Abstract
The nephron is the functional unit that executes the homeostatic roles of the kidney in vertebrates. Critical to this function is the physical arrangement of the glomerular blood filter attached to a tubular epithelium that is subdivided into specialized proximal and distal segments. During embryogenesis, nephron progenitors undergo a mesenchymal-epithelial transition (MET) and adopt different segment-specific cell fates along the proximo-distal axis of the nephron. The molecular basis of how these segments arise remains largely unknown. Recent studies using the zebrafish identified the Hnf1beta transcription factor (Hnf1b) as a major regulator of tubular segmentation. In Hnf1b-deficient zebrafish embryos, nephron progenitors fail to adopt the proximo-distal segmentation pattern of the nephron, yet still undergo MET. This observation suggests that the functional segmentation of renal tubular epithelial cells is independent of pathways that induce their epithelialization. Here we review this new role of Hnf1b for nephron segmentation during zebrafish and mouse kidney development.
Keywords: kidney, embryo, nephrogenesis, zebrafish, mouse, HNF1beta, transcription factor
Introduction
Nephrogenesis refers to the formation of the blood filtering tubules (nephrons) from mesenchymal progenitor cells. This process involves changes in cell morphology (mesenchymal to epithelial) and the adoption of distinct cell fates along the proximo-distal axis of the nephron. How is process is controlled is poorly understood, however recent studies in zebrafish have identified the Hnf1beta transcription factor (Hnf1b) as a major player [1]. This article reviews this new function of HNF1b during zebrafish as well as mouse nephrogenesis.
Nephrogenesis in mammals and zebrafish
Vertebrate kidney formation progresses through two or three stages, depending on the species, giving rise to the pronephric, mesonephric, and metanephric kidneys. Common to each is the nephron, comprising a glomerular blood filter attached to a tubular epithelium. The metanephric nephron can be broadly subdivided into proximal, intermediate, and distal segments that have the general functions of bulk filtrate reabsorption (proximal tubule), urine concentration (intermediate segments) and acid-base and salt ‘fine-tuning’ (distal nephron).
By contrast, zebrafish pronephric nephrons are simpler, comprising a single glomerulus attached to two tubules that are subdivided into two proximal tubule segments and two distal tubule segments (Figure 1B)[2,3]. Whilst there are notable differences between the genesis of nephrons in the mammalian metanephros and the zebrafish pronephros, the overall process is very similar with mesenchymal nephron progenitors activating conserved transcriptional and signaling pathways and undergoing MET into segmented nephrons [2,4,5]. The nephrons of freshwater fish such as zebrafish, primarily function to reclaim filtered solutes, balance ions, and void water taken