Abstract
Objective
The effectiveness of the combination therapy of triazole and echinocandin in treatment of invasive aspergillosis (IA) remains controversial. The objective of this systematic review was to assess the efficacy of combination therapy of triazole and echinocandin in treatment of IA.
Methods
Relevant articles on the combination therapy of triazole and echinocandin in IA, including the animal studies and clinical studies from January 1966 to October 2013, were searched on Web of Science, PubMed and Cochrane Library. The prolongation of survival of the combination therapy of triazole and echinocandin in IA was performed as risk ratio (RR) with 95% confidence interval (95% CI).
Results
Nine animal studies with a total of 1,582 animals and five clinical trials totaling 872 patients were included. The survival of the included animal studies with combination therapy was significantly prolonged compared with echinocandin alone [RR =2.26, (95% CI, 1.79-2.87; P<0.00001)], but no statistical difference compared with monotherapy of triazole [RR =1.19, (95% CI, 0.98-1.44; P=0.08)]. Of the four human cohort studies, two studies observed that the combination therapy of triazole and echinocandin was associated with a significant reduction in mortality compared with other treatments, and one study might be considered as a preferable therapy [HR =0.58, (95% CI, 0.3-1.14; P=0.117)]. While another study revealed that there was no significant difference among the combination therapy of triazole and echinocandin and either of the monotherapy. In the randomized clinical trial (RCT), of the 135 patients who received the combination therapy, 39 died, while 55 died out of 142 patients who received monotherapy (P=0.08, 95% CI, –21.4, 1.09) by week 12.
Conclusions
The combination therapy of triazole and echinocandin in treating IA results in a trend towards improved overall survival in animals’ studies and clinical studies. Well-designed RCTs and further improved clinical trials are necessary to study the effectiveness of the combination therapy.
Keywords: Triazole, echinocandin, invasive aspergillosis (IA), systematic review
Introduction
Invasive aspergillosis (IA) is an opportunistic infection caused by fungi of the genus Aspergillus. Due to increasing number of people with compromised immunity (most as a results of AIDS and organ transplantation), IA has been on a sharp rise for the past few decades. Aspergillus fumigatus is widely present in environment and the most common species recovered from cases of IA among which 90% are involved into the lung (1). Other commonly recovered species are Aspergillus flavus, Aspergillus niger, and Aspergillus terreus.
Invasive pulmonary aspergillosis (IPA) is a life-threatening infection associated with severe mortality. Voriconazole is considered to be the primary therapy for IPA based on the results of randomized clinical trials (RCTs) (2,3) and alternatives are liposomal amphotericin B, amphotericin B lipid complex, caspofungin, micafungin, posaconazole and itraconazole. Despite these treatment options, the outcomes of IPA remain poor, with mortality rates of 25% to 35% 12 weeks after diagnosis (4).
The target of triazole is at cell-membrane, and the target of echinocandin is at cell-wall (2), so that the combination therapy of triazole and echinocandin may result in synergistic function against Aspergillus spp. strains with a wider spectrum of efficacy and lower toxicity (5-7). However, some studies showed that the combination therapy of triazole and echinocandin did not significantly improve the therapeutic outcome (8), or they might even be potentially antagonistic to each other (9). Furthermore, the combination of antifungal drugs for primary therapy of IPA is not routinely recommended by the Infectious Diseases Society of America due to lack of enough clinical data (2). Therefore, our objective was to evaluate the evidences for the combination therapy of triazole and echinocandin in treatment of IA in animal and clinical studies.
Materials and methods
Literature search
Relevant articles from January 1966 to October 2013 were searched on Web of Science, PubMed and Cochrane Library by two researchers. Keywords or text words in medical subjects heading (MeSH) included: “invasive aspergillosis” OR “invasive pulmonary aspergillosis”, “triazole” OR “itraconazole” OR “voriconazole” OR “posaconazole” OR “ravuconazole”, “echinocandin” OR “caspofungin” OR “micafungin” OR “anidulafungin”. We also did hand searching of reviews, guidelines and citations of all included studies for complete references.
Selection criteria for studies
Animal studies
Inclusion criteria: animal models were in line with IA standard. Appropriate control groups were set, and uniform evaluation indexs were included.
Exclusion criteria: any study which was only related to pharmacokinetic study, combination of triazole or echinocandin with amphotericin B, not set with a blank or a placebo-control or repeatedly published data, was excluded.
