Abstract
A 58-year-old woman underwent radical proctectomy 19 months prior to admission. The initial diagnosis was rectal adenocarcinoma of pathological stage T2N0M0. She was discharged five days after the operation. She was followed by abdominal computed tomographic (CT) scan at 3, 9 and 18 months after the operation. Eighteen months after the operation, follow-up abdominal CT scan revealed tiny nodules in the bilateral lower lobes. Subsequent CT scan of the chest showed two tiny nodules in the right lower lobe and a single tiny nodule in left lower lobe. She then underwent single port thoracoscopic surgery through the right side for resection of the nodules. Using a single port wound, we excised the two tiny nodules on the right side and the one tiny nodule in the left lower lobe across the mediastinum. She was discharged four days later. The final pathology report showed those three nodules were metastases from an adenocarcinoma in the colon.
Keywords: Empyema, single-incision thoracoscopic surgery (SITS), uniportal, thoracoscopy/video-assisted thoracic surgery (VATS), metastectomy
Introduction
The current trend of management of metastatic colon cancer is to a more aggressive approach. Along with the advancement of endoscopic techniques, metastectomy of the lung is performed using an endoscopic approach. Conventional thoracoscopic surgery typically utilizes three or more incisions for an operation. An endoscopic approach has many advantages, but one potential problem of pulmonary metastectomy is the difficulty of identifying very tiny nodules during the course of the operation. Because direct palpation of the lung is not amenable, instrument palpation has become an alternative method to confirm the location of such nodules. At present, thoracoscopic surgery can be performed with a certain single port techniques (1-3). In the past, the single-port techniques were limited to the management of unilateral pleural space. In this case, we have extended the range to the bilateral pleural space. We used a unilateral single-port approach to excise bilateral lung lesions, a procedure which has hardly ever been performed, even with conventional multi-port techniques.
Case
A 58-year-old woman was found to have rectal cancer on the performance of an annual health exam two years ago. At that time, she underwent radical proctectomy after complete cancer survey. The final pathology report showed adenocarcinoma with a depth of invasion to the level of the submucosa and muscularis propria. Lymph nodes were metastasis free. The pathology stage was T2N0M0. Her postoperative recovery was adequate and she was discharged five days after the operation. Because of the stage I status, she did not receive any chemotherapy. She was followed in the outpatient department.
In the later period of follow-up, she regularly underwent chest radiograph (CXR), whole abdominal computed tomographic (CT) scan, and various blood tests, including an evaluation of the serum level of carcinoembryonic antigen (CEA) at 3, 9 and 18 months after the operation. The CEA level was normal. CXR also displayed a normal pattern. The whole abdominal CT scan at 18 months revealed suspicious tiny lesions in the bilateral lower lung fields. One month later, she was examined with a CT scan of the chest, which showed two tiny nodules in the right lower lobe (Figure 1A,B) and another tiny lesion in the left lower lobe (Figure 1C). The size of the two peripheral lesions in the right lower lobe was 0.5 cm × 0.3 cm and 0.3 cm × 0.2 cm, respectively. The lesion in the left lower lobe was 0.3 cm × 0.3 cm. Because of the presence of newly developed neoplasms, lung metastasis was considered. After discussing with the patient about the choices of either follow-up three months later or thoracoscopic biopsy, she preferred immediate surgical biopsy.
Figure 1.
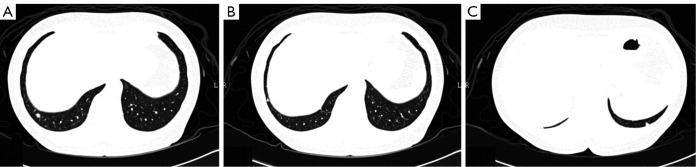
CT scan of the chest showed that there was one 5 mm nodule (A) and one 2-3 mm nodule (B) in the periphery of the right lower lobe. In the contralateral left lower lobe, there was a 3 mm nodule near the pleural surface (C).
The preoperative evaluation revealed the forced expiratory volume in the span of one second to be 2.21 liters, and the blood tests were all normal. The CEA level was 2.1. CT scan of the abdomen showed no local recurrence and there was no evidence of liver metastasis. After excluding the possibility of extra-pulmonary metastasis, she was prepared for surgical biopsy.
