Abstract
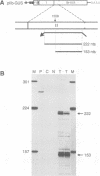
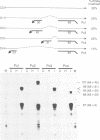
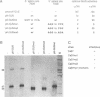
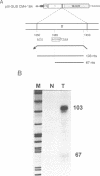
A splicing event essential for the infectivity of a plant pararetrovirus has been characterized. Transient expression experiments using reporter constructs revealed a splice donor site in the leader sequence of the cauliflower mosaic virus (CaMV) 35S RNA and three additional splice donor sites within open reading frame (ORF) I. All four donors use the same splice acceptor within ORF II. Splicing between the leader and ORF II produces an mRNA from which ORF III and, in the presence of the CaMV translational transactivator, ORF IV can be translated efficiently. The other three splicing events produce RNAs encoding ORF I-II in-frame fusions. All four spliced CaMV RNAs were detected in CaMV-infected plants. Virus mutants in which the splice acceptor site in ORF II is inactivated are not infectious, indicating that splicing plays an essential role in the CaMV life cycle. The results presented here suggest a model for viral gene expression in which RNA splicing is required to provide appropriate substrate mRNAs for the specialized translation mechanisms of CaMV.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berthomieu P., Meyer C. Direct amplification of plant genomic DNA from leaf and root pieces using PCR. Plant Mol Biol. 1991 Sep;17(3):555–557. doi: 10.1007/BF00040656. [DOI] [PubMed] [Google Scholar]
- Blanc S., Cerutti M., Usmany M., Vlak J. M., Hull R. Biological activity of cauliflower mosaic virus aphid transmission factor expressed in a heterologous system. Virology. 1993 Feb;192(2):643–650. doi: 10.1006/viro.1993.1080. [DOI] [PubMed] [Google Scholar]
- Bonneville J. M., Sanfaçon H., Fütterer J., Hohn T. Posttranscriptional trans-activation in cauliflower mosaic virus. Cell. 1989 Dec 22;59(6):1135–1143. doi: 10.1016/0092-8674(89)90769-1. [DOI] [PubMed] [Google Scholar]
- Chen G., Müller M., Potrykus I., Hohn T., Fütterer J. Rice tungro bacilliform virus: transcription and translation in protoplasts. Virology. 1994 Oct;204(1):91–100. doi: 10.1006/viro.1994.1513. [DOI] [PubMed] [Google Scholar]
- Chen P. J., Chen C. R., Sung J. L., Chen D. S. Identification of a doubly spliced viral transcript joining the separated domains for putative protease and reverse transcriptase of hepatitis B virus. J Virol. 1989 Oct;63(10):4165–4171. doi: 10.1128/jvi.63.10.4165-4171.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Tapia M., Himmelbach A., Hohn T. Molecular dissection of the cauliflower mosaic virus translation transactivator. EMBO J. 1993 Aug;12(8):3305–3314. doi: 10.1002/j.1460-2075.1993.tb06000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon L. K., Hohn T. Initiation of translation of the cauliflower mosaic virus genome from a polycistronic mRNA: evidence from deletion mutagenesis. EMBO J. 1984 Dec 1;3(12):2731–2736. doi: 10.1002/j.1460-2075.1984.tb02203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driesen M., Benito-Moreno R. M., Hohn T., Fütterer J. Transcription from the CaMV 19 S promoter and autocatalysis of translation from CaMV RNA. Virology. 1993 Jul;195(1):203–210. doi: 10.1006/viro.1993.1361. [DOI] [PubMed] [Google Scholar]
