Abstract
Background. Canine babesiosis is a clinically important hemoprotozoan parasite affecting dogs. The goal of this present study was to determine the clinical symptoms and to establish its hematological and microscopic detection and compare it with the PCR findings attained from dogs infected with Babesia canis vogeli. Methodology/Principal Findings. 13-PCR confirmed Babesia-infected dogs were examined; seminested PCR was used to discover the precise type of Babesia and Babesia canis vogeli was the only subspecies detected. The most consistent clinical signs were elevated rectal temperature and a pale mucous membrane. Thrombocytopenia, monocytosis, and lymphocytosis, along with a significant reduction in red cell parameters, were the most commonly recorded hematologic alterations. Microscopic examination revealed the presence of typical large merozoites and trophozoites of B. canis in the ratio 76.92%. Conclusions/Significance. The presumptive diagnosis of canine babesiosis should be based on a fever and anemia, while thrombocytopenia is considered the hallmark of the disease; microscopic examination may not be very revealing in the detection at low parasitemia, but it remains the most rapid confirmatory method. Seminested PCR turned out to be a sensitive and accurate method for diagnosis; during the process of differentiation between Babesia subspecies, only B. canis subsp. vogeli was detected.
1. Introduction
Canine babesiosis is a clinically noteworthy and well-known hemoprotozoan parasite of dogs [1]. The first incidence of Babesia was reported in South Africa by Hutcheon in 1885 but was recognized much later in 1896 by Purvis and in 1897 by Koch [2]. Babesia normally causes massive injuries to the host depending on the virulence and pathogenicity of the parasite. The extent of the injuries also depends on the age and the hygiene of the host [3]. The commonly occurring Babesia species in dogs are B. canis and B. gibsoni [4]. B. canis is endemic in Southern Europe, America, Asia, and South Africa while B. gibsoni is found in the Middle East, Northern Africa, and South Asia [5]. In Egypt, only B. canis has been detected [6].
B. canis is a typical intraerythrocyte pear-shaped large piroplasm, frequently found in pairs within the RBCs as identified among dogs worldwide [7–9]. There are three different subspecies, B. canis, B. rossi, and B. Vogeli, which are morphologically alike but have different vectors and pathogenicity [10]. R. sanguineus is the main global vector, though D. reticulatus may serve as a vector for B. canis in Europe. Other Ixodid ticks belonging to the genera, namely, Dermacentor, Haemaphysalis, and Hyalomma are also capable of serving as vectors [11]. The life cycle of Babesia includes 2 stages: inside the host RBCs, in which the sporozoites convert into piroplasms, and the other inside the tick vector [10]. Clinically, the illness is characterized by fever, pale mucous membrane, anorexia, anemia, icterus, lymphadenopathy, and splenomegaly [12–15]. Clinical signs are exceedingly variable; the classical presentation is a febrile illness with perceptible anemia [16]. The severity of babesiosis varies from subclinical infection to extensive organ failure and death [1].
The most common hematologic alterations associated with B. canis infections are anemia and thrombocytopenia [13, 16–19].
Discrimination between subspecies of B. canis on the basis of blood smear examination is almost impractical. Today, polymerase chain reaction is being utilized to diagnose and distinguish between the different infections caused by various Babesia subspecies [20–22].
In Egypt, canines are infected by numerous infections, of which Ehrlichiosis and Babesiosis are the most significant enzootic ailments. In these areas though, dogs are pre-immunized against piroplasmosis and act as a reservoir of infection. However, this immunity may collapse under unfavorable conditions and all such animals become clinically infected [23].
The objectives of this study were to describe clinical signs and hematological alterations and to verify the results obtained by blood smear examination using molecular recognition to identify the subspecies of Babesia involved.
2. Material and Methods
Thirteen dogs of different age, sex, and breed were used in this study; the dogs were referred to a small animal medicine teaching hospital, faculty of veterinary medicine, Cairo University, Egypt, with signs attuned with babesial infection. The age, sex, breed, and locality of both control and infected dogs are listed in Table 1. The survey was conducted from March 2011 to March 2012.
