INTRODUCTION
The prevalence of dietary supplement use has increased over time in the United States.1–3 In addition, dietary supplements have diversified to include herbals, botanicals, amino acids, and enzymes, in addition to vitamin and mineral supplements.3,4 The increase in the prevalence and variety of dietary supplement use has continued together with exponential growth of the supplement industry5 and convenient purchase through supermarkets and the internet. However, unexpected negative effects of supplements of vitamin A, β-carotene, vitamin C, and α-tocopherol on lung cancer and cardiovascular disease have been reported in recent decades.6–8 Therefore, longitudinal trends in the use of various supplements need to be characterized among U.S. adults. Most previous studies on the prevalence of supplement use in the U.S. population were cross-sectional based on national surveys, including the National Health and Nutrition Examination Survey (NHANES) and Continuing Survey of Food Intakes by Individuals.9–16 Evaluations of trends in supplement use using data from these national surveys is limited by inconsistencies in definitions of dietary supplements, survey questions, reference periods, and methodologies over time.17 The few studies evaluating trends in supplement use have been subject to limitations such as limited variety of supplements,2 cross-sectional comparison,1–3 or exclusive focus on elderly women.18 In addition, some studies reported trends only until 20001–3 or compared only two surveys.18 Therefore, trends after 2000 and fluctuations in supplement use after major publications on supplements need to be examined using repeated data from cohort studies.
The surveys on supplement use in the Nurses’ Health Study (NHS) and Health Professionals Follow-up Study (HPFS) were launched in 1980 and 1986 (respectively) and have been repeated every two years. Because these cohort participants are health care workers, trends in their supplement use may reflect consumer response to the results of scientific research. Since longitudinal changes in supplement use may be partly attributed to the aging of cohort participants, examining secular trends in supplement use among participants of the same age group at different times can provide insights into the potential effect of factors other than age on supplement use.
The aim of this study was to explore the secular and longitudinal trend of dietary supplement use in US health professionals using repeated measurements of supplement use over 20 years.
METHODS
Study population
The NHS is a prospective cohort study of 121,700 female registered nurses aged 30–55 years in 1976. The HPFS included 51,529 male health professionals (dentists, veterinarians, pharmacists, optometrists, osteopathic physicians, and podiatrists) aged 40–75 years in 1986. The follow-up rates of the participants in these cohorts have exceeded 90%.19
The questions on supplement use were first asked in 1980 for the NHS and 1986 for the HPFS, and were repeated every 2 years thereafter. The assessment methods on supplement use, including daily dose, were consistent across the studies and time periods of every 4 years since 1986. Thus, to maintain consistency across the cohorts, we analyzed data on supplement use every 4 years between 1986 and 2006. We censored those who did not respond to a questionnaire on supplement use for that questionnaire cycle. We did not exclude the participants based on any particular health conditions. The procedures and protocols of the study were approved by the Institutional Review Boards of Brigham and Women’ Hospital and the Harvard School of Public Health. The reply to the self-administered questionnaire was considered to imply informed consent.
Assessment of supplement use
Participants were asked whether they currently took any supplements, such as multivitamins, vitamin A, β-carotene, vitamin C, vitamin D, vitamin E, vitamin B6, folic acid, vitamin B complex, calcium, iron, selenium, zinc, and magnesium. In addition, for multivitamins, they were asked to report the number of pills per week (4 choices; ≤2, 3–5, 6–9, or ≥ 10/week) and brand name of multivitamins. For some individual vitamin (vitamin A, vitamin C, vitamin E, and vitamin B6) and mineral supplements (selenium, calcium, and zinc), information on daily dose (5 choices; for example, vitamin C < 400 mg, 400–700 mg, 750–1250 mg, ≥ 1300 mg, or don't know) were asked. For vitamin A and vitamin C, participants were queried whether they took it seasonally or during most months. Frequency of use of these supplements was not queried. We defined all respondents who reported taking supplements (including seasonal use) as supplement users. For β-carotene, B complex, vitamin D, folic acid, iron, magnesium, and fish oil, dosage information was not asked. Fish oil was asked since 1988. In the two cohort studies, the reproducibility and validity of the questionnaire for total vitamin and mineral intake which included intake from food and supplements were previously documented;20,21 Intraclass correlation coefficients for total vitamin and mineral intakes assessed by two food frequency questionnaires (FFQs) one year apart ranged from 0.58 to 0.60 in the NHS and from 0.57 to 0.80 in the HPFS, supporting reproducibility of the intake measure. The correlation coefficients between the energy-adjusted vitamin intakes measured by diet records and the FFQ ranged from 0.49 to 0.75 in the NHS and from 0.41 to 0.87 in the HPFS, supporting validity of the intake measure.
