Abstract
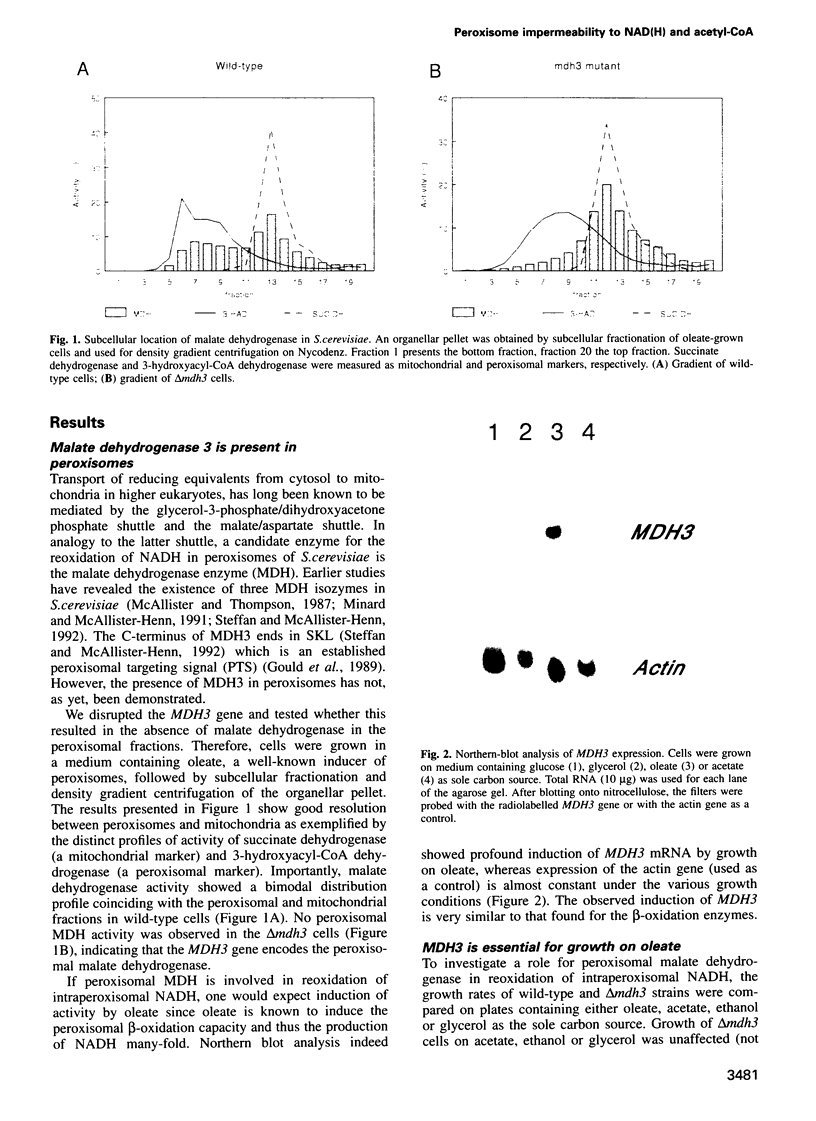
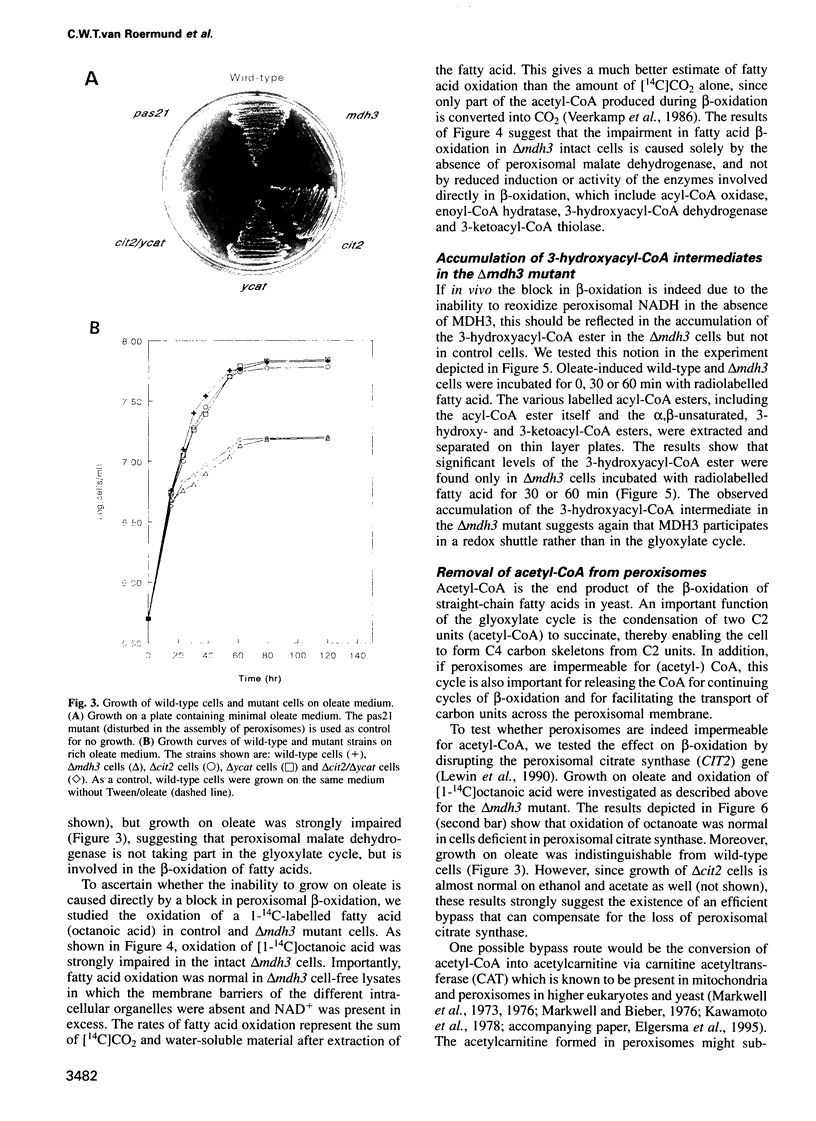
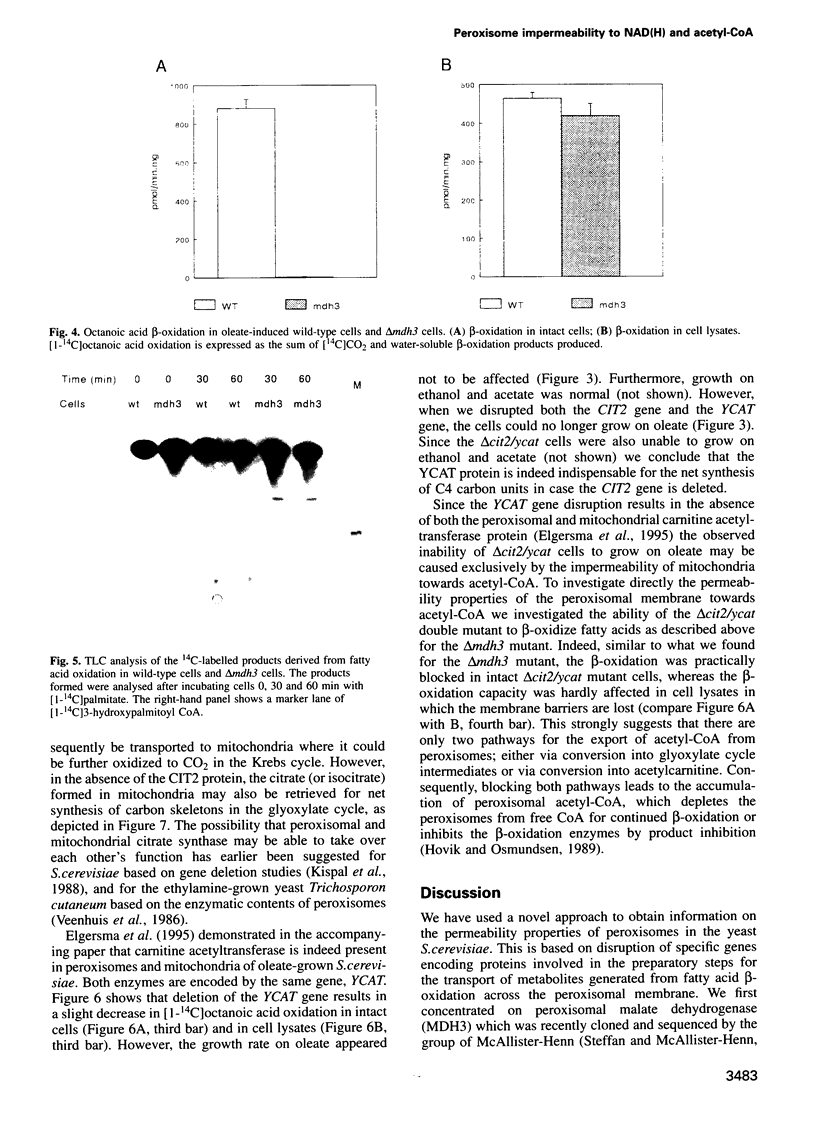
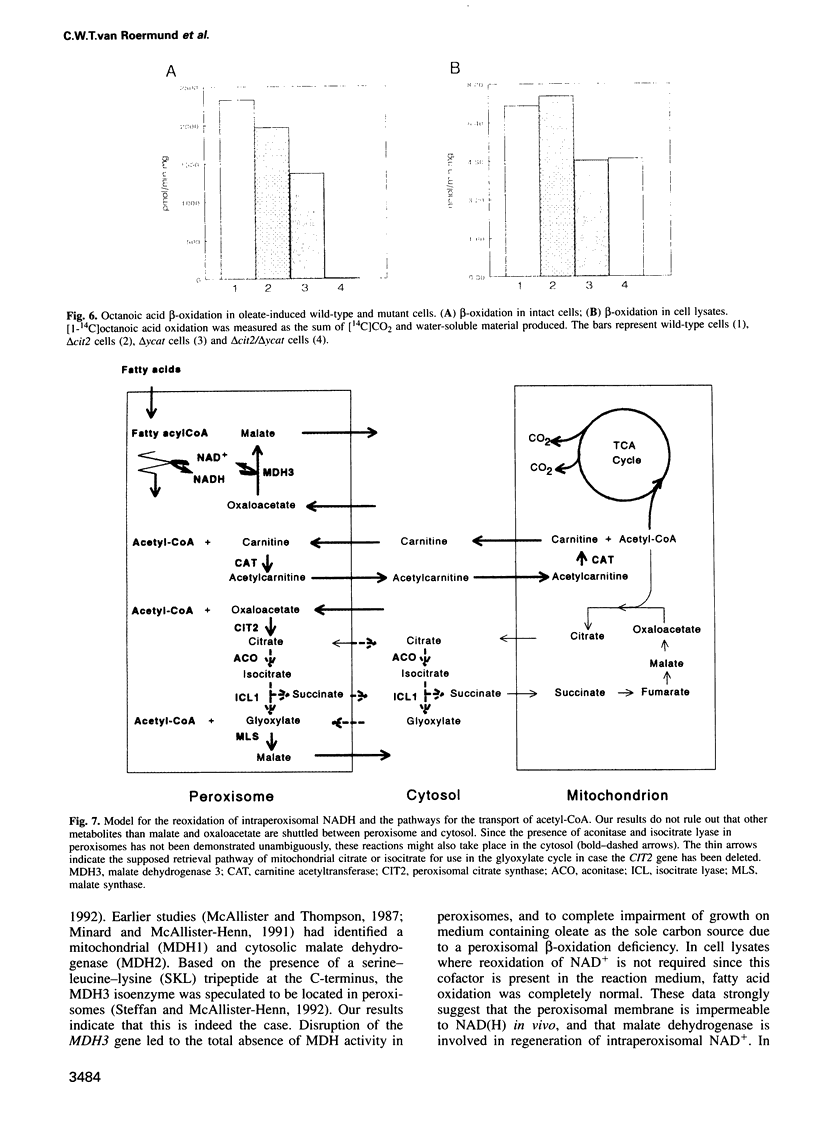
We investigated how NADH generated during peroxisomal beta-oxidation is reoxidized to NAD+ and how the end product of beta-oxidation, acetyl-CoA, is transported from peroxisomes to mitochondria in Saccharomyces cerevisiae. Disruption of the peroxisomal malate dehydrogenase 3 gene (MDH3) resulted in impaired beta-oxidation capacity as measured in intact cells, whereas beta-oxidation was perfectly normal in cell lysates. In addition, mdh3-disrupted cells were unable to grow on oleate whereas growth on other non-fermentable carbon sources was normal, suggesting that MDH3 is involved in the reoxidation of NADH generated during fatty acid beta-oxidation rather than functioning as part of the glyoxylate cycle. To study the transport of acetyl units from peroxisomes, we disrupted the peroxisomal citrate synthase gene (CIT2). The lack of phenotype of the cit2 mutant indicated the presence of an alternative pathway for transport of acetyl units, formed by the carnitine acetyltransferase protein (YCAT). Disruption of both the CIT2 and YCAT gene blocked the beta-oxidation in intact cells, but not in lysates. Our data strongly suggest that the peroxisomal membrane is impermeable to NAD(H) and acetyl-CoA in vivo, and predict the existence of metabolite carriers in the peroxisomal membrane to shuttle metabolites from peroxisomes to cytoplasm and vice versa.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bieber L. L. Carnitine. Annu Rev Biochem. 1988;57:261–283. doi: 10.1146/annurev.bi.57.070188.001401. [DOI] [PubMed] [Google Scholar]
- Borst P. Peroxisome biogenesis revisited. Biochim Biophys Acta. 1989 Jun 1;1008(1):1–13. doi: 10.1016/0167-4781(89)90163-2. [DOI] [PubMed] [Google Scholar]