Table 1.
Age, sex, breed, and locality of control and infected dogs.
| Criteria | Infected dogs | Control dogs |
|---|---|---|
| Breed | German shepherd [10] | German shepherd [7] |
| Great dane [1] | Great dane [2] | |
| Labrador [1] | Labrador [2] | |
| Malino [1] | Malino [1] Rotweiler [1] |
|
|
| ||
| Age | <3 yrs [4] | <3 yrs [6] |
| 3–5 yrs [6] | 3–5 yrs [5] | |
| >5 yrs [3] | >5 yrs [2] | |
|
| ||
| Sex | Male [10] | Male [6] |
| Female [3] | Female [7] | |
|
| ||
| Locality | Giza | Giza |
The selection of control dogs depended mainly on these considerations: no prior or current tick presentation, absence of clinical signs, failure to develop the specific Babesia DNA amplicon on two consecutive seminested PCRs 1-month apart, and the absence of IgG in ELISA test; these animals were free from internal parasites and vaccinated.
Each dog was subjected to inclusive physical examination; the rectal temperature at the time of admission and clinical signs were recorded. During clinical testing, the presence of ticks on the coat of dogs was determined. Ticks were collected and identified [24]. Capillary blood samples collected from the ear vein and Giemsa-stained thin blood films were produced and examined under the microscope for direct detection of intraerythrocytic stages of the hemoparasite [25].
3. Hematologic and Molecular Study
Venous blood was collected from the cephalic vein with appropriate restrain; clinical hematology was done within 2 hours after this collection according to methods described by Feldman et al. [26]. DNA extraction was performed on the whole blood sample using Genomic DNA purification (Jena Bioscience GmbH, Jena, Germany) and DNA extraction was performed according to manufacturer's instructions. DNA extraction and PCR were performed in separate rooms.
Oligonucleotide primers (Table 2) were designed based on the canine Babesia 18S rRNA genes as described by Benson et al. [27]. PCR was performed according to Birkenheuer et al. [28]. The thermal cycler (Primus MWG, Bio tech, Germany) was performed for DNA amplification of the outer primer pair at the following temperatures: 95°C for 5 min, followed by 50 amplification cycles (95°C for 1 min, 56°C for 1 min, and 72°C for 1 min), and a final extension step at 72°C for 5 minutes. The seminested PCRs (i.e., specific forward primers paired with the outer reverse primer) were each carried out in separate tubes under the same conditions as the outer primer pair, except for the following: 0.5 μL from the initial reaction was used as a DNA template and the reactions were amplified for 35 cycles.
Table 2.
Primers sequence for canine babesiosis according to Benson et al., (2002) [27].
| Primer | Sequence | Reaction and/or use | Expected amplicon |
|---|---|---|---|
| 455-479F | GTCTTGTAATTGGAATGATGGTGAC | Seminested PCR outer forward primer | 340 bp |
| 793-772R | ATGCCCCCAACCGTTCCTATTA | Seminested PCR outer reverse primer | |
| BgibAsia-F | ACTCGGCTACTTGCCTTGTC | Seminested PCR B. gibsoni (Asian genotype) specific forward primer | 185 bp |
| BCV-F | GTTCGAGTTTGCCATTCGTT | Seminested PCR B. c. vogeli specific forward primer | 192 bp |
| BCC-F | TGCGTTGACGGTTTGACC | Seminested PCR B. c. canis specific forward primer | 198 bp |
| BCR-F | GCTTGGCGGTTTGTTGC | Seminested PCR B. c. rossi specific forward primer | 197 bp |
A descriptive analysis using Student's t-test (statistica for Windows, version 5.1., StatSoft, Inc. 1984–1996) to compare between infected and control dogs was obtained; P value of 0.05 or lower was considered to be significant.