Statistical analysis
We calculated the prevalence of use of each supplement in each follow-up cycle. To observe the secular trend of the prevalence of supplement use, we grouped the participants based on age in 1986 (<50, 50–59, and ≥ 60 y) and then shift the line of each age group to the same median age over time. Generalized estimating equation (GEE) models for repeated analysis were fitted by the GENMOD procedure and adjusted for age in 1986 and current smoking status in each year. We conducted separate analyses for each cohort. As the NHS was launched in 1976, the participants who did not fill in 1986 could be included in later. So we conducted a sensitivity analysis by just keeping the participants who filled in questionnaire in 1986. P-values less than 0.05 were considered statistically significant. All analyses were conducted using the SAS statistical package (version 9.2; SAS Institute, Inc., Cary, NC, USA).
RESULTS AND DISCUSSION
The number of participants included in this analysis in 1986 was 74,194 for the NHS and 50,497 for the HPFS. The average age of participants in 1986 was 53.0 ± 7.2 years for the NHS and 54.8± 9.9 years for the HPFS.
Prevalence of any supplement use
The percentage of participants who used at least one supplement including multivitamins was 71.3% among women and 56.4% among men in 1986. This increased to 88.3% among women and 80.7% among men in 2006 (Table 1). The prevalence of use of at least one supplement was higher in our populations than in recent national surveys (65.0% in the NHANES in 2003–2006;16 73.0% in the Health and Diet Survey in 2002;15 50.4% in the National Health Interview Survey in 20003) or a cohort study among postmenopausal women (85.4% in the Iowa Women’s Health Study (IWHS) in 200418). Because our participants were health care professionals, they might be more knowledgeable regarding dietary supplements and use them more often than the general population. However, the prevalence of use of vitamin C supplement in the NHS (28.3%) was similar to that of the IWHS (28.9%).18 Studies using the national surveys did not provide any data on trends of supplement use over time.
Table 1.
Prevalence of use of one or more supplements including multivitamins at baseline (1986) and follow-up (2006) in the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS)
| NHS |
HPFS |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1986 | 2006 | 1986 | 2006 | |||||||||
| Total no. of supplements |
Any supplementa |
Multivitamins useb |
Any supplement |
Multivitamins use |
Any supplement |
Multivitamins use |
Any supplement |
Multivitamins use |
||||
| No | Yes | No | Yes | No | Yes | No | Yes | |||||
| 0 | 21,261 (28.7) | 21,261 (28.7) | 5,640 ( 7.6) | 7,747 (11.7) | 7,747 (11.7) | 8,614 (13.0) | 22,010 (43.6) | 22,010 (43.6) | 6,126 (12.1) | 5,463 (19.3) | 5,463 (19.3) | 7,384 (26.0) |
| 1 | 18,399 (24.8) | 12,759 (17.2) | 9,856 (13.3) | 11,945 (18.0) | 3,331 ( 5.0) | 12,083 (18.3) | 10,666 (21.1) | 4,540 ( 9.0) | 5,122 (10.1) | 8,522 (30.0) | 1,138 ( 4.0) | 4,457 (15.7) |
| 2–3 | 22,285 (30.0) | 5,868 ( 7.9) | 10,200 (13.8) | 28,105 (42.4) | 4,177 ( 6.3) | 18,951 (28.6) | 9,817 (19.4) | 1,650 ( 3.3) | 4,931 ( 9.8) | 8,288 (29.2) | 888 ( 3.1) | 4,828 (17.0) |
| 4–5 | 7,101 ( 9.6) | 1,300 ( 1.7) | 3,581 ( 4.8) | 12,232 (18.5) | 1,201 ( 1.8) | 6,145 ( 9.3) | 3,479 ( 6.9) | 375 ( 0.7) | 2,054 ( 4.1) | 3,454 (12.2) | 356 ( 1.3) | 1,961 ( 6.9) |
| ≥ 6 | 5,148 ( 6.9) | 881 ( 1.2) | 2,848 ( 3.8) | 6,193 ( 9.4) | 853 ( 1.3) | 3,120 ( 4.7) | 4,525 ( 9.0) | 357 ( 0.7) | 3,332 ( 6.6) | 2,632 ( 9.3) | 338 ( 1.2) | 1,546 ( 5.5) |
| Total | 74,194 (100) | 42,069 (56.7) | 32,125 (43.3) | 66,222 (100) | 17,309 (26.1) | 48,913 (73.9) | 50,497 (100) | 28,932 (57.3) | 21,565 (42.7) | 28,359 (100) | 8,183 (28.9) | 20,176 (71.1) |
no. (%)
including multivitamins, vitamin A, β-carotene, vitamin C, vitamin D, vitamin E, vitamin B6, folic acid, vitamin B complex, calcium, iron, selenium, zinc, and magnesium
multivitamins was excluded in total number of supplements.