- Bremer J., Wojtczak A. B. Factors controlling the rate of fatty acid -oxidation in rat liver mitochondria. Biochim Biophys Acta. 1972 Dec 8;280(4):515–530. doi: 10.1016/0005-2760(72)90131-2. [DOI] [PubMed] [Google Scholar]
- De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles). Physiol Rev. 1966 Apr;46(2):323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- Elgersma Y., van Roermund C. W., Wanders R. J., Tabak H. F. Peroxisomal and mitochondrial carnitine acetyltransferases of Saccharomyces cerevisiae are encoded by a single gene. EMBO J. 1995 Jul 17;14(14):3472–3479. doi: 10.1002/j.1460-2075.1995.tb07353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. J., Keller G. A., Hosken N., Wilkinson J., Subramani S. A conserved tripeptide sorts proteins to peroxisomes. J Cell Biol. 1989 May;108(5):1657–1664. doi: 10.1083/jcb.108.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikoop J. C., van Roermund C. W., Just W. W., Ofman R., Schutgens R. B., Heymans H. S., Wanders R. J., Tager J. M. Rhizomelic chondrodysplasia punctata. Deficiency of 3-oxoacyl-coenzyme A thiolase in peroxisomes and impaired processing of the enzyme. J Clin Invest. 1990 Jul;86(1):126–130. doi: 10.1172/JCI114674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovik R., Osmundsen H. A kinetic investigation of the acyl-CoA oxidase reaction with the use of a novel spectrophotometric assay. Inhibition by acetyl-CoA, CoA and FMN. Biochem J. 1989 Oct 1;263(1):297–299. doi: 10.1042/bj2630297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto S., Ueda M., Nozaki C., Yamamura M., Tanaka A., Fukui S. Localization of carnitine acetyltransferase in peroxisomes and in mitochondria of n-alkane-grown Candida tropicalis. FEBS Lett. 1978 Dec 1;96(1):37–40. doi: 10.1016/0014-5793(78)81057-6. [DOI] [PubMed] [Google Scholar]