4. Results
Pertinent signalment (age, sex, and breed of the patients) are listed in Table 1. The highest infection was found in the age group of 3–5 yrs, followed by the age group of less than 3 years. German shepherd dogs were the most affected breed, and the male dogs appeared to be more infected than the female dogs.
The ticks were identified as Rhipicephalus sanguineus (on the basis of morphological characteristics).
The most consistent clinical signs observed during the examination were elevated rectal temperature (above 39.6°C) in 12 out of 13 dogs (92.07%), pale mucous membrane in 8/13 dogs (61.5%), anorexia 12/13 dogs (92.07%), enlargement of lymph nodes 10/13 dogs (76.92%), splenomegaly 12/13 dogs (92.07%), and presence of ticks 10/13 dogs (76.92%). Less common clinical signs included haematuria in 2/13 dogs (15.38%) and icterus in 2/13 dogs (15.38%).
Examination of Giemsa-stained thin blood smear revealed the presence of typical large merozoites and trophozoites of B. canis (Figure 1) in 10/13 dogs (75.9%).
Figure 1.
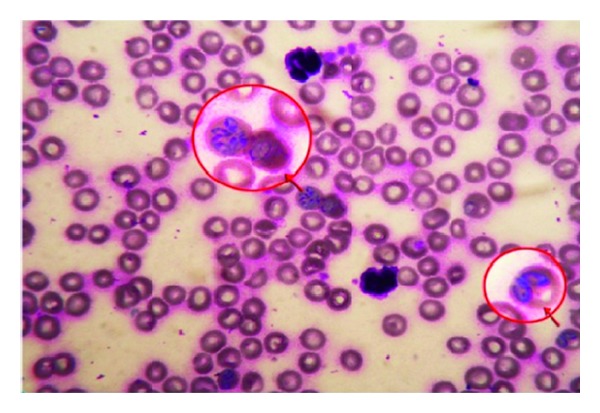
Giemsa-stained blood smear of infected dog showing the pear-shaped large B. canis inside the RBCs (×1000).
Hematologic findings were compared to hematologic parameters of control dogs from the same locality (Table 3). Significant reduction in RBCs, HB content, and PCV percentage (P < 0.05) were noted among infected dogs compared to control dogs; 61.5% of dogs showed RBCs count below reference values, while RBCs count was within reference values in 38.5% of the dogs. Haematocrite percentage was below reference values in 53.8% of dogs, while 46.2% of dogs had haematocrite within reference range. The content of hemoglobin was below normal reference values in 53.8% of dogs, while 46.2% of dogs came up with hemoglobin content within normal values.
Table 3.
Hematologic findings of 13 dogs with babesiosis from small animal internal medicine teaching hospital, faculty of veterinary medicine, Cairo University.
| Parameter | Patient data | Control data1 | Reference range2 |
|---|---|---|---|
| RBCs (×106/μL) | 4.05 ± 0.8a | 6.41 ± 1.09b | 5.5–8.5 |
| Hemoglobin (g/dL) | 11.375 ± 0.61a | 14.74 ± 2.07b | 12.0–18.0 |
| PCV (%) | 32.33 ± 7.00a | 43.14 ± 6.44b | 37–55 |
| MCV (fL) | 74.67 ± 13.2 | 68.14 ± 6.86 | 66–77 |
| MCH (pg) | 25.25 ± 5.46 | 23.01 ± 2.92 | 21.0–26.2 |
| MCHC (%) | 32.17 ± 3.72 | 33.56 ± 1.77 | 32.0–36.3 |
| WBCs (×103/μL) | 9.650 ± 1.4a | 12.850 ± 1.61b | 6,000–17,000 |
| Neutrophil (×103/μL) | 3.787 ± 0.92a | 6.728 ± 0.89b | 3,000–11,500 |
| Lymphocyte (×103/μL) | 4.583 ± 1.33 | 4.928 ± 0.37 | 1,000–4,800 |
| Monocyte (×103/μL) | 1.062 ± 0.196a | 0.771 ± 0.13b | 0.1–1.4 |
| Platelets (×103/μL) | 93.667 ± 15.0a | 268.93 ± 14.2b | 200,000–500,000 |
(ab)Different letters on the same line indicate statistically different means (P < 0.05); confidence interval: 95%.
