Abstract
The need for an intact immune system for cancer radiation therapy to be effective suggests that radiation not only acts directly on the tumor but also indirectly, through the activation of host immune components. Recent studies demonstrated that endogenous type I interferons (type I IFNs) play a role in radiation-mediated anti-tumor immunity by enhancing the ability of dendritic cells to cross-prime CD8+ T cells. However, it is still unclear to what extent endogenous type I IFNs contribute to the recruitment and function of CD8+ T cells. Little is also known about the effects of type I IFNs on myeloid cells. In the current study, we demonstrate that type I and type II IFNs (IFN-γ) are both required for the increased production of CXCL10 (IP-10) chemokine by myeloid cells within the tumor after radiation treatment. Radiation-induced intratumoral IP-10 levels in turn correlate with tumor-infiltrating CD8+ T cell numbers. Moreover, type I IFNs promote potent tumor-reactive CD8+ T cells by directly affecting the phenotype, effector molecule production, and enhancing cytolytic activity. Using a unique inducible expression system to increase local levels of IFN-α exogenously, we show here that the capacity of radiation therapy to result in tumor control can be enhanced. Our preclinical approach to study the effects of local increase in IFN-α levels can be used to further optimize the combination therapy strategy in terms of dosing and scheduling, which may lead to better clinical outcome.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-013-1506-7) contains supplementary material, which is available to authorized users.
Keywords: Radiation therapy, Interferon, B16 melanoma, CXCR3, CD8+ T cells
Introduction
Ionizing radiation has been used for the treatment for cancer for more than 100 years. Although radiation therapy (RT) is best known to directly kill tumor cells by the induction of lethal DNA damage, it is now appreciated that RT induces an anti-tumor immune response that is essential for its therapeutic effects including tumor growth delay, tumor eradication, and even long-term tumor-free survival. Since the 1940s, patients have been treated with low dose (typically 1–3 Gy) of daily fractions of radiation delivered over weeks or months [1]. However, with the advent of improved tumor imaging technology and highly targeted radiation delivery systems, there has been increasing interest in the use of hypofractionated, high-dose RT, also known as stereotactic body radiation therapy (SBRT). Despite promising data from many clinical trials that demonstrated the effectiveness of SBRT in a variety of cancers [2–5], the mechanisms by which the treatment reduces tumor growth are still unclear. This lack of understanding is currently a limiting factor in optimizing the treatment strategy in the clinic.
We previously reported, using the murine melanoma B16-OVA model, that the frequency and number of CD8+ T cells increased after RT [6]. In a similar model (B16-SIY), the depletion of CD8+ T cells abrogated the ability of RT to delay tumor growth [7]. Data from our laboratory and other investigators suggested that RT causes the release of tumor antigens and “danger signals” [8], which enhance the ability of dendritic cells (DCs) both in tumor-draining lymph nodes (tdLN) [6] as well as in tumors [9], to present antigens. These effects are in turn required for priming of antigen-specific T cells, and the resulting T cell proliferation, activation, cytokine release, and cytolytic activity cause effective killing of tumor cells.
Soon after its discovery as an anti-viral factor, exogenous type I IFNs were shown to exhibit anti-tumor effects. IFN-α has been FDA-approved for the treatment for various types of cancer since 1997 [10, 11]. In the last decade, studies revealed that endogenous type I IFNs play a critical role in tumor growth control by the host immune system [12–14]. More recently, type I IFNs were implicated in radiation-mediated delay of tumor growth. Although it is well established that type I IFNs have direct anti-proliferative effects, the main target of RT-induced type I IFNs is the hematopoietic compartment [9]. Strikingly, deficiency in type I IFN signaling abrogated the capacity of DCs to cross-prime antigens, which may result in less efficient priming of CD8+ T cell-mediated anti-tumor responses. However, type I IFNs may affect CD8+ T cells in other ways, for example, by providing “signal 3” that T cells require to acquire full effector functions [15]. These possible direct effects of type I IFNs on T cells are still unclear. Our current study reveals that radiation-induced type I IFNs play a role in increasing CXCR3 chemokine levels within the tumor, which affects the initial recruitment of immune cells into the tumor microenvironment. In addition, type I IFNs are also required for CD8+ T cells to acquire an activated phenotype and tumor cell-killing activity, at least partly through direct effects of type I IFNs on T cells. Using an inducible expression system, we show that increasing intratumoral IFN-α levels exogenously after RT can further improve the ability of the therapy to bring about tumor control. Therefore, SBRT is a moderately effective therapy that activates immune responses against tumor, and better clinical outcomes can be achieved by using SBRT in combination with immunotherapies like exogenous IFN-α.
Materials and methods
Mice
C57BL/6J wild-type (WT), B6.129S7-Ifngtm1ts (IFN-γKO), B6.129P2-Cxcr3 tm1Dgen/J (CXCR3 KO), and B6-SJL-PtpreaPepeb/BoyJ (B6-SJL) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Breeding pairs of IFN-α/β receptor-deficient (IFNABRKO) mice in the C57BL/6 background were kindly provided by T. Randall and F. Lund (University of Alabama, Birmingham, AL). These transgenic mice were bred in the animal facility at the University of Rochester, where all other mice were also housed. Mice used for experiments were 6–12 weeks old. Guidelines for the humane treatment of animals were followed, and experiments were performed using protocols approved by the University Committee on Animal Resources.
Tumor cell lines and animal models
B16-F0, a cell line derived from the spontaneous melanoma of a C57BL/6 mouse, was obtained from the American Type Culture Collection (CRL 6322). E0771 murine breast cancer cell line was kindly provided by Edward Brown (University of Rochester, Rochester, NY). Cells were maintained as previously described [16]. On the day of tumor cell implantation, tumor cells were washed with saline and injected intramuscularly into the lower left thigh of mice at 1 × 105 cells per mouse. Tumor growth was monitored by measuring thigh diameters of mice using digital calipers and expressing the values as mean thigh diameter, as described before [16].
Local single high-dose radiation treatment
In all experiments, mice received a single local dose of 15 Gy on day 7 of post-tumor implantation. Irradiation was performed on nonanesthetized mice using a 137Cs source as previously described [6].
Flow cytometry and cell sorting
Tumors were excised from mice, mechanically cut into smaller pieces, digested with collagenase as previously described [17], and single-cell suspensions were incubated with LIVE/DEAD® aqua dead cell stain (Invitrogen, Grand Island, NY) before surface staining. Antibodies to mouse CD3 (145-2C11), CD4 (GK1.5), CD8 (Ly-2, 53-6.7), CD16/CD32 (Fc Block) (2.4G2), CD45 (30-F11), CD45.1 (A20), CD45.2 (104), Gr-1 (RB6/8C5), CD11b (M1/70), CD11c (HL3), NK1.1 (PK136), H-2 Kb (AF6-88.5), CD69 (H1.2F3), Ly6C (AL-21), F4/80 (BM8), and isotype controls were from BD Biosciences (San Jose, CA) and eBioscience (San Diego, CA). For intracellular staining of granzyme B, surface-stained cells were fixed and permeabilized using Foxp3/Transcription Factor Staining Buffer Set (eBioscience, San Diego, CA) before incubation with anti-granzyme B antibody (NGZB, eBioscience, San Diego, CA).
ELISA
Tumor pieces were snap-frozen in lysis buffer [18] containing protease inhibitors (BioVision, Milpitas, CA). Samples were thawed on ice, processed using a motorized tissue homogenizer (PRO Scientific, Oxford, CT), and cell debris was pelleted by centrifugation. Supernatants from the samples were used for protein analysis. ELISA kits for murine IFN-α from PBL InterferonSource (Piscataway, NJ), IFN-γ from eBioscience, and IP-10 from Peprotech (Rocky Hill, NJ) were used according to the manufacturers’ protocol. The IFN-α ELISA kit detects all 14 murine IFN-α subtypes and is not cross-reactive with IFN-β. Concentration value of each sample was normalized to the total protein level in the sample, which was quantified using BCA assay (Pierce Biotechnology, Rockford, IL).