up from their freshwater environment. Evidence to date indicates that the two proximal tubule segments in zebrafish, with their well-developed apical brush borders and transporter expression profiles, are analogous to the proximal tubule of metanephric nephrons. The first distal segment (‘distal early’) shares a gene signature with the thick ascending limb segment in mammals, while the ‘distal late’ segment has molecular hallmarks of the distal convoluted segment in mammals [3].
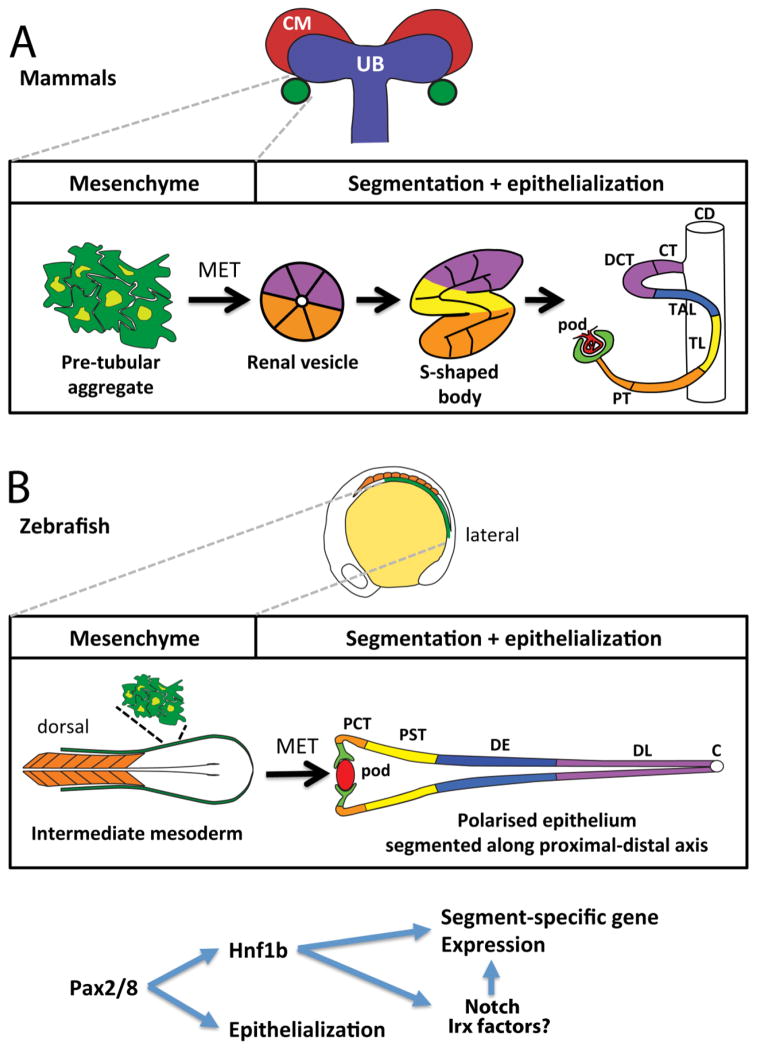
Figure 1.
A) Top panel shows the stages of mammalian metanephric nephron development from a pretubular aggregate to an early nephron. Bottom panel shows an overview of zebrafish pronephros formation from the intermediate mesoderm. B) Postulated genetic pathway during early nephrogenesis showing the hierarchical relationship between Pax2/8, Hnf1b, Notch pathway, and Irx genes and their proposed roles in directing the mesenchymal-to-epithelial transition (MET) of nephron progenitors and proximo-distal segmentation. Abbreviations: CM, cap mesenchyme; UB, ureteric bud; pod, podocytes; PT, proximal tubule; TL, thin limb; TAL, thick ascending limb; DCT, distal convoluted tubule; CT, connecting tubule; CD, collecting duct. PCT, proximal convoluted tubule; PST, proximal straight tubule; DE, distal early tubule; DL, distal late tubule; C, Cloaca.
During development of the mammalian metanephric kidney an outgrowth of the nephric duct called the ureteric bud (UB) interacts closely with a population of nephron progenitors and reciprocal communication coordinates progressive UB branching and nephron induction. Nephrogenesis is characterized by the epithelialization of nephron progenitors, close to the UB branches, which pass through stereotypical stages of maturation (pretubular aggregate, renal vesicle, comma-shaped body, and S-shaped body; SSB). The developing nephron fuses with the UB-derived collecting duct at the late renal vesicle stage [6,7]. The S-shaped body is subdivided into lower, mid, and upper limb compartments, with the glomerulus arising from the lower limb, the proximal tubule and loop of Henle most likely deriving from the mid limb, and the distal nephron from the upper limb (Figure 1A).
In the zebrafish, the pronephros arises from bilateral stripes of intermediate mesoderm that epithelializes into a simple two-nephron kidney (Figure 1B). Unlike in mammals, where the intermediate mesoderm differentiates into the nephric duct that migrates a significant distance to the cloaca, most of the zebrafish intermediate mesoderm differentiates in situ to form nephron progenitors. Because of this, the distal pronephros only migrates a short distance to the cloaca where it fuses [8]. The linear morphology of pronephric nephrons is a useful feature of the zebrafish model, as it means dynamic gene expression patterns [9,10] can be readily mapped during nephrogenesis and correlated to the final segmentation pattern of the nephron [2].