Clinical studies
Inclusion criteria: any study in which IA was diagnosed according to the European Organization for Research and Treatment of Cancer and the Mycoses Study Group consensus criteria was included (10). We included studies in which patients were diagnosed with either proven or probable IA. We included cohort or RCT studies that assessed the efficacy of combination therapy of triazole and echinocandin with appropriate control groups.
Exclusion criteria: any study with only a case report or repeatedly published data, without control group or lacking uniform diagnostic criteria, was excluded.
Data extraction
Two reviewers independently applied selection criteria, performed quality assessment, and extracted data, including the sample size, antifungal dose, duration of treatment (days), the observed indicators and evaluation criteria. If we found that the information provided in a literature is not comprehensive, we contacted the author to get detailed information. Disagreement on whether some specific studies should be included into this study between the two reviewers was attempted to be reached a consensus in a subsequent discussion between the two reviewers, which otherwise was resolved by a third researcher.
Study quality assessment
A quality assessment of all selected full-text articles of animal studies was performed according to the ARRIVE guidelines (11,12). The Newcastle-Ottawa Quality Assessment Scales (13) for cohort clinical studies was applied to assess selection bias, comparability of exposed and unexposed groups of each cohort, outcome assessment, and attrition bias. The quality of the RCT was assessed according to modified Jadad score (14), including details of randomization, generation of random numbers, implementation of double-blinding, information on withdrawals, and allocation concealment. Two reviewers independently evaluated these components of the scale. Disagreements among reviewers were resolved by discussion until a consensus was reached.
Statistical methods and data analysis
The survival was reported as risk ratio (RR) with 95% confidence interval (95% CI). A heterogeneity test was performed to examine the homogeneity. If there was homogeneity, the fixed-effect model was used; if there was heterogeneity, the random-effect model was used. Z-statistic test for over effect was done, P≤0.05 was considered to be statistically significant. All statistical analyses were performed using Review Manager Version 5.1 (The Nordic Cochrane Centre, Cochrane Collaboration, 2011) software.
Results
Database searched results
The search process, the number of initially searched studies, and the number of excluded studies are illustrated in Figure 1. Nine animal studies (5-9,15-18) and five clinical studies, including one RCT and four cohort studies (19-24) were eligible for final review. Tables 1 and 2 show that the included studies were of high quality. The Jadad scale score of the RCT was five.
Figure 1.
Flow chart of included and excluded studies.
Table 1. Quality assessment of combination therapy in animal models (Kilkenny et al. 2010a).
| Studies | Items |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
| Kirkpatrick WR et al., 2002 (7) | 1 | 2 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 1 | 2 |
| Luque JC et al., 2003 (6) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 2 | 2 | 0 | 2 | 2 | 0 | 1 | 0 | 2 |
| Petraitis V et al., 2003 (5) | 1 | 2 | 2 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 2 | 2 | 0 | 2 | 2 | 0 | 1 | 1 | 2 |
| MacCallum DM et al., 2005 (15) | 1 | 2 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 2 | 2 | 0 | 2 | 2 | 0 | 1 | 0 | 1 |
| Clemons KV et al., 2006 (9) | 1 | 2 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 2 | 2 | 0 | 2 | 2 | 0 | 1 | 0 | 1 |
| van de Sande WW et al., 2009 (8) | 1 | 2 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 2 | 2 | 0 | 2 | 2 | 0 | 1 | 0 | 1 |
| Petraitis V et al., 2009 (16) | 1 | 2 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 2 | 2 | 0 | 2 | 2 | 0 | 1 | 0 | 2 |
| Calvo E et al., 2012 (17) | 1 | 2 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 2 | 2 | 0 | 2 | 2 | 0 | 1 | 1 | 2 |
| Seyedmousavi S et al., 2013 (18) | 1 | 2 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 2 | 2 | 0 | 2 | 2 | 0 | 1 | 0 | 2 |
Table 2. Newcastle-Ottawa quality assessment scale for cohort studies included in this review.
