Abstract
Phosphate plays many essential roles in our body. To accomplish these functions, serum phosphate needs to be maintained in a certain range. Serum phosphate level is regulated by intestinal phosphate absorption, renal phosphate handling and equilibrium of extracellular phosphate with that in bone or intracellular fluid. Several hormones such as parathyroid hormone, 1,25-dihydroxyvitamin D (1,25(OH)2D) and fibroblast growth factor 23 (FGF23) regulate serum phosphate by modulating intestinal phosphate absorption, renal phosphate reabsorption and/or bone metabolism. In addition, dietary phosphate rapidly enhances renal phosphate excretion, although detailed mechanisms of this adaptation remain to be clarified. Physiologically, extracellular concentrations of phosphate and these hormones are maintained by several negative feedback loops. For example, 1,25(OH)2D enhances FGF23 production and FGF23 reduces 1,25(OH)2D level. In addition, phosphate affects 1,25(OH)2D and FGF23 levels. Dysfunction of these negative feedback loops results in several diseases with abnormal phosphate and 1,25(OH)2D levels. Especially, excess actions of FGF23 cause several hypophosphatemic rickets/osteomalacia with relatively low level of 1,25(OH)2D that had been classified as vitamin D-resistant rickets/osteomalacia. In contrast, deficient actions of FGF23 cause hyperphosphatemic familial tumoral calcinosis. However, there still remain several unanswered questions regarding phosphate and vitamin D metabolism.
Introduction
Phosphate plays several essential roles in our body.1 Phosphate is necessary for proper mineralization of bone as a constituent of hydroxyapatite crystal. In addition, several phosphorylated proteins like osteopontin and dentin matrix protein 1 (DMP1) have been shown to regulate bone mineralization.2 Phosphate is also a constituent of biomembranes and nucleic acids. Furthermore, many phosphorylated metabolites such as adenosine triphosphate, 2,3-diphosphoglycerate, glucose-6-phosphate and phosphorylated proteins are necessary for diverse actions of all cells such as energy metabolism, differentiation, proliferation and specific function of differentiated cells. In order to accomplish at least some of these functions, it seems to be necessary that concentration of extracellular phosphate is maintained in a certain range. Actually, hypophosphatemia can cause several abnormalities like muscle weakness, rhabdomyolysis, consciousness disturbance and rickets/osteomalacia characterized by impaired mineralization of bone matrix. On the contrary, hyperphosphatemia can result in ectopic calcification. Although it is not entirely clear how intracellular phosphate level is regulated, extracellular phosphate seems to affect it to a certain degree as hypophosphatemia is known to induce tissue hypoxia by lowering 2,3-diphosphoglycerate level in red blood cells.3 Serum phosphate level is regulated by several hormones including parathyroid hormone (PTH), 1,25-dihydroxyvitamin D (1,25(OH)2D) and fibroblast growth factor 23 (FGF23). In this review, regulatory mechanisms of serum phosphate and disorders of phosphate and vitamin D metabolism are summarized with emphasis on the contribution of FGF23.