Longitudinal trends of supplement use
As shown in Table 2, the use of multivitamins, vitamin D, folic acid, calcium, magnesium, and fish oil supplements has continued to increase since 1990. Notably, the proportions of participants who took vitamin D (2.2 to 32.2% for women and 1.1 to 6.7% for men), folic acid (0.8 to 10.7% for women and 1.1 to 13.8% for men), and fish oil (1.6 to 18.1% for women and 3.3 to 22.2% for men) supplements increased substantially from 1990 to 2006.
Table 2.
Longitudinal trends in prevalence of supplement use in the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS), 1986–2006
| 1986 | 1990 | 1994 | 1998 | 2002 | 2006 | |
|---|---|---|---|---|---|---|
| NHS | ||||||
| No. | 74,194 | 79,428 | 84,571 | 82,599 | 77,600 | 66,222 |
| Multivitamins (%) | 43.3 | 38.4 | 48.3 | 62.7 | 70.1 | 73.9 |
| Vitamin A (%) | 5.0 | 3.3 | 4.8 | 4.6 | 4.1 | 3.0 |
| β-carotene (%) | 1.4 | 2.1 | 9.7 | 6.3 | 2.6 | 1.6 |
| Vitamin C (%) | 29.3 | 24.5 | 32.8 | 37.1 | 32.2 | 28.3 |
| Vitamin D (%) | 4.2 | 2.2 | 3.1 | 7.8 | 25.4 | 32.2 |
| Vitamin E (%) | 16.1 | 14.7 | 30.1 | 46.2 | 44.3 | 19.8 |
| Vitamin B6 (%) | 8.1 | 5.4 | 6.5 | 9.1 | 8.4 | 7.9 |
| Folic acid (%) | 1.3 | 0.8 | 1.4 | 6.7 | 9.5 | 10.7 |
| B complex (%) | 9.5 | 6.6 | 6.6 | 7.5 | 7.5 | 8.1 |
| Calcium(%) | 52.1 | 34.4 | 43.1 | 55.0 | 63.4 | 64.6 |
| Iron (%) | 6.8 | 5.1 | 4.1 | 2.2 | 2.0 | 2.6 |
| Selenium (%) | 3.9 | 2.7 | 4.7 | 7.0 | 5.8 | 4.3 |
| Magnesium (%) | 4.1 | 2.8 | 4.8 | 7.4 | 7.9 | 8.6 |
| Zinc (%) | 6.7 | 4.8 | 6.9 | 8.8 | 7.7 | 6.8 |
| Fish oil (%) | -a | 1.6 | 1.7 | 2.1 | 5.0 | 18.1 |
| HPFS | ||||||
| No. | 50,497 | 38,382 | 36,272 | 34,281 | 31,707 | 28,359 |
| Multivitamins (%) | 42.7 | 39.3 | 49.9 | 62.0 | 68.3 | 71.1 |
| Vitamin A (%) | 8.1 | 4.4 | 6.5 | 7.4 | 6.3 | 4.8 |
| β-carotene (%) | 2.1 | 2.9 | 14.6 | 12.2 | 7.8 | 3.2 |
| Vitamin C (%) | 35.6 | 26.9 | 37.6 | 44.7 | 37.9 | 32.4 |
| Vitamin D (%) | 3.5 | 1.1 | 1.6 | 3.1 | 3.7 | 6.7 |
| Vitamin E (%) | 18.9 | 16.4 | 34.1 | 52.0 | 49.4 | 24.5 |
| Vitamin B6 (%) | 9.1 | 4.6 | 6.7 | 9.7 | 10.6 | 9.6 |
| Folic acid (%) | 2.8 | 1.1 | 1.9 | 7.7 | 12.7 | 13.8 |
| B complex (%) | 10.0 | 5.6 | 6.0 | 6.8 | 7.8 | 8.0 |
| Calcium (%) | 15.8 | 10.9 | 13.8 | 20.6 | 22.6 | 23.8 |
| Iron (%) | 5.8 | 2.7 | 3.1 | 2.1 | 1.7 | 1.6 |
| Selenium (%) | 6.5 | 4.2 | 7.8 | 13.7 | 14.3 | 11.0 |
| Magnesium (%) | 4.3 | 2.5 | 4.1 | 6.7 | 6.9 | 6.8 |
| Zinc (%) | 11.6 | 7.8 | 10.7 | 14.8 | 12.3 | 8.8 |
| Fish oil (%) | -a | 3.3 | 3.3 | 3.9 | 6.9 | 22.2 |
Fish oil was not surveyed in 1986.