| Studies | Selection |
Comparability | Outcome |
Total score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Outcome of Interest not present at start of study | Assessment of outcome | Adequacy of duration of follow-up | Adequacy of completeness of follow-up | |||
| Marr KA et al., 2004 (19) | A | A | A | A | A | B | A | A | 7 |
| Singh N et al., 2006 (20) | A | A | A | A | A | B | A | A | 7 |
| Upton A et al., 2007 (21) | A | A | A | A | A | B | A | A | 7 |
| Rieger CT et al., 2008 (22) | A | A | A | A | A | B | A | A | 7 |
Animal study characteristics
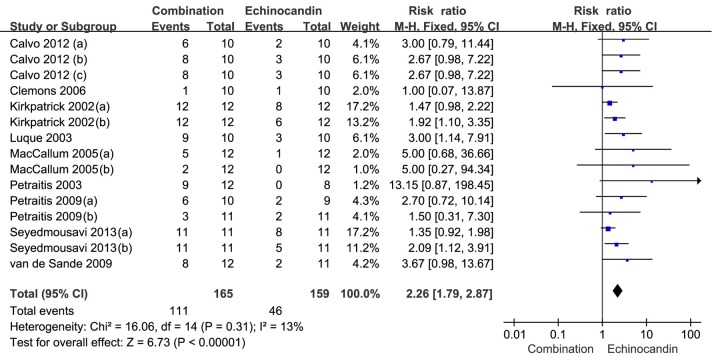
The main characteristics of the analyzed animal studies are summarized in Table 3. The survival of the included animal studies with combination therapy was significantly prolonged compared with echinocandin alone [67.3% versus 28.9%; RR =2.26, (95% CI, 1.79-2.87; P<0.00001); Figure 2], but no statistical difference compared with triazole alone [67.2% versus 52.3%; RR =1.19, (95% CI, 0.98-1.44; P=0.08); Figure 3].
Table 3. Characteristics of included animal studies.
| Studies | Types of animals | Sample sizes | Aspergillus | MIC(µg/mL) | Infective doses | Treatments |
Duration of treatment (days) | Findings | |
|---|---|---|---|---|---|---|---|---|---|
| Combination therapy | Monotherapy | ||||||||
| Kirkpatrick WR et al., 2002 (7) | Male guinea pigs | 72 | A. fumigatus | VRC 0.5, CAS 32 | 1×106 conidia | CAS 1 or 2.5 mg/kg/day IP + VRC 5 mg/kg/day PO | CAS 1 or 2.5 mg/kg/day IP or VRC 5 mg/kg/day PO | 5 | Mortality↓ (P<0.0025 compared to CAS); no differences compared to VRC |
| Luque JC et al., 2003 (6) | Female mice | 40 | A. fumigatus | ITZ 1.56, MICA >16 | 8×106 conidia | MICA 3 mg/kg q12h + ICZ 100 mg/kg/day | MICA 3 mg/kg q12h or ICZ 100 mg/kg/day or no drug | 12 | Survival↑ (P<0.05 compared to MICA); no differences compared to ICZ |
| Petraitis V et al., 2003 (5) | Female rabbits | 36 | A. fumigatus | RAV 1.0, MICA 0.25 | 1×108-1.25×108 conidia | MICA 1 mg/kg/day IV + RAV 2.5 mg/kg/day IV | MICA 1 mg/kg IV or RAV 2.5 mg/kg IV or no drug | 12 | Mortality↓ (P≤0.001); residual fungal burden↓ (P≤0.05); galactomannan indexes↓ (P≤0.01) |
| MacCallum DM et al., 2005 (15) | Male Guinea pigs | 90 | A. fumigatus | VRC 0.032 to 0.5, CAS 0.125 | 104 or 103 conidia/g | CAS 1 mg/kg/day IP + VRC 1 mg/kg PO q12h | VRC 1 mg/kg PO q12h or CAS 1 mg/kg/day IP | 7 | Survival↑ (P=0.048 compared to CAS with 103 conidia/g) |
| Clemons KV et al., 2006 (9) | Female mice | 40 | A. fumigatus | NR | 3.96×104 conidia | MICA 1 mg/kg/day + ICZ 100 mg/kg/day | MICA 1 mg/kg/day or ICZ 100 mg/kg/day or no drug | 10 | Survival↓ (P>0.05) |
| van de Sande WW et al., 2009 (8) | Female rats | 58 | A. fumigatus | NR | NR | AFG 20 mg/kg/day on day 1, followed 5 mg/kg/day + VRC 7.5, 10, 12.5, and 15 mg/kg on days 0, 1, 2, and 3 and 17.5 mg/kg on day 4 and beyond, IP q12h | AFG or VRC or no drug | 5-10 | Survival↓ (P=0.3290); galactomannan indexes (P=0.0238 and P=0.0357) |
| Petraitis V et al., 2009 (16) | Female rabbits | 70 | A. fumigatus | VRC 0.5 to 1.0, AFG 0.25 | 1.0×108-1.25×108 conidia | AFG 5 or 10 mg/kg/day IV + VRC 10 mg/kg q8h IV | AFG 5 or 10 mg/kg/day IV or VRC 10 mg/kg q8h IV or no drug | 12 | Survival↑ (P<0.001) (AFG 5 mg/kg/day); survival↓ (P>0.05) (AFG 10 mg/kg/day); residual fungal burden↓ (P<0.05); galactomannan indexes↓ (P<0.05) |
