Abstract
This study investigated the influence of different durations of aerobic exercise on fuel utilization, lactate levels and antioxidative status in trained rats. Forty rats underwent physical training (T, n = 20) or non- training (NT, n = 20) for 6 weeks. For physical training, animals exercised on a treadmill for 30 min 5 days per week. At the end of week 6, the animals in each group were subdivided into BE, DE-0.5, DE-1 and DE-2, which were sacrificed at the end of week 6 without having performed exercise or after exercise on a treadmill for 0.5h, 1h and 2h, respectively, immediately before being sacrificed. The plasma glucose level in DE-2 of the NT group was significantly lower than in the other groups. Muscle and liver glycogen levels were significantly lower in DE-1 and DE-2, but there were no significant differences between DE-1 and DE-2 in the T group. Liver protein in DE-2 of the NT group was significantly lower. Muscle TG levels were decreased in DE-0.5 of the T group, while those of the NT group were decreased in DE-1. FFA levels were increased in DE-0.5 of the T group and in DE-1 of the NT group. Lactate levels were increased in DE-0.5 of the NT group, while they were increased in DE-1 of the T group. Catalase activity of the T group was lower in BE but higher in DE-0.5, DE-1 and DE-2. SOD activities were higher in trained rats, while the GSH/GSSG ratios were higher in BE, DE-0.5 and DE-1 in the T group, and there was no difference in that of DE-2. There were no differences in MDA levels in BE and DE-0.5, but they were significantly lower in DE-1 and DE-2 of the T group. Overall, the results of this study, suggest that training may improve exercise performance by facilitating the mobilization and oxidation of fat and conserving limited carbohydrate storage, and that it may delay the onset of fatigue and enhance the antioxidative defense system, but cannot support two hours of vigorous exercise.
Keywords: Duration of exercise, physical training, energy source, antioxidative system, lactate
Introduction
The positive effects of physical training on animals have been generally accepted. A number of mechanisms have been investigated to explain the physiological effects of aerobic exercise on fuel utilization, antioxidant defense system and lactate levels [1-3]. However, the effects of training in the state of different durations of aerobic exercise have not been fully elucidated. When the body is involved in physical training, certain metabolic processes occur to assure that adequate energy is provided to the exercising muscles [4,5] via increased oxidation of fat and conservation carbohydrate storage [1]. As an adaptive response, antioxidant defense systems are up regulated [3] and physical training shifts the maximum lactate steady state to a higher intensity [2]. At the skeletal muscle level, such training in humans is able to reduce the release of lactate from contracting muscle, mainly by improving mitochondrial respiratory capacity [6]. There is also accumulating evidence that strenuous exercise induces negative effects between free radical production and the body's antioxidant defense systems [3]. Therefore, this study was conducted to examine the influence of different durations of exercise on fuel utilization, lactate levels and antioxidant defense system in trained rats.
Materials and Methods
Experimental diets
Forty 4-wk old male Sprague-Dawley rats (Daehanbiolink Co., South Korea) weighing 95-105 g were fed, a diet that met the AIN-93 recommendations (American Institute of Nutrition). The study protocol was approved by the Committee on Animal Welfare Regulations of Duksung Women's University (2011-108), Seoul 132-714, South Korea.