Mixed bone marrow chimera
C57BL/6 recipient mice (CD45.2) were given 10 Gy whole body irradiation. Bone marrow cell suspensions were made using tibias and femurs of B6-SJL (CD45.1) and B6-IFNABRKO (CD45.2) mice, and mixed 1:1. 3 × 106 cells from each donor type were injected i.v. into every host mouse. Mice were allowed to reconstitute for at least 35–50 days before tumor cells were inoculated.
In vitro cytotoxicity assay
Tumor-infiltrating lymphocytes (TILs) were purified from collagenase-dissociated tumor suspensions using magnetic beads conjugated to anti-Thy-1 (clone T24/40.7) and used as effector cells. B16 cells were cultured in vitro in the presence of recombinant mouse IFN-γ at 5 ng/ml for 48 h to increase surface expression levels of MHC class I, labeled with 51Cr and used as target cells. Effector and target cells were cocultured in 96-well plates at a range of E:T ratios and 51Cr released by killed target cells into supernatant was measured after 6 h.
Construction of plasmids for inducible expression of IFN-α in B16.F0 cells
Plasmids required for inducible control of IFN-α expression by the rapamycin analog, A/C heterodimerizer, were constructed using vectors from iDimerize™ inducible heterodimer system (Clontech Laboratories, Mountain View, CA). pIRESpuro3 (Clontech Laboratories) was cloned into pHet-Act2-1 (transcription factor plasmid, Online Resource 3a), and successfully transfected B16.F0 cells were selected by the addition of puromycin (1 μg/mL) in the tissue culture medium. Single-cell clones were obtained using limiting dilution cloning method. Murine Ifnα2 DNA was subcloned from pCMV-A-mIFNα2 plasmid (from Dr. Thomas Tüting, University of Bonn, Bonn, Germany) into the pZFHD1-1 (target gene plasmid, Online Resource 3b). B16 clones that had been selected for transcription factor plasmid were subsequently co-transfected with target gene plasmid and pcDNA3.1, which allowed for selection based on G418 resistance. Double-transfected cells were screened for inducibility of IFN-α expression upon A/C heterodimerizer treatment using ELISA. All transfections were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol.
Intravenous administration of A/C heterodimerizer
A/C heterodimerizer (inducer) was purchased in powdered form and reconstituted with N,N-dimethylacetamide (DMA, Sigma-Aldrich) to obtain a 10 mg/mL stock solution. Immediately before injection, the stock solution was further diluted [19] and the resulting 0.5 mg/mL solution was injected i.v. in the tail vein of each mouse so that the final dose of the inducer was 50 μg per animal.
Real-time quantitative PCR (qRT-PCR)
Tumors were excised and pieces were snap-frozen in buffer RLT (QIAGEN). RNA was isolated, and qRT-PCR was performed as described previously [20]. The intron-spanning forward and reverse primers used were designed in-house and obtained from Eurofins MWG Operon. Sequences of primers used are as follows. For GAPDH: Forward, 5′-CATTGCTCTCAATGACAACT-3′, Reverse, 5′-GGGTTTCTTACTCCTTGGAG-3′. For IFN-γ: Forward, 5′-TACTACCTTCTTCAGCAACAG-3′, Reverse, 5′-GATGAGCTCATTGAAT GCTT-3′. For IP-10: Forward, 5′-CCATAGGGAAGCTTGAAATC-3′, Reverse, 5′-GATGGTC TTAGATTCCGGAT-3′. For GranzymeB: Forward, 5′-GGAAGATGAAGATCCTCCTG-3′, Reverse, 5′-ATCGAAAGTAAGGCCATGTA-3′. For FasL: Forward, 5′-CACCAACCAAAGC CTTAAAG-3′, Reverse, 5′-CATATGTGTCTTCCCATTCC-3′. For relative qRT-PCR, all samples were normalized based on GAPDH threshold cycle values and fold changes over a randomly chosen sample in the control group were calculated using formulae by comparative C t method. For absolute qRT-PCR, DNA standards were generated using PCR-amplified cloned target sequences.
Statistical analysis
Multiple group comparisons were done using one-way ANOVA, and p values were adjusted using Bonferroni correction.
Results
Endogenous IFN-α/β is needed to support radiation-mediated anti-tumor immunity
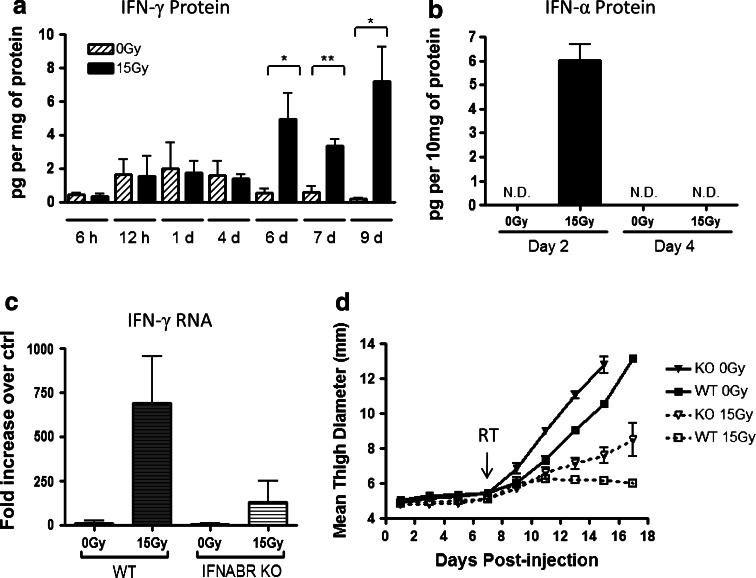
Our laboratory has previously shown that the capacity of RT to reduce tumor growth is partly dependent on the induction of IFN-γ and downstream IFN-γ-inducible genes [17, 21]. Using the intramuscular B16 murine melanoma model in autologous hosts, we treated tumors 7 days after inoculation, with single local high-dose RT of 15 Gy. Untreated tumors had low levels of IFN-γ, which further decreased as tumors grew larger in size. In mice given treatment, a significant increase in radiation-mediated IFN-γ was first detected in tumor homogenates after 6 days and remained elevated even at 9 days post-treatment (Fig. 1a). Intracellular IFN-γ staining identified that a proportion of CD8+ T cells, CD4+ T cells, and NK cells contribute to the production of IFN-γ in B16 tumors, and that the increase in IFN-γ+ cells following RT was greatest among CD8+ T cells (data not shown).
Fig. 1.