Phosphate metabolism
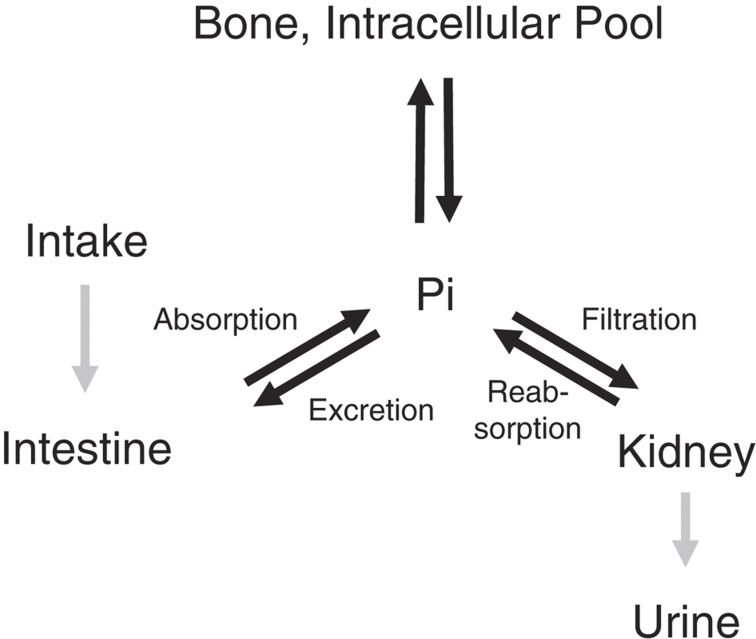
There are several hundred grams of phosphate in adult human body. Approximately 85% of phosphate is present in bone or teeth as hydroxyapatite and ∼15% is present within cells. Therefore, extracellular inorganic phosphate is <1% of total phosphate.4 Serum phosphate is maintained by intestinal phosphate absorption, renal phosphate handling and equilibrium of extracellular phosphate with that in bone or intracellular fluid (Figure 1). In healthy adults, several hundred milligrams of phosphate are daily absorbed in intestine and nearly the same amount is excreted into urine, thereby maintaining phosphate balance.1 It is not clear how this balance is maintained. Although intestinal phosphate was reported to rapidly modulate renal phosphate handling,5 the responsible signals for this regulation of renal phosphate handling are unidentified. In addition, there is a movement of several hundred milligrams of phosphate per day between extracellular fluid and intracellular pool or bone (Figure 1). Phosphate shift into cells is enhanced by insulin and respiratory alkalosis, and occurs within minutes to hours. Respiratory alkalosis is considered to enhance glycolysis by increasing intracellular pH and cause uptake of phosphate by cells. This shift of phosphate into cells is less evident in metabolic alkalosis. In contrast, serum phosphate level is mainly regulated by renal handling of phosphate in a chronic state. In renal proximal tubules, 80–90% of phosphate filtered through glomeruli is reabsorbed by type 2a and 2c sodium–phosphate cotransporters.6 These cotransporters are expressed in brush border membrane of proximal tubular cells. The expression levels rather than the activity of the expressed transporters are considered to regulate proximal tubular phosphate reabsorption.
Figure 1.
Phosphate homeostasis. Serum phosphate is maintained by intestinal phosphate absorption, renal phosphate handling and equilibrium of phosphate between extracellular fluid and phosphate in bone or intracellular pool. PTH, 1,25(OH)2D and FGF23 regulate serum phosphate by modulating intestinal phosphate absorption, renal phosphate reabsorption and/or bone metabolism. Pi, inorganic phosphate.
Several studies indicate that there is a circadian rhythm of serum phosphate.7,8 Phosphate peak is observed after midnight, between 0200 and 0400 h, and the lowest phosphate is seen between 0800 and 1000 h. Because prolonged fasting abolished nocturnal peak of phosphate,7,8 intestinal phosphate absorption contributes to this circadian change of serum phosphate. However, changes of PTH, growth hormone, 1,25(OH)2D or FGF23 cannot fully explain the circadian rhythm of phosphate and the mechanism of this diurnal variation remains to be clarified.7,9,10
Regulatory mechanisms of serum phosphate level
PTH
Several hormones regulate serum phosphate levels. PTH and 1,25(OH)2D are considered to be calciotropic hormones. Ionized Ca concentration is the primary determinant of PTH secretion in subjects with normal renal function. Ionized extracellular Ca modulates PTH secretion and synthesis through Ca-sensing receptor.11 However, phosphate also modulates PTH synthesis especially in patients with chronic kidney disease. It was shown that phosphate enhances PTH production by posttranscriptionally stabilizing PTH mRNA.12 PTH reduces serum phosphate by decreasing the abundance of type 2a and 2c sodium–phosphate cotransporters by promoting their internalization in proximal tubular cells.13 Therefore, there seems to be a negative feedback between serum phosphate and PTH production (Figure 2), although it is not clear whether this feedback is important in subjects with normal renal function. PTH also enhances production of 1,25(OH)2D and FGF23 that can regulate serum phosphate as shown below.14,15,16
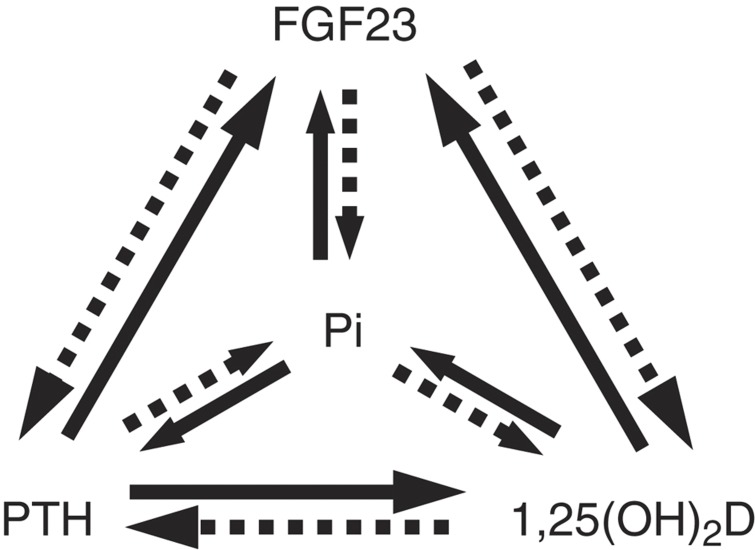
Figure 2.