Exercise regime and sample collection
Forty animals underwent either physical training (T, n = 20) or were sedentary (NT, n = 20) for 6 weeks. For physical training, the rats exercised on a treadmill (Jungdo Bio & Plant, JD-A-09, Korea, 30 min/d, 15° incline, 0.5-0.8 km/h) 5 days per week. Rats received light electric shocks if they did not comply with running on the treadmill [7,8]. At the end of week 6, the animals were subdivided into four groups (five rats per group) based on exercise: before exercise (BE, n = 5) and during exercise (DE-0.5, n = 5; DE-1, n = 5; DE-2, n = 5). The BE groups were sacrificed without having performed exercise at the end of week 4. The three DE groups were sacrificed immediately after exercising on a treadmill (15° incline, 0.5-0.8 km/h) for 30 min (DE-0.5), 1 h (DE-1), or 2h (DE-2), respectively. At each of the respective time points, the animals were sacrificed by decapitation while under light ether anesthesia. Immediately following decapitation, blood samples were collected in heparinized tubes (BD Vacutainer®). All blood samples were immediately centrifuged (1300 RCF for 20 min at 4℃) to separate the plasma and erythrocytes. Additionally, the heart, kidney, liver, visceral fat and skeletal muscle from the medial red gastrocnemius were rapidly removed and stored at -70℃ until analysis.
Biochemical analysis
Glycogen was measured using a colorimetric procedure as previously described [9]. After tissue samples were homogenized (Omni THQ Digital Tissue Homogenizer) in cold sodium phosphate buffer (2 mL, 0.02 M, pH 7.0), aliquots of the homogenates were analyzed for protein and triglycerides. Specifically, the total protein concentration was determined using a commercial kit (Asan Pharmaceutical Co., South Korea) [10], while triglyceride were measured using a commercial kit (Asan Pharmaceutical Co.) that employed the glycerol phosphate oxidase-quinoneimine colorimetric method [11]. Plasma glucose level was determined using a commercial kit (Youndong Pharmaceutical Co., South Korea) based on an enzymatic method [12]. Free fatty acid (FFA) level was measured with a commercial kit (NEFAZYME-S, Eiken Chemical Co., Japan) utilizing acyl CoA synthetase-Acyl CoA oxidase [13]. Plasma triglyceride (TG) was analyzed with a commercial kit (Youngdong Pharmaceutical Co., Seoul, Korea). Plasma lactate levels were analyzed with a Lactate Colorimetric Assay Kit II (Bio Vision K627-100). The activity of plasma catalase was determined with a commercial kit based on the method described by Zamocky (Bioxytech Catalase-520). The activity of superoxide dismutase (SOD), ratio of reduced glutathione to oxidized glutathione (GSH/GSSG), and levels of malondialdehyde (MDA) were determined in liver cytosol. Briefly liver was homogenized in cold Tris-KCl buffer (0.1M), after which the homogenized solution was centrifuged (8,000 ×g, 4℃, 30 min) and the supernatant was removed and further centrifuged (10,000 ×g, 4℃, 30 min). The supernatant was then ultra-centrifuged (105,000 ×g, 4℃, 90 min) to separate the cytosol. The SOD activity subsequently determined with a commercial kit based on the method described by Nebot (Bioxytech SOD-525). GSH/GSSG was determined using a commercial kit based on the method described by Anderson (Bioxytech GSH/GSSG-412). The levels of MDA were measured with a commercial kit based on the method described by Gerard-Monnier (Bioxytech MDA-586).
Statistical analysis
Data were subjected to analysis of variance (ANOVA) followed by Duncan's multiple range test (SAS Institute, Cary, NC). Additionally, differences between the control group and trained group were identified using a t-test. A P < 0.05 was considered to indicate significance.
Results
Body and organ weights
As shown in table 1, the non-training group showed significantly higher body weights than the trained group, but there were no differences in the organ weights and food feed efficiency (FFA) ratio between the non-training group and the training group.
Table 1.