Endogenous IFN-α/β receptor signaling plays a role in reducing tumor growth and supporting radiation treatment (RT) efficacy. a, b C57BL/6 mice were injected with 1 × 105 B16 cells intramuscularly (i.m.) in the left thigh. Seven days later, mice were either given 15 Gy local radiation or left untreated. At the indicated time points after RT, mice were killed and tumors were excised. IFN-α and IFN-γ protein levels in the tumor homogenates were determined by ELISA, and values were normalized by total protein in each sample. ND not detectable. c, d C57BL/6 and IFNABR KO mice were injected and treated with RT as described in (a). c Total RNA was isolated from tumors on day 6 post-RT. Relative qRT-PCR analysis was performed as described in Materials and Methods. d Every other day throughout the time course, tumor growth was monitored by measuring mean thigh diameter. Each data point is an average of 4 mice, and the data are representative of two experiments. On day 15, all groups were significantly different by one-way ANOVA comparison with Bonferroni post hoc test
The regulatory mechanism of IFN-γ induction in response to RT is unclear. Since endogenous type I IFNs have been recently implicated in the efficacy of RT and type I IFNs have the capacity to amplify other cytokine responses [22, 23], we were interested in investigating the role of type I IFNs in influencing IFN-γ responses within the tumor. First, we examined the effects of RT on intratumoral IFN-α levels in the B16 model at 6, 12, 24, 48 h, and 4 days post-treatment. IFN-α protein levels were first detectable at 2 days post-treatment (Fig. 1b and data not shown), but by 4 days, IFN-α had returned to undetectable levels and remained undetectable at 7 and 9 days post-treatment (data not shown). Our data thus far demonstrated that the induction of IFN-α was transient and occurred early in response to radiation, whereas IFN-γ protein levels within the tumor were not up-regulated until 6 days after RT.
Next, we sought to determine whether engagement of IFN-α/β receptors (IFNABR) on host cells is necessary for IFN-γ responses after RT. Wild-type (WT) and IFNABR-deficient mice (IFNABRKO) were injected with B16 cells. When untreated, tumors in both WT and IFNABRKO had similar levels of IFN-γ mRNA within the tumors on day 6 following RT (Fig. 1c). However, among the radiation-treated groups, IFNABRKO had a significantly lower induction of IFN-γ compared to WT. To investigate whether the absence of IFNABR signaling in host cells also affects tumor growth, we monitored the tumor sizes by measuring thigh diameters of these mice. Untreated B16 tumors started growing progressively in vivo by day 7 post-tumor inoculation but tumor growth was faster in IFNABRKO than in WT mice (Fig. 1d). After RT, tumor growth in WT mice slowed down and the thigh measurements remained about 6 mm until day 18, whereas in mice that lacked type I IFN signaling, tumor growth slowed only slightly. A similar trend was observed when growth of E0771 tumors was evaluated (data not shown). These data suggest that tumor growth delay in response to RT is not merely due to direct effects of radiation on tumor cells but is also dependent on the host immune system. Taken together, endogenous type I IFN signaling plays a role in suppressing growth of B16 melanoma tumors. Moreover, the ability of host cells to respond to type I IFN is imperative for RT to achieve its optimal therapeutic potential, and the lack of tumor control in radiation-treated IFNABRKO may be partly due to reduced IFN-γ-mediated anti-tumor responses.
Radiation-mediated increase in immune cell infiltration requires IFN-α/β signaling in host cells
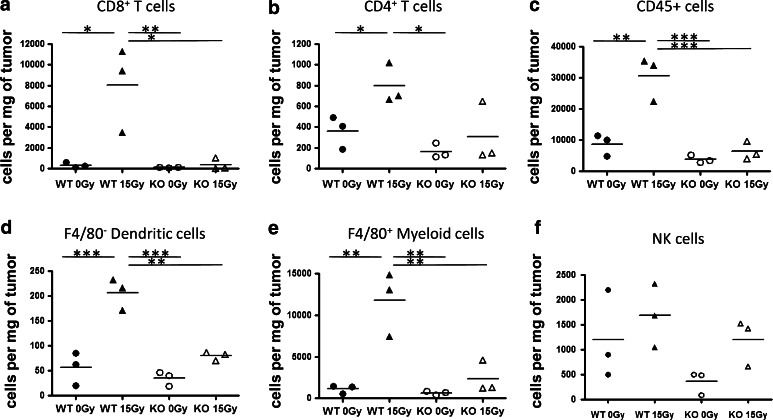
We hypothesized that the poor induction of IFN-γ post-RT in IFNABRKO mice is due to reduced numbers of IFN-γ-producing effector T cells within tumors. To test this, tumor-bearing WT and IFNABRKO mice were euthanized 6 days post-RT and the immune cell components of each tumor were analyzed by flow cytometry. Indeed, in WT mice where we observed elevated levels of IFN-γ, there was a striking increase in the numbers of CD8+ T cells within radiation-treated tumors compared to untreated tumors (Fig. 2a). On the other hand, both untreated and radiation-treated tumors in IFNABR-deficient mice had comparable numbers of CD8+ T cells, again correlating with the relative levels of IFN-γ in the tumors. The numbers of CD4+ T cells from these tumors showed a similar trend but the magnitudes of these differences were less drastic (Fig. 2b). On the other hand, there was an overall reduction in the numbers of CD45+ cells in radiation-treated tumors grown in IFNABR-deficient mice compared to WT mice (Fig. 2c). Besides CD4+ and CD8+ T cells, the radiation-mediated increase in numbers of DCs and macrophages were also diminished in mice that lacked IFNABR (Fig. 2d, e). NK cells, however, were similar between WT and IFNABR-deficient mice for the respective treatment groups (Fig. 2f).
Fig. 2.
Tumor infiltration by CD8+ T cells, CD4+ T cells, and myeloid cells was diminished in mice that lack IFN-α/β signaling. On day 13 post-tumor inoculation (day 6 post-RT), tumors were removed from C57BL/6 or IFNABR KO mice that were either untreated or given 15 Gy RT. Single-cell suspensions were obtained using collagenase digestion, and cells were incubated with Live/Dead™ reagent, followed by fluorescently conjugated antibodies. The graphs show absolute numbers of a CD8+ CD3+ T cells, b CD4+ CD3+ T cells, c CD45+ cells, d F4/80− CD11c+ CD11b+ DCs, and e F4/80+ myeloid cells f CD3−NK1.1 + cells, from individual tumors calculated after gating out dead cells and pre-gating on CD45+ cells. Data shown are representative of two experiments (*p < 0.05, **p < 0.01, ***p < 0.001)
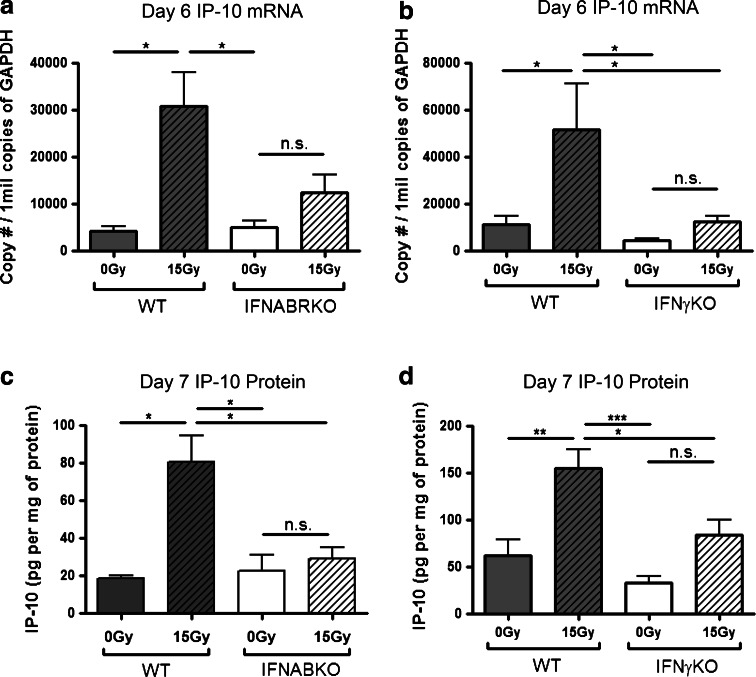
It has been previously shown in the B16 melanoma model that CXCR3 family of chemokines, particularly CXCL10 (IP-10), is important in the recruitment of immune cells into the tumor [24]. To address whether the decreased numbers of T cells in radiation-treated tumors of IFNABRKO mice may be due to lack of chemokine-mediated recruitment, we assessed the levels of IP-10 within the tumor microenvironments of WT and IFNABRKO mice using qRT-PCR. Consistent with the pattern observed for the numbers of tumor-infiltrating CD8+ T cells, IP-10 mRNA levels remained at basal levels within tumors of IFNABRKO mice after radiation treatment, whereas these levels were drastically increased in WT mice in response to treatment (Fig. 3a).