Negative feedback loops regulating serum phosphate level. PTH, 1,25(OH)2D and FGF23 reciprocally regulate their own synthesis and modulate serum phosphate level. In contrast, serum phosphate or phosphate load can regulate production of PTH, 1,25(OH)2D and FGF23. Solid lines indicate stimulation of production or increase in serum level. Broken lines mean inhibition of production or decrease in serum level. PTH and 1,25(OH)2D are also regulated by serum calcium (not shown in the figure).
1,25(OH)2D
Intestinal phosphate absorption is mediated by both transcellular and paracellular routes. The 1,25(OH)2D increases intestinal transcellular phosphate absorption at least in part by enhancing expression of type 2b sodium–phosphate cotransporter.17 Although vitamin D-responsive elements were reported in human genes encoding type 2a and 2c sodium–phosphate cotransporters,18 1,25(OH)2D was shown to rather decrease expression of renal sodium–phosphate cotransporter in a certain experimental condition.19 In addition, phosphate deprivation enhanced expression of type 2a sodium–phosphate cotransporter in the kidneys of both wild-type and vitamin D receptor-null mice.20 Therefore, it has not been established whether 1,25(OH)2D has a physiologically essential role in the regulation of renal phosphate reabsorption. The 1,25(OH)2D works to increase serum phosphate mainly by enhancing intestinal phosphate absorption and phosphate suppresses 1,25(OH)2D production.21,22,23,24 Therefore, there is another negative feedback system between phosphate and 1,25(OH)2D. The 1,25(OH)2D also suppresses synthesis of PTH and enhances FGF23 production.25,26
FGF23
FGF23 is produced by osteocytes and osteoblasts. Human FGF23 produces a peptide with 251 amino acids. There is a signal peptide with N-terminal 24 amino acids and secreted full-length FGF23 is considered to comprise 227 amino acids.27 FGF23 has a FGF homology region with β-trefoil structure as other members of FGF family in its N-terminal portion. There are 22 members of FGF family and these FGF family members are divided into several subfamilies. FGF23 belongs to FGF19 subfamily together with FGF19 and FGF21.28 A part of FGF23 protein is proteolytically cleaved between 179Arg and 180Ser before or during the process of secretion. The processed N-terminal and C-terminal fragments do not have activities shown below.29
Currently, several assays are available to evaluate FGF23 levels. Intact assays use two kinds of antibodies that recognize N-terminal and C-terminal portion of the processing site of FGF23, respectively, and recognize only full-length, biologically active FGF23.30 In contrast, C-terminal assay detects both full-length and processed C-terminal fragment of FGF23.31 The performance of these assays is not the same,32,33 and these assays can produce completely discrepant results in some particular cases.34 Therefore, results obtained by one assay may not be always reproducible by other assays.