Effects of physical training on body weight and organ weight
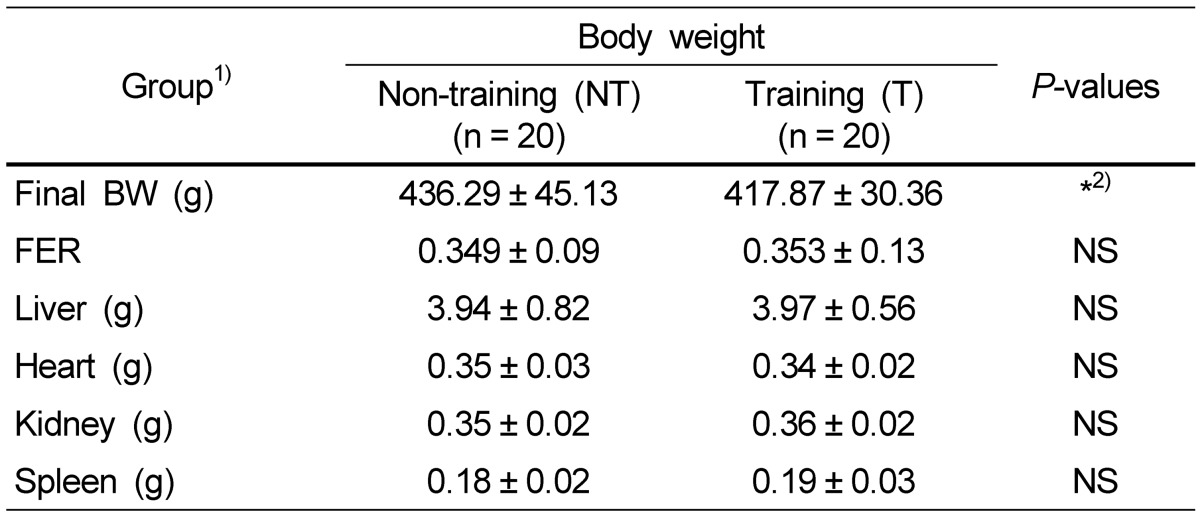
1)NT: non-training group, T: 30min training group (5 d per week, 15° incline, 0.5-0.8 km/h)
2)Significant difference between non-training group and training group at P < 0.05 by t-test.
Carbohydrate utilization and lactate concentration
Table 2 shows the effects of physical training on the levels of plasma glucose, liver glycogen and muscle glycogen. Plasma glucose levels were not significantly different at the time of BE, DE-0.5 and DE-1 by training groups, but in the non-training group those level was significantly decreased at the time of DE-2 compared to other time points. However, muscle glycogen levels of the training group were significantly higher than those of the non-training group in BE, DE-0.5, DE-1 and DE-2. DE-1 and DE-2 were significantly lower than BE and DE-0.5 in the non-training group, but in the training group, DE-2 was significantly lower than BE, DE-0.5 and DE-1. The liver glycogen levels of the training group were also significantly higher than those of the non-training group in DE-0.5 and DE-1, but were not significantly different from those of the non-training group in BE and DE-2. The liver glycogen levels in DE-2 were significantly lower than BE, DE-0.5 and DE-1 in the non-training group, and the training group. The lactate levels were higher in DE-0.5 in the non-training group, while the levels increased in DE-2 in the training group.
Table 2.
Effects of physical training on the levels of plasma glucose, liver glycogen and muscle glycogen levels
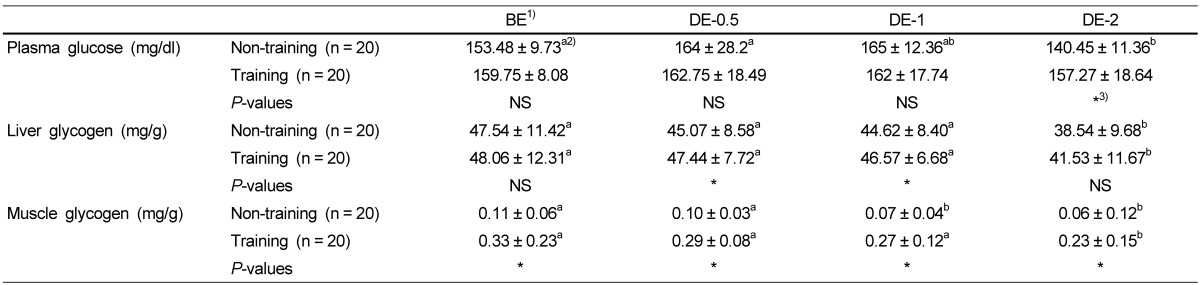
1)BE: before-exercise, DE-0.5: exercise for 30 min, DE-1: exercise for 1h, DE-2: exercise for 2h
2)Group means and standard deviations were presented, ANOVA was used to compare mean between groups and Duncan's multiple range test (SAS Institute, Cary, NC) was used to determine group was different from each other with in row. P value < 0.05 was considered to be significant.