Role of Hnf1b in nephron development
Hepatocyte nuclear factor-1 beta (Hnf1b) encodes a POU homeodomain transcription factor that is expressed in tubular portions of the nephron and the metanephric collecting duct system, starting at the renal vesicle stage [1,11–13]. In humans, mutations in HNF1B are a common cause of congenital anomalies of the kidney [14–16] and heterozygous mutations are associated with the ‘renal cysts and diabetes’ syndrome (RCAD) in young adults [17]. Despite obvious links to kidney development and function, defining the exact role of Hnf1b in the kidney has been hampered by the early embryonic lethality (E7.5) of the constitutive knock-out mouse, due to extra-embryonic visceral endoderm defects [18,19]. Various strategies have been employed to try to overcome this difficulty. Of these, transgenic overexpression of a dominant negative variant of Hnf1b in already formed, but still elongating, tubular segments leads to a polycystic kidney phenotype [20]. Further studies revealed that Hnf1b functions as a ‘bookmarking factor’ that is required after cell division to re-establish the expression of a number of genes associated with cystic kidney disease [21]. Utilization of tetraploid complementation experiments, which rescues the visceral endoderm defects while leaving the ‘embryo proper’ deficient in Hnf1b, demonstrated a requirement for Hnf1b in UB branching [12]. In addition, expression of Wnt9b, encoding a critical UB-derived inducer of nephrogenesis, was absent in the UB tips of Hnf1b-deficient embryos [12]. While this study demonstrated the functional importance of Hnf1b in the UB, it precluded an assessment of its role during later stages of nephrogenesis.
In zebrafish there are two orthologues of Hnf1b (hnf1ba and hnf1bb) that are expressed in the intermediate mesoderm and developing pronephric tubules [1,22]. In hnf1ba/b-deficient zebrafish embryos (herein referred to as hnf1b-deficient), the pronephric tubules fail to adopt a proximo-distal segmentation pattern and mature markers of proximal and distal segment identity, such as solute transporter genes, are not expressed [1] This result provided the first evidence that Hnf1b factors play a role in establishing the tubular segmentation pattern of the nephron. This new function was confirmed in the mouse by two independent conditional knockout strategies utilizing the Six2-Cre and Wnt4-Cre drivers and floxed Hnf1b alleles [11,13]. Early nephrogenesis is relatively unaffected in these mutants, with polarized renal vesicles and comma-shaped bodies developing normally. However, morphological defects become apparent at the SSB stage with mutants failing to expand presumptive proximal tubule and loop of Henle precursors that are believed to be located in the mid-limb of the SSB. This region shows reduced proliferation and increased apoptosis compared to wild-type SSBs, providing one explanation for the altered SSB morphology in the mutants. Mid-limb markers are significantly reduced in Hnf1b-deficient embryos, including Notch components such as the Dll1 ligand. Given that Dll1 hypomorphs and Notch2 nulls display proximal tubule defects that overlap with Hnf1b mutants, it is likely that disrupted Notch signaling plays a major role in the Hnf1b-deficient kidney phenotype [23]. In both zebrafish and mouse mutants, persistent expression of Pax2 and Lhx1 expression was noted, suggesting that Hnf1b-deficient tubular epithelial cells do not fully differentiate and retain the expression of genes that characterize earlier stages of nephrogenesis. Consistent with this, expression of mature proximal and distal tubule markers, which initiate later in nephrogenesis, is absent in Hnf1b mutant kidneys and the nephrons that ultimately form are comprised of dilated glomeruli connected to collecting ducts via short tubules. Taken together, the fish and mouse studies support a model in which Hnf1b acts during early nephrogenesis to regulate genes involved in establishing the maturation of all nephron tubular segments. The mammalian studies suggest that Hnf1b is not required for the initial patterning of the renal vesicle but instead acts during the transition to the SSB stage, when distinct tubular precursors are arising. In addition, Hnf1b may have a direct role in regulating the expansion and survival of tubular precursors, or alternatively the defects in proliferation and apoptosis seen in the mouse mutants (but not zebrafish) may be secondary to failed differentiation.
The zebrafish study revealed an unexpected role for Hnf1b in regulating the formation of podocytes, the highly specialized epithelial cells that contribute to the glomerular blood filter. Hnf1b-deficient zebrafish embryos display ectopic podocyte marker expression in regions of the intermediate mesoderm where the first proximal tubule segment would normally form. This result implicates Hnf1b factors in the developmental pathways that regulate the partitioning of podocyte and tubule fates from the intermediate mesoderm [1]. This function may not be conserved in mammals, as glomerular development appears largely unaffected in the conditional Hnf1b mutants [11,13]. During zebrafish pronephros formation, transcripts for the hnf1b genes are excluded from podocyte progenitors, suggesting that Hnf1b factors may act in proximal tubule progenitors to inhibit podocyte fate.