She received general anesthesia in a supine position and was intubated with a Fr. 35 double-lumen endotracheal tube. Then she was changed to a left lateral position and the operative field on the right side was disinfected and draped. We used the single-port approach reported by our team previously (1,2). The basic tools used included a plastic wound protector, a 5-mm endoscope with a 30-degree field-of-view, an endoscopic grasp and a stapler. An L-hook electrical cautery was used for dissection of the mediastinum. In consideration of an effective contralateral dissection, the selection of incision is critically important. We made the 2.5 cm incision in the 6th intercostal space and the incision was slightly medial to the anterior axillary line. We initially approached the right lower lobe lesion from an anterior approach, that is, the surgeon stood on the ventral site of the patient, from which position it was easier to carry out single port surgery. A 5 mm right lower lobe lesion (of 5-mm size) in the periphery was identified (the green arrow in Figure 2A), but the 3-mm nodule could not be seen on an endoscopic view. Because the two nodules were very close to each other, we used a stapler to excise the lung tissues that were thought to contain the two tiny lesions (Figure 2B). After the specimen extracted, the 5- and 3-mm nodules were palpated and then sent for frozen sectioning, which indicated metastatic adenocarcinoma. Before approaching the contralateral site, we changed the operator’s position to the patient’s back and then the operative table was slightly tilted backwards by approximately 30 degrees for a better angle of approach. Then we dissected the retrosternal soft tissues at the junction of heart, diaphragm and sternum (Figure 2C). With assistance of a ring forceps to push down the soft tissues and pericardial fat (Figure 2D), an L-hook electrical cautery was used to dissect the mediastinal pleura that is just beneath the sternum (Figure 2E). Using both electrical cauterization and blunt dissection, we created a safe entry route to the contralateral pleura (Figure 2F). Then the pleura was opened and we entered the left side pleural space (Figure 2G). The 3-mm nodule in the periphery of the left lower lobe was not identified endoscopically. Based on the CT scan of the lung, we excised the part of left lower lobe that was considered to contain the lesion (Figure 2H). After pull-out of the specimen, careful palpation showed a very tiny nodule. Frozen sectioning of the surface of the nodule displayed metastatic adenocarcinoma. After confirming lung metastasis, distilled water was used to irrigate the pleural space. Then a Fr. 24 tube was placed in the left side pleural space through the mediastinum, after which a Fr. 28 chest tube was place in the right side pleural space (Figure 3A).
Figure 2.
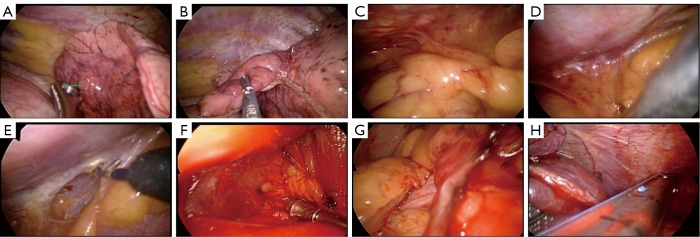
A peripheral nodule was palpated with an instrument in the right lower lobe (the green arrow in A). However, a second tiny nodule could not be palpated. We excised the related part of the lung because we knew the smaller nodule was adjacent to the larger nodule (B). After the completion of a wedge resection of the right lower lobe, we then sought a safe location at the junction of the heart, sternum and diaphragm (C). A ring-forceps was used to hold the mediastinal pleura downwards (D), and then an L-hook electrical cautery was used to open the mediastinal pleura (E). Blunt dissection was performed with either a ring-forceps or suction tube (F) until the contralateral pleura was opened (G). We then identified the suspicious lung tissues for resection with a stapler (H).
Figure 3.