3)Significant difference between non-training group and training group at P < 0.05 by t-test.
Lipid utilization
Table 4 shows the effects of physical training on the levels of plasma FFA and triglyceride levels of liver and muscle. Plasma FFA levels were significantly higher in the training group than the non-training group. FFA levels were increased in DE-0.5 in the training group and in DE-1 in the non-training group. Liver triglyceride levels of the training group were lower than those of the non-training group in BE, D-30, DE-1 and DE-2. However, muscle triglyceride levels of the training group were significantly lower than those of the non-training group in DE-1 and DE-2.
Table 4.
Effects of physical training on the free fatty acid and liver and muscle triglyceride levels
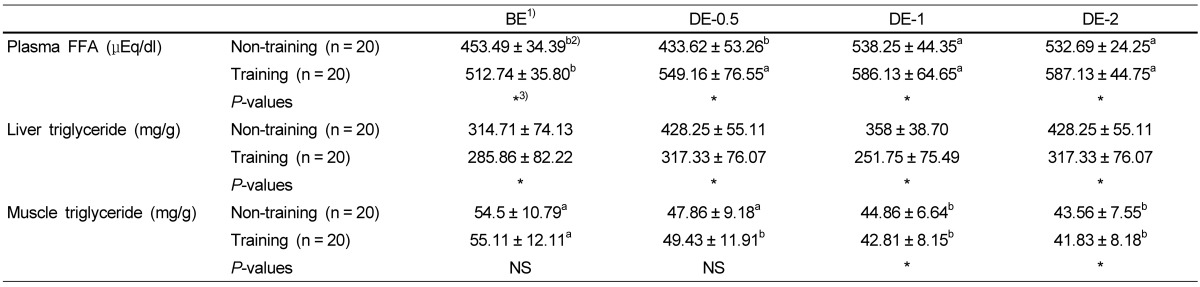
1)BE: before-exercise, DE-0.5: exercise for 30 min, DE-1: exercise for 1h, DE-2: exercise for 2h
2)Group means and standard deviations were presented, ANOVA was used to compare mean between groups and Duncan's multiple range test (SAS Institute, Cary, NC) was used to determine group was different from each other with in row. P value < 0.05 was considered to be significant.
3)Significant difference between non-training group and training group at P < 0.05 by t-test.
Protein utilization
Table 5 shows the effects of physical training on the protein levels of plasma, liver and muscle. Plasma and muscle protein levels of the training group were not significantly different from those of the non-training group in BE, DE-0.5 DE-1 and DE-2. Liver protein levels of the training group were not significantly different from those of the non-training group in BE, DE-0.5 and DE-1, but were significantly higher than those of the non-training group in DE-2.
Table 5.
Effects of physical training on plasma, liver and muscle protein levels.