P values for trend over time of the prevalence of use of all supplements adjusting for age (<50, 50–60, ≥60 y) and smoking status (smokers vs. nonsmokers) were < 0.01 except for zinc supplement for the NHS and vitamin C supplement for the HPFS.
The use of vitamin D supplements increased primarily in the 2000’s. It is widely recognized that vitamin D plays an important role in calcium absorption for bone health, especially in postmenopausal women.22 Despite fortification of milk with vitamin D, one-thirds of the U.S. population had vitamin D inadequacy or deficiency in 2001–2006.23 Vitamin D deficiency is known to be more common among the elderly due to malabsorption, lower intake, and limited sun exposure.22 Only since the 2000’s has vitamin D deficiency became widely recognized as a potential risk factor for various chronic diseases such as hypertension, cardiovascular disease, and cancer.24,25 In the 2000’s, most participants in the NHS became postmenopausal, which might have also contributed to the sharp increase in the use of vitamin D supplement for the prevention and treatment of osteoporosis. The prevalence of vitamin D supplement use was significantly higher in the NHS than in the HPFS across the time periods, probably due to gender-specific concern about osteoporosis.
The use of folic acid supplements increased notably in 1998. This coincided with the U.S. Food and Drug Administration (FDA)’s requirement that folic acid be added to enriched grain products in 1996; mandatory compliance took effect in January 1998.26 In addition to the national interest in the prevention of neural tube defects, an awareness of potential beneficial effects of folic acid on chronic diseases such as cardiovascular disease and cancer might have encouraged increases in the voluntary use of folic acid supplements. However, it has been suggested that excess folate may promote tumor initiation,27 and other health benefits of folic acid fortification or supplementation (except prevention of neural tube defects) have not yet been proven.28
Fish oil has attracted considerable attention recently because of the cardioprotective effects of the omega-3 fatty acids contained in fish oil, including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA).29 The American Heart Association (AHA) initiated recommendation of fish and/or fish oil supplements for patients with heart disease in 2002.30 In our cohorts, the use of fish oil supplements substantially increased in 2006. However, a recent meta-analysis reported that omega-3 polyunsaturated fatty acids supplementation was not associated with risk of major cardiovascular disease events.31 More studies are needed on the effect of fish oil on cardiovascular disease including practical questions, such as dose and optimal ratio of EPA and DHA.
Among supplements, iron was the only one that showed consistent decrease in use (from 6.8% to 2.6% in women and from 5.8% to 1.6% in men from 1986 to 2006). The prevalence of iron supplement use was significantly higher in the NHS than in the HPFS across the time periods except 1998. Iron is important to the growth and development of infants and children and the prevention of iron-deficiency anemia.2 Since the 1990s, iron deficiency has been relatively uncommon, and the prevalence of iron-deficiency anemia declined in the general population in the U.S..32 In 2001, the tolerable upper intake level (UL) for iron was reduced from 60 mg/d to 45 mg/d,32 based on the rationale that excessive iron intake from supplements may reduce the absorption of zinc and certain prescription medications, cause gastrointestinal distress, and increase the risk of colorectal cancer, cardiovascular disease, and infection.33,34 Possibly, for these reasons, the use of iron supplements among our adult participants might have declined for the past few decades. In the women, the transition from pre- to post-menopausal status also probably contributed.