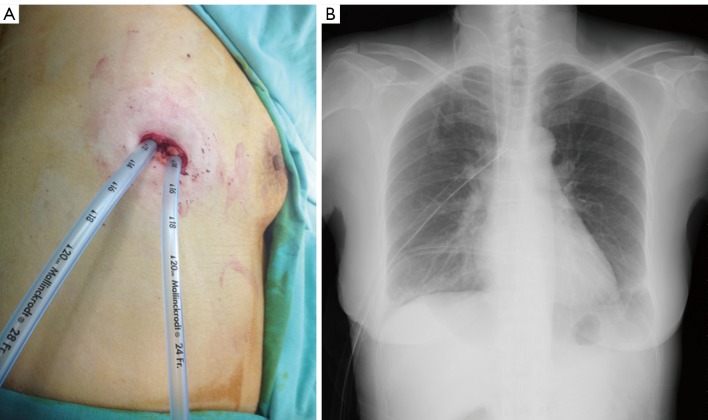
After the procedure, we placed two chest tubes in the respective bilateral pleural spaces. The two tubes were fixed in the same single wound (A). A chest radiograph was taken to confirm their location and function (B).
Immediate postoperative CXR indicated adequate lung expansion that was effusion free (Figure 3B). The postoperative course was uneventful. The Fr. 24 chest tube was removed 48 hours after the operation and the Fr. 28 chest tube was removed 72 hours after the operation. She was then discharged for subsequent chemotherapy.
Discussion
The management of colorectal cancer with distal metastasis has come to be aggressive, because the overall outcome has improved as a result. The cases reviewed by Kawano et al. showed that metastectomy may be of benefit for selected cases with better long-term survival (4). In some patients with very early metastasis to the lung, the lesions may be extremely small and not easy to identify by imaging alone (5). Therefore, surgical biopsy has a role in deciding the management plan of colorectal cancer. Follow-up at a certain interval may be an option, but may also delay treatment planning.
Conventional thoracoscopic surgery utilizes three or more incisions. In this case, we used the single-port approach proposed by us previously (1,6). This technique is comprised of four parts or concepts. The first is the use of a minimal incision to carry out procedures whenever possible. The theoretically smallest incision is just small enough to allow the specimen to be pulled out or to allow the instruments to be placed into the pleural space if there is no specimen to be extracted. The second guiding concept is to use an anterior incision. An incision in the 5th to 6th intercostal space (ICS) on the anterior axillary line is an advantageous site for a single-port approach because of the wider ICS (2,3). However, in considering the approach to the contralateral site, the surgeon must change positions to the patient’s back. We tilted the operating table in order to create a more comfortable approaching angle for the surgeon (Figure 4). The third concept is to avoid the use of a rigid trocar, so we used a plastic wound protector instead (Figure 4). A rigid trocar limits the freedom of instrument handling. A wound protector may afford the maximal degree of freedom for instrument manipulation. The fourth concept or principle is to always try a single port approach at first. If necessary, there is always the option of converting to a conventional multi-port approach or even an open method. Using these same guiding principles, we extended the application of single-port techniques to the treatment bilateral disease.
Figure 4.
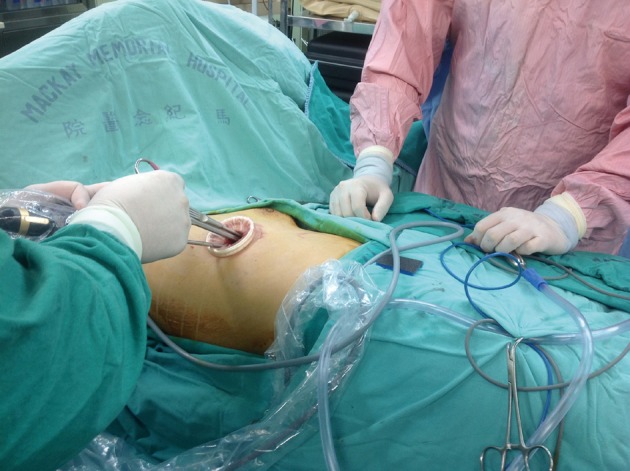
In contrast to the single-port techniques proposed by us that using a ventral location for the operation, the position was changed to the patient’s back in order to provide an adequate approach to the contralateral lesion.