FGF23 is considered to bind to FGF receptor–Klotho complex to exert its function.35,36 FGF23 suppresses the expression of type 2a and 2c sodium–phosphate cotransporters and thereby inhibits proximal tubular phosphate reabsorption.37 FGF23 also reduces the expression of CYP27B1 that encodes 25-hydoxyvitamin D[25(OH)D]-1α-hydroxylase and enhances CYP24 expression that produces 25(OH)D-24-hydroxylase.37 By these actions, FGF23 reduces circulatory level of 1,25(OH)2D. Several studies indicated that 1,25(OH)2D enhances FGF23 production.25,26 In addition, oral phosphate administration increases FGF23 level in both humans and animals.38,39 However, it has not been shown that phosphate directly modulates FGF23 expression so far. It was reported that increase or decrease of serum phosphate did not alter FGF23 levels during several hours, indicating that there is no acute regulation of FGF23 by phosphate.40 On the other hand, FGF23 levels are low in patients with chronic hypophosphatemia such as Fanconi syndrome,41 and it was shown that serum phosphate can regulate FGF23 level.39 These results suggest that phosphate chronically regulates FGF23 production. In addition, FGF23 was shown to inhibit both production and secretion of PTH.42
Therefore, there are multiple negative feedback loops involving 1,25(OH)2D, PTH, FGF23 and phosphate (Figure 2). Furthermore, as calciotropic hormones, 1,25(OH)2D and PTH levels are also regulated by serum Ca and it is suggested that Ca enhances FGF23 production.43 Although there remain several unanswered questions regarding the regulatory mechanisms of the production of these hormones, serum phosphate level seems to be maintained at least in part by these feedback loops.
Other regulators
In addition to these hormones, glucocorticoid suppresses and growth hormone enhances renal phosphate reabsorption, respectively, by modulating expression of sodium–phosphate cotransporters.44,45 Furthermore, intestinal phosphate absorption, renal phosphate handling and shift of phosphate can happen independently of these hormone actions. For example, ingestion of a large amount of phosphate can increase serum phosphate by increased phosphate absorption through paracellular route. In addition, proximal tubular damage can cause phosphaturia and hypophosphatemia as seen in patients with Fanconi syndrome. Therefore, although there is a hormonal negative feedback system that maintains serum phosphate in a certain range, dysregulation of this feedback system as well as hormone-independent deranged phosphate handling in several organs can result in abnormal phosphate levels.
Regulation of vitamin D metabolism by phosphate and FGF23
Conversion of 25(OH)D to 1,25(OH)2D is the key step in the production of 1,25(OH)2D and is tightly regulated by several factors. It is well known that PTH enhances 1,25(OH)2D synthesis and 1,25(OH)2D itself inhibits its own production.46 In addition, although the detailed molecular mechanism is not clear, it was shown that phosphate depletion enhances and conversely hyperphosphatemia suppresses 1,25(OH)2D production.21,22,23,24 Furthermore, hypercalcemia reduces 1,25(OH)2D synthesis.47
Klotho mice were created by transgenic method and show severely reduced expression of Klotho.48 Klotho and FGF23-null mice show similar phenotypes such as hyperphosphatemia, high 1,25(OH)2D level and ectopic calcification.48,49 These similar phenotypes can be explained by the findings that Klotho works as a coreceptor for FGF23.35,36 These mice provide unique opportunity to consider the regulatory mechanism of 1,25(OH)2D production. Namely, these mice show hyperphosphatemia, hypercalcemia and low PTH. All these factors are expected to suppress 1,25(OH)2D production. However, these mice show quite high level of 1,25(OH)2D and enhanced expression of CYP27B1.49,50 These results indicate that the suppressive action of FGF23 is dominant over inhibitory effects of hyperphosphatemia, hypercalcemia and low PTH on 1,25(OH)2D production. Therefore, deficient action of FGF23 causes hyperphosphatemic diseases with high 1,25(OH)2D levels as observed in Klotho and FGF23-null mice. Conversely, excess action of FGF23 results in hypophosphatemic diseases with relatively low 1,25(OH)2D as shown below.