| Calvo E et al., 2012 (17) | Male mice | 240 | A. flavus | VRC 0.5 to 1.0, AFG > 32 | 8×103 CFU | AFG 1 mg/kg/day IP + VRC 12.5 mg/kg PO q12h | AFG 1 mg/kg/day IP or VRC 12.5 mg/kg PO q12h or no drug | 7 | Survival↑ (P<0.05); residual fungal burden↓ (P<0.05); galactomannan indexes↓ (P<0.05) |
| Seyedmousavi S et al., 2013 (18) | Female mice | 882 | VRC-S and VRC-R A. fumigatus | VRC 0.25 and 4, AFG 0.031 | 2.4×107 and 2.5×107 conidia | AFG 20 mg/kg/day + VRC 20 mg/kg | AFG 10 mg/kg/day or VRC 20 mg/kg | 7 | Synergistic in VRC-S; additive in VRC-R |
AFG, anidulafungin; AMB, amphotericin B; CAS, caspofungin; ICZ, itraconazole; L-AMB, liposomal amphotericin B; MICA, micafungin; POC, posaconazole; RAV, ravuconazole; VRC, voriconazole; VRC-S, voriconazole-susceptible; VRC-R, voriconazole-resistant; IP, intraperitoneal; IV, intravenous; PO, peros (oral); q12h, every 12 h; NR, not reported; MIC, minimal inhibitory concentration.
Figure 2.
Forest plot showing the survival of the combination therapy of triazole and echinocandin compared with monotherapy of echinocandin in animal studies.
Figure 3.
Forest plot showing the survival of the combination therapy of triazole and echinocandin compared with monotherapy of triazole in animal studies.
IA models infected by A. fumigatus (8,16,18) or A. flavus (17) were treated with combination therapy of voriconazole and anidulafungin or either of monotherapy of voriconazole or anidulafungin. The efficacy of the combination therapy was synergistic compared with either of the monotherapy (16-18) (survival, P<0.05). Meanwhile, Petraitis et al. (16) concluded that anidulafungin at a dosage of 10 mg/kg/day was antagonistic to voriconazole. Seyedmousavi et al. (18) showed that the combination therapy was additive in treatment of voriconazole-resistant IA. However, Van de Sande et al. (8) showed that the monotherapy of voriconazole was therapeutically effective and superior to the monotherapy of anidulafungin and that the combination therapy did not significantly improve the therapeutic outcome of either of the monotherapy.
Combination therapy of voriconazole and caspofungin in male Guinea pig IA model was demonstrated to be highly effective compared with caspofungin monotherapy, but no differences compared to voriconazole (7). However, another study showed highly effective (15) (survival, P=0.048). Combination therapy of itraconazole and micafungin in female mice IA model significantly improved the efficacy in prolonging survival compared with either of the monotherapy of micafungin (6), while traconazole and micafungin might be antagonistic to each other (9). Petraitis et al. (5) found that combination therapy of ravuconazole and micafungin might increase efficacy, sparing toxicity, or both (P<0.05).
Human study characteristics
A summary of the human study characteristics included in this review is presented in Table 4. The sample sizes of the reviewed human studies varied widely [47-405]. Five of the studies had treatment duration of 12 weeks or 90 days and used mortality as the endpoint.