There are multiple technical considerations which must be borne in mind. The first one is the entry route through the mediastinum must be kept safe. In the case described here, we created a retrosternal route slightly above the level of diaphragm while avoiding compression of the heart. If the entry route is higher in the level of heart, direct compression of the cardiac chambers may carry an additional risk of arrhythmia and/or hypotension during the procedure. The bilateral internal mammary arteries should not be transected. If the plane is correct, blunt dissection with minimal electrical cauterization is sufficient to create the space within a matter of minutes. The potential space across the mediastinum created in this way is prone to close if the endoscopic instruments are not in place. We still lack an effective tool for keeping the space open without any additional instruments to push the soft tissues downwards. This makes the procedures more complex and difficult in the single-port approach than is optimal. For the lesion in the contralateral lower lobe, the space we created in this case was sufficient. However, if the lesion is in another location that is more challenging, a different entry site should be considered.
A prospective study performed in 1996 showed that thoracoscopic identification metastatic lung nodules may be difficult and had higher failure rate (7). However, the techniques of thoracoscopic surgery and resolution of CT improved a lot in recent 15 years. At present time, high-resolution CT scan can detect pulmonary lesion around 2 to 3 mm in diameter. Endoscopic techniques also allowed us to detect superficial lesion more than 3 mm in diameter. However, for deep parenchymal lesions, detection may be very difficult. Even in ipsilateral side, CT-guided needle localization may be required.
The identification of tiny nodules in the contralateral lung using our method may be usefully assisted by CT-guided needle localization. In the absence of a needle localization method, the identification of a tumor less than 3 mm is very difficult. Endoscopic inspection and instrument palpation are the only two means of confirming the location. Based on our experience, contralateral small nodules should be localized under CT-guidance.
The field-of-view is limited in this procedure. With some amount of improvement in the design of the endoscope, the field-of-view may be greatly improved (8). The manipulation is also limited by the current straight endoscopic instruments as well as the stapler. Therefore, the approach should be considered best suited to highly selected cases. Not all patients with bilateral lung tumors can be treated by this method. Another consideration is that when malignant effusion is encountered, the opening of the mediastinum may make the possibility of mediastinal metastasis a serious concern. Moreover, in a particularly complicated condition, such as adhesion, the approach may not be feasible and additional incisions or a contralateral incision may be required.
Conclusions
This report demonstrates the value of single-port thoracoscopic surgery in treating bilateral pleural and lung disease. It should be considered as an alternative time-saving method for certain conditions in which the simultaneous treatment of bilateral disease is required.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Chen CH, Chang H, Lee SY, et al. Single-port thoracoscopic surgery can be a first-line approach for elective thoracoscopic surgery. Rev Port Pneumol 2012;18:278-84 [DOI] [PubMed] [Google Scholar]
- 2.Chen CH, Lee SY, Chang H, et al. Technical aspects of single-port thoracoscopic surgery for lobectomy. J Cardiothorac Surg 2012;7:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen CH, Lee SY, Chang H, et al. The adequacy of single-incisional thoracoscopic surgery as a first-line endoscopic approach for the management of recurrent primary spontaneous pneumothorax: a retrospective study. J Cardiothorac Surg 2012;7:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawano D, Takeo S, Tsukamoto S, et al. Prediction of the prognosis and surgical indications for pulmonary metastectomy from colorectal carcinoma in patients with combined hepatic metastases. Lung Cancer 2012;75:209-12 [DOI] [PubMed] [Google Scholar]
- 5.McLeish AR, Lee ST, Byrne AJ, et al. Impact of 18F-FDG-PET in decision making for liver metastectomy of colorectal cancer. ANZ J Surg 2012;82:30-5 [DOI] [PubMed] [Google Scholar]
- 6.Chen CH, Chang H, Tseng PY, et al. A rare case of dysphagia and palpitation caused by the compression exerted by an enormous mediastinal lipoma. Rev Port Pneumol 2012;18:149-52 [DOI] [PubMed] [Google Scholar]
- 7.McCormack PM, Bains MS, Begg CB, et al. Role of video-assisted thoracic surgery in the treatment of pulmonary metastases: results of a prospective trial. Ann Thorac Surg 1996;62:213-6; discussion 216-7 [DOI] [PubMed] [Google Scholar]
- 8.Chen CH, Chang H, Yang LY, et al. A preliminary report of a disposable electrical non-fiberoptic endoscope in thoracoscopic surgery. Int J Surg 2012;10:20-4 [DOI] [PubMed] [Google Scholar]