Disorders of phosphate and vitamin D metabolism
Vitamin D was discovered as an antirachitic factor. However, it was known that vitamin D cannot cure all kinds of rickets. Vitamin D-resistant rickets is a somewhat ambiguous name. It can indicate rickets that is not cured by native vitamin D. In this sense, vitamin D-resistant rickets can include various diseases such as vitamin D-dependent rickets type 2 caused by inactivating mutations in vitamin D receptor in addition to X-linked hypophosphatemic rickets (XLHR).51 On the other hand, vitamin D-resistant rickets is sometimes used as a synonym for XLHR partly because this disease is the most frequent cause of vitamin D-resistant rickets.52 However, since the identification of FGF23, several kinds of rickets with similar clinical and biochemical features to XLHR were shown to be caused by excess actions of FGF23.
Responsible genes for XLHR, autosomal dominant hypophosphatemic rickets and autosomal recessive hypophosphatemic rickets 1 and 2 are phosphate-regulating gene with homologies to endopeptidases on the X chromosome (PHEX), FGF23, dentin matrix protein 1 (DMP1) and ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1), respectively.53 These types of hypophosphatemic rickets are characterized by impaired renal tubular phosphate reabsorption, low to normal 1,25(OH)2D and high circulatory FGF23 levels. Measurement of FGF23 was shown to be useful for the differential diagnosis of these FGF23-related hypophosphatemic diseases and other causes of chronic hypophosphatemia.41 Although overexpression of FGF23 in bone is considered to cause high FGF23 in these diseases, it is largely unknown how mutations in these genes result in enhanced production of FGF23. In addition to these congenital hypophosphatemic rickets, hypophosphatemic rickets/osteomalacia associated with McCune–Albright syndrome/fibrous dysplasia, tumor-induced osteomalacia and hypophosphatemic disease caused by intravenous administration of saccharated ferric oxide or iron polymaltose are also caused by excess actions of FGF23 and associated with elevated FGF23.53 Recently, mutations in family with sequence similarity 20, member C (FAM20C) were shown to cause hypophosphatemic disease with high FGF23 levels.54 Inactivating mutations in FAM20C have been known to cause Raine syndrome characterized by osteosclerotic bone dysplasia.55 Although Raine syndrome was originally reported to be a lethal disease,55 it became clear that there are some surviving patients.56 In addition, mice that lack FAM20C were shown to present hypophosphatemic rickets with high FGF23.57 A patient with compound heterozygous mutations in FAM20C was reported to show hypophosphatemia and osteosclerosis without rickets.54 Therefore, dysfunction of FAM20C seems to cause different phenotypes in mice and humans. However, these results suggest that FAM20C is involved in both mineralization of bone and regulation of FGF23 production.
In contrast to these hypophosphatemic diseases, deficient action of FGF23 causes hyperphosphatemic familial tumoral calcinosis characterized by enhanced tubular phosphate reabsorption, high 1,25(OH)2D and ectopic calcification. Three genes, UDP-N-acetyl-alpha-D- galactosamine:polypeptide N-acetylgalactosaminyltransferase 3 (GALNT3), FGF23 and Klotho, were identified as responsible genes for hyperphosphatemic familial tumoral calcinosis.53 Of these, mutations in GALNT3 and FGF23 cause impaired secretion of full-length FGF23 and low circulatory level of full-length FGF23. In contrast, a mutation in Klotho was reported to result in resistance to FGF23 and this patient showed high full-length FGF23 level. These diseases caused by aberrant actions of FGF23 indicate that FGF23 is a physiological regulator of vitamin D and phosphate metabolism.
Conclusion
The 1,25(OH)2D can regulate serum phosphate level either directly or indirectly through modulating expression of FGF23 as well as working as a calciotropic hormone. Therefore, phosphate and vitamin D metabolism are highly interconnected. However, there still remain several important unanswered questions. Especially, we do not know the detailed mechanisms by which phosphate regulates production of 1,25(OH)2D and FGF23. In addition, it is not clear either how phosphate is sensed in our body. Still, recent findings concerning phosphate metabolism and diseases with abnormal phosphate levels created new perspective of vitamin D metabolism and actions.
Footnotes
The author declares no conflict of interest.
