphtheria toxin (DT, (D0564, Sigma)) was administered to mice (control animals received saline vehicle alone) by injection (1.5ng/μl (i.p.)) at a final dosage of 15ng/gram body weight. Kinetic assessments of eosinophil ablation were completed by i.p. injection of DT (15ng/gram body weight) on protocol days 0 and day 1. Ablation of eosinophils during the sensitization phase of the OVA provocation protocol was completed by administration of DT ((i.p.) - 15ng/gram body weight) on days -5,-4 and 0, 1, 2, 3, and 4 of the 17 day protocol. Ablation of eosinophils during the airway challenge phase was accomplished by DT administration ((i.p.) - 15ng/gram body weight) on days 20, 21, 24, 25, 26, and 27 of the 28 protocol. Wild type controls also received DT injections. Long-term eosinophil ablation studies were performed by initially administering DT ((i.p.) - 15ng/gram body weight) on protocol days 0 and 1 followed by additional administration of DT ((i.p.) - 15ng/gram body weight) every 3 days thereafter for up to 28 days total.
Collection of airway luminal cells and bronchoalveolar lavage fluid
Isolation of bronchoalveolar lavage (BAL) fluid, determination of total cell numbers, and cell differential assessments were performed as previously described (8).
Histology and MBP-immunohistochemistry
Femurs and lungs were collected as described previously (22, 23). Serial sections of mouse lungs were either stained with hematoxylin and eosin (HE) or Periodic Acid Schiff (PAS) or immunohistochemistry using an eosinophil-specific rat anti-mouse MBP monoclonal antibody also as described previously (24, 25).
Assessments of Cytokines
Mouse IL-17, IL-13, and IFN-γ levels were assessed using immunoassay kits (R&D Systems) according to the manufacturer’s instructions. The limits of detection for each ELISA assay were 5–10pg/mL.
Assessments of Lung Function
Airway hyperresponsiveness (AHR) in response to increasing doses of nebulized methacholine (0, 6, 12, 25, 50 mg/mL) was determined on day 28 of the OVA allergen protocol as previously described (24) using a small animal mechanical ventilator (flexivent; SCIREQ).
Statistical analysis
All data are derived from ≥2 independent experiments with (n) of two to six mice per experiment. Data were analyzed using GraphPad Prism 5 statistics program using Student’s t-tests with error bars representing the mean ± SEM. Differences between means were considered significant when p<0.05.
RESULTS
Generation of iPHIL
A strain of mice (iPHIL) capable of conditional (i.e., “on-demand”) eosinophil deficiency was created by a gene knock in strategy that inserted the complete open reading frame encoding the human DTR at the endogenous start codon of the eosinophil peroxidase (epx) gene on chromosome 11 (Supplemental Figure 1(A)). iPHIL-derived eosinophil progenitors showed eosinophil-specific expression of DTR-EPX transcripts (Supplementary Figure 1(B)), but failed to replicate EPX protein expression despite the inclusion of an IRES element designed to mediate EPX expression from the dicistronic mRNA from the knock in locus (Supplementary Figure 1(C)). Thus, all subsequent studies described here were performed with iPHIL heterozygous (+/−) mice, which displayed EPX expression levels comparable to wild type C57BL/6J animals.
Eosinophil lineage-committed progenitors but not mature terminally differentiated metamyelocytes are targeted by DT
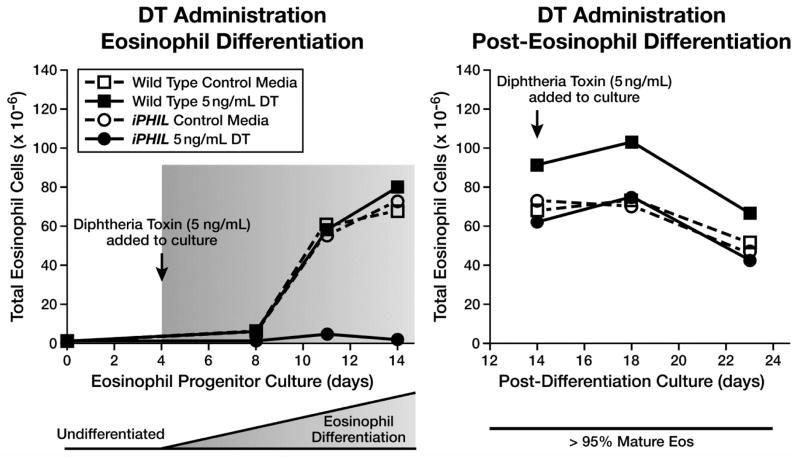
The ability of DT to mediate the killing of eosinophil lineage committed progenitors in iPHIL mice was tested by exposing ex vivo bone marrow cultures to DT during selected windows of time. In these cultures, eosinophil progenitors expand through culture-day 12–14 upon which 90–95% of the cells present are terminally differentiated eosinophils (20, 21). DT (5ng/mL) was added to and maintained in the culture media in two distinct time frames to target immature (day 4–14) versus differentiated (day 14–23) eosinophils. iPHIL bone marrow cultures exposed to DT during the eosinophil progenitor expansion phase (days 4–14) failed to expand and were unable to survive this culture period (Figure 1). Doses up to 5μg/mL were tested and gave similar results (data not shown). In contrast, cultures of mature terminally-differentiated eosinophils (day 14–23) were unaffected by DT exposure relative to control groups.
Figure 1. Diphtheria Toxin (DT) mediated cell toxicity is limited to eosinophil progenitor cell populations with mature eosinophils unresponsive to DT exposure.
Ex vivo bone marrow cultures from iPHIL and wild type mice were exposed to DT (5ng/mL) during select culture time periods to assess for the differential ablation of eosinophil progenitors vs. terminally differentiated metamyelocytes. In the left panel (DT Administration – Eosinophil Differentiation), DT exposure was performed from protocol days 4–14 during the time of eosinophil progenitor expansion. In the right panel (DT Administration – Post-Eosinophil Differentiation), DT exposure was performed from protocol days 14–23 during the time when the cultures were >95% terminally differentiated metamyelocytes (Mature Eos).