1Control data was done from apparently healthy dogs tested negative on two PCR cycles with 1-month period; 2Rizzi et al. [29].
Red cell indices showed a significant increase in MCV in 38.4% of the dogs, and 15.4% of dogs showed MCV below the normal range with 46.2% of the dogs having MCV within normal reference values. Decrease in MCHC values was noted in 38.4% of the dogs, and an increase in MCHC values in 15.4% of dogs with 46.2% of the dogs having MCHC within the normal reference values was noted.
Significant decrease in WBCs count and neutrophils along with an increase in monocyte count was noted in infected dogs compared to control dogs. Leukocytes abnormalities include neutropenia in 84.6% of dogs, lymphocytosis in 69.2% of dogs, and monocytosis in 69.2% of the dogs.
Significant decrease in platelet count was noted in infected dogs compared to control dogs. Thrombocytopenia is the most consistent hematologic abnormality observed in 100% of the affected dogs.
During the primary reaction of seminested PCR, a 339 bp product (Figure 2) was amplified from infected canine whole blood samples (n = 13). During the secondary seminested reaction, the test was able to differentiate between B. gibsoni (Asian genotype), B. canis subsp. vogeli, B. canis subsp. canis, and B. canis subsp. rossi. After the second round only, B. canis subsp. vageli and 200 bp amplicon were visualized on the gel in all 13 samples.
Figure 2.
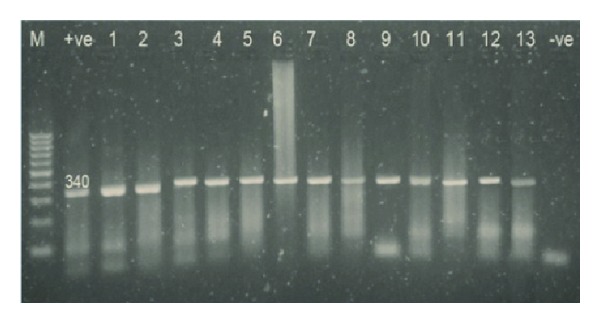
Analysis of seminested PCR products (outer primer pair only) by 1.5% agarose gel electrophoresis and ethidium bromide staining showing the positive samples after the primary amplification cycle.
5. Discussion
Until now, especially in Egypt, canines did not receive much attention from scientists as compared to other species, and many findings about them are still unfinished studies. Canines in this country are infected by numerous infections, of which Ehrlichiosis and Babesiosis are the most significant enzootic illnesses [23].
In this study, highest occurrence of canine babesiosis was in the age group 3–5 years followed by those below 3 years; Babesia infection has been known to rise with the age, reaching its peak between the age of 3 and 5 years and then peter out [30]. The male dogs reported higher infection rate than the female ones, though Martinod et al. [31] found no difference in sex susceptibility between males and females; The aggressiveness and hormonal status of male dogs may be a contributory factor here [32]. German shepherd dogs appeared to be the breed with the highest infection rate. The popularity of this breed in Egypt could be the cause for it [33].
Rhipicephalus sanguineus was the only tick detected in our study; R. sanguineus is a three-host tick and well tailored to rural areas [34] and it thrives as the biological vector of canine babesiosis [35].
The main clinical signs observed were fever, lymphadenopathy, splenomegaly, and anorexia; these signs were reported by other authors in different studies too [4, 13]. Though the clinical presentation of canine babesiosis can be highly variable, the classical presentation can be safely described as febrile illness with apparent anemia [16]. Babesia subspecies can cause different clinical presentations. B. rossi is the most virulent subspecies causing acute renal failure and acute respiratory distress syndrome [36], and B. vogeli is the least virulent causing mild clinical illness that may develop to severe fatal haemolytic anemia in puppies [37]. Several blood borne infections may share one or more clinical presentation(s) of babesia, such as Ehrlichia spp., Anaplasma spp., Bartonella spp., Rickettsia spp., and Leishmania [1]; thus, relying solely on the clinical signs will lead to an inaccurate diagnosis.