References
- Berndt T, Kumar R. Phosphatonins and the regulation of phosphate homeostasis. Annu Rev Physiol 2007;69:341–359. [DOI] [PubMed] [Google Scholar]
- George A, Veis A. Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chem Rev 2008;108:4670–4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian R, Khardori R. Severe hypophosphatemia. Pathophysiologic implications, clinical presentations, and treatment. Medicine (Baltimore) 2000;79:1–8. [DOI] [PubMed] [Google Scholar]
- Penido MG, Alon US. Phosphate homeostasis and its role in bone health. Pediatr Nephrol 2012;27:2039–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt T, Thomas LF, Craig TA, Sommer S, Li X, Bergstralh EJ et al. Evidence for a signaling axis by which intestinal phosphate rapidly modulates renal phosphate reabsorption. Proc Natl Acad Sci USA 2007;104:11085–11090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatschek S, Haller M, Oberbauer R. Renal phosphate handling in human--what can we learn from hereditary hypophosphataemias? Eur J Clin Invest 2010;40:552–560. [DOI] [PubMed] [Google Scholar]
- Jubiz W, Canterbury JM, Reiss E, Tyler FH. Circadian rhythm in serum parathyroid hormone concentration in human subjects: correlation with serum calcium, phosphate, albumin, and growth hormone levels. J Clin Invest 1972;51:2040–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker GJ, Walker RG, Hewitson TD, Pedagogos E. Phosphate levels--time for a rethink? Nephrol Dial Transplant 2009;24:2321–2324. [DOI] [PubMed] [Google Scholar]
- Carpenter TO, Insogna KL, Zhang JH, Ellis B, Nieman S, Simpson C et al. Circulating levels of soluble klotho and FGF23 in X-linked hypophosphatemia: circadian variance, effects of treatment, and relationship to parathyroid status. J Clin Endocrinol Metab 2010;95:E352–E357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halloran BP, Portale AA, Castro M, Morris RC Jr, Goldsmith RS. Serum concentration of 1,25-dihydroxyvitamin D in the human: diurnal variation. J Clin Endocrinol Metab 1985;60:1104–1110. [DOI] [PubMed] [Google Scholar]
- Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev 2001;81:239–297. [DOI] [PubMed] [Google Scholar]
- Silver J, Naveh-Many T. Phosphate and the parathyroid. Kidney Int. 2009;75:898–905. [DOI] [PubMed] [Google Scholar]
- Lanzano L, Lei T, Okamura K, Giral H, Caldas Y, Masihzadeh O et al. Differential modulation of the molecular dynamics of the type IIa and IIc sodium phosphate cotransporters by parathyroid hormone. Am J Physiol Cell Physiol 2011;301:C850–C861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett-Bowie SM, Henao MP, Dere ME, Lee H, Leder BZ. Effects of hPTH(1-34) infusion on circulating serum phosphate, 1,25-Dihydroxyvitamin D and FGF23 levels in healthy men. J Bone Miner Res 2009;24:1681–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawata T, Imanishi Y, Kobayashi K, Miki T, Arnold A, Inaba M et al. Parathyroid hormone regulates fibroblast growth factor-23 in a mouse model of primary hyperparathyroidism. J Am Soc Nephrol 2007;18:2683–2688. [DOI] [PubMed] [Google Scholar]
- Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, Naveh-Many T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol 2010;299:F882–F889. [DOI] [PubMed] [Google Scholar]
- Xu H, Bai L, Collins JF, Ghishan FK. Age-dependent regulation of rat intestinal type IIb sodium-phosphate cotransporter by 1,25-(OH)(2) vitamin D(3). Am J Physiol Cell Physiol 2002;282:C487–C493. [DOI] [PubMed] [Google Scholar]
- Kido S, Kaneko I, Tatsumi S, Segawa H, Miyamoto K. Vitamin D and type II sodium-dependent phosphate cotransporters. Contrib Nephrol 2013;180:86–97. [DOI] [PubMed] [Google Scholar]