- Kispal G., Rosenkrantz M., Guarente L., Srere P. A. Metabolic changes in Saccharomyces cerevisiae strains lacking citrate synthases. J Biol Chem. 1988 Aug 15;263(23):11145–11149. [PubMed] [Google Scholar]
- Lewin A. S., Hines V., Small G. M. Citrate synthase encoded by the CIT2 gene of Saccharomyces cerevisiae is peroxisomal. Mol Cell Biol. 1990 Apr;10(4):1399–1405. doi: 10.1128/mcb.10.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannaerts G. P., Van Veldhoven P. P. Metabolic pathways in mammalian peroxisomes. Biochimie. 1993;75(3-4):147–158. doi: 10.1016/0300-9084(93)90072-z. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Bieber L. L. Localization and solubilization of a rat liver microsomal carnitine acetyltransferase. Arch Biochem Biophys. 1976 Feb;172(2):502–509. doi: 10.1016/0003-9861(76)90103-x. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., McGroarty E. J., Bieber L. L., Tolbert N. E. The subcellular distribution of carnitine acyltransferases in mammalian liver and kidney. A new peroxisomal enzyme. J Biol Chem. 1973 May 25;248(10):3426–3432. [PubMed] [Google Scholar]
- Markwell M. A., Tolbert N. E., Bieber L. L. Comparison of the carnitine acyltransferase activites from rat liver peroxisomes and microsomes. Arch Biochem Biophys. 1976 Oct;176(2):497–488. doi: 10.1016/0003-9861(76)90191-0. [DOI] [PubMed] [Google Scholar]
- McAlister-Henn L., Thompson L. M. Isolation and expression of the gene encoding yeast mitochondrial malate dehydrogenase. J Bacteriol. 1987 Nov;169(11):5157–5166. doi: 10.1128/jb.169.11.5157-5166.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettler I. J., Beevers H. Oxidation of NADH in Glyoxysomes by a Malate-Aspartate Shuttle. Plant Physiol. 