Febrile illness was observed in canine babesiosis-infected dogs; this may be attributed to the release of endogenous pyrogens from erythrolysis, parasitic destruction, and activation of inflammatory mediators [6]. Administration of 2 doses of imidocarb (2 weeks apart) is effective in clearing the organism from blood [14]. However, in these areas dogs are preimmunized against piroplasmosis and act as a reservoir of infection. This immunity though may collapse under unfavorable circumstances and such animals could become clinically infected [23].
When studied microscopically, a typical pear-shaped large B. canis was detected inside the RBCs in 10 out of the 13 blood smears; The diagnosis of canine babesiosis mainly relies on microscopic examination and a trained personnel can differentiate between B. gibsoni and B. canis based on morphology in Giemsa-stained blood films [9, 11, 38–40]. Thus, the parasite was not detected in 3 blood smears (23.07%). The divergence among the microscopic results observed may be a case of the lower sensitivity of the microscopic examination method [41]. The tick population, climatic condition, geographic region, and individual response to hemoprotozoan play important roles in the incidence of the infection [42].
Anemia, thrombocytopenia, and monocytosis were the most hematologic alterations observed. The destruction of circulating red cells by auto antibodies is directed against infected and noninfected red cell membranes resulting in intravascular and extravascular haemolysis [43–45]. However, Taboada and Lobetti [46] proposed that direct parasitic damage contributes to anemia. Nevertheless, induction of serum hemolytic factors increased erythrophagocytic activity of macrophages and damage induced by secondary immune system after the formation of antierythrocyte membrane antibodies also proved important in the pathogenesis of anemia.
Thrombocytopenia with no obvious hemorrhage observed either by the owner or during the examination, platelet destruction, increase platelet sequestration, and decrease platelet production, could be linked to thrombocytopenia [47, 48].
In Egypt, only B. canis was detected via PCR; B. canis was the common Babesia subspecies recorded in Egyptian dogs [6, 23, 33, 42]. B. canis subsp. vogeli was detected using seminested PCR, when compared to microscopic examination results; PCR proved the superiority and the sensitivity in the diagnosis and differentiation of Babesia infection in suspected dogs [1, 37, 49, 50]; however, the cost, equipment, and time may be a major limitation for the use of PCR in clinic-based practice.
The results of this study showed dogs with fever, lymphadenopathy, splenomegaly, anorexia, and pale mucous membrane can be suspected to carry infection related to Babesia. However, microscopic examination may not detect low parasitemia though; it remains the most rapid confirmatory method. Thrombocytopenia is considered the hallmark of the disease, and seminested PCR was a sensitive and accurate method for diagnosis and differentiation between Babesia subspecies. Only B. canis subsp. vogeli was detected in the course of this study.
Acknowledgment
The authors thank members of the Biotechnology Research Unit (ARRI), Egypt, for supplying facilities for molecular analysis.
Conflict of Interests
The authors whose names are listed on this paper certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements) or nonfinancial interest in the subject matter or materials discussed in this paper.