- Friedlaender MM, Wald H, Dranitzki-Elhalel M, Zajicek HK, Levi M, Popovtzer MM. Vitamin D reduces renal NaPi-2 in PTH-infused rats: complexity of vitamin D action on renal P(i) handling. Am J Physiol Renal Physiol 2001;281:F428–F433. [DOI] [PubMed] [Google Scholar]
- Capuano P, Radanovic T, Wagner CA, Bacic D, Kato S, Uchiyama Y et al. Intestinal and renal adaptation to a low-Pi diet of type II NaPi cotransporters in vitamin D receptor- and 1alphaOHase-deficient mice. Am J Physiol Cell Physiol 2005;288:C429–C434. [DOI] [PubMed] [Google Scholar]
- Baxter LA, DeLuca HF. Stimulation of 25-hydroxyvitamin D3-1alpha-hydroxylase by phosphate depletion. J Biol Chem 1976;251:3158–3161. [PubMed] [Google Scholar]
- Hughes MR, Brumbaugh PF, Hussler MR, Wergedal JE, Baylink DJ. Regulation of serum 1alpha,25-dihydroxyvitamin D3 by calcium and phosphate in the rat. Science 1975;190:578–580. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Deluca HF. The control of 25-hydroxyvitamin D metabolism by inorganic phosphorus. Arch Biochem Biophys 1973;154:566–574. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Yoshida N, Monkawa T, Hayashi M, Saruta T. Dietary phosphorus deprivation induces 25-hydroxyvitamin D(3) 1alpha-hydroxylase gene expression. Endocrinology 2001;142:1720–1726. [DOI] [PubMed] [Google Scholar]
- Collins MT, Lindsay JR, Jain A, Kelly MH, Cutler CM, Weinstein LS et al. Fibroblast growth factor-23 is regulated by 1alpha,25-dihydroxyvitamin D. J Bone Miner Res 2005;20:1944–1950. [DOI] [PubMed] [Google Scholar]
- Saito H, Maeda A, Ohtomo S, Hirata M, Kusano K, Kato S et al. Circulating FGF-23 is regulated by 1alpha,25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem 2005;280:2543–2549. [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Martin TJ. Bone as an endocrine organ. Trends Endocrinol Metab 2009;20:230–236. [DOI] [PubMed] [Google Scholar]
- Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genet 2004;20:563–569. [DOI] [PubMed] [Google Scholar]
- Shimada T, Muto T, Urakawa I, Yoneya T, Yamazaki Y, Okawa K et al. Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology 2002;143:3179–3182. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T et al. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab 2002;87:4957–4960. [DOI] [PubMed] [Google Scholar]
- Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med 2003;348:1656–1663. [DOI] [PubMed] [Google Scholar]
- Imel EA, Peacock M, Pitukcheewanont P, Heller HJ, Ward LM, Shulman D et al. Sensitivity of fibroblast growth factor 23 measurements in tumor-induced osteomalacia. J Clin Endocrinol Metab 2006;91:2055–2061. [DOI] [PubMed] [Google Scholar]
- Ito N, Fukumoto S, Takeuchi Y, Yasuda T, Hasegawa Y, Takemoto F et al. Comparison of two assays for fibroblast growth factor (FGF)-23. J Bone Miner Metab 2005;23:435–440. [DOI] [PubMed] [Google Scholar]
- Frishberg Y, Ito N, Rinat C, Yamazaki Y, Feinstein S, Urakawa I et al. Hyperostosis-hyperphosphatemia syndrome: a congenital disorder of O-glycosylation associated with augmented processing of fibroblast growth factor 23. J Bone Miner Res 2007;22:235–242. [DOI] [PubMed] [Google Scholar]
- Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 2006;444:770–774. [DOI] [PubMed] [Google Scholar]
- Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 2006;281:6120–6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 2004;19:429–435. [DOI] [PubMed] [Google Scholar]
- Ferrari SL, Bonjour JP, Rizzoli R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab 2005;90:1519–1524. [DOI] [PubMed] [Google Scholar]
- Perwad F, Azam N, Zhang MY, Yamashita T, Tenenhouse HS, Portale AA. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology 2005;146:5358–5364. [DOI] [PubMed] [Google Scholar]
- Ito N, Fukumoto S, Takeuchi Y, Takeda S, Suzuki H, Yamashita T et al. Effect of acute changes of serum phosphate on fibroblast growth factor (FGF)23 levels in humans. J Bone Miner Metab 2007;25:419–422. [DOI] [PubMed] [Google Scholar]