1980 Oct;66(4):555–560. doi: 10.1104/pp.66.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minard K. I., McAlister-Henn L. Isolation, nucleotide sequence analysis, and disruption of the MDH2 gene from Saccharomyces cerevisiae: evidence for three isozymes of yeast malate dehydrogenase. Mol Cell Biol. 1991 Jan;11(1):370–380. doi: 10.1128/mcb.11.1.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser H. W. Peroxisomal disorders. Clin Biochem. 1991 Aug;24(4):343–351. doi: 10.1016/0009-9120(91)80009-r. [DOI] [PubMed] [Google Scholar]
- Munujos P., Coll-Cantí J., González-Sastre F., Gella F. J. Assay of succinate dehydrogenase activity by a colorimetric-continuous method using iodonitrotetrazolium chloride as electron acceptor. Anal Biochem. 1993 Aug 1;212(2):506–509. doi: 10.1006/abio.1993.1360. [DOI] [PubMed] [Google Scholar]
- Nicolay K., Veenhuis M., Douma A. C., Harder W. A 31P NMR study of the internal pH of yeast peroxisomes. Arch Microbiol. 1987 Feb;147(1):37–41. doi: 10.1007/BF00492902. [DOI] [PubMed] [Google Scholar]
- Opperdoes F. R., Borst P. Localization of nine glycolytic enzymes in a microbody-like organelle in Trypanosoma brucei: the glycosome. FEBS Lett. 1977 Aug 15;80(2):360–364. doi: 10.1016/0014-5793(77)80476-6. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Steffan J. S., McAlister-Henn L. Isolation and characterization of the yeast gene encoding the MDH3 isozyme of malate dehydrogenase. J Biol Chem. 1992 Dec 5;267(34):24708–24715. [PubMed] [Google Scholar]
- Van Veldhoven P. P., Just W. W., Mannaerts G. P. Permeability of the peroxisomal membrane to cofactors of beta-oxidation. Evidence for the presence of a pore-forming protein. J Biol Chem. 1987 Mar 25;262(9):4310–4318. [PubMed] [Google Scholar]