Eosinophil-specific ablation in iPHIL mice in vivo
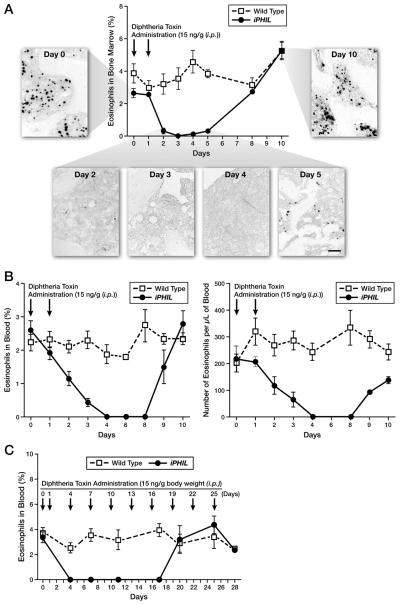
Kinetic assessments of eosinophil ablation were completed in iPHIL mice to determine the ability of DT targeted killing of eosinophils in vivo. DT administration to iPHIL mice on days 0 and 1 showed that eosinophils were ablated from the bone marrow (Figure 2(A)) and blood (Figure 2(B)) of iPHIL mice 3–4 days post-injection. The ablation of peripheral blood eosinophils is particularly noteworthy as circulating levels dropped to 0 ± 0 by day 4 and remained at this level for five days before returning to pre-DT treatment baseline levels by protocol day 10. Given that DT administration does not target terminally differentiated eosinophils, these data predict (assuming stochastic decay from circulation) that once they exit the marrow the half-life of eosinophils in peripheral blood is 1.16 days. Continued DT administration (i.e., every 3 days) showed that it was also possible to maintain the complete ablation of circulating blood eosinophils in iPHIL mice for >14 days (Figure 2(C)) before host humoral immune responses neutralize the effectiveness of this ablation strategy (26). Assessments of blood, lymphatic, and bone marrow compartments of iPHIL mice treated with DT (Supplementary Figure 2) demonstrated that the ablation of eosinophils was cell specific and did not affect the levels of any other cell types (i.e., basophils, neutrophils, NK cells, T cells, B cells, dendritic cells, and macrophages).
Figure 2. Intraperitoneal (i.p.) injections of DT of iPHIL mice on consecutive days are sufficient to deplete circulating eosinophils and continued DT administration elicits eosinophil ablation over an extended period of time.
Wild type and iPHIL mice were administered DT ((i.p.) - 15ng/gram body weight) on days 0 and 1 (arrows) and then followed ten days assessing eosinophil levels (mean ± SEM). (A) Bone marrow eosinophils were assessed in femur smears stained with Diff-Quick (counting >900 cells per sample) and plotted as function of time post-DT administration. In addition, femoral eosinophils were identified by immunohistochemistry using a rat anti-mouse MBP monoclonal antibody. Scale bar = 50μm. (B) The percent of eosinophils in blood (left panel) as well as the absolute number of blood eosinophils (right panel) were determined from Diff-Quick stained blood films and manual cell counts using a hemocytometer, respectively, plotting these data as functions of time post-DT administration. (C) In an extended DT administration study, wild type and iPHIL mice were administered DT ((i.p.) - 15ng/gram body weight) on days 0, 1 and every three days thereafter (arrows). The percent of eosinophils in blood was plotted as a function of time post-DT administration throughout the extended protocol period, assessing cell counts from blood smears stained with Diff-Quick (counting >900 cells/sample).
Allergen sensitization/airway challenge of iPHIL mice demonstrated that the presence of eosinophils is not required during allergen sensitization phase of these protocols for the development of Th2 pulmonary inflammation
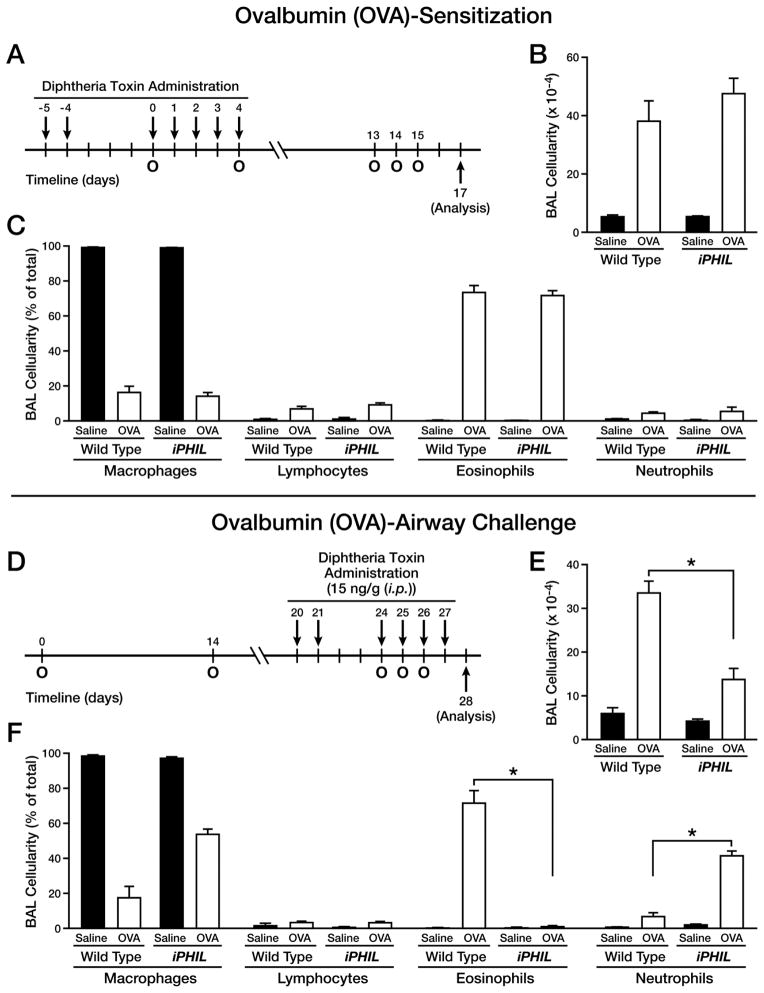
Two widely used allergen-specific models were examined for eosinophil-dependence during allergen sensitization/airway challenge: an acute OVA model (Figure 3(A)) and an acute HDM provocation strategy (Supplementary Figure 3(A)). Depletion of eosinophils specifically during allergen sensitization in either model did not alter subsequent airway inflammatory responses of iPHIL mice relative to wild type controls. That is, following allergen challenge of these mice the eosinophilic infiltration into the airways of iPHIL was similar to wild type mice in both OVA (Figure 3(B, C)) and HDM (Supplementary Figure 3(B, C)) models. Allergen (OVA or HDM)-mediated elaboration of Th2 cytokines (e.g., IL-13) in the bronchoalveolar lavage (BAL) fluid also remained the same regardless of the presence or absence of eosinophils during allergen sensitization (Supplementary Figure 4(A, B)); IFN-γ and IL-17 were below detection levels. A cursory examination of the OVA-induced airway histopathologies, including goblet cell metaplasia/airway epithelial mucin accumulation also showed no differences between allergen sensitized/airway challenged wild type and iPHIL mice rendered eosinophil-deficient only during the sensitization phase (representative photographs from each group of mice are shown in Supplementary Figure 5).