Despite the lack of proximo-distal segmentation in hnf1b-deficient zebrafish embryos, the mesenchyme-to-epithelial transition (MET) of tubule progenitors is relatively unaffected. Electron microscopy shows tubular epithelial cells in hnf1b-deficient animals with apical junctional complexes and a surrounding basement membrane. In support of this, the expression of epithelial markers such as cdh1, epcam, and laminin5 are maintained in hnf1b-deficient embryos [1]. No effect on MET was seen in the mouse studies either, however this was not unexpected as the timing of Hnf1b expression differs between zebrafish and mouse with transcripts appearing in the metanephros after the epithelialization of nephron progenitors into renal vesicles [11,13]. The zebrafish findings suggest that the patterning of nephron progenitors into distinct segments is independent of the MET that occurs during nephron development. In further support of this, genes that are critical for segment functionality, such as the Na+K+ATPase subunit genes and solute transporters genes like slc4a4 (proximal tubule), and slc12a3 (distal late tubule), are expressed in zebrafish nephron progenitors prior to the onset of epithelial-associated genes such as cdh1 and epcam (our observations and reference [10]).
Interactions between Pax2/8 and Hnf1b
If Hnf1b does not drive the MET of nephron progenitors then what factors do? Pax2 and the closely related Pax8 are among the earliest acting transcription factors in the intermediate mesoderm and likely candidates for inducing MET [24–28]. Both in vivo and in vitro studies have implicated Pax2/8 in the epithelialization of the intermediate mesoderm and metanephric nephron progenitors [29,30]. However, the downstream targets of Pax2/8 in this process are unclear. A role for Pax2/8 in directing the MET of zebrafish nephron progenitors is consistent with the observation that expression of pax2a and pax8 persists in hnf1b-deficient zebrafish embryos. Interestingly, in pax2a/8-deficient embryos the intermediate mesoderm initially forms but fails to activate expression of hnf1ba (our observations). These results suggest a model in which Pax2a and Pax8 act upstream of the Hnf1b genes, resulting in the initiation of segment-specific gene expression programs, in addition to inducing MET through Hnf1b-independent pathways. Thus, we propose Pax2/8 control both tubular epithelialization and segment identity (Figure 1B).
Interactions between the Hnf1b and Iroquois transcription factors
Functional studies in zebrafish and Xenopus have implicated orthologues of the Iroquois (Irx) transcription factors Irx1 and Irx3 in the development of the pronephros [10,31,32]. In the mouse Irx1, Irx2, and Irx3 are expressed in the mid-limb of SSBs, however a functional requirement of these factors for mammalian nephrogenesis has yet to be shown [11]. Chromatin-immunoprecipitation experiments demonstrated that Hnf1b is recruited to potential regulatory elements around the Irx1 and Irx2 gene cluster [13]. Consistent with being direct targets of Hnf1b, expression of Irx1 and Irx2 are virtually undetectable in Hnf1b mutants. Similarly, there is a loss of irx3b expression in the pronephros of hnf1b-deficient zebrafish embryos [1]. Work in zebrafish implicates Irx3b in the morphogenesis of the first distal tubule segment although this role is poorly understood [10]. Whether the Irx genes directly regulate segment identity and/or maturation downstream of Hnf1b remains unclear and requires more study.
Conclusions and future directions
Recent discoveries using the zebrafish and mouse models have extended our knowledge of the multiple roles Hnf1b factors play during kidney development. In nephron progenitors, Hnf1b is required for the activation of segment-specific gene expression programs that are central to the function of the nephron. The direct targets of Hnf1b are beginning to be elucidated and while prior studies have focused predominantly on genes in the proximal tubule [33–38], it is now clear that the search needs to be broadened. The recent studies discussed here have identified new targets including key regulatory genes such as components of the Notch pathway and the Iroquois transcription factors that are expressed in tubule precursors [11,13,23,31,32,39,40]. More work is needed to understand how these genes act downstream of Hnf1b to regulate the proximo-distal patterning of the nephron. If Hnf1b acts in all tubule segments then additional layers of regulation must exist to ensure that Hnf1b only activates targets appropriate to each segment. Solving this mystery will depend on further studies utilizing ChIP-Seq and the ability to examine Hnf1b binding to chromatin in epithelial cells from each segment. An additional challenge will be to understand how Hnf1b interacts with the pathways that initially establish the segmental pattern of the nephron. In zebrafish, retinoic acid signaling has been identified as a major regulator of proximo-distal patterning in the pronephros [2,3,10], whereas in mammals the Notch pathway is implicated in the establishment of proximal nephron fates [5,23,41–43]. How these pathways interface with the Hnf1b factors and whether they play a role in determining the targets that are available for activation in each segment remains to be determined.