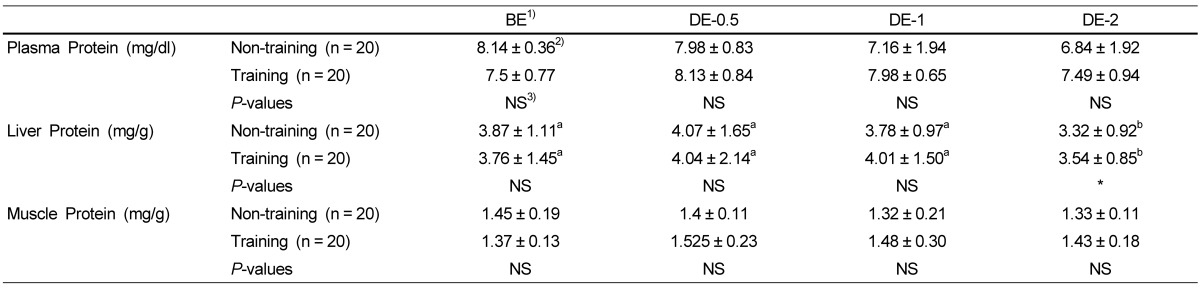
1)BE: before-exercise, DE-0.5: exercise for 30 min, DE-1: exercise for 1h, DE-2: exercise for 2h
2)Group means and standard deviations were presented, ANOVA was used to compare mean between groups and Duncan's multiple range test (SAS Institute, Cary, NC) was used to determine group was different from each other with in row. P value < 0.05 was considered to be significant.
3)No significant difference between non-training group and training group at P < 0.05 by t-test.
Anti-oxidative defense system
Table 6 shows the effects of physical training on catalase activities. Catalase activities of the training group were significantly lower in BE, but higher in DE-0.5, DE-1 and DE-2 than those of the non-training group. This was because catalase activities of the non-training group were significantly decreased in DE-0.5, while those of training group were not changed in DE-0.5 and DE-1. As shown in Table 3, the SOD activities of the training group were significantly higher than those of non-training group in BE as well as in DE-0.5, DE-1 and DE-2. SOD activities were not changed in BE, DE-0.5 and DE-1 in either the training group or the non-training group, but they were significantly lower in DE-2 in both groups. Table 4 shows the effects of moderate physical training on the GSH/GSSG ratio. The GSH/GSSG ratio of the training group was significantly higher in BE, DE-0.5 and DE-1 but was not significantly different in DE-2. Table 5 shows the effects of moderate physical training on liver MDA levels. There were no differences between the non-training group and training group in BE or DE-0.5. MDA levels were increased in DE-1 and DE-2.
Table 6.
Effects of moderate physical training on superoxide dismutase (SOD) and catalase activities, the ratio of reduced glutathione to oxidized glutathione (GSH/GSSG) and malondialdehyde (MDA) concentrations
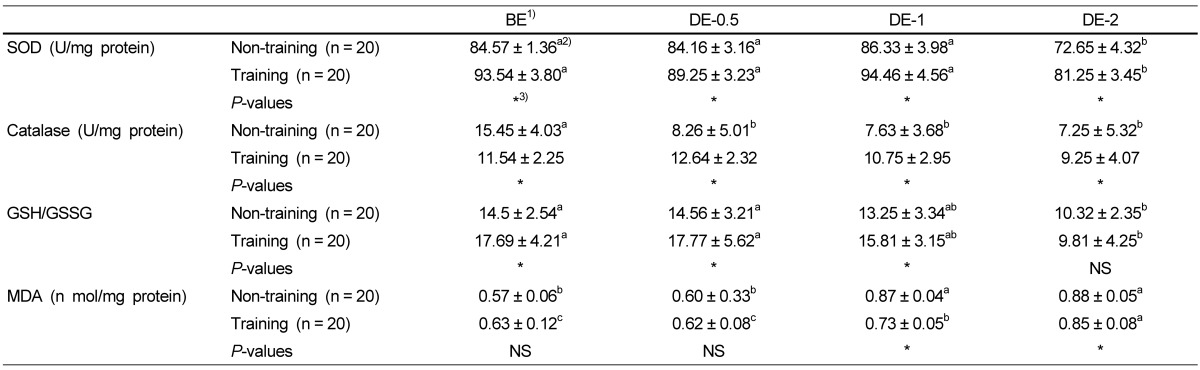
1)BE: before-exercise, DE-0.5: exercise for 30 min, DE-1: exercise for 1h, DE-2: exercise for 2h
2)Group means and standard deviations were presented, ANOVA was used to compare mean between groups and Duncan's multiple range test (SAS Institute, Cary, NC) was used to determine group was different from each other with in row. P value < 0.05 was considered to be significant.