Figure 3. Eosinophil ablation during OVA challenge, but not the sensitization, phase resulted in a neutrophilic airway phenotype.
Wild type and iPHIL mice were administered DT ((i.p.) - 15ng/gram body weight) prior to and during the sensitization and airway challenge phases of an acute OVA allergen provocation protocol to assess the consequences of eosinophil ablation. (A) Eosinophil ablation during OVA sensitization: DT was administered (i.p.) on days -5, -4, and 0–4 (arrows) with day 0 representing the first of two (day 0 and 4) OVA sensitizations (O). These mice were then challenged with an OVA nebulant generated from a 1% OVA solution in saline (control mice received saline vehicle alone) on protocol days 13–15 (O) and assessed on day 17. (B) Total BAL cell counts as well as (C) cell differential analyses were performed on cytospins preparations of recovered airway cells (mean ± SEM), counting >300 cells/sample. *P<0.05. (D) Eosinophil ablation during OVA airway challenge: DT was administered on days 20, 21, and 24–27 (arrows) with day 0 representing the first of two (day 0 and 14) OVA sensitizations (intraperitoneal (i.p.) administration of OVA/Imject® adjuvant (o)) and days 24–26 representing the airway OVA challenge phase of this acute protocol (OVA nebulant generated from a 1% OVA solution in saline (o)) with mice assessed on day 28; control mice received saline vehicle alone. (E) Total BAL cell counts as well as (F) cell differential analyses were performed on cytospins preparations of recovered airway cells (mean ± SEM), counting >300 cells/sample. *P<0.05.
Eosinophil ablation during allergen challenge of previously sensitized mice leads to neutrophilic asthma in mice
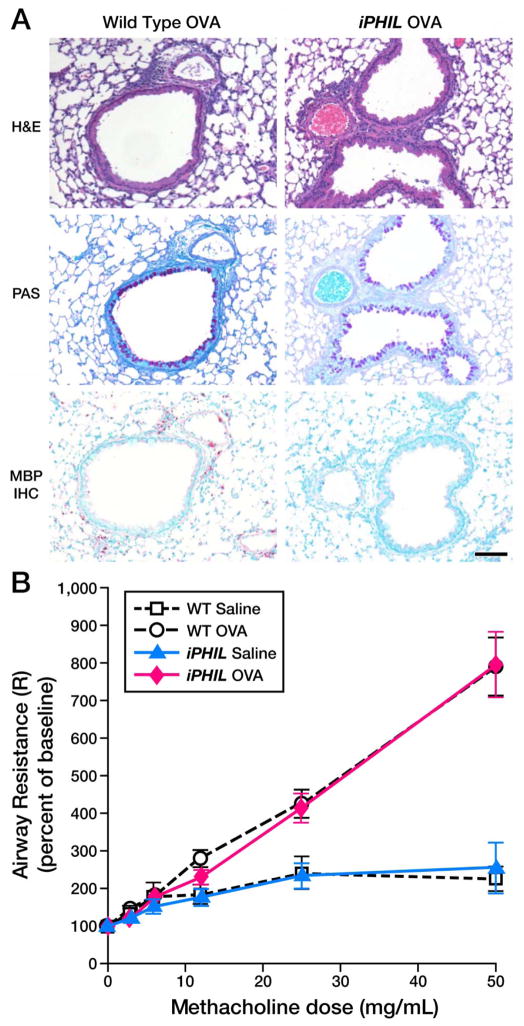
The importance of eosinophils during allergen airway challenge was assessed in iPHIL mice by administration of DT prior to, and then throughout, the allergen challenge phase of an OVA (Figure 3(D)) or HDM (Supplementary Figure 3(D)) provocation model. The depletion of eosinophils in DT-treated iPHIL mice led as expected to significant reductions in the cellular airway infiltrates (Figure 3(E) and Supplementary Figure 3(E)). Nonetheless, airway cell differentials of OVA challenged iPHIL mice revealed eosinophil-deficient iPHIL mice (>90% of treated animals) displayed a significant neutrophil inflammatory infiltrate (>15% neutrophils) with <0.1% eosinophils in the airways (Figure 3(F) and Supplementary Figure 3(F)). In both models this allergen-induced neutrophil infiltrate was a 250% increase in airway neutrophil numbers relative to DT-treated wild type mice. Surprisingly, BAL IL-13 levels in either OVA or HDM challenged neutrophilic iPHIL mice were elevated and were similar in magnitude to those observed in allergen provoked wild type mice (Supplementary Figure 4(C, D)). Significantly, the neutrophilic inflammation in the lungs of iPHIL mice accompanying the ablation of eosinophils during the allergen airway challenged phase of either the OVA (Figure 4(A)) or the HDM (Supplementary Figure 6) models was also associated with increased airway histopathologies and goblet cell metaplasia/airway epithelial mucin accumulation that were equivalent to the changes observed in allergen sensitized/airway challenged wild type animals. In addition, lung function assessments showed that iPHIL mice displaying the neutrophilic airway phenotype also displayed airway hyperresponsiveness that was comparable to wild type mice (Figure 4(B)). These data collectively demonstrate that the absence of eosinophils appears to enable the development of an allergic neutrophilic phenotype with histopathological and physiological similarities to allergen-mediated eosinophilic inflammation in wild type animals.