References
- 1.Naylor RW, Przepiorski A, Ren Q, Yu J, Davidson AJ. HNF1beta is essential for nephron segmentation during nephrogenesis. J Am Society of Nephrol. 2013;24:77–87. doi: 10.1681/ASN.2012070756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wingert RA, Davidson AJ. The zebrafish pronephros: a model to study nephron segmentation. Kidney Int. 2008;73:1120–1127. doi: 10.1038/ki.2008.37. [DOI] [PubMed] [Google Scholar]
- 3.Wingert RA, Selleck R, Yu J, Song HD, Chen Z, Song A, Zhou Y, Thisse B, Thisse C, McMahon AP, Davidson AJ. The cdx genes and retinoic acid control the positioning and segmentation of the zebrafish pronephros. PLoS Genet. 2007;3:1922–1938. doi: 10.1371/journal.pgen.0030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dressler GR. The cellular basis of kidney development. Annual review of cell and developmental biology. 2006;22:509–529. doi: 10.1146/annurev.cellbio.22.010305.104340. [DOI] [PubMed] [Google Scholar]
- 5.Costantini F, Kopan R. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev Cell. 2010;18:698–712. doi: 10.1016/j.devcel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mah SP, Saueressig H, Goulding M, Kintner C, Dressler GR. Kidney development in cadherin-6 mutants: delayed mesenchyme-to-epithelial conversion and loss of nephrons. Dev Biol. 2000;223:38–53. doi: 10.1006/dbio.2000.9738. [DOI] [PubMed] [Google Scholar]
- 7.Davies JA, Bard JB. Inductive interactions between the mesenchyme and the ureteric bud. Exp Nephrol. 1996;4:77–85. [PubMed] [Google Scholar]
- 8.Slanchev K, Putz M, Schmitt A, Kramer-Zucker A, Walz G. Nephrocystin-4 is required for pronephric duct-dependent cloaca formation in zebrafish. Hum Mol Genet. 2011;20:3119–3128. doi: 10.1093/hmg/ddr214. [DOI] [PubMed] [Google Scholar]
- 9.Brunskill EW, Aronow BJ, Georgas K, Rumballe B, Valerius MT, Aronow J, Kaimal V, Jegga AG, Yu J, Grimmond S, McMahon AP, Patterson LT, Little MH, Potter SS. Atlas of gene expression in the developing kidney at microanatomic resolution. Dev Cell. 2008;15:781–791. doi: 10.1016/j.devcel.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wingert RA, Davidson AJ. Zebrafish nephrogenesis involves dynamic spatiotemporal expression changes in renal progenitors and essential signals from retinoic acid and irx3b. Dev Dyna. 2011;240:2011–2027. doi: 10.1002/dvdy.22691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heliot C, Desgrange A, Buisson I, Prunskaite-Hyyrylainen R, Shan J, Vainio S, Umbhauer M, Cereghini S. HNF1B controls proximal-intermediate nephron segment identity in vertebrates by regulating Notch signalling components and Irx1/2. Development. 2013;140:873–885. doi: 10.1242/dev.086538. [DOI] [PubMed] [Google Scholar]
- 12.Lokmane L, Heliot C, Garcia-Villalba P, Fabre M, Cereghini S. vHNF1 functions in distinct regulatory circuits to control ureteric bud branching and early nephrogenesis. Development. 2010;137:347–357. doi: 10.1242/dev.042226. [DOI] [PubMed] [Google Scholar]
- 13.Massa F, Garbay S, Bouvier R, Sugitani Y, Noda T, Gubler MC, Heidet L, Pontoglio M, Fischer E. Hepatocyte nuclear factor 1beta controls nephron tubular development. Development. 2013;140:886–896. doi: 10.1242/dev.086546. [DOI] [PubMed] [Google Scholar]
- 14.Nakanishi K, Yoshikawa N. Genetic disorders of human congenital anomalies of the kidney and urinary tract (CAKUT) Pediatr Int. 2003;45:610–616. doi: 10.1046/j.1442-200x.2003.01779.x. [DOI] [PubMed] [Google Scholar]