Fig. 3.
Up-regulation of IFN-γ-dependent CXCR3 chemokine, IP-10, in response to radiation treatment is dependent on endogenous type I IFNs and IFN-γ. WT and IFNABR KO (a, c) or IFNγKO (b, d) mice were inoculated with B16 melanoma cells and treated with 15 Gy local radiation as before. a, b Total RNA was isolated from tumors on day 6 post-RT. Absolute qRT-PCR was performed as described in Materials and Methods. c, d Tumors were excised on day 7 post-RT, homogenized, and IP-10 ELISA was used to determine IP-10 protein levels within each tumor. Each value was normalized to total protein concentration in each individual sample. Data shown are representative of two independent experiments (n = 4, *p < 0.05, **p < 0.01, ***p < 0.001)
Both type I IFNs and IFN-γ have been implicated in the regulation of CXCR3 family of chemokines. To examine whether the radiation-mediated induction of IP-10 is also dependent on IFN-γ, we repeated the experiment using IFN-γKO mice. Interestingly, the absence of IFN-γ signaling also caused the up-regulation of IP-10 to be drastically reduced (Fig. 3b). Data from our WT versus IFNABRKO and WT versus IFN-γKO analysis using qRT-PCR were confirmed by measuring IP-10 protein levels using ELISA (Fig. 3c, d). Altogether, these data suggest that the increased infiltration of immune cells after RT is mediated by CXCR3 chemokines, through type I IFN and IFN-γ-dependent mechanisms.
Myeloid cells are major producers of intratumoral IP-10 following RT
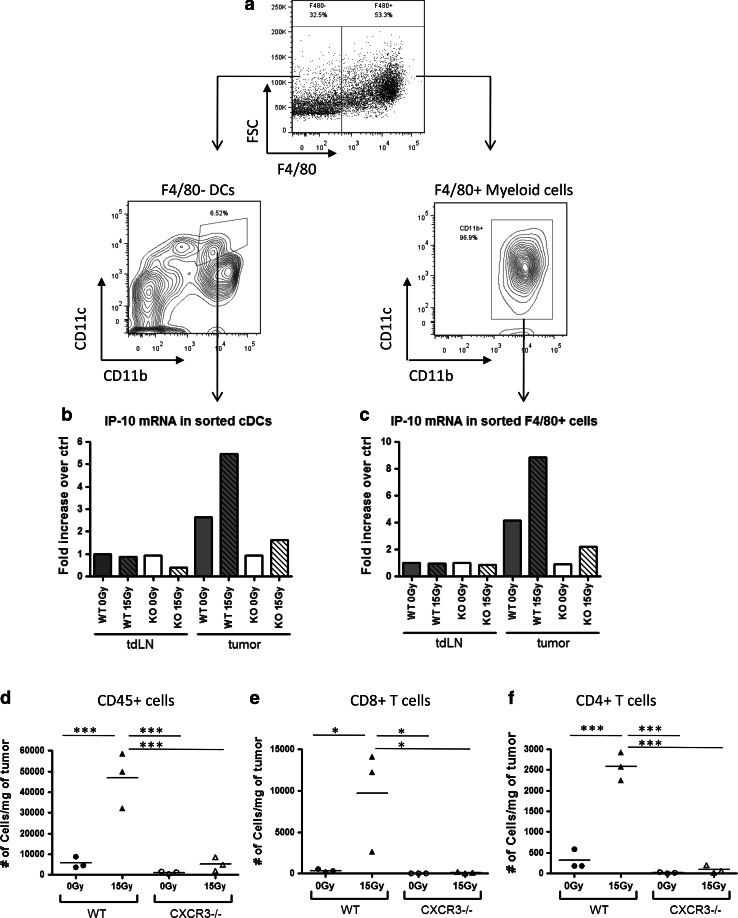
Myeloid cells are among the first immune cell types to migrate into the B16 tumors after RT (data not shown). It is possible that the production of IP-10 by the initial influx of myeloid cells generates a strong chemokine gradient that in turn enhances the recruitment of more immune cells including tumor-reactive CD8+ T cells. To test this, we sorted for various cell types within the tumor and analyzed their mRNA levels of IP-10. The two major myeloid cell subsets within B16 tumors include CD45+ F4/80+ macrophages and CD45+ F4/80− CD11c+ CD11b+ DCs (Fig. 4a). As a control, we also sorted these cell populations from tdLN, and as expected, IP-10 mRNA levels of both macrophages and F4/80− CD11c+ CD11b+ DCs from tdLNs were similar in all treated and untreated, WT and IFNABR-deficient mice. In contrast, compared to tdLN macrophages and DCs, tumor-associated macrophages and DCs from untreated WT mice had about two- and four-fold higher IP-10 mRNA levels, respectively (Fig. 4b, c). These levels were dramatically increased in macrophages from tumors that were treated with 15 Gy radiation. Among the IFNABR-deficient mice, however, untreated tumors were infiltrated by cells that express IP-10 levels similar to that of tdLN cells for both the myeloid populations, and RT induced only a small increase in IP-10 mRNA levels. Similar trends were observed when mRNA levels of CXCL9 (MIG), the other member of CXCR3 chemokine family, were analyzed (data not shown).
Fig. 4.
Tumor-associated myeloid cells up-regulate CXCR3 chemokines after RT by a type I IFN-dependent mechanism, and CXCR3 plays an essential role in the recruitment of immune cells into B16 tumors. Tumors and tdLN were excised on day 13 (4 days after RT) and made into single-cell suspensions, which were then pooled (3 mice per group). a Gating scheme for F4/80− DCs and F4/80+ myeloid cells in tumors. b F4/80− DCs and c F4/80+ myeloid cells were sorted from tumor suspensions using a cell sorter. RNA was isolated from the sorted cell populations, and IP-10 mRNA was quantified by qRT-PCR. RNA from cells in tumor-draining lymph nodes of untreated WT mice was used as comparison controls. Data shown are representative of two independent experiments. d, e, f C57BL/6 or CXCR3−/− mice were inoculated with B16 melanoma cells and either treated with 15 Gy RT or left untreated 7 days later. On day 13, tumors were excised and flow cytometry was used to enumerate tumor-infiltrating d CD45+ cells e CD8+ T cells and f CD4+ T cells into the tumor. Data shown are representative of two independent experiments (*p < 0.05, **p < 0.01, ***p < 0.001)
To demonstrate that CXCR3 chemokines play a critical role in the recruitment of T cells and other immune cells into tumors, CXCR3-deficient (CXCR3−/−) mice were inoculated with B16 cells and immune cell subsets were analyzed on day 13 as before. The immune cells of these mice lack the receptor CXCR3, which renders them unresponsive to chemoattractive gradients of IP-10 and MIG. Indeed, B16 tumors grown in CXCR3−/− mice had very few tumor-infiltrating T cells, and unlike WT mice, the numbers of CD4+ and CD8+ T cells did not increase in response to RT, suggesting that CXCR3 chemokines are indeed essential for the recruitment of both CD4+ and CD8+ T cells (Fig. 4d–f).