Figure 4. iPHIL mice rendered eosinophil-deficient during the OVA airway challenge phase displayed allergen-induced pulmonary histopathologies and lung dysfunction equivalent to OVA-treated wild type animals.
(A) Representative photomicrographs of lung sections are presented from wild type as compared iPHIL mice whose eosinophils were ablated exclusively during the airway challenge phase of the OVA provocation protocol outlined earlier (see Figure 3(D)). Lung sections were stained with H&E and PAS as well as immunohistochemistry for the identification of infiltrating eosinophils (MBP-IHC). These data demonstrated that the lungs of iPHIL mice rendered eosinophil deficient during airway OVA challenge (iPHIL OVA) displayed equivalent levels (relative to OVA-treated wild type controls (Wild Type OVA)) of airway epithelial hypertrophy (H&E), goblet cell metaplasia/airway epithelial cell mucin accumulation ((PAS) – dark purple staining cells)) despite the lack of eosinophils (MBP IHC – red staining cells infiltrating the parenchyma). Scale bar = 100μm. (B) OVA-treated wild type and iPHIL mice rendered eosinophil-deficient during the airway challenge phase of the OVA provocation protocol displayed equivalent levels of airway hyperresponsiveness (AHR) relative to saline-treated control groups. *P<0.05.
Interestingly, the remaining small groups of allergen challenged iPHIL mice (~10%) that did not display a neutrophilic inflammatory phenotype displayed the nominal inflammatory variant previously linked with congenitally eosinophil deficient mice (4, 5). That is, these small groups of OVA (Supplementary Figure 7(A–D)) or HDM (Supplementary Figure 7(E–H))-treated iPHIL mice displayed neither an induced pulmonary eosinophilia nor neutrophilia, low allergen-induced BAL levels of IL-13, and minimal histopathologies. This discrepancy necessitated side-by-side comparisons of allergen-treated iPHIL mice with similarly treated of congenitally eosinophil-deficient PHIL and ΔdblGATA mice. OVA sensitization and challenge of both PHIL (Supplementary Figure 8) and ΔdblGATA mice (Supplementary Figure 9) also resulted in the appearance of the majority of mice (80–90% of mice) with a neutrophilic (>15% neutrophilia) phenotype that was associated with increased histopathologies, including goblet cell metaplasia/airway epithelial mucin accumulation. The observation that the allergen-induced neutrophilic phenotype occurring in iPHIL animals also occurred following allergen sensitization/airway challenge of both established strains of congenitally eosinophil-deficient mice demonstrated that the occurrence of this phenotype is a consequence of eosinophil ablation in the challenge phases of these models; that is, this observation was not an unintended result linked with the genetic manipulations or DT administration of iPHIL.
The neutrophilic phenotype of OVA-treated eosinophil-deficient iPHIL mice is reversed following secondary allergen challenge by restoration of blood eosinophil levels
The inducible nature of iPHIL mice allowed the opportunity to determine whether the neutrophilic phenotype in these mice was an artifact or a reversible phenotype specific to the loss of eosinophils. In these studies, iPHIL mice were rendered eosinophil deficient by administration of DT during the first allergen challenge phase (days 24–26) of previously sensitized mice (Figure 5(A)). Subsets of these mice were allowed to “rest” 12 days (i.e., DT administration was discontinued) allowing eosinophil levels in these mice to return to baseline before a final allergen challenge on protocol day 39 (Figure 5(A)). As expected, eosinophil depletion during the initial allergen challenge phase, resulted in an airways neutrophilia (Figure 5(B)) and increased goblet cell metaplasia/airway epithelial mucin accumulation relative to saline controls (Figure 5(C)) following OVA challenge (protocol day 28) that paralleled increases in airway IL-13 levels. The restoration of eosinophil levels prior to a subsequent second allergen challenge resulted in a phenotype (protocol day 41) characterized by increased airway and lung eosinophils (Figure 5(B)) that was also accompanied by airway histopathologies including increased goblet cell metaplasia/airway epithelial mucin accumulation that were comparable to the neutrophilic inflammation occurring during DT administration of these mice at day 28 (Figure 5(C)). These data demonstrated that the induced neutrophilic pulmonary inflammation of eosinophil-deficient iPHIL mice is a reversible mixed immune phenotype contingent on the presence of peripheral eosinophils.
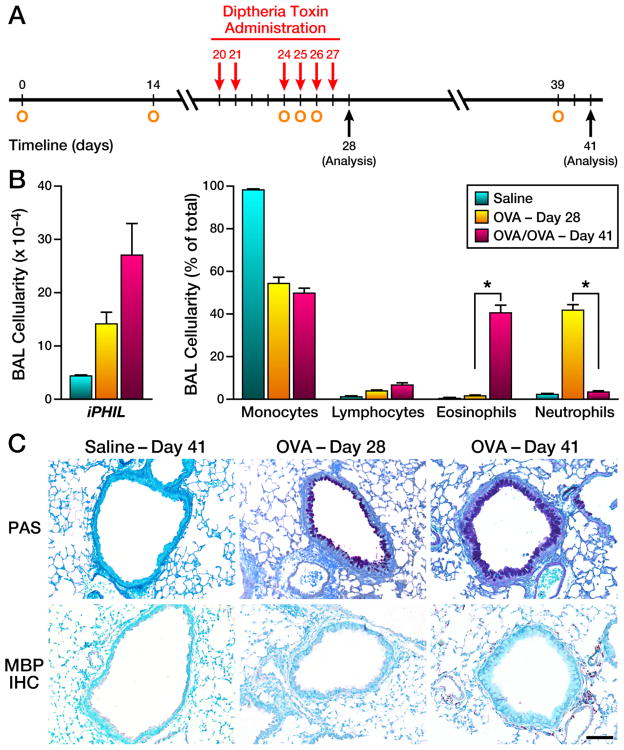
Figure 5. The neutrophilic phenotype of OVA-treated iPHIL mice rendered eosinophil-deficient during an initial airway challenge is reversed upon subsequent OVA airway challenges by restoration of peripheral eosinophils.