Table 4. Characteristics of included human studies.
| Studies | Sample sizes | Age mean (years) | Study population | Types of studies |
Treatments |
Treatment duration (days) | End-point | Outcome measure | |
|---|---|---|---|---|---|---|---|---|---|
| Combination | Monotherapy | ||||||||
| Marr KA et al., 2004 (19) | 47 | 45 | HSCT | Cohort | VRC 6 mg/kg q12h IV for 1 day and then 4 mg/kg q12h + CAS 70 mg IV for 1 day and then 50 mg/d | VRC 4 mg/kg q12h IV, AMB 1 mg/kg/day | 90 | Mortality | HR =0.28 (95% CI, 0.1-0.92); P=0.01 |
| Singh N et al., 2006 (20) | 87 | 50 | Organ transplant recipients |
Cohort | VRC 6 mg/kg q12h IV for 1 day and then 4 mg/kg q12h + CAS 70 mg IV for 1 day and then 50 mg/d | L-AMB 5.2 mg/kg/d | 90 | Mortality | HR =0.58 (95% CI, 0.3-1.14); P=0.12 |
| Upton A et al., 2007 (21) | 405 | 40.7 | HSCT | Cohort | VRC + CAS | VRC (before 1996: AMB 0.5 mg/kg/day; after 1996: L-AMB 5 mg/kg/day) | 90 | Mortality | HR =2.3 (95% CI, 0.6-9.4); P=0.23 |
| Rieger CT et al., 2008 (22) | 56 | 46 | Haematological cancer | Cohort | VRC 6 mg/kg q12h IV for 1 day and then 4 mg/kg q12h + CAS 70 mg IV for 1 day and then 50 mg/d | L-AMB 3 mg/kg/d ± CAS 70 mg IV for 1 day and then 50 mg/d or VRC 6 mg/kg q12h IV for 1 day and then 4 mg/kg q12h | 90 | Efficacy; survival | No adjusted analysis |
| Marr KA et al., 2012 (24) | 277 | 51.9 | HSCT and haematological malignancies | RCT | VRC 6 mg/kg q12h IV for 1 day and then 4 mg/kg q12h + AFG 200 mg IV for 1 day and then 100 mg/d | VRC 6 mg/kg q12h IV for 1 day and then 4 mg/kg q12h + placebo | 42 or 84 | Mortality | P=0.09; 95% CI, –19, 1.5 (42 days); P=0.08; 95% CI, –21.4, 1.09 (84 days) |
HSCT, hematopoietic stem cell transplant; AFG, anidulafungin; AMB, amphotericin B; CAS, caspofungin; ICZ, itraconazole; L-AMB, liposomal amphotericin B; MICA, micafungin; POC, posaconazole; RAV, ravuconazole; VRC, voriconazole; IV, intravenous; IP, intraperitoneal; PO, peros (oral); q12h, every 12 h; HR, hazard ratio.
Four studies (19-22) compared the combination therapy of voriconazole and caspofungin with voriconazole, caspofungin, or lipid formulation of amphotericin B. Marr et al. (19) found lower mortality in the combination therapy of voriconazole and caspofungin than monotherapy of voriconazole. Rieger et al. (22) showed that the mortality at the end of treatment of the combination of voriconazole and caspofungin and other treatment was 11% and 34% three months after initiation of combination therapy. Meanwhile, Singh et al. (20) considered that the combination therapy of voriconazole and caspofungin might be a preferable therapy. However, Upton et al. (21) did not observe any significant difference between this combination therapy and either of the monotherapy.
In the RCT (24), 277 patients enrolled from 93 sites in 24 countries were randomised to receive either voriconazole plus placebo (monotherapy) or voriconazole plus anidulafungin (combination therapy). Of the 135 patients who received this combination therapy, 26 (19.3%) died by week 6, compared to 39/142 (27.5%) recipients receiving either of the monotherapy (P=0.09; 95% CI, –18.99, 1.51); 39 (28.9%) died by week 12, compared to 55/142 (38.7%) recipients receiving either of the monotherapy (P=0.08; 95% CI, –21.4, 1.09). The combination therapy of voriconazole and anidulafungin results in a trend towards improved overall survivals compared with monotherapy of voriconazole in patients with proven or probable IA.
Discussion
In this review, to assess the efficacy of the combination therapy of triazole and echinocandin in treatment of IA, we systematically assessed publications on the combination therapy of triazole and echinocandin in treatment of IA, including the animal studies and clinical studies. We found that the survival in the combined therapy groups were significantly improved in the animal studies compared with monotherapy of echinocandin [RR =2.26, (95% CI, 1.79-2.78; P<0.00001)], but no statistical difference compared with monotherapy of triazole [RR =1.19, (95% CI, 0.98-1.44; P=0.08)]. It only suggests that the addition of triazole to echinocandin results in a trend towards improved overall survival in animals with IA. Meanwhile, we also found that the combination therapy of triazole and echinocandin in treating IA also results in a trend towards improved the survival in clinical studies.