3)Significant difference between non-training group and training group at P < 0.05 by t-test.
Table 3.
Effects of physical training on the lactate levels of plasma

1)BE: before-exercise, DE-0.5: exercise for 30 min, DE-1: exercise for 1h, DE-2: exercise for 2h
2)Group means and standard deviations were presented, ANOVA was used to compare mean between groups and Duncan's multiple range test (SAS Institute, Cary, NC) was used to determine group was different from each other with in row. P value < 0.05 was considered to be significant.
3)Significant difference between non-training group and training group at P < 0.05 by t-test.
Discussion
This study showed that physical training had a positive effect on utilization of fuel, lactate levels and antioxidative status under different durations of exercise (BE, DE-0.5 and DE-1). However, moderate physical training could not support two hours of vigorous exercise (DE-2).
Higher levels of muscle glycogen were observed regardless of exercise, and higher levels of liver glycogen were observed in BE, DE-0.5 and DE-1 in the training group relative to the non-training group, however, no differences were observed in the DE-2 groups. These findings indicate that moderate physical training resulted in the animal adapting to store more glycogen and reduce glycogen depletion for one hour [1]. The plasma glucose levels of the training group were not significantly different among the BE, DE-0.5, DE-1 and DE-2 groups. However, in the non-training group, the glucose level of DE-2 was significantly lower than that of other groups. These findings indicate that decreased liver glycogens were associated with low glucose levels in the non-training group. There were no differences in plasma and muscle protein levels between the non-training group and the training group, regardless of exercise, but liver protein in DE-2 of the non-training group was significantly lower than those of the other group. Liver protein appears to be a source of energy during exercise for less than two hours in the non-training group. It has been reported that, as endurance exercise depletes endogenous carbohydrate stores, the body starts to catabolize protein for energy; therefore, it is eventually converted to glucose [14,15]. When exercise is started, energy turnover increases with rapid mobilization and oxidation of both carbohydrates and lipids stored within contracting muscle, and increased fat oxidation is most important in low intensity exercise [16,17]. The plasma FFA levels of the training group were significantly higher than those of non-training group, indicating that the training group might efficiently consume FFA as a fuel source [1]. Intramuscular fat utilization could also be a good fuel sources during prolonged exercise, and increased content and use of muscle triglyceride may be the primary adaptive mechanism underlying the greater capacity of trained muscle to oxidize FFA to energy during exercise [18]. A relative increase in the availability of free fatty acids during exercise has been shown to delay the onset of exhaustion [19], and maintaining adequate fuel stores helps prevent fatigue during exercise [20]. Moreover, it has been shown that physical training does not alter the maximum lactate steady state, but shifts it to a higher exercise intensity [2]. This likely leads to lower lactate production for the same relative and absolute workload [21] and/or increased blood lactate removal [22,23] in trained animals. Therefore blood lactate concentrations are not the only a result of lactate release, since there are several pathways for blood lactate removal [2]. The liver appears to play an important role, using lactate as a substrate for glucose production by means of gluconeogenesis [24,25]. The heart is another organ that contributes to blood lactate removal since it uses lactate as an energy source [26], but skeletal muscle itself seems to play the major role in lactate removal during and after exercise [22,23,25]. In this study lactate levels increase in DE-0.5 in non-training groups, while they increased in DE-1 in trained groups, indicating that physical training delayed the increase of lactate levels. These results suggested that moderate physical training may delay the onset of fatigue and improve usage of energy by facilitating the mobilization and oxidation fat and conserving limited carbohydrate stores. However, moderate physical training cannot lead to sufficient storage of energy sources to support two hours of exercise.