(A) OVA sensitized mice (O) were administered DT ((i.p.) - 15ng/gram body weight) prior to, and during, an initial airway challenge phase on days 20, 21, and 24–27 ((O) arrows). Mice were assessed at two time points in this protocol: (i) Two days following the last of the initial OVA airway challenges - day 28. (ii) OVA sensitized mice that were previously rendered eosinophil-deficient during the initial airway challenge phase were allowed to “rest” (i.e., DT administration was discontinued) 12 days to allow restoration of the peripheral eosinophils prior to re-challenge with 1% OVA (control mice received saline alone) on day 39. (B) Assessments of total BAL cell counts as well as cell differential analyses were performed on cytospins preparations of recovered airway cells at OVA Day 28 vs. Day 41. *P<0.05. (C) Representative photomicrographs of lung sections from iPHIL mice at OVA Day 28 vs. Day 41 are presented relative to lung sections from saline-treated control Day 41 mice. These lungs sections were stained with PAS (dark purple staining cells) as well as immunohistochemistry using an anti-MBP monoclonal antibody (MBP IHC – red staining cells infiltrating the parenchyma), assessing goblet cell metaplasia/airway epithelial cell mucin accumulation and eosinophil infiltration, respectively. These data demonstrated that while both OVA-Day 28 and OVA-Day 41 lungs displayed goblet cell metaplasia/airway epithelial cell mucin accumulation (PAS), iPHIL mice at OVA Day 41 (i.e., after secondary allergen challenge) developed a prominent pulmonary eosinophilia (MBP IHC) in contrast to the complete absence of eosinophils in iPHIL at OVA Day 28. Scale bar = 100μm.
The neutrophilic pulmonary responses in eosinophil-deficient iPHIL mice are a steroid-resistant form of allergic respiratory inflammation
The responsiveness of the neutrophilic inflammatory phenotype linked with allergen-treated eosinophil-deficient iPHIL mice to steroid-treatment was assessed relative to allergic eosinophil-associated inflammation of control mice; i.e., wild type or iPHIL treated following DT administration with or without dexamethasone (Figure 6). Administration of the steroid dexamethasone immediately after allergen challenge (i.e., day 27 (Figure 6(A)) led to a significant reduction in BAL cellularity (Figure 6(B)), airway eosinophil numbers in wild type mice (Figure 6(C)), and reduced goblet cell metaplasia/airway epithelial mucin accumulation (representative photomicrographs are shown in Figure 6(D)). In contrast, dexamethasone treatment of eosinophil-deficient iPHIL mice displayed no increase in total BAL cellularity (Figure 6(B)) and retained both a significant allergen-associated airways neutrophilia (Figure 6(C)) and levels of goblet cell metaplasia/airway epithelial mucin accumulation equivalent to vehicle control mice (Figure 6(D)). These data demonstrated that eosinophil-depletion in iPHIL mice resulted in allergen-mediated neutrophilic pulmonary inflammatory variant that was resistant to steroid-treatment.
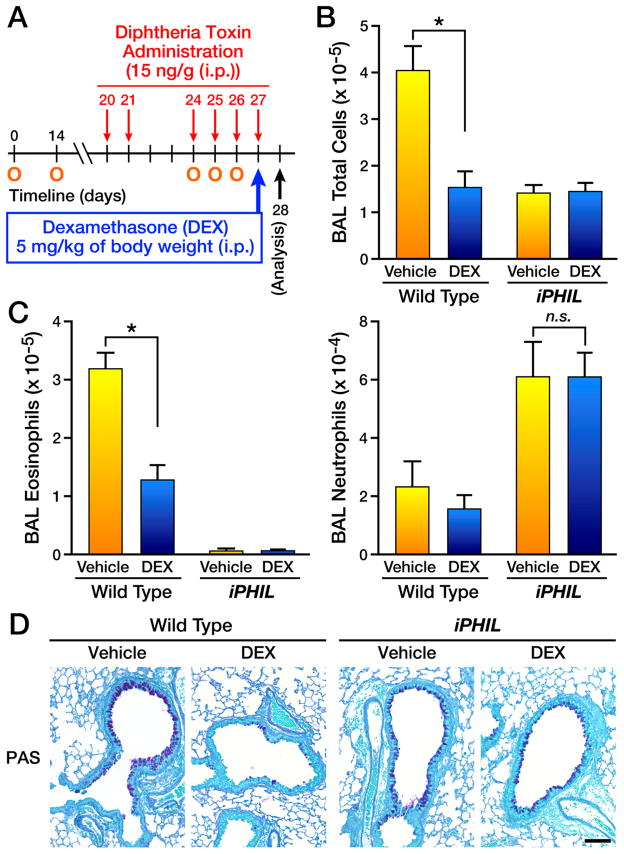
Figure 6. Eosinophil ablation during allergen challenge leads to a steroid-resistant neutrophilic phenotype.