Type I IFNs are required to generate fully functional tumor-reactive T cells
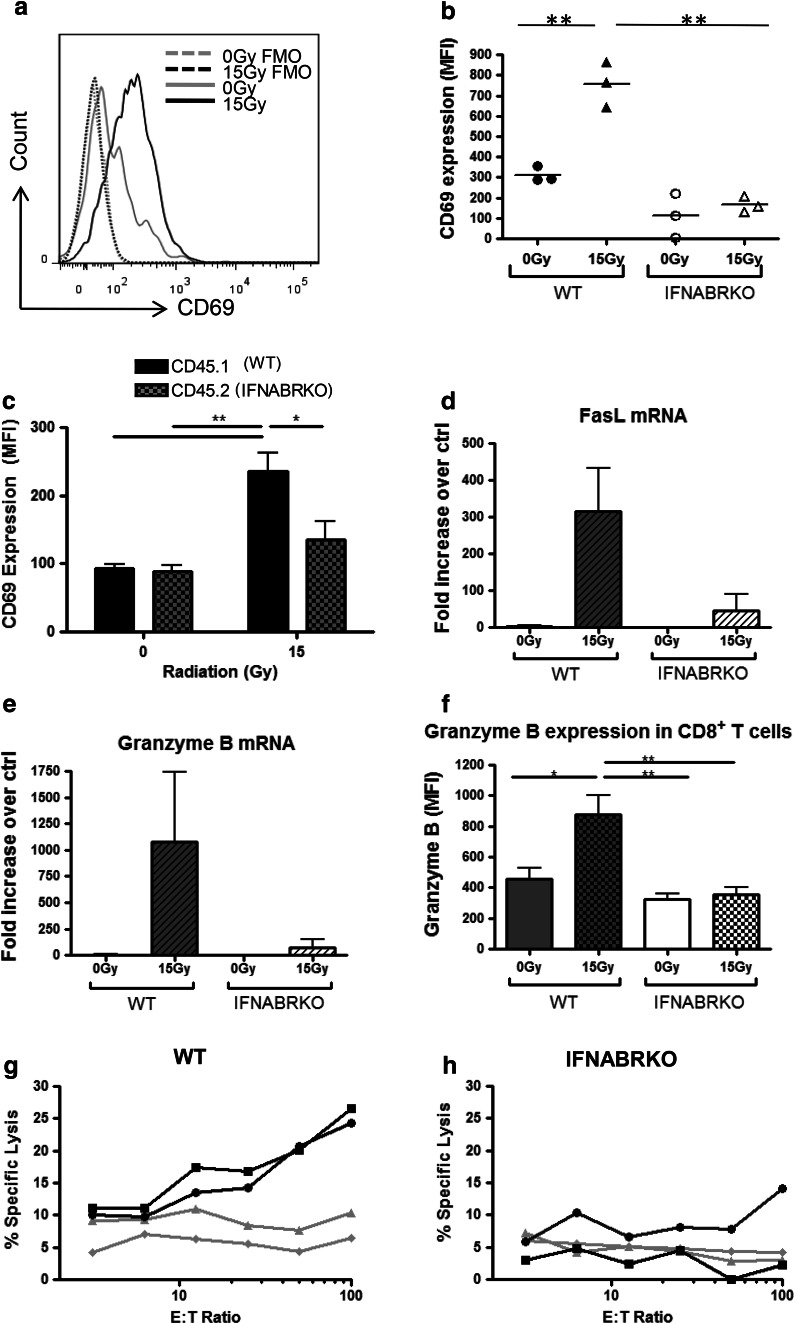
Since RT requires endogenous type I IFNs to be effective in controlling tumor growth, we wanted to investigate whether type I IFNs are also involved in the generation of activated effector T cells in response to RT. First, we examined whether RT up-regulates CD69 expression on the surface of CD8+ T cells. CD69 is an early activation marker known to be indicative of the activation status of T cells. Four days after RT (day 11 post-tumor inoculation), we detected a significantly higher CD69 expression on the surface of tumor-infiltrating CD8+ T cells from WT mice that were treated with radiation compared to cells from untreated mice (Fig. 5a, b). This increase was dependent on IFNABR signaling, because up-regulation of CD69 was not detected on tumor-infiltrating CD8+ T cells from IFNABR-deficient mice that had received radiation treatment. A similar trend was observed for tumor-infiltrating CD4+ T cells (data not shown). Intriguingly, assessment of CD69 expression on T cells from tumors grown in IFN-γKO mice indicated that IFN-γ plays a less critical role in promoting the surface expression of CD69 (Online Resource 1). Therefore, type I IFNs are uniquely required for up-regulating the early activation marker, CD69, on tumor-infiltrating T cells.
Fig. 5.
Tumor-infiltrating lymphocytes from mice that lack IFN-α/β signaling are less activated and have decreased overall ability to kill tumor cells. WT and IFNABR-deficient C57BL/6 mice were inoculated with B16 cells and treated as described before. Four days post-RT, CD69 expression on CD45+ CD3+ CD8+ cells was quantified using mean fluorescence intensity (MFI) values. a Representative histograms demonstrating the shift in CD69 expression in WT mice that were treated with 15 Gy RT (FMO fluorescence minus one control). b MFI of CD69 staining on CD8+ T cells from individual mice. c Lethally irradiated C57BL/6 hosts were reconstituted with 1:1 mixture of BL/6.SJL (CD45.1+, black bars) and IFNABR KO (CD45.2+, gray bars) bone marrow cells. Mice were rested for 40 days, inoculated with B16 tumors i.m., and were either untreated as described earlier. Four days post-RT, tumors were excised. WT and IFNABR KO hematopoietic cells were distinguished using congenic markers CD45.2 and CD45.1, respectively. Data shown are representative of two independent experiments. d, e Tumors were excised six days post-treatment, tumors pieces were homogenized, and RNA was extracted. qRT-PCR was used to quantify the levels of d FasL and e granzyme B mRNA in each sample relative to one random sample in the WT 0 Gy group. The data are representative of two experiments (n = 3). f Using intracellular granzyme B staining and flow cytometry analysis, expression levels of granzyme B in tumor-infiltrating CD8+ T cells from were quantified and expressed as MFI values. g, h Tumors were processed into single-cell suspensions with collagenase digestion. Thy1.1+ cells were enriched using antibody-coated magnetic beads and incubated with 51Cr-labeled B16 cells at various E:T ratios for 6 h. 51Cr released into supernatant by killed cells was measured and expressed as percentage of lysis over positive control. Each line represents one sample containing cells from three or four pooled 0 Gy (gray lines) or 15 Gy (black lines) tumors (*p < 0.05, **p < 0.01)
To ensure that the lack of CD69 expression on IFNABR-deficient T cells was not due to a general defect in these T cells to up-regulate CD69, we stimulated splenocytes from WT and IFNABR-deficient mice in vitro with PMA/Ionomycin and tested for any differences in their CD69 expression levels. Our data demonstrated that both WT and IFNABR-deficient T cells up-regulate their CD69 surface expression to a similar extent (Online Resource 2). This indicates that IFNABR-deficient T cells are equally capable of up-regulating cell surface expression of CD69 compared to WT T cells in response to activation, and the lack of CD69 expression that we observed in vivo was most likely due to inadequate activation signals.