- Van der Leij I., Van den Berg M., Boot R., Franse M., Distel B., Tabak H. F. Isolation of peroxisome assembly mutants from Saccharomyces cerevisiae with different morphologies using a novel positive selection procedure. J Cell Biol. 1992 Oct;119(1):153–162. doi: 10.1083/jcb.119.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerkamp J. H., van Moerkerk T. B., Glatz J. F., Zuurveld J. G., Jacobs A. E., Wagenmakers A. J. 14CO2 production is no adequate measure of [14C]fatty acid oxidation. Biochem Med Metab Biol. 1986 Jun;35(3):248–259. doi: 10.1016/0885-4505(86)90080-0. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Runswick M. J. The mitochondrial transport protein superfamily. J Bioenerg Biomembr. 1993 Oct;25(5):435–446. doi: 10.1007/BF01108401. [DOI] [PubMed] [Google Scholar]
- Wanders R. J., Heymans H. S., Schutgens R. B., Barth P. G., van den Bosch H., Tager J. M. Peroxisomal disorders in neurology. J Neurol Sci. 1988 Dec;88(1-3):1–39. doi: 10.1016/0022-510x(88)90203-1. [DOI] [PubMed] [Google Scholar]
- Wanders R. J., IJlst L., van Gennip A. H., Jakobs C., de Jager J. P., Dorland L., van Sprang F. J., Duran M. Long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency: identification of a new inborn error of mitochondrial fatty acid beta-oxidation. J Inherit Metab Dis. 1990;13(3):311–314. doi: 10.1007/BF01799383. [DOI] [PubMed] [Google Scholar]
- Waterham H. R., Keizer-Gunnink I., Goodman J. M., Harder W., Veenhuis M. Immunocytochemical evidence for the acidic nature of peroxisomes in methylotrophic yeasts. FEBS Lett. 1990 Mar 12;262(1):17–19. doi: 10.1016/0014-5793(90)80142-6. [DOI] [PubMed] [Google Scholar]
- van den Bosch H., Schutgens R. B., Wanders R. J., Tager J. M. Biochemistry of peroxisomes. Annu Rev Biochem. 1992;61:157–197. doi: 10.1146/annurev.bi.61.070192.001105. [DOI] [PubMed] [Google Scholar]