(A) Wild type and iPHIL mice were sensitized (i.p.) with OVA/Imject® adjuvant protocol days 0 and 14 (O) and administered DT ((i.p.) - 15ng/gram body weight) prior to and during the airway challenge phases on days 20, 21, and 24–27 (arrows) with the mice receiving an OVA nebulant generated from a 1% OVA solution in saline (control mice received saline alone) on protocol days 24–26 (O) and assessed on day 28. Some mice received saline (Vehicle) while other mice received (i.p.) 5mg/kg body weight dexamethasone (DEX) on day 27 of the protocol. (B) Total bronchoalveolar lavage (BAL) cellularity in wild type vs. DT-treated iPHIL mice following treatment with DEX (control animals received saline vehicle alone (Vehicle)). *p<0.05 (C) Cell counts and differentials were used to determine the number (mean ± SEM) of airway eosinophils and neutrophils following treatment with DEX (control animals received saline vehicle alone (Vehicle)). *p<0.05; n.s., not significant. (D) Representative photomicrographs of lung sections are presented from wild type as compared to iPHIL mice whose eosinophils were ablated during the airway challenge phase. Lung sections were stained with PAS and showed that although wild type allergen-treated lungs displayed a reduction in goblet cell metaplasia/airway epithelial cell mucin accumulation ((PAS) – black staining cells) upon DEX treatment, allergen-treated iPHIL mice receiving dexamethasone displayed inflammation levels similar to saline injected animals; control animals received saline vehicle alone. Scale bar = 100μm.
DISCUSSION
The absence of eosinophils in congenitally deficient strains of mice during development of the immune system (i.e., thymus (12) and bone marrow (13)) is a confounding issue for studies of eosinophil-mediated events in health and disease (for review (15)). Furthermore, the inability to target transiently eosinophils within defined periods of time of a given experimental protocol has prevented studies examining the consequences of eosinophil-targeted therapies and their potential clinical utility in subjects with established disease/inflammation (27). We developed an inducible strain of mouse, iPHIL, as a logistical solution to these issues, promoting the reversible ablation of eosinophils. This depletion was entirely specific for eosinophils and did not target any other leukocyte subtype.
Previous reports have suggested that eosinophils were unlikely to participate in allergen sensitization, including antigen priming of T cells (28, 29) or the development of significant humoral responses (9). However, some studies suggested that IL-4+/Gr1+ eosinophils were specifically recruited to the site of OVA/alum injections (30, 31), leaving open the possibility that in addition to effector functions mediated upon recruitment to the lung following airway allergen challenge, eosinophils may have a contributory role in sensitization (at least in typical acute models). However, the studies reported here were definitive and showed that in both OVA and HDM provocation models the loss of eosinophils specifically during the respective allergen sensitization phases of these protocols (i.e., with or without the use of an immune adjuvant) had no effects on the subsequent development of Th2 immune responses and inflammatory metrics (including the development of a robust airway eosinophilia) following allergen challenge.
To date, a significant effort has been undertaken to determine the relative importance of eosinophils as immune modulatory cells during allergen challenge when lung pathologies are greatest (6–8, 32). The ability to ablate eosinophils “on demand” in iPHIL mice provides a strategy circumventing many logistical issues associated with congenital eosinophil-deficiency and eosinophil adoptive cell transfer techniques used to define potential immunomodulatory roles. The ablation of eosinophils specifically in the time frame of airway challenge of both OVA and HDM models reproducibly resulted in a dominant group of mice (>90%) displaying a neutrophilic/Th2 inflammatory airway phenotype and only a small portion of mice having no significant inflammatory response(s). Our previous studies (see for example (4)) showed that congenitally eosinophil-deficient PHIL mice displayed this non-inflammatory phenotype as the dominant group with the neutrophilic inflammatory variant being an occasionally observed event, suggesting a potential immune developmental defect associated with congenital eosinophil deficiency. However, parallel studies with both of the available congenitally eosinophil-deficient strains of mice (i.e., PHIL and ΔdblGATA) revealed that each of these strains also displayed the dominant neutrophilic airway phenotype following allergen challenge with a small group of mice with the inflammatory null phenotype. This observation suggests that the advent of the allergen-mediated neutrophilic phenotype in these mice is not a strain-specific phenomenon. The reversibility of this phenotype upon restoration of the peripheral eosinophilia in iPHIL mice also suggested that this neutrophilic respiratory variant is not an artifact and is specifically linked to the presence vs. absence of eosinophils. Thus, it appears that together with one or more additional environmental cues (e.g., commensal bacteria (11, 33–35)) eosinophils may have a significant, yet underappreciated, role in the development of the immune responses to aeroallergens. The eosinophil-dependent mechanisms leading to this neutrophilic phenotype are many and likely include potential direct effects on neutrophil recruitment/accumulation in the lung (36), eosinophil-induced suppression of Th1/Th17 polarization possibly mediated by eosinophil dependent activation of dendritic cells (8), and eosinophil-derived expression of factors modulating T cell polarizing events (e.g., eosinophil-derived expression of IL-4/13 (37, 38), IDO (12), and IL-25 (39)).
The studies presented also showed that the allergen-induced neutrophilic airway phenotype was linked with both histopathology and lung dysfunction (i.e., AHR) that was virtually identical to that observed in eosinophilic allergen-mediated responses. However, unlike allergen-mediated eosinophilic pulmonary inflammation, the pathologies linked with the neutrophilic phenotype are resistant to steroid treatment. Many reports have previously highlighted that the steroid refractory pathologies occurring in animal models (e.g., the adoptive transfer of ex vivo polarized Th17 cells (40) or models in which dendritic cells are polarized with Th17 inducing adjuvants such as zymosan (41)) resulted as a consequence of glucocorticosteroid-resistant Th17 mediated immune responses. Significantly, our previous studies have demonstrated that eosinophils suppress allergen-mediated Th17 responses in airway challenged mice (8), suggesting that steroid resistance of the neutrophilic phenotype occurring in iPHIL mice may be a direct consequence of eosinophil-mediated modulation of Th17-mediated allergic immune responses. These data highlight the complexity and heterogeneity of the inflammatory phenotypes possible, including the concurrent presence of multiple concurrent and independent airway immune/inflammatory responses; potentially reflective of a severe and often difficult to treat subset of human asthma patients (reviewed in (42)). Collectively, the studies presented here suggest that while eosinophil-mediated activities may not be the singular cause of acute asthma symptoms, these granulocytes display several activities that nonetheless shape the character of allergic respiratory disease. Thus, as opposed to terminally differentiated end stage effector cells whose activities are exclusively limited to the causation of pathology(ies), eosinophils are likely important contributors to the multiple overlapping and independent immune pathways that occur in the varied responses to allergen provocation observed in both mouse models and human patients.