To the best of our knowledge, this is the first study to assess the efficacy of the combination therapy of triazole and echinocandin in IA in both animal studies and clinical studies. However, there are some limitations in this review. First, the animal species, infective dosages of Aspergillus, route of infections, and antifungal drugs and doses are different among some animal studies. Second, there may be difference between animals and humans in drug metabolism rate. For an example, the metabolic rate in rodents is faster than in humans. Third, the clinical studies contained only one RCT.
Due to the different targets of triazole and echinocandin, simultaneous inhibition of fungal cell-wall and cell-membrane biosynthesis may result in a synergistic or additive function against Aspergillus. However, we did not find this expected outcome in some animal studies and clinical studies. The possible causes may be ascribed to that the doses of triazole or echinocandin used in animal studies are different.
The area under the curve (AUC)/MIC ratio, a pharmacokinetic/pharmacodynamic (PK/PD) index, is used to predict triazole therapeutic efficacy (25,26) while both the AUC/MIC and the Cmax/MIC are used to predict echinocandin therapeutic efficacy (27,28). However, according to Petraitis et al. (16), anidulafungin was synergistic at a dosage of 5 mg/kg/day but antagonistic at 10 mg/kg/day in the combination with voriconazole, suggesting that a higher dosage of echinocandin may be deleterious to the combination therapy. The reason for this phenomenon may be paradoxical echinocandin activity (29).
The resistance of Aspergillus to triazole may result in decrease of efficacy. According to a study by Seyedmousavi et al. (18), combination therapy of voriconazole and anidulafungin for IA was synergistic in voriconazole-susceptible A. fumigatus, but additive in voriconazole-resistant A. fumigatus.
In the RCT (24), the prolongation of survival, either six weeks (P=0.09) or 12 weeks (P=0.08), results in a trend towards improved in the combination therapy of voriconazole and anidulafungin compared with monotherapy of voriconazole. Of the four human cohort studies, two studies (19,22) observed that the combination therapy of triazole or echinocandin was associated with a significant reduction in mortality compared with other treatments and another study (20) might be a preferable therapy; However, one study (21) revealed that there was no significant difference between the combination therapy and either of the monotherapy. It suggested that the effectiveness of the combination therapy of triazole and echinocandin may be better than either of the monotherapy or other combination. Well-designed RCTs and further improved clinical trials are necessary to study the effectiveness of the combination therapy.
Acknowledgements
Funding: This work was supported by grants from the National Natural Science Foundation of China [grant number 81270064, 81200063].
Disclosure: The authors declare no conflict of interest.
References
- 1.Perfect JR, Cox GM, Lee JY, et al. The impact of culture isolation of Aspergillus species: a hospital-based survey of aspergillosis. Clin Infect Dis 2001;33:1824-33 [DOI] [PubMed] [Google Scholar]
- 2.Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2008;46:327-60 [DOI] [PubMed] [Google Scholar]
- 3.Zmeili OS, Soubani AO. Pulmonary aspergillosis: a clinical update. QJM 2007;100:317-34 [DOI] [PubMed] [Google Scholar]
- 4.Greene RE, Mauskopf J, Roberts CS, et al. Comparative cost-effectiveness of voriconazole and amphotericin B in treatment of invasive pulmonary aspergillosis. Am J Health Syst Pharm 2007;64:2561-8 [DOI] [PubMed] [Google Scholar]
- 5.Petraitis V, Petraitiene R, Sarafandi AA, et al. Combination therapy in treatment of experimental pulmonary aspergillosis: synergistic interaction between an antifungal triazole and an echinocandin. J Infect Dis 2003;187:1834-43 [DOI] [PubMed] [Google Scholar]
- 6.Luque JC, Clemons KV, Stevens DA. Efficacy of micafungin alone or in combination against systemic murine aspergillosis. Antimicrob Agents Chemother 2003;47:1452-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirkpatrick WR, Perea S, Coco BJ, et al. Efficacy of caspofungin alone and in combination with voriconazole in a Guinea pig model of invasive aspergillosis. Antimicrob Agents Chemother 2002;46:2564-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van de Sande WW, Mathot RA, ten Kate MT, et al. Combination therapy of advanced invasive pulmonary aspergillosis in transiently neutropenic rats using human pharmacokinetic equivalent doses of voriconazole and anidulafungin. Antimicrob Agents Chemother 2009;53:2005-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemons KV, Stevens DA. Efficacy of micafungin alone or in combination against experimental pulmonary aspergillosis. Med Mycol 2006;44:69-73 [DOI] [PubMed] [Google Scholar]