The use of vitamin A, β-carotene, vitamin C, vitamin E, and zinc supplements peaked in 1994 or 1998 but since then has significantly declined with time in both men and women. This change coincides with the publications of clinical trials since 1996 suggesting that these antioxidant nutrients have no effect35 or increase the risk of lung cancer and cardiovascular disease.6,7 Since our participants work in the health care setting, they may be more sensitive to the findings of scientific research than the general population.
As the NHS was launched in 1976, the participants who did not fill in 1986 questionnaire could be included in the later years. However, in a sensitivity analysis of just keeping the participants who filled in questionnaire in 1986, the prevalence of use of all supplements did not materially change (data not shown).
Prevalence of supplement use by smoking status
It is generally recognized that dietary intakes and plasma concentrations of antioxidants, especially vitamin C and β-carotene, are lower in smokers than in non-smokers.36 Also, the detrimental effect of beta-carotene supplementation on cardiovascular and cancer endpoints was particularly evident in smokers in clinical trials.6,7 Thus, we conducted a stratified analysis of the use of antioxidant supplements according to smoking status. We found that smokers had a significantly lower frequency of use of supplements of vitamin A, β-carotene, vitamin C, and vitamin E compared to nonsmokers after adjusting for age group and time (Supplementary Figure 1). These differences by smoking status were consistent across the time periods and were not particularly evident following the publication of the clinical trials. It appeared that these publications led a drop in use of antioxidant supplements among both of smokers and non-smokers.
Secular trends of supplement use
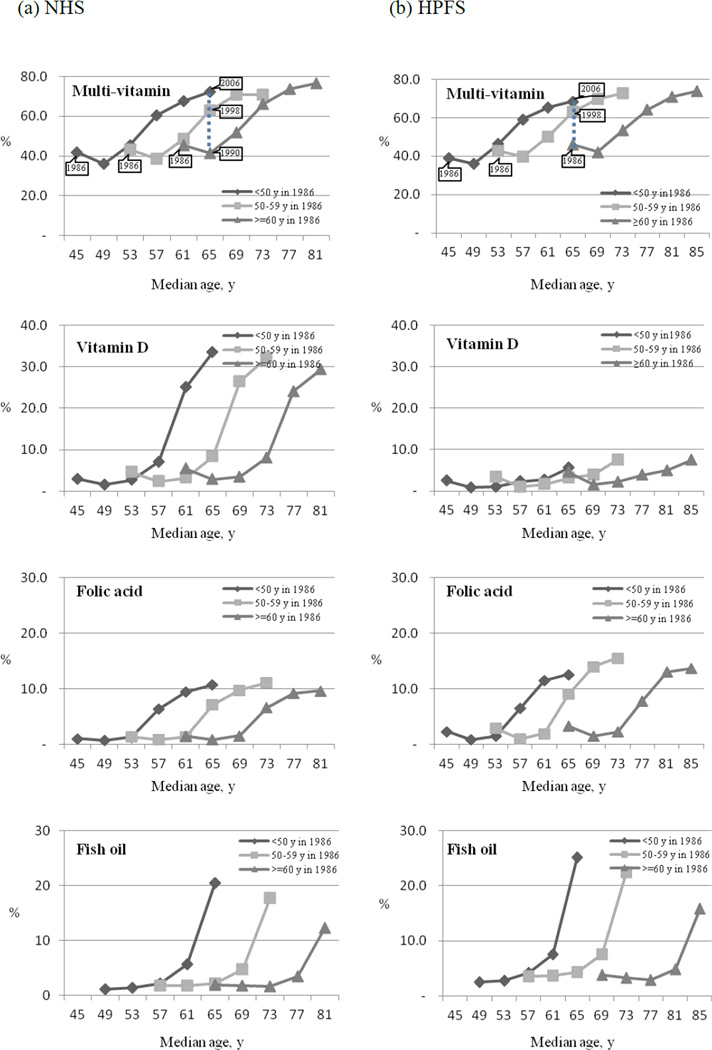
In general, the use of dietary supplements has increased with age.2,3,13,16 Because of the prospective design of our study and repeated assessment of supplement use, we were able to evaluate both age effects and secular trends of supplement use. We evaluated secular trends in the use of several supplements, which had substantial longitudinal increase of use, according to age group (<50, 50–59, and ≥60 years) in 1986 (Figure 1). The percentages of participants taking multivitamins, vitamin D, folic acid, and fish oil across the same age group increased by time. For example, the prevalence of multivitamins use was respectively 41.4%, 63.0%, and 72.3% among women aged 65 years in 1990, in 1998, and in 2006 and 46.2%, 63.1%, and 68.5% among men aged 65 years in 1986, in 1998, and in 2006. Also, the use of multivitamins slightly increased with age. This effect may be attributable to age-related factors, such as individual attempts to correct a nutritional deficiency or concerns about disease prevention with advancing age. However, the secular change in supplement use was greater than the change due to age. This suggests that changes in trends of supplement use may be only partially attributable to aging, and that a great part of this change may be due to other factors, such as changes in government policies or dietary guidelines, scientific research reports, or growth of supplement industry with aggressive marketing strategies.