The type I IFN-dependent up-regulation of CD69 molecules on T cells may be through a direct or indirect mechanism. For example, type I IFNs have been found to be required for the activation and maturation of DCs, particularly CD8α+ DCs that are important in cross-presenting tumor antigens to CD8+ T cells [25]. The defect in CD69 up-regulation on T cells in IFNABR-deficient mice may be due to poor stimulation by DCs. To address this possibility, we generated mixed bone marrow (BM) chimeras by reconstituting lethally irradiated WT mice with a 1:1 mixture of WT cells with the congenic marker CD45.1 and IFNABR-deficient cells with the congenic marker CD45.2. In these chimeric mice, both WT and IFNABR-deficient T cells were exposed to the same activation signals, in the tdLN as well as the tumor microenvironment. When we assessed the CD69 expression of WT and IFNABR-deficient T cells from B16 tumors, we observed that IFNABR-deficient T cells have much lower CD69 expression on their cell surface than WT T cells from the same tumor microenvironment (Fig. 5c). Moreover, the increase in CD69 expression in response to RT was detected only on T cells that have IFNABR. In conclusion, type I IFNs play a direct role in the activation status of tumor-associated T cells.
Activated CD8+ T cells have been shown to kill B16 melanoma cells via both the Fas/FasL and the perforin/granzyme B pathways [26]. Next, we sought to confirm that type I IFN not only affects the expression of activation marker CD69 on tumor-associated T cells, but also their effector functions. First, we examined the overall tumor microenvironment for the expression of genes related to effector T cell functions. Intratumoral FasL and granzyme B mRNA levels were similar between WT and IFNABRKO mice that were untreated. As expected, FasL and granzyme B mRNA levels were both markedly increased in radiation-treated tumors of WT mice compared to untreated tumors (Fig. 5d, e). In mice that lack IFNABR signaling, these levels were only slightly elevated. mRNA levels of these genes reflect the overall effector molecules in the tumor microenvironment. The increase in granzyme B mRNA levels in tumors that were treated with 15 Gy radiation was further verified directly in CD8+ T cells using flow cytometry. Whereas CD8+ T cells in radiation-treated tumors from WT mice had significantly increased expression of granzyme B, these levels were not increased in IFNABRKO mice (Fig. 5f). This agrees with our proposal that not only are the numbers of tumor-infiltrating effector cells increased in response to RT, these effector cells have higher expression of genes associated with cytolytic function.
To directly compare cytolytic function using standardized numbers of tumor-associated T cells from the different tumors, 51Cr-release assays were performed. B16 cells were pre-treated with IFN-γ in vitro prior to labeling with 51Cr to overcome the fact that these cells have low levels of MHC class I and hence are poor targets of CD8+ cytotoxic T lymphocytes. Target cell lysis resulting from 6-h co-culture with tumor-infiltrating Thy1+ cells from untreated WT mice was barely above background levels and remained unaltered with increasing E:T ratio (Fig. 5g). On the other hand, WT TILs from radiation-treated tumors gradually increased with increasing number of effector cells, with the percentage of lysis reaching about 25 % at E:T ratio of 100:1. Target cell lysis was completely abrogated when anti-CD8 antibody was added to block CD8/peptide-MHC class I interactions during co-culture (data not shown). Therefore, tumor-infiltrating CD8+ T cells elicited by RT have enhanced B16 cell-killing abilities compared to that of cells in untreated tumors. Corresponding to the reduced expression of FasL and granzyme B expression in tumors of radiation-treated IFNABR-deficient mice compared to WT mice, tumor-infiltrating Thy1+ cells isolated from these tumors were also less able to induce killing of target cells (Fig. 5h). Collectively, these data strongly suggest that RT elicits effector T cells that are more activated, have greater cytolytic activity, and that these characteristics are at least partially dependent on direct effects of type I IFNs on T cells.
Increasing IFN-α levels within the tumor exogenously improve the efficacy of RT
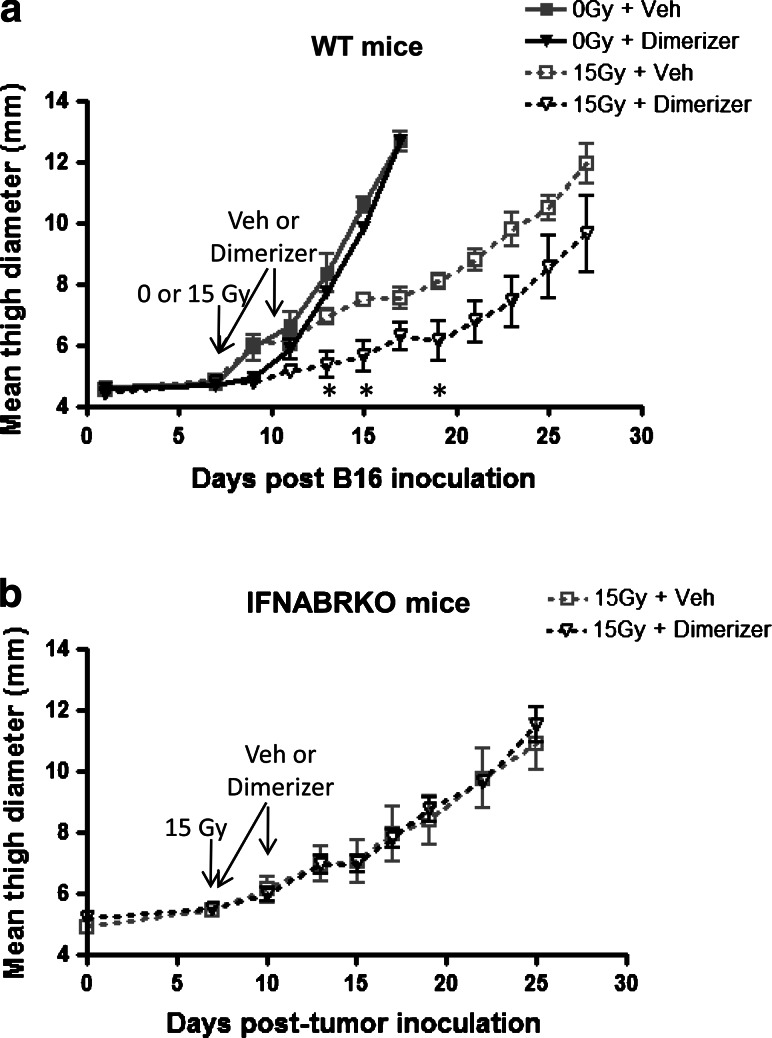
Since endogenous type I IFNs are essential for RT to achieve maximal therapeutic potential, we hypothesized that increasing intratumoral levels of IFN-α exogenously will further enhance anti-tumor responses induced by the therapy. A common approach used to increase local levels of a protein of interest in murine tumor models is to generate a clone of the tumor cell line that stably carries a transgene that codes for the protein. While this method has been previously successful for studying many other cytokines, it is not an ideal way to directly simulate immunotherapy in a clinical setting. Tumor cells that constitutively overexpress cytokines often establish tumors in vivo differently than parental cells and the tumor microenvironments may be altered. To circumvent this, we engineered a B16 clone that has inducible expression of IFN-α (B16-iIFN-α). B16-iIFN-α cells make undetectable levels of IFN-α but when treated with a rapamycin analog [27, 28], two components of a transcription factor form a dimer and the expression of IFN-α is induced (Online Resource 3). Using this system, tumors grow normally in mice after inoculation, and IFN-α production can be turned on at specific time points. This strategy enables the direct comparison of the effects of elevated IFN-α levels within the tumor on RT efficacy, at different time points.
Our in vitro characterization of B16-iIFN-α cells indicated that as little as 5nM of the inducer was sufficient to activate the expression of IFN-α (Online Resource 4a). The expression demonstrated dose response up to 25nM of dimerizer but surprisingly, a further increase to 250nM resulted in decreased induction. In addition, IFN-α production can be detected as early as 12-h post-treatment with the inducer. When we tested the cells in vitro for the durability of IFN-α production, we were able to detect IFN-α up to 72-h post-inducer treatment, even when the inducer was removed at 12 h (Online Resource 4b). This observation verified the feasibility of using these cells for our in vivo studies because daily doses of the inducer would not be necessary to induce continued production of IFN-α over several days. Importantly, the addition of inducer did not inhibit proliferation of parental B16 cells (Online Resource 4c). When cells were treated with up to 1 ng/mL of IFN-α, there was no effect on cell expansion of either of the cell lines, suggesting that the amount of IFN-α produced may not directly affect the tumor cells themselves.