- 10.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008;46:1813-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGrath JC, Drummond GB, McLachlan EM, et al. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol 2010;160:1573-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwarz F, Iglhaut G, Becker J.Quality assessment of reporting of animal studies on pathogenesis and treatment of peri-implant mucositis and peri-implantitis. A systematic review using the ARRIVE guidelines. J Clin Periodontol 2012;39Suppl 12:63-72 [DOI] [PubMed] [Google Scholar]
- 13.Wells GA, Shea B, O’connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 3rd Symposium on Systematic Reviews: Beyond the Basics, 2000. [Google Scholar]
- 14.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12 [DOI] [PubMed] [Google Scholar]
- 15.MacCallum DM, Whyte JA, Odds FC. Efficacy of caspofungin and voriconazole combinations in experimental aspergillosis. Antimicrob Agents Chemother 2005;49:3697-701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petraitis V, Petraitiene R, Hope WW, et al. Combination therapy in treatment of experimental pulmonary aspergillosis: in vitro and in vivo correlations of the concentration- and dose- dependent interactions between anidulafungin and voriconazole by Bliss independence drug interaction analysis. Antimicrob Agents Chemother 2009;53:2382-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calvo E, Pastor FJ, Salas V, et al. Combined therapy of voriconazole and anidulafungin in murine infections by Aspergillus flavus. Mycopathologia 2012;173:251-7 [DOI] [PubMed] [Google Scholar]
- 18.Seyedmousavi S, Brüggemann RJ, Melchers WJ, et al. Efficacy and pharmacodynamics of voriconazole combined with anidulafungin in azole-resistant invasive aspergillosis. J Antimicrob Chemother 2013;68:385-93 [DOI] [PubMed] [Google Scholar]
- 19.Marr KA, Boeckh M, Carter RA, et al. Combination antifungal therapy for invasive aspergillosis. Clin Infect Dis 2004;39:797-802 [DOI] [PubMed] [Google Scholar]
- 20.Singh N, Limaye AP, Forrest G, et al. Combination of voriconazole and caspofungin as primary therapy for invasive aspergillosis in solid organ transplant recipients: a prospective, multicenter, observational study. Transplantation 2006;81:320-6 [DOI] [PubMed] [Google Scholar]
- 21.Upton A, Kirby KA, Carpenter P, et al. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis 2007;44:531-40 [DOI] [PubMed] [Google Scholar]
- 22.Rieger CT, Ostermann H, Kolb HJ, et al. A clinical cohort trial of antifungal combination therapy: efficacy and toxicity in haematological cancer patients. Ann Hematol 2008;87:915-22 [DOI] [PubMed] [Google Scholar]
- 23.Rojas R, Molina JR, Jarque I, et al. Outcome of Antifungal Combination Therapy for Invasive Mold Infections in Hematological Patients is Independent of the Chosen Combination. Mediterr J Hematol Infect Dis 2012;4:e2012011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marr KA, Schlamm H, Rottinghaus ST, et al. A randomised, double-blind study of combination antifungal therapy with voriconazole and anidulafungin versus voriconazole monotherapy for primary treatment of invasive aspergillosis. 22nd European Congress of Clinical Microbiology and Infectious Diseases. [Google Scholar]
- 25.Andes D, Marchillo K, Conklin R, et al. Pharmacodynamics of a new triazole, posaconazole, in a murine model of disseminated candidiasis. Antimicrob Agents Chemother 2004;48:137-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andes D, Marchillo K, Stamstad T, et al. In vivo pharmacokinetics and pharmacodynamics of a new triazole, voriconazole, in a murine candidiasis model. Antimicrob Agents Chemother 2003;47:3165-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andes D.Pharmacokinetics and pharmacodynamics in the development of antifungal compounds. Curr Opin Investig Drugs 2003;4:991-8 [PubMed] [Google Scholar]
- 28.Andes D, Diekema DJ, Pfaller MA, et al. In vivo pharmacodynamic characterization of anidulafungin in a neutropenic murine candidiasis model. Antimicrob Agents Chemother 2008;52:539-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiederhold NP. Paradoxical echinocandin activity: a limited in vitro phenomenon? Med Mycol 2009;47Suppl 1:S369-75 [DOI] [PubMed] [Google Scholar]