Figure 1.
Secular trends in prevalence of supplement use according to baseline age groups and secular time in the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS), 1986–2006
X-axis shows the median age for each age group in each survey year. Points represent the prevalence of supplement use at the median age for the age group in each year (1986, 1990, 1994, 1998, 2002, 2006). The vertical dotted line illustrates the differences in prevalence of supplement use for participants aged 65 y in 1990, 65 y in 1998, and 65 y in 2006.
Strengths and Limitations
A major strength of our study is its large size and prospective design with repeated measures on supplement use, which enabled us to explore both longitudinal and secular trends of supplement use over time as well as age effects. One limitation is that the participants in the NHS and the HPFS are not a representative sample of the U.S. population. However, we focused on trends of supplement use over time rather than absolute prevalence of supplement use, which may be comparable to those in the general population. Given that few data exist in trend of use in the general population, we believe that our data are still useful and important for society and policy making related to supplement use.
CONCLUSIONS
The use of many types of dietary vitamin and mineral supplements has increased substantially over the past 20 years. However, the use of vitamin A, β-carotene, vitamin C, and vitamin E supplements peaked in 1994 or 1998 but has continued to decline with time. The secular increase in the prevalence of use of supplements across same age group suggests that aging of the population is not the primary reason for the increase. The findings in these health professionals need to be replicated in the general populations. Moreover, more studies on the benefits, risks, and safety of supplements must be conducted, and evidence-based guidelines for supplement use are desirable.
Supplementary Material
ACKNOWLEGEMENT
The authors thank Meir Stampfer, MD, DrPH for his support and helpful advice on the preparation of this manuscript.
FUNDING/SUPPORT
This study was supported by research grant CA87969 and CA55075 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
STATEMENT OF POTENTIAL CONFLICT OF INTEREST
No potential conflict of interest was reported by the authors.
Contributor Information
Hyun Ja Kim, Visiting assistant professor, Channing Division of Network Medicine, Brigham and Women’s Hospital and Harvard Medical School, 181 Longwood Avenue, Boston, MA 02115, Tel: (617) 525-2026, Fax: (617) 525-2008, hyun.kim@channing.harvard.edu. And she is also a research associate professor, Department of Preventive Medicine, Hanyang University College of Medicine, Seoul, Korea..
Edward Giovannucci, Professor, Channing Division of Network Medicine, Brigham and Women’s Hospital and Harvard Medical School, Department of Nutrition, and Department of Epidemiology, Harvard School of Public Health, 655 Huntington Avenue, Building II Room 319, Boston, MA 02115, Tel: (617) 432-4648, Fax: (617) 432-2435, egiovann@hsph.harvard.edu.
Bernard Rosner, Professor, Channing Division of Network Medicine, Brigham and Women’s Hospital and Harvard Medical School, Department of Biostatistics, Harvard School of Public Health, 181 Longwood Avenue, Boston, MA 02115, Tel: (617) 525-2743, Fax: (617) 731-1541, stbar@channing.harvard.edu..
Walter C. Willett, Professor, Channing Division of Network Medicine, Brigham and Women’s Hospital and Harvard Medical School, Department of Nutrition, and Department of Epidemiology, Harvard School of Public Health, 651 Huntington Avenue, Building II Room 311, Boston, MA 02115, Tel: (617) 432-4680, Fax: (617) 432-0464, wwillett@hsph.harvard.edu..
Eunyoung Cho, Assistant professor, Channing Division of Network Medicine, Brigham and Women’s Hospital and Harvard Medical School, 181 Longwood Avenue, Boston, MA 02115, Tel: (617) 525-2091; Fax: (617) 525-2008, hpeyc@channing.harvard.edu..