- 15.Thomas R, Sanna-Cherchi S, Warady BA, Furth SL, Kaskel FJ, Gharavi AG. HNF1B and PAX2 mutations are a common cause of renal hypodysplasia in the CKiD cohort. Pediatr Nephrol. 2011;26:897–903. doi: 10.1007/s00467-011-1826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber S, Moriniere V, Knuppel T, Charbit M, Dusek J, Ghiggeri GM, Jankauskiene A, Mir S, Montini G, Peco-Antic A, Wuhl E, Zurowska AM, Mehls O, Antignac C, Schaefer F, Salomon R. Prevalence of mutations in renal developmental genes in children with renal hypodysplasia: results of the ESCAPE study. J Am Soc Nephrol. 2006;17:2864–2870. doi: 10.1681/ASN.2006030277. [DOI] [PubMed] [Google Scholar]
- 17.Edghill EL, Stals K, Oram RA, Shepherd MH, Hattersley AT, Ellard S. HNF1B deletions in patients with young-onset diabetes but no known renal disease. Diabet Med. 2013;30:114–117. doi: 10.1111/j.1464-5491.2012.03709.x. [DOI] [PubMed] [Google Scholar]
- 18.Barbacci E, Reber M, Ott MO, Breillat C, Huetz F, Cereghini S. Variant hepatocyte nuclear factor 1 is required for visceral endoderm specification. Development. 1999;126:4795–4805. doi: 10.1242/dev.126.21.4795. [DOI] [PubMed] [Google Scholar]
- 19.Coffinier C, Thepot D, Babinet C, Yaniv M, Barra J. Essential role for the homeoprotein vHNF1/HNF1beta in visceral endoderm differentiation. Development. 1999;126:4785–4794. doi: 10.1242/dev.126.21.4785. [DOI] [PubMed] [Google Scholar]
- 20.Gresh L, Fischer E, Reimann A, Tanguy M, Garbay S, Shao X, Hiesberger T, Fiette L, Igarashi P, Yaniv M, Pontoglio M. A transcriptional network in polycystic kidney disease. EMBO J. 2004;23:1657–1668. doi: 10.1038/sj.emboj.7600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verdeguer F, Le Corre S, Fischer E, Callens C, Garbay S, Doyen A, Igarashi P, Terzi F, Pontoglio M. A mitotic transcriptional switch in polycystic kidney disease. Nat Med. 2010;16:106–110. doi: 10.1038/nm.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun Z, Hopkins N. vhnf1, the MODY5 and familial GCKD-associated gene, regulates regional specification of the zebrafish gut, pronephros, and hindbrain. Genes Dev. 2001;15:3217–3229. doi: 10.1101/gad946701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng HT, Kim M, Valerius MT, Surendran K, Schuster-Gossler K, Gossler A, McMahon AP, Kopan R. Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development. 2007;134:801–811. doi: 10.1242/dev.02773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouchard M, Souabni A, Mandler M, Neubuser A, Busslinger M. Nephric lineage specification by Pax2 and Pax8. Genes Dev. 2002;16:2958–2970. doi: 10.1101/gad.240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dressler GR, Deutsch U, Chowdhury K, Nornes HO, Gruss P. Pax2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development. 1990;109:787–795. doi: 10.1242/dev.109.4.787. [DOI] [PubMed] [Google Scholar]
- 26.Majumdar A, Lun K, Brand M, Drummond IA. Zebrafish no isthmus reveals a role for pax2.1 in tubule differentiation and patterning events in the pronephric primordia. Development. 2000;127:2089–2098. doi: 10.1242/dev.127.10.2089. [DOI] [PubMed] [Google Scholar]
- 27.Pfeffer PL, Gerster T, Lun K, Brand M, Busslinger M. Characterization of three novel members of the zebrafish Pax2/5/8 family: dependency of Pax5 and Pax8 expression on the Pax2.1 (noi) function. Development. 1998;125:3063–3074. doi: 10.1242/dev.125.16.3063. [DOI] [PubMed] [Google Scholar]
- 28.Serluca FC, Fishman MC. Pre-pattern in the pronephric kidney field of zebrafish. Development. 2001;128:2233–2241. doi: 10.1242/dev.128.12.2233. [DOI] [PubMed] [Google Scholar]