References
- 1.Irwin PJ. Blood, Bull terriers and babesiosis: a review of canine babesiosis. Proceedings of the 32nd World Small Animal Veterinary Congress Association (WSAVA '07); August 2007; Sydney, Australia. [Google Scholar]
- 2.Lobetti RG. Canine babesiosis. Compendium on Continuing Education for the Practicing Veterinarian. 1998;20(4):418–430. [Google Scholar]
- 3.Leschnik M, Kirtz G, Leidinger E. Seasonal occurrence of canine babesiosis is influenced by local climate conditions. Proceedings of the 9th International Jena Symposium on Tick-Borne Diseases; 2007; Jena, Germany. [Google Scholar]
- 4.Taboada J, Merchant SR. Babesiosis of companion animals and man. Veterinary Clinics of North America. 1991;21(1):103–123. doi: 10.1016/s0195-5616(91)50011-5. [DOI] [PubMed] [Google Scholar]
- 5.Macintire DK. DVM News Magazine. 2003. Canine babesiosis continues to create challenges for practitioners; pp. 1–4. [Google Scholar]
- 6.Sakla AA. Studies on Ticks in Assuit Governorate, with special reference to their role in transmission of parasitic diseases [Ph.D. thesis] Egypt: Faculty of Medicine, Assuit University; 1975. [Google Scholar]
- 7.Uilenberg G, Franssen FF, Perié NM, Spanjer AA. Three groups of Babesia canis distinguished and a proposal for nomenclature. Veterinary Quarterly. 1989;11(1):33–40. doi: 10.1080/01652176.1989.9694194. [DOI] [PubMed] [Google Scholar]
- 8.Zahler M, Schein E, Rinder H, Gothe R. Characteristic genotypes discriminate between Babesia canis isolates of differing vector specificity and pathogenicity to dogs. Parasitology Research. 1998;84(7):544–548. doi: 10.1007/s004360050445. [DOI] [PubMed] [Google Scholar]
- 9.Homer MJ, Aguilar-Delfin I, Telford SR, III, Krause PJ, Persing DH. Babesiosis. Clinical Microbiology Reviews. 2000;13(3):451–469. doi: 10.1128/cmr.13.3.451-469.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uilenberg G. Babesia—a historical overview. Veterinary Parasitology. 2006;138(1-2):3–10. doi: 10.1016/j.vetpar.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 11.Georgi JR, Georgi ME. Canine Clinical Parasitology. Philadelphia, Pa, USA: Lea & Febiger; 1992. [Google Scholar]
- 12.Breitschwerdt EB, Malone JB, MacWilliams P, Levy MG, Qualls CW, Jr., Prudich MJ. Babesiosis in the Greyhound. Journal of the American Veterinary Medical Association. 1983;182(9):978–982. [PubMed] [Google Scholar]
- 13.Boozer AL, Macintire DK. Canine babesiosis. Veterinary Clinics of North America. 2003;33(4):885–904. doi: 10.1016/s0195-5616(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 14.Alleman AR. The diagnosis and treatment of tickborne disease in dogs. Proceedings of the North American Veterinary Conference (NAVC '05); Orlando, Fla, USA. pp. 472–477. [Google Scholar]
- 15.Lobetti R. Canine and feline babesiosis. Proceedings of the 33rd World Small Animal Veterinary Association Congress (WSAVA '08); August 2008; Bryanston, South Africa. Bryanston Veterinary Hospital; [Google Scholar]
- 16.Schetters TPM, Kleuskens JAGM, van de Crommert J, de Leeuw PWJ, Finizio A-L, Gorenflot A. Systemic inflammatory responses in dogs experimentally infected with Babesia canis; a haematological study. Veterinary Parasitology. 2009;162(1-2):7–15. doi: 10.1016/j.vetpar.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Lobetti R. Hematological changes associated with tick-borne diseases. Proceedings of the World Animal Veterinary Association World Congress; 2004; Rhodes, Greece. [Google Scholar]