REFERENCES
- 1.Slesinski MJ, Subar AF, Kahle LL. Trends in use of vitamin and mineral supplements in the United State: the 1987 and 1992 National Health Interview Surveys. J Am Diet Assoc. 1995;95(8):921–923. doi: 10.1016/s0002-8223(95)00255-3. [DOI] [PubMed] [Google Scholar]
- 2.Briefel RR, Johnson CL. Secular trends in dietary intake in the United States. Annu Rev Nutr. 2004;24:401–431. doi: 10.1146/annurev.nutr.23.011702.073349. [DOI] [PubMed] [Google Scholar]
- 3.Millen AE, Dodd KW, Subar AF. Use of vitamin, mineral, nonvitamin, and nonmineral supplements in the United States: The 1987, 1992, and 2000 National Health Interview Survey results. J Am Diet Assoc. 2004;104(6):942–950. doi: 10.1016/j.jada.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 4.Radimer KL, Subar AF, Thompson FE. Nonvitamin, nonmineral dietary supplements: issues and findings from NHANES III. J Am Diet Assoc. 2000;100(4):447–454. doi: 10.1016/S0002-8223(00)00137-1. [DOI] [PubMed] [Google Scholar]
- 5.NBJ's Supplement Business Report 2011. San Diego, CA: Nutrition Business Journal; 2011. [Google Scholar]
- 6.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, Barnhart S, Hammar S. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334(18):1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 7.Albanes D, Heinonen OP, Taylor PR, Virtamo J, Edwards BK, Rautalahti M, Hartman AM, Palmgren J, Freedman LS, Haapakoski J, Barrett MJ, Pietinen P, Malila N, Tala E, Liippo K, Salomaa ER, Tangrea JA, Teppo L, Askin FB, Taskinen E, Erozan Y, Greenwald P, Huttunen JK. Alpha-Tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: effects of base-line characteristics and study compliance. J Natl Cancer Inst. 1996;88(21):1560–1570. doi: 10.1093/jnci/88.21.1560. [DOI] [PubMed] [Google Scholar]
- 8.Lee DH, Folsom AR, Harnack L, Halliwell B, Jacobs DR., Jr. Does supplemental vitamin C increase cardiovascular disease risk in women with diabetes? Am J Clin Nutr. 2004;80(5):1194–1200. doi: 10.1093/ajcn/80.5.1194. [DOI] [PubMed] [Google Scholar]
- 9.Stewart ML, McDonald JT, Levy AS, Schucker RE, Henderson DP. Vitamin/mineral supplement use: a telephone survey of adults in the United States. J Am Diet Assoc. 1985;85(12):1585–1590. [PubMed] [Google Scholar]
- 10.Subar AF, Block G. Use of vitamin and mineral supplements: demographics and amounts of nutrients consumed. The 1987 Health Interview Survey. Am J Epidemiol. 1990;132(6):1091–1101. doi: 10.1093/oxfordjournals.aje.a115752. [DOI] [PubMed] [Google Scholar]
- 11.Ervin RB, Wright JD, Kennedy-Stephenson J. Use of dietary supplements in the United States, 1988–94. Vital Health Stat 11. 1999;(244):i–iii. 1–14. [PubMed] [Google Scholar]
- 12.Balluz LS, Kieszak SM, Philen RM, Mulinare J. Vitamin and mineral supplement use in the United States. Results from the third National Health and Nutrition Examination Survey. Arch Fam Med. 2000;9(3):258–262. doi: 10.1001/archfami.9.3.258. [DOI] [PubMed] [Google Scholar]
- 13.Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Picciano MF. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999–2000. Am J Epidemiol. 2004;160(4):339–349. doi: 10.1093/aje/kwh207. [DOI] [PubMed] [Google Scholar]
- 14.Balluz LS, Okoro CA, Bowman BA, Serdula MK, Mokdad AH. Vitamin or supplement use among adults, behavioral risk factor surveillance system, 13 states, 2001. Public Health Rep. 2005;120(2):117–123. doi: 10.1177/003335490512000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Timbo BB, Ross MP, McCarthy PV, Lin CT. Dietary supplements in a national survey: Prevalence of use and reports of adverse events. J Am Diet Assoc. 2006;106(12):1966–1974. doi: 10.1016/j.jada.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, Engel JS, Thomas PR, Betz JM, Sempos CT, Picciano MF. Dietary supplement use in the United States, 2003–2006. J Nutr. 2011;141(2):261–266. doi: 10.3945/jn.110.133025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Briefel RR. National Nutrition Data: Contributions and Challenges. Nutr Today. 