The catalase activities of the training group were significantly lower in BE, but higher in DE-0.5, DE-1 and DE-2 than those of the non-training group. These findings are consistent with those of a previous study. It has been suggested that, in training group, exercise-induced oxidative stress did not affect the catalase activities [3]. In the present study, the SOD activities of the training group were significantly higher than those of the non-training group, but in DE-2, SOD activities decreased to the same levels as in the non-training group. The GSH/GSSG ratios of the training group were significantly higher in DE-0.5, DE-1 and DE-2, but not in BE. MDA levels increased in DE-1 and DE-2 in the training group, suggesting that moderate physical training, and repetition of short-term generation of increased vascular oxidative stress; induced an increase in antioxidative enzyme activity and antioxidant status, but did not lead to a significant change in liver MDA levels. These results indicate that moderate physical training can activate antioxidant defenses, but that moderate physical training cannot effectively provide defense against free radicals during two hours of exercise. Accordingly, moderate physical training may improve exercise performance by facilitating the mobilization and oxidation of fat and conserving limited carbohydrate store. Additionally, moderate physical training may delay the onset of fatigue associated with low lactate levels and enhance antioxidative defense systems, but these effects will not last for two hours of vigorous exercise.
Footnotes
This study was supported by a 2012 Research Grant from Duksung Women's University.
References
- 1.Choi EY, Cho YO. Moderate physical training can increase muscle glycogen levels but does not alter protein levels with exercise in rats. Nutr Sci. 2006;9:112–116. [Google Scholar]
- 2.Gobatto CA, de Mello MA, Sibuya CY, de Azevedo JR, dos Santos LA, Kokubun E. Maximal lactate steady state in rats submitted to swimming exercise. Comp Biochem Physiol A Mol Integr Physiol. 2001;130:21–27. doi: 10.1016/s1095-6433(01)00362-2. [DOI] [PubMed] [Google Scholar]
- 3.Choi EY, Cho YO. The effects of physical training on antioxidative status under exercise-induced oxidative stress. Nutr Res Pract. 2007;1:14–18. doi: 10.4162/nrp.2007.1.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnett C, Carey M, Proietto J, Cerin E, Febbraio MA, Jenkins D. Muscle metabolism during sprint exercise in man: influence of sprint training. J Sci Med Sport. 2004;7:314–322. doi: 10.1016/s1440-2440(04)80026-4. [DOI] [PubMed] [Google Scholar]
- 5.Stuewe SR, Gwirtz PA, Agarwal N, Mallet RT. Exercise training enhances glycolytic and oxidative enzymes in canine ventricular myocardium. J Mol Cell Cardiol. 2000;32:903–913. doi: 10.1006/jmcc.2000.1131. [DOI] [PubMed] [Google Scholar]
- 6.Green HJ. How important is endogenous muscle glycogen to fatigue in prolonged exercise? Can J Physiol Pharmacol. 1991;69:290–297. doi: 10.1139/y91-045. [DOI] [PubMed] [Google Scholar]
- 7.Fulk LJ, Stock HS, Lynn A, Marshall J, Wilson MA, Hand GA. Chronic physical exercise reduces anxiety-like behavior in rats. Int J Sports Med. 2004;25:78–82. doi: 10.1055/s-2003-45235. [DOI] [PubMed] [Google Scholar]
- 8.Hand GA, Hewitt CB, Fulk LJ, Stock HS, Carson JA, Davis JM, Wilson MA. Differential release of corticotropin-releasing hormone (CRH) in the amygdala during different types of stressors. Brain Res. 2002;949:122–130. doi: 10.1016/s0006-8993(02)02972-4. [DOI] [PubMed] [Google Scholar]