- 18.Zygner W, Gójska O, Rapacka G, Jaros D, Wędrychowicz H. Hematological changes during the course of canine babesiosis caused by large Babesia in domestic dogs in Warsaw (Poland) Veterinary Parasitology. 2007;145(1-2):146–151. doi: 10.1016/j.vetpar.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 19.van de Maele I, Savary-Bataille K, Gielen I, Daminet S. Case report: an unusual form of canine babesiosis. Canadian Veterinary Journal. 2008;49(3):283–286. [PMC free article] [PubMed] [Google Scholar]
- 20.Cacciò SM, Antunovic B, Moretti A, et al. Molecular characterization of Babesia canis canis and Babesia canis vogeli from naturally infected European dog. Veterinary Parasitology. 2002;106(4):285–292. doi: 10.1016/s0304-4017(02)00112-7. [DOI] [PubMed] [Google Scholar]
- 21.Inokuma H, Yoshizaki Y, Matsumoto K, et al. Molecular survey of Babesia infection in dogs in Okinawa, Japan. Veterinary Parasitology. 2004;121(3-4):341–346. doi: 10.1016/j.vetpar.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Oyamada M, Davoust B, Boni M, et al. Detection of Babesia canis rossi, B. canis vogeli and Hepatozoon canis in dogs in a village of Eastern Sudan by using a screening PCR and sequencing methodologies. Clinical and Diagnostic Laboratory Immunology. 2005;12(11):1343–1346. doi: 10.1128/CDLI.12.11.1343-1346.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Safey AA. Studies on some blood parasites in dogs [M.S. thesis] Egypt: Faculty of Veterinary Medicine, Cairo University; 2009. [Google Scholar]
- 24.Nuttal GHF, Warburton C, Cooper WF, Robinson LE. Ticks. part 1. Cambridge University Press; 1908. (A Monograph of the Ixodoidea). [Google Scholar]
- 25.Brown AB. Hematology: Principles and Procedures. 6th edition. Philadelphia, Pa, USA: Lea & Feibiger; 1993. [Google Scholar]
- 26.Feldman BF, Zinkl JG, Jain NC, Gasper PE. Schalm's Veterinary Hematology. 5th edition. Ames, Iowa, USA: Wiley Blackwell Publishing; 2000. [Google Scholar]
- 27.Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Rapp BA, Wheeler DL. GenBank. Nucleic Acids Research. 2002;30(1):17–20. doi: 10.1093/nar/30.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birkenheuer AJ, Levy MG, Breitschwerdt EB. Development and evaluation of a seminested PCR for detection and differentiation of Babesia gibsoni (Asian genotype) and B. canis DNA in canine blood samples. Journal of Clinical Microbiology. 2003;41(9):4172–4177. doi: 10.1128/JCM.41.9.4172-4177.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rizzi TE, Meinkoth JH, Clinkenbeard KD. Normal hematology of the dogs. In: Weiss DJ, Wardrop KJ, editors. Schalm's Veterinary Hematology. Weiley-Blackwell Publishing; 2010. pp. 799–810. [Google Scholar]
- 30.Hornok S, Edelhofer R, Farkas R. Seroprevalence of canine babesiosis in Hungary suggesting breed predisposition. Parasitology Research. 2006;99(6):638–642. doi: 10.1007/s00436-006-0218-8. [DOI] [PubMed] [Google Scholar]
- 31.Martinod S, Laurent N, Moreau Y. Resistance and immunity of dogs against Babesia canis in an endemic area. Veterinary Parasitology. 1986;19(3-4):245–254. doi: 10.1016/0304-4017(86)90072-5. [DOI] [PubMed] [Google Scholar]
- 32.van Zyl M. Prediction of survival in hospitalized cases of canine babesiosis: a retrospective investigation employing serum biochemical parameters and signalment data [M.S. thesis] Pretoria, South Africa: University of Pretoria; 1995. [Google Scholar]
- 33.Salem NY. Diagnostic and therapeutic studies on fevers and dehydration in dogs [Ph.D. thesis] Giza, Egypt: Faculty of Veterinary Medicine, Cairo University; 2012. [Google Scholar]