Supplementary Material
Acknowledgments
The performance of these studies, including data analysis and manuscript preparation, was supported by resources from the Mayo Foundation and grants from the United States National Institutes of Health [NAL (HL058723), JJL (HL065228)], the American Heart Association [EAJ (11SDG7510043)], the Canadian Institutes of Health Research [RM (MOP89748)], and the Lung Association of Alberta [LWW]. These funding sources had no involvement in study design, data collection (including analysis and interpretation), the writing of the manuscript, or the decision to submit for publication.
The authors wish to thank Dr. Michael McGarry and Alfred Doyle for the many insightful discussions of the data and other members of Lee Laboratories who provided assistance in data collection, the necessary organization/infrastructure needed to complete the studies presented, and the review of various drafts of this manuscript. We also wish to acknowledge the invaluable assistance of the Mayo Clinic Arizona medical graphic artist, Marv Ruona, and the excellent administrative support provided to Lee Laboratories by Linda Mardel and Shirley (“Charlie”) Kern.
Footnotes
The authors have no conflicting financial interests.
References
- 1.Gonem S, Desai D, Siddiqui S, Brightling CC. Evidence for phenotype-driven treatment in asthmatic patients. Current opinion in allergy and clinical immunology. 2011;11(4):381–5. doi: 10.1097/ACI.0b013e328348a8f9. [DOI] [PubMed] [Google Scholar]
- 2.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nature Medicine. 2012;18(5):716–25. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 3.Lee JJ, Rosenberg HF, editors. Eosinophils in Health and Disease. Waltham, MA: Elsevier; 2012. [Google Scholar]
- 4.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O’Neill KR, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305(5691):1773–6. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 5.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, et al. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305(5691):1776–9. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 6.Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, et al. Allergic Pulmonary Inflammation in Mice is Dependent on Eosinophil-induced Recruitment of Effector T Cells. Journal of Experimental Medicine. 2008;205(3):699–710. doi: 10.1084/jem.20071840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh ER, Sahu N, Kearley J, Benjamin E, Kang BH, Humbles A, et al. Strain-specific requirement for eosinophils in the recruitment of T cells to the lung during the development of allergic asthma. J Exp Med. 2008;205(6):1285–1292. doi: 10.1084/jem.20071836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobsen EA, Zellner KR, Colbert D, Lee NA, Lee JJ. Eosinophils regulate dendritic cells and Th2 pulmonary immune responses following allergen provocation. Journal of Immunology. 2011;187(11):6059–68. doi: 10.4049/jimmunol.1102299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fattouh R, Al-Garawi A, Fattouh M, Arias K, Walker TD, Goncharova S, et al. Eosinophils are dispensable for allergic remodeling and immunity in a model of house dust mite-induced airway disease. American Journal of Respiratory and Critical Care Medicine. 2011;183(2):179–88. doi: 10.1164/rccm.200905-0736OC. [DOI] [PubMed] [Google Scholar]
- 10.Willis-Owen SA, Valdar W. Deciphering gene-environment interactions through mouse models of allergic asthma. The Journal of allergy and clinical immunology. 2009;123(1):14–23. doi: 10.1016/j.jaci.2008.09.016. quiz 24-5. [DOI] [PubMed] [Google Scholar]
- 11.Ma BW, Bokulich NA, Castillo PA, Kananurak A, Underwood MA, Mills DA, et al. Routine habitat change: a source of unrecognized transient alteration of intestinal microbiota in laboratory mice. PLoS ONE. 2012;7(10):e47416. doi: 10.1371/journal.pone.0047416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tulic MK, Sly PD, Andrews D, Crook M, Davoine F, Odemuyiwa SO, et al. Thymic Indoleamine 2,3-Dioxygenase-Positive Eosinophils in Young Children: Potential Role In Maturation of the Naive Immune System 10.2353/ajpath.2009. 090015. American Journal of Pathology. 2009;175(5):2043–2052. doi: 10.2353/ajpath.2009.090015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu VT, Frohlich A, Steinhauser G, Scheel T, Roch T, Fillatreau S, et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nature Immunology. 2011 doi: 10.1038/ni.1981. advance online publication. [DOI] [PubMed] [Google Scholar]
- 14.Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA. Eosinophils in Health and Disease: The LIAR Hypothesis. Clinical and Experimental Allergy. 2010;40(4):563–575. doi: 10.1111/j.1365-2222.2010.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobsen EA, Helmers RA, Lee JJ, Lee NA. The expanding role(s) of eosinophils in health and disease. Blood. 2012;120(19):3882–90. doi: 10.1182/blood-2012-06-330845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fahy JV. Eosinophilic and neutrophilic inflammation in asthma: insights from clinical studies. Proc Am Thorac Soc. 2009;6(3):256–9. doi: 10.1513/pats.200808-087RM. [DOI] [PubMed] [Google Scholar]
- 17.Denzler KL, Levin WJ, Lee JJ, Lee NA. The murine eosinophil peroxidase gene (Epx) maps to chromosome 11. Mammalian Genome. 1997;8(5):381–2. doi: 10.1007/s003359900448. [DOI] [PubMed] [Google Scholar]
- 18.McGarry MP, Protheroe CA, Lee JJ. A Laboratory Manual with accompanying CD of training videos and Poster of Mouse Peripheral Blood Cells. 1. New York: Cold Spring Harbor Laboratory Press; 2010. Mouse Hematology. [Google Scholar]