- 9.Hassid WZ, Abraham S. Chemical procedures for analysis of polysaccharides. In: Colowick SP, Kaplan NO, editors. Methods in Enzymology. Vol. 3. New York (NY): Academic Press, Inc.; 1957. pp. 34–50. [Google Scholar]
- 10.Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- 11.Giegel JL, Ham AB, Clema W. Manual and semi-automated procedures for measurements of triglycerides in serum. Clin Chem. 1975;21:1575–1581. [PubMed] [Google Scholar]
- 12.Raabo E, Terkildsen TC. On the enzymatic determination of blood glucose. Scand J Clin Lab Invest. 1960;12:402–407. doi: 10.3109/00365516009065404. [DOI] [PubMed] [Google Scholar]
- 13.Rogiers V. Stability of the long chain non-esterified fatty acid pattern in plasma and blood during different storage conditions. Clin Chim Acta. 1978;84:49–54. [Google Scholar]
- 14.Lemon PW. Is increased dietary protein necessary or beneficial for individuals with a physically active lifestyle? Nutr Rev. 1996;54:S169–S175. doi: 10.1111/j.1753-4887.1996.tb03913.x. [DOI] [PubMed] [Google Scholar]
- 15.Andersen LL, Tufekovic G, Zebis MK, Crameri RM, Verlaan G, Kjaer M, Suetta C, Magnusson P, Aagaard P. The effect of resistance training combined with timed ingestion of protein on muscle fiber size and muscle strength. Metabolism. 2005;54:151–156. doi: 10.1016/j.metabol.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Van Aggel-Leijssen DP, Saris WH, Hul GB, Van Baak MA. Long-term effects of low-intensity exercise training on fat metabolism in weight-reduced obese men. Metabolism. 2002;51:1003–1010. doi: 10.1053/meta.2002.34028. [DOI] [PubMed] [Google Scholar]
- 17.Rowlands DS, Hopkins WG. Effects of high-fat and high-carbohydrate diets on metabolism and performance in cycling. Metabolism. 2002;51:678–690. doi: 10.1053/meta.2002.32723. [DOI] [PubMed] [Google Scholar]
- 18.Martin WH., 3rd Effects of acute and chronic exercise on fat metabolism. Exerc Sport Sci Rev. 1996;24:203–231. [PubMed] [Google Scholar]
- 19.Jesek JK, Martin NB, Broeder CE, Thomas EL, Wambsgans KC, Hofman Z, Ivy JL, Wilmore JH. Changes in plasma free fatty acids and glycerols during prolonged exercise in trained and hypertensive persons taking propranolol and pindolol. Am J Cardiol. 1990;66:1336–1341. doi: 10.1016/0002-9149(90)91164-2. [DOI] [PubMed] [Google Scholar]
- 20.Williams C. Carbohydrate intake and recovery from exercise. Sci Sports. 2004;19:239–244. [Google Scholar]
- 21.Jones AM, Carter H. The effect of endurance training on parameters of aerobic fitness. Sports Med. 2000;29:373–386. doi: 10.2165/00007256-200029060-00001. [DOI] [PubMed] [Google Scholar]
- 22.Gladden LB. Muscle as a consumer of lactate. Med Sci Sports Exerc. 2000;32:764–771. doi: 10.1097/00005768-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Donovan CM, Pagliassotti MJ. Quantitative assessment of pathways for lactate disposal in skeletal muscle fiber types. Med Sci Sports Exerc. 2000;32:772–777. doi: 10.1097/00005768-200004000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Ryan C, Ferguson K, Radziuk J. Glucose dynamics and gluconeogenesis during and after prolonged swimming in rats. J Appl Physiol (1985) 1993;74:2404–2411. doi: 10.1152/jappl.1993.74.5.2404. [DOI] [PubMed] [Google Scholar]
- 25.Brooks GA. Intra- and extra-cellular lactate shuttles. Med Sci Sports Exerc. 2000;32:790–799. doi: 10.1097/00005768-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Bonen A. Lactate transporters (MCT proteins) in heart and skeletal muscles. Med Sci Sports Exerc. 2000;32:778–789. doi: 10.1097/00005768-200004000-00010. [DOI] [PubMed] [Google Scholar]