- 34.Ribeiro MFB, Passos LMF, Lima JD, Guimaraes AM. Frequency of anti-Babesia canis antibodies in dogs, in Belo Horizonte Minas Gerais. Arquivo Brasileiro de Medicina Veterinaria e Zootecnia. 1990;42:511–517. [Google Scholar]
- 35.Maia MG, Costa RT, Haddad JPA, Passos LMF, Ribeiro MFB. Epidemiological aspects of canine babesiosis in the semiarid area of the state of Minas Gerais, Brazil. Preventive Veterinary Medicine. 2007;79(2–4):155–162. doi: 10.1016/j.prevetmed.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 36.Jacobson LS, Clark IA. The pathophysiology of canine babesiosis: new approaches to an old puzzle. Journal of the South African Veterinary Association. 1994;65(3):134–145. [PubMed] [Google Scholar]
- 37.Gallego SL, Trotta M, Carli E, Carcy B, Caldin M, Furlanello T. Babesia canis canis and Babesia canis vogeli clinicopathological findings and DNA detection by means of PCR-RFLP in blood from Italian dogs suspected of tick-borne disease. Veterinary Parasitology. 2008;157(3-4):211–221. doi: 10.1016/j.vetpar.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 38.Kuttler KL. World-wide impact of babesiosis. In: Ristic M, editor. Babesiosis of Domestic Animals and Man. Boca Raton, Fla, USA: CRC Press; 1988. pp. 1–22. [Google Scholar]
- 39.Böse R, Jorgensen WK, Dalgliesh RJ, Friedhoff KT, de Vos AJ. Current state and future trends in the diagnosis of babesiosis. Veterinary Parasitology. 1995;57(1–3):61–74. doi: 10.1016/0304-4017(94)03111-9. [DOI] [PubMed] [Google Scholar]
- 40.Comazzi S, Paltrinieri S, Manfredi MT, Agnes F. Diagnosis of canine babesiosis by Percoll gradient separation of parasitized erythrocytes. Journal of Veterinary Diagnostic Investigation. 1999;11(1):102–104. doi: 10.1177/104063879901100119. [DOI] [PubMed] [Google Scholar]
- 41.Posnett ES, Fehrsen J, de Waal DT, Ambrosio RE. Detection of babesia equi in infected horses and carrier animals using a DNA probe. Veterinary Parasitology. 1991;39(1-2):19–32. doi: 10.1016/0304-4017(91)90058-4. [DOI] [PubMed] [Google Scholar]
- 42.Farag HS. Epidemiological, diagnostic and therapeutic studies on canine babesiosis [M.S. thesis] Giza, Egypt: Faculty of Veterinary Medicine, Cairo University; 2012. [Google Scholar]
- 43.Day MJ. Antigen specificity in canine autoimmune hemolytic anemia. Veterinary Immunology and Immunopathology. 1999;69:215–224. doi: 10.1016/s0165-2427(99)00055-0. [DOI] [PubMed] [Google Scholar]
- 44.Pederson NC. A review of immunologic diseases of the dog. Veterinary Immunology and Immunopathology. 1999;69:251–342. doi: 10.1016/S0165-2427(99)00059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Irwin PJ. Babesiosis and Cytauxzoonsis. Arthropode-Borne Infectious Diseases of Dogs and Cats. 1st edition. Barcelona, Spain: Manson Publishing Ltd.; 2005. [Google Scholar]
- 46.Taboada J, Lobetti R. Babesiosis. In: Greene CG, editor. Infectious Diseases of the Dog and Cat. 3rd edition. Elsevier; 2006. [Google Scholar]
- 47.Stappendel RJ. Hemolytic anemia in the dog [Ph.D. thesis] Utrecht, The Netherland: Utrecht University; 1978. [Google Scholar]
- 48.Feldman BF, Thomason KJ, Jain NC. Quantitative platelet disorders. Veterinary Clinics of North America. 1988;18(1):35–49. doi: 10.1016/s0195-5616(88)50005-0. [DOI] [PubMed] [Google Scholar]
- 49.Schoeman JP. Canine babesiosis an update. Proceedings of the 33rd World Small Animal Veterinary Association Congress; August 2008; Dublin, Ireland. [Google Scholar]
- 50.Iqbal A, Wazir VS, Malik MA, Singh R. Canine babesiosis-an emerging vector-borne disease. Indian Pet Journal. 2011;10:34–38. [Google Scholar]