- 19.Doyle AD, Jacobsen EA, Ochkur SI, McGarry MP, Shim KG, Nguyen DTC, et al. Blood 2013. 1. Jun 4, 2013. Expression of the Secondary Granule Proteins Major Basic Protein (MBP)-1 and Eosinophil Peroxidase (EPX) is Required for Eosinophilopoiesis in Mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dyer KD, Moser JM, Czapiga M, Siegel SJ, Percopo CM, Rosenberg HF. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J Immunol. 2008;181(6):4004–9. doi: 10.4049/jimmunol.181.6.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doyle AD, Jacobsen EA, Ochkur SI, McGarry MP, Shim KG, Nguyen DT, et al. Expression of the secondary granule proteins major basic protein (MBP)-1 and eosinophil peroxidase (EPX) is required for eosinophilopoiesis in mice. Blood. 2013 doi: 10.1182/blood-2013-01-473405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee NA, McGarry MP, Larson KA, Horton MA, Kristensen AB, Lee JJ. Expression of IL-5 in thymocytes/T cells leads to the development of a massive eosinophilia, extramedullary eosinophilopoiesis, and unique histopathologies. J Immunol. 1997;158(3):1332–1344. [PubMed] [Google Scholar]
- 23.Masterson JC, McNamee EN, Jedlicka P, Fillon S, Ruybal J, Hosford L, et al. CCR3 Blockade Attenuates Eosinophilic Ileitis and Associated Remodeling. The American journal of pathology. 2011;179(5):2302–14. doi: 10.1016/j.ajpath.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ochkur SI, Jacobsen EA, Protheroe CA, Biechele TL, Pero RS, McGarry MP, et al. Co-Expression of IL-5 and Eotaxin-2 in Mice Creates an Eosinophil-Dependent Model of Respiratory Inflammation with Characteristics of Severe Asthma. Journal of Immunology. 2007;178(12):7879–89. doi: 10.4049/jimmunol.178.12.7879. [DOI] [PubMed] [Google Scholar]
- 25.Ochkur SI, Protheroe CA, Li W, Colbert DC, Zellner KR, Shen H, et al. Cys-Leukotrienes Promote Fibrosis in a Mouse Model of Eosinophil-mediated Respiratory Inflammation and atherosclerosis. American Journal of Physiology and Cell Molecular Physiology. 2013 doi: 10.1165/rcmb.2013-0009OC. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buch T, Heppner FL, Tertilt C, Heinen TJ, Kremer M, Wunderlich FT, et al. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods. 2005;2(6):419–26. doi: 10.1038/nmeth762. [DOI] [PubMed] [Google Scholar]
- 27.Fulkerson PC, Rothenberg ME. Targeting eosinophils in allergy, inflammation and beyond. Nature reviews Drug discovery. 2013;12(2):117–29. doi: 10.1038/nrd3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambrecht BN, De Veerman M, Coyle AJ, Gutierrez-Ramos JC, Thielemans K, Pauwels RA. Myeloid dendritic cells induce Th2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J Clin Invest. 2000;106(4):551–9. doi: 10.1172/JCI8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKee AS, Munks MW, MacLeod MK, Fleenor CJ, Van Rooijen N, Kappler JW, et al. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J Immunol. 2009;183(7):4403–14. doi: 10.4049/jimmunol.0900164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kool M, Soullie T, van Nimwegen M, Willart MA, Muskens F, Jung S, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205(4):869–82. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang HB, Weller PF. Pivotal Advance: Eosinophils mediate early alum adjuvant-elicited B cell priming and IgM production. Journal of Leukocyte Biology. 2008;83(4):817–21. doi: 10.1189/jlb.0607392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fulkerson PC, Fischetti CA, McBride ML, Hassman LM, Hogan SP, Rothenberg ME. A central regulatory role for eosinophils and the eotaxin/CCR3 axis in chronic experimental allergic airway inflammation. Proc Natl Acad Sci U S A. 2006;103(44):16418–23. doi: 10.1073/pnas.0607863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansen CH, Nielsen DS, Kverka M, Zakostelska Z, Klimesova K, Hudcovic T, et al. Patterns of early gut colonization shape future immune responses of the host. PLoS ONE. 2012;7(3):e34043. doi: 10.1371/journal.pone.0034043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sczesnak A, Segata N, Qin X, Gevers D, Petrosino JF, Huttenhower C, et al. The genome of th17 cell-inducing segmented filamentous bacteria reveals extensive auxotrophy and adaptations to the intestinal environment. Cell host & microbe. 2011;10(3):260–72. doi: 10.1016/j.chom.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herbst T, Sichelstiel A, Schar C, Yadava K, Burki K, Cahenzli J, et al. Dysregulation of allergic airway inflammation in the absence of microbial colonization. American Journal of Respiratory and Critical Care Medicine. 2011;184(2):198–205. doi: 10.1164/rccm.201010-1574OC. [DOI] [PubMed] [Google Scholar]
- 36.Isobe Y, Kato T, Arita M. Emerging roles of eosinophils and eosinophil-derived lipid mediators in the resolution of inflammation. Frontiers in immunology. 2012;3:270. doi: 10.3389/fimmu.2012.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Justice JP, Borchers MT, Lee JJ, Rowan WH, Shibata Y, Scott MRV. Ragweed-induced expression of GATA-3, IL-4, and IL-5 by eosinophils in the lungs of allergic C57BL/6 mice. American Journal of Physiology -LCMP. 2002;282(2):L302–L309. doi: 10.1152/ajplung.00158.2001. [DOI] [PubMed] [Google Scholar]
- 38.Spencer LA, Melo RC, Perez SA, Bafford SP, Dvorak AM, Weller PF. Cytokine receptor-mediated trafficking of preformed IL-4 in eosinophils identifies an innate immune mechanism of cytokine secretion. Proc Natl Acad Sci U S A. 2006;103(9):3333–8. doi: 10.1073/pnas.0508946103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204(8):1837–47. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181(6):4089–97. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyakh L, Trinchieri G, Provezza L, Carra G, Gerosa F. Regulation of interleukin-12/interleukin-23 production and the T-helper 17 response in humans. Immunol Rev. 2008;226:112–31. doi: 10.1111/j.1600-065X.2008.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wenzel SE. Complex phenotypes in asthma: Current definitions. Pulm Pharmacol Ther. 2013 doi: 10.1016/j.pupt.2013.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.