- 29.Bouchard M, Souabni A, Mandler M, Neubüser A, Busslinger M. Nephric lineage specification by Pax2 and Pax8. Genes Dev. 2002;16:2958–2970. doi: 10.1101/gad.240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothenpieler UW, Dressler GR. Pax-2 is required for mesenchyme-to-epithelium conversion during kidney development. Development. 1993;119:711–720. doi: 10.1242/dev.119.3.711. [DOI] [PubMed] [Google Scholar]
- 31.Alarcon P, Rodriguez-Seguel E, Fernandez-Gonzalez A, Rubio R, Gomez-Skarmeta JL. A dual requirement for Iroquois genes during Xenopus kidney development. Development. 2008;135:3197–3207. doi: 10.1242/dev.023697. [DOI] [PubMed] [Google Scholar]
- 32.Reggiani L, Raciti D, Airik R, Kispert A, Brandli AW. The prepattern transcription factor Irx3 directs nephron segment identity. Genes Dev. 2007;21:2358–2370. doi: 10.1101/gad.450707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brunskill EW, Aronow BJ, Georgas K, Rumballe B, Valerius MT, Aronow J, Kaimal V, Jegga AG, Grimmond S, McMahon AP, Patterson LT, Little MH, Potter SS. Atlas of Gene Expression in the Developing Kidney at Microanatomic Resolution. Dev Cell. 2008;15:781–791. doi: 10.1016/j.devcel.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Auyeung DJ, Kessler FK, Ritter JK. Differential regulation of alternate UDP-glucuronosyltransferase 1A6 gene promoters by hepatic nuclear factor-1. Toxicol Appl Pharmaco. 2003;191:156–166. doi: 10.1016/s0041-008x(03)00230-8. [DOI] [PubMed] [Google Scholar]
- 35.Ferre S, Veenstra GJ, Bouwmeester R, Hoenderop JG, Bindels RJ. HNF-1B specifically regulates the transcription of the gammaa-subunit of the Na+/K+-ATPase. Biochem Biophys Res Commun. 2011;404:284–290. doi: 10.1016/j.bbrc.2010.11.108. [DOI] [PubMed] [Google Scholar]
- 36.Kikuchi R, Kusuhara H, Hattori N, Kim I, Shiota K, Gonzalez FJ, Sugiyama Y. Regulation of tissue-specific expression of the human and mouse urate transporter 1 gene by hepatocyte nuclear factor 1 alpha/beta and DNA methylation. Mol Pharmacol. 2007;72:1619–1625. doi: 10.1124/mol.107.039701. [DOI] [PubMed] [Google Scholar]
- 37.Ma Z, Gong Y, Patel V, Karner CM, Fischer E, Hiesberger T, Carroll TJ, Pontoglio M, Igarashi P. Mutations of HNF-1beta inhibit epithelial morphogenesis through dysregulation of SOCS-3. Pro Natl Acad Sci U S A. 2007;104:20386–20391. doi: 10.1073/pnas.0705957104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saji T, Kikuchi R, Kusuhara H, Kim I, Gonzalez FJ, Sugiyama Y. Transcriptional regulation of human and mouse organic anion transporter 1 by hepatocyte nuclear factor 1 alpha/beta. J Pharmacol and Exp Thera. 2008;324:784–790. doi: 10.1124/jpet.107.128249. [DOI] [PubMed] [Google Scholar]
- 39.Naylor RW, Jones EA. Notch activates Wnt-4 signalling to control medio-lateral patterning of the pronephros. Development. 2009;136:3585–3595. doi: 10.1242/dev.042606. [DOI] [PubMed] [Google Scholar]
- 40.McCright B. Notch signaling in kidney development. Curr Opin Nephrol Hypertens. 2003;12:5–10. doi: 10.1097/00041552-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Boyle SC, Kim M, Valerius MT, McMahon AP, Kopan R. Notch pathway activation can replace the requirement for Wnt4 and Wnt9b in mesenchymal-to-epithelial transition of nephron stem cells. Development. 2011;138:4245–4254. doi: 10.1242/dev.070433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng HT, Kopan R. The role of Notch signaling in specification of podocyte and proximal tubules within the developing mouse kidney. Kidney Int. 2005;68:1951–1952. doi: 10.1111/j.1523-1755.2005.00627.x. [DOI] [PubMed] [Google Scholar]
- 43.Kopan R, Cheng HT, Surendran K. Molecular insights into segmentation along the proximal-distal axis of the nephron. J Am Soc Nephrol. 2007;18:2014–2020. doi: 10.1681/ASN.2007040453. [DOI] [PMC free article] [PubMed] [Google Scholar]