When injected into mice, B16-iIFN-α established solid melanoma tumors that grew similar to that of parental B16 (Fig. 1d and 6). The treatment for these tumors with RT also resulted in comparable tumor growth delay as in parental B16. Although the induction of exogenous IFN-α production alone did not significantly improve tumor control, tumor growth was significantly delayed in mice that were given both RT and inducer. The further delay in tumor growth after combination therapy compared to RT alone is abrogated in mice that lack IFNABR, confirming that IFN-α acts on host cells and not directly on tumor cells (Fig. 6b). In conclusion, our data suggest that exogenous IFN-α indeed enhances the anti-tumor effect of RT, and this combination therapy strategy should be explored in the clinic to improve RT.
Fig. 6.
Increasing IFN-α levels within the tumor using an inducible expression system improve tumor control mediated by RT. C57BL/6 a WT and b IFNABRKO mice were injected i.m. with 1 × 105 B16-iIFNα cells. Seven days post-tumor cell injection, tumors were treated locally with 15 Gy radiation. At 3 h and 3 days after RT, mice were either given vehicle or 0.3 mg dimerizer i.v. in the tail vein. Tumor growth was monitored over time and expressed as mean thigh diameter (n = 4, *p < 0.05 using unpaired t test comparing 15 Gy + vehicle versus 15 Gy + dimerizer groups). Data shown are representative of two experiments with similar results
Discussion
Although high-dose RT and IFN-α therapy are both currently used in the clinic to treat melanoma patients, the therapeutic effects of each of these treatments alone result in only partial responses [13]. Many recent tumor studies have provided strong evidence that combining conventional treatment strategies with an immunotherapy is necessary to result in better clinical outcome [29]. In this study, we explored the combination of IFN-α treatment with RT and found that endogenous type I IFNs do indeed play a pertinent role in the efficacy of RT for cancer.
First, our data indicate that intratumoral levels of IFN-α protein spiked 48 h after 15 Gy RT, consistent with a previous study that demonstrated using a subcutaneous B16-SIY model that mRNA levels of IFN-β in the tumor are elevated after 20 Gy RT [9]. Despite the transient nature of this induction, it may be a critical process as the lack of IFNABR on host cells impaired the capacity of RT to increase IFN-γ production and control tumor growth. With regard to the mechanism for type I IFNs induction after RT, it has been shown that radiation causes DNA damage and recent studies suggested that the activation of DNA damage sensors may be involved in the induction of type I IFNs through the STING-dependent pathway [30]. This potential mechanism is very interesting and is currently being investigated by other laboratories.
Second, when we examined the tumor microenvironment to determine the effects of RT on interferon-dependent genes, we observed that tumors grown in IFNABR-deficient mice had poor induction of IP-10, as well as MIG (data not shown). However, this trend was also observed in IFN-γKO mice. Therefore, whether type I IFNs act directly or indirectly through their effects on IFN-γ remains to be determined. Since cross talk between the two cytokines occurs in many other systems [31–34], it is conceivable that type I IFNs and IFN-γ function cooperatively in the up-regulation of CXCR3 chemokines.
Based on our results, we propose that following RT, surviving cells that remain in the tumor receive “danger signals” such as DNA damage signals, which induce type I IFN production. Our preliminary data by intracellular cytokine staining for IFN-α and flow cytometry analysis showed that the cytokine is produced by F4/80+ CD11b+ CD11c− macrophages within the tumor (data not shown). This suggests that the intratumoral cell type producing type I IFNs following RT is neither myeloid DC nor plasmacytoid DCs (pDCs), which are known for their high expression of IFN-α upon activation. pDCs, identified by the expression of the surface markers CD11c+, B220+ PDCA-1+, and lack of CD11b, are present in B16 tumors albeit at low frequencies and did not stain positive for IFN-α (data not shown). Further characterization of IFN-α+ myeloid cells in B16 tumors revealed that they have low-to-mid expression levels of Ly6C on the cell surface and are negative for Gr-1, which suggests that they are not myeloid-derived suppressor cells. Based on these phenotypic markers, the cells producing IFN-α in B16 tumors are likely to be a subset of macrophages.
In response to high-dose RT, a first wave of myeloid cells repopulates the tumor and is activated locally by type I IFNs to up-regulate CXCR3 chemokines. As a result, a second wave of immune cells is recruited, including CD8+ T cells that were primed and expanded in the tdLN by DCs that had trafficked to tdLN after being activated following RT. While endogenous type I IFNs enhance the ability of DCs in the tdLN and tumor to cross-present antigens [9], type I IFN signaling is also needed to act directly on T cells to optimally activate them. Besides elevating the production of type I IFNs in the tumor, treatment with RT alters the tumor microenvironment. T cells associated with irradiated tumors express higher levels of CD69 on their cell surface compared to those in untreated tumors. Moreover, these cells are active producers of IFN-γ and granzyme B, and have increased cytolytic function. Further investigation is needed to confirm whether tumor-reactive T cells are primed, expanded, and fully activated in the tdLN or if primed T cells proliferate in the tdLN, but become fully activated in the tumor following RT. Interestingly, the increase in IFN-γ within the tumor 6 days post-RT was not detectable in the circulation when we analyzed serum levels of IFN-γ four and 6 days post-RT (data not shown). This supports the idea that tumor-reactive T cells may become fully activated effector cells after infiltrating the tumor. We speculate that the tumor microenvironment plays a pivotal role in influencing the functional activity of effector T cells.
A team of investigators recently showed that the delivery of IFN-β into B16-SIY tumors in vivo using an adenoviral vector approach is able to enhance anti-tumor effects of high-dose RT, and that T cells are required for tumor responsiveness to this combination treatment [9]. Although both IFN-α and IFN-β are members of the type I IFN family, it has been shown in multiple disease models that they have distinct downstream effects. The unique approach that we used, a system with inducible expression of IFN-α, enables the local increase in IFN-α within the tumor at time points that we define. One of the caveats to this strategy is that it is not directly applicable in the clinic. However, it serves as an efficient and economical method for preclinical, proof-of-concept research that involves exogenous treatment with cytokines or any other proteins, which may be expensive or difficult to obtain. The clone of B16-iIFN-α cells used in this study produced low amounts of IFN-α. Although the induction of intratumoral exogenous IFN-α production alone did not result in complete tumor regression, it effectively delayed the in vivo growth of an aggressive tumor cell line when administered in combination with RT. We believe that the dose regimen and scheduling of this combination treatment approach can be further optimized to achieve better therapeutic outcome. Moreover, the promising data obtained using IFN-β and RT to treat B16 [9] suggest that combining IFN-α, IFN-β, and RT may be a potential strategy to explore in the future.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors thank Dr. John G. Frelinger for suggestions and thoughtful discussions pertinent to this study. This project was financially supported by National Institutes of Health, Grant CA 28332.
Conflict of interest
The authors disclose no conflicts of interest.