2002;37(3):126–127. doi: 10.1097/00017285-200205000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Park K, Harnack L, Jacobs DR., Jr. Trends in dietary supplement use in a cohort of postmenopausal women from Iowa. Am J Epidemiol. 2009;169(7):887–892. doi: 10.1093/aje/kwn410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith-Warner SA, Spiegelman D, Ritz J, Albanes D, Beeson WL, Bernstein L, Berrino F, van den Brandt PA, Buring JE, Cho E, Colditz GA, Folsom AR, Freudenheim JL, Giovannucci E, Goldbohm RA, Graham S, Harnack L, Horn-Ross PL, Krogh V, Leitzmann MF, McCullough ML, Miller AB, Rodriguez C, Rohan TE, Schatzkin A, Shore R, Virtanen M, Willett WC, Wolk A, Zeleniuch-Jacquotte A, Zhang SM, Hunter DJ. Methods for pooling results of epidemiologic studies: the Pooling Project of Prospective Studies of Diet and Cancer. Am J Epidemiol. 2006;163(11):1053–1064. doi: 10.1093/aje/kwj127. [DOI] [PubMed] [Google Scholar]
- 20.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 21.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. discussion 1127–1136. [DOI] [PubMed] [Google Scholar]
- 22.National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. NW, Washington, DC: National Osteoporosis Foundation; 2008. [Google Scholar]
- 23.Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, Sempos CT. Vitamin D status: United States, 2001–2006. NCHS Data Brief. 2011;(59):1–8. [PubMed] [Google Scholar]
- 24.Nadir MA, Szwejkowski BR, Witham MD. Vitamin D and cardiovascular prevention. Cardiovasc Ther. 2010;28(4):e5–e12. doi: 10.1111/j.1755-5922.2010.00192.x. [DOI] [PubMed] [Google Scholar]
- 25.Makariou S, Liberopoulos EN, Elisaf M, Challa A. Novel roles of vitamin D in disease: what is new in 2011? Eur J Intern Med. 2011;22(4):355–362. doi: 10.1016/j.ejim.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Food and Drug Administration. Health and Human Services. Food and Drug Administration; 1996. Food Standards: Amendment of Standards of Identity For Enriched Grain Products to Require Addition of Folic Acid. Federal Register 61; pp. 8781–8797. editor: [Google Scholar]
- 27.Kim YI. Will mandatory folic acid fortification prevent or promote cancer? Am J Clin Nutr. 2004;80(5):1123–1128. doi: 10.1093/ajcn/80.5.1123. [DOI] [PubMed] [Google Scholar]
- 28.Eichholzer M, Tonz O, Zimmermann R. Folic acid: a public-health challenge. Lancet. 2006;367(9519):1352–1361. doi: 10.1016/S0140-6736(06)68582-6. [DOI] [PubMed] [Google Scholar]
- 29.Holub DJ, Holub BJ. Omega-3 fatty acids from fish oils and cardiovascular disease. Mol Cell Biochem. 2004;263(1–2):217–225. doi: 10.1023/B:MCBI.0000041863.11248.8d. [DOI] [PubMed] [Google Scholar]
- 30.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106(21):2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 31.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012;308(10):1024–1033. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- 32.Institute of Medicine. Institute of Medicine. Washington, DC: National Academy Press; 2001. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. editor. [PubMed] [Google Scholar]
- 33.Fung EB, Ritchie LD, Woodhouse LR, Roehl R, King JC. Zinc absorption in women during pregnancy and lactation: a longitudinal study. Am J Clin Nutr. 1997;66(1):80–88. doi: 10.1093/ajcn/66.1.80. [DOI] [PubMed] [Google Scholar]
- 34.Tuomainen TP, Punnonen K, Nyyssonen K, Salonen JT. Association between body iron stores and the risk of acute myocardial infarction in men. Circulation. 1998;97(15):1461–1466. doi: 10.1161/01.cir.97.15.1461. [DOI] [PubMed] [Google Scholar]
- 35.Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, Belanger C, LaMotte F, Gaziano JM, Ridker PM, Willett W, Peto R. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334(18):1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 36.Northrop-Clewes CA, Thurnham DI. Monitoring micronutrients in cigarette smokers. Clin Chim Acta. 2007;377(1–2):14–38. doi: 10.1016/j.cca.2006.08.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.