- Endo I, Fukumoto S, Ozono K, Namba N, Tanaka H, Inoue D et al. Clinical usefulness of measurement of fibroblast growth factor 23 (FGF23) in hypophosphatemic patients: proposal of diagnostic criteria using FGF23 measurement. Bone 2008;42:1235–1239. [DOI] [PubMed] [Google Scholar]
- Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest 2007;117:4003–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Yamazaki Y, Takahashi M, Hasegawa H, Urakawa I, Oshima T et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol 2005;289:F1088–F1095. [DOI] [PubMed] [Google Scholar]
- Loffing J, Lotscher M, Kaissling B, Biber J, Murer H, Seikaly M et al. Renal Na/H exchanger NHE-3 and Na-PO4 cotransporter NaPi-2 protein expression in glucocorticoid excess and deficient states. J Am Soc Nephrol 1998;9:1560–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woda CB, Halaihel N, Wilson PV, Haramati A, Levi M, Mulroney SE. Regulation of renal NaPi-2 expression and tubular phosphate reabsorption by growth hormone in the juvenile rat. Am J Physiol Renal Physiol 2004;287:F117–F123. [DOI] [PubMed] [Google Scholar]
- Murayama A, Takeyama K, Kitanaka S, Kodera Y, Kawaguchi Y, Hosoya T et al. Positive and negative regulations of the renal 25-hydroxyvitamin D3 1alpha-hydroxylase gene by parathyroid hormone, calcitonin, and 1alpha,25(OH)2D3 in intact animals. Endocrinology 1999;140:2224–2231. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Ikeda K, Morita K, Fukumoto S, Takahashi H, Ogata E. Blood Ca2+ modulates responsiveness of renal 25(OH)D3-1 alpha-hydroxylase to PTH in rats. Am J Physiol 1987;253:E503–E507. [DOI] [PubMed] [Google Scholar]
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997;390:45–51. [DOI] [PubMed] [Google Scholar]
- Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 2004;113:561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Fujimori T, Nabeshima Y. Mediation of unusually high concentrations of 1,25-dihydroxyvitamin D in homozygous klotho mutant mice by increased expression of renal 1alpha-hydroxylase gene. Endocrinology 2002;143:683–689. [DOI] [PubMed] [Google Scholar]
- Liberman UA, Eil C, Marx SJ. Clinical features of hereditary resistance to 1,25-dihydroxyvitamin D (hereditary hypocalcemic vitamin D resistant rickets type II). Adv Exp Med Biol 1986;196:391–406. [DOI] [PubMed] [Google Scholar]
- Levine BS, Kleeman CR, Felsenfeld AJ. The journey from vitamin D-resistant rickets to the regulation of renal phosphate transport. Clin J Am Soc Nephrol 2009;4:1866–1877. [DOI] [PubMed] [Google Scholar]
- Hori M, Shimizu Y, Fukumoto S. Minireview: fibroblast growth factor 23 in phosphate homeostasis and bone metabolism. Endocrinology 2011;152:4–10. [DOI] [PubMed] [Google Scholar]
- Rafaelsen SH, Raeder H, Fagerheim AK, Knappskog P, Carpenter TO, Johansson S et al. Exome sequencing reveals FAM20c mutations associated with fibroblast growth factor 23-related hypophosphatemia, dental anomalies, and ectopic calcification. J Bone Miner Res 2013;28:1378–1385. [DOI] [PubMed] [Google Scholar]
- Simpson MA, Hsu R, Keir LS, Hao J, Sivapalan G, Ernst LM et al. Mutations in FAM20C are associated with lethal osteosclerotic bone dysplasia (Raine syndrome), highlighting a crucial molecule in bone development. Am J Hum Genet 2007;81:906–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson MA, Scheuerle A, Hurst J, Patton MA, Stewart H, Crosby AH. Mutations in FAM20C also identified in non-lethal osteosclerotic bone dysplasia. Clin Genet 2009;75:271–276. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang S, Li C, Gao T, Liu Y, Rangiani A et al. Inactivation of a novel FGF23 regulator, FAM20C, leads to hypophosphatemic rickets in mice. PLoS Genet 2012;8:e1002708. [DOI] [PMC free article] [PubMed] [Google Scholar]