Abbreviations
- IFN
Interferon
- MFI
Mean fluorescence intensity
- RT
Radiation therapy
- SBRT
Stereotactic body radiation therapy
- tdLN
Tumor-draining lymph nodes
- TILs
Tumor-infiltrating lymphocytes
- Veh
Vehicle control
References
- 1.Connell PP, Hellman S. Advances in radiotherapy and implications for the next century: a historical perspective. Cancer Res. 2009;69:383–392. doi: 10.1158/0008-5472.CAN-07-6871. [DOI] [PubMed] [Google Scholar]
- 2.Hoopes DJ, Tann M, Fletcher JW, Forquer JA, Lin PF, Lo SS, Timmerman RD, McGarry RC. FDG-PET and stereotactic body radiotherapy (SBRT) for stage I non-small-cell lung cancer. Lung Cancer. 2007;56:229–234. doi: 10.1016/j.lungcan.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Nedzi LA. The implementation of ablative hypofractionated radiotherapy for stereotactic treatments in the brain and body: observations on efficacy and toxicity in clinical practice. Semin Radiat Oncol. 2008;18:265–272. doi: 10.1016/j.semradonc.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Ritter M. Rationale, conduct, and outcome using hypofractionated radiotherapy in prostate cancer. Semin Radiat Oncol. 2008;18:249–256. doi: 10.1016/j.semradonc.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamada Y, Bilsky MH, Lovelock DM, Venkatraman ES, Toner S, Johnson J, Zatcky J, Zelefsky MJ, Fuks Z. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. 2008;71:484–490. doi: 10.1016/j.ijrobp.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 6.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 7.Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8 + T cells: changing strategies for cancer treatment. Blood. 2009;114:589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiao SL, Coussens LM. The tumor-immune microenvironment and response to radiation therapy. J Mammary Gland Biol Neoplasia. 2010;15:411–421. doi: 10.1007/s10911-010-9194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, Fu YX, Auh SL. The efficacy of radiotherapy relies upon induction of type I interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71:2488–2496. doi: 10.1158/0008-5472.CAN-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrantini M, Capone I, Belardelli F. Interferon-alpha and cancer: mechanisms of action and new perspectives of clinical use. Biochimie. 2007;89:884–893. doi: 10.1016/j.biochi.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Gresser I, Bourali C, Levy JP, Fontaine-Brouty-Boye D, Thomas MT. Increased survival in mice inoculated with tumor cells and treated with interferon preparations. Proc Natl Acad Sci USA. 1969;63:51–57. doi: 10.1073/pnas.63.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Picaud S, Bardot B, Maeyer E, Seif I. Enhanced tumor development in mice lacking a functional type I interferon receptor. J Interferon Cytokine Res. 2002;22(4):457–462. doi: 10.1089/10799900252952244. [DOI] [PubMed] [Google Scholar]
- 13.Belardelli F, Ferrantini M, Proietti E, Kirkwood JM. Interferon-alpha in tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:119–134. doi: 10.1016/S1359-6101(01)00022-3. [DOI] [PubMed] [Google Scholar]
- 14.Gresser I, Belardelli F. Endogenous type I interferons as a defense against tumors. Cytokine Growth Factor Rev. 2002;13:111–118. doi: 10.1016/S1359-6101(01)00035-1. [DOI] [PubMed] [Google Scholar]
- 15.Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol. 2010;22:333–340. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorensen EW, Gerber SA, Frelinger JG, Lord EM. IL-12 suppresses vascular endothelial growth factor receptor 3 expression on tumor vessels by two distinct IFN-gamma-dependent mechanisms. J Immunol. 2010;184:1858–1866. doi: 10.4049/jimmunol.0903210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol. 2008;180:3132–3139. doi: 10.4049/jimmunol.180.5.3132. [DOI] [PubMed] [Google Scholar]
- 18.Gerber SA, Pober JS. IFN-alpha induces transcription of hypoxia-inducible factor-1alpha to inhibit proliferation of human endothelial cells. J Immunol. 2008;181:1052–1062. doi: 10.4049/jimmunol.181.2.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogel R, Mammeri H, Mallet J. Lentiviral vectors mediate nonimmunosuppressive rapamycin analog-induced production of secreted therapeutic factors in the brain: regulation at the level of transcription and exocytosis. Hum Gene Ther. 2008;19:167–178. doi: 10.1089/hum.2007.125. [DOI] [PubMed] [Google Scholar]
- 20.Gerber SA, Sorensen EW, Sedlacek AL, Lim JY, Skrombolas D, Frelinger JG, Lord EM. Local expression of interleukin-2 by B16 melanoma cells results in decreased tumour growth and long-term tumour dormancy. Immunology. 2013;138:280–292. doi: 10.1111/imm.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerber SA, Sedlacek AL, Cron KR, Murphy SP, Frelinger JG, Lord EM. IFN-gamma mediates the antitumor effects of radiation therapy in a murine colon tumor. Am J Pathol. 2013 doi: 10.1016/j.ajpath.2013.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brassard DL, Grace MJ, Bordens RW. Interferon-alpha as an immunotherapeutic protein. J Leukoc Biol. 2002;71:565–581. [PubMed] [Google Scholar]
- 23.Taylor JL, Grossberg SE. The effects of interferon-alpha on the production and action of other cytokines. Semin Oncol. 1998;25:23–29. [PubMed] [Google Scholar]
- 24.Hong M, Puaux AL, Huang C, et al. Chemotherapy induces intratumoral expression of chemokines in cutaneous melanoma, favoring T-cell infiltration and tumor control. Cancer Res. 2011;71:6997–7009. doi: 10.1158/0008-5472.CAN-11-1466. [DOI] [PubMed] [Google Scholar]
- 25.Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, Gajewski TF. Host type I IFN signals are required for antitumor CD8 + T cell responses through CD8{alpha} + dendritic cells. J Exp Med. 2011;208:2005–2016. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kline J, Zhang L, Battaglia L, Cohen KS, Gajewski TF. Cellular and molecular requirements for rejection of B16 melanoma in the setting of regulatory T cell depletion and homeostatic proliferation. J Immunol. 2012;188:2630–2642. doi: 10.4049/jimmunol.1100845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klemm JD, Schreiber SL, Crabtree GR. Dimerization as a regulatory mechanism in signal transduction. Annu Rev Immunol. 1998;16:569–592. doi: 10.1146/annurev.immunol.16.1.569. [DOI] [PubMed] [Google Scholar]
- 28.Pollock R, Clackson T. Dimerizer-regulated gene expression. Curr Opin Biotechnol. 2002;13:459–467. doi: 10.1016/S0958-1669(02)00373-7. [DOI] [PubMed] [Google Scholar]
- 29.Voloshin T, Voest EE, Shaked Y. The host immunological response to cancer therapy: an emerging concept in tumor biology. Exp Cell Res. 2013 doi: 10.1016/j.yexcr.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Kondo T, Kobayashi J, Saitoh T, Maruyama K, Ishii KJ, Barber GN, Komatsu K, Akira S, Kawai T. DNA damage sensor MRE11 recognizes cytosolic double-stranded DNA and induces type I interferon by regulating STING trafficking. Proc Natl Acad Sci USA. 2013;110:2969–2974. doi: 10.1073/pnas.1222694110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 32.Wong LH, Hatzinisiriou I, Devenish RJ, Ralph SJ. IFN-gamma priming up-regulates IFN-stimulated gene factor 3 (ISGF3) components, augmenting responsiveness of IFN-resistant melanoma cells to type I IFNs. J Immunol. 1998;160:5475–5484. [PubMed] [Google Scholar]
- 33.Taniguchi T, Takaoka A. A weak signal for strong responses: interferon-alpha/beta revisited. Nat Rev Mol Cell Biol. 2001;2:378–386. doi: 10.1038/35073080. [DOI] [PubMed] [Google Scholar]
- 34.Rayamajhi M, Humann J, Kearney S, Hill KK, Lenz LL. Antagonistic crosstalk between type I and II interferons and increased host susceptibility to bacterial infections. Virulence. 2010;1:418–422. doi: 10.4161/viru.1.5.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.