- Edwards J. B., Delort J., Mallet J. Oligodeoxyribonucleotide ligation to single-stranded cDNAs: a new tool for cloning 5' ends of mRNAs and for constructing cDNA libraries by in vitro amplification. Nucleic Acids Res. 1991 Oct 11;19(19):5227–5232. doi: 10.1093/nar/19.19.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck A., Guilley H., Jonard G., Richards K., Hirth L. Nucleotide sequence of cauliflower mosaic virus DNA. Cell. 1980 Aug;21(1):285–294. doi: 10.1016/0092-8674(80)90136-1. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm M., Taylor L. P., Walbot V. Expression of genes transferred into monocot and dicot plant cells by electroporation. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5824–5828. doi: 10.1073/pnas.82.17.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fütterer J., Gordon K., Pfeiffer P., Sanfaçon H., Pisan B., Bonneville J. M., Hohn T. Differential inhibition of downstream gene expression by the cauliflower mosaic virus 35S RNA leader. Virus Genes. 1989 Sep;3(1):45–55. doi: 10.1007/BF00301986. [DOI] [PubMed] [Google Scholar]
- Fütterer J., Gordon K., Sanfaçon H., Bonneville J. M., Hohn T. Positive and negative control of translation by the leader sequence of cauliflower mosaic virus pregenomic 35S RNA. EMBO J. 1990 Jun;9(6):1697–1707. doi: 10.1002/j.1460-2075.1990.tb08293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fütterer J., Hohn T. Role of an upstream open reading frame in the translation of polycistronic mRNAs in plant cells. Nucleic Acids Res. 1992 Aug 11;20(15):3851–3857. doi: 10.1093/nar/20.15.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fütterer J., Hohn T. Translation of a polycistronic mRNA in the presence of the cauliflower mosaic virus transactivator protein. EMBO J. 1991 Dec;10(12):3887–3896. doi: 10.1002/j.1460-2075.1991.tb04958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fütterer J., Kiss-László Z., Hohn T. Nonlinear ribosome migration on cauliflower mosaic virus 35S RNA. Cell. 1993 May 21;73(4):789–802. doi: 10.1016/0092-8674(93)90257-q. [DOI] [PubMed] [Google Scholar]
- Fütterer J., Potrykus I., Valles Brau M. P., Dasgupta I., Hull R., Hohn T. Splicing in a plant pararetrovirus. Virology. 1994 Feb;198(2):663–670. doi: 10.1006/viro.1994.1078. [DOI] [PubMed] [Google Scholar]
- Giband M., Mesnard J. M., Lebeurier G. The gene III product (P15) of cauliflower mosaic virus is a DNA-binding protein while an immunologically related P11 polypeptide is associated with virions. EMBO J. 1986 Oct;5(10):2433–2438. doi: 10.1002/j.1460-2075.1986.tb04518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall G. J., Filipowicz W. The AU-rich sequences present in the introns of plant nuclear pre-mRNAs are required for splicing. Cell. 1989 Aug 11;58(3):473–483. doi: 10.1016/0092-8674(89)90428-5. [DOI] [PubMed] [Google Scholar]
- Goodall G. J., Wiebauer K., Filipowicz W. Analysis of pre-mRNA processing in transfected plant protoplasts. Methods Enzymol. 1990;181:148–161. doi: 10.1016/0076-6879(90)81117-d. [DOI] [PubMed] [Google Scholar]
- Gowda S., Wu F. C., Scholthof H. B., Shepherd R. J. Gene VI of figwort mosaic virus (caulimovirus group) functions in posttranscriptional expression of genes on the full-length RNA transcript. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9203–9207. doi: 10.1073/pnas.86.23.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilley H., Dudley R. K., Jonard G., Balàzs E., Richards K. E. Transcription of Cauliflower mosaic virus DNA: detection of promoter sequences, and characterization of transcripts. Cell. 1982 Oct;30(3):763–773. doi: 10.1016/0092-8674(82)90281-1. [DOI] [PubMed] [Google Scholar]
- Hinnebusch A. G. Novel mechanisms of translational control in Saccharomyces cerevisiae. Trends Genet. 1988 Jun;4(6):169–174. doi: 10.1016/0168-9525(88)90023-6. [DOI] [PubMed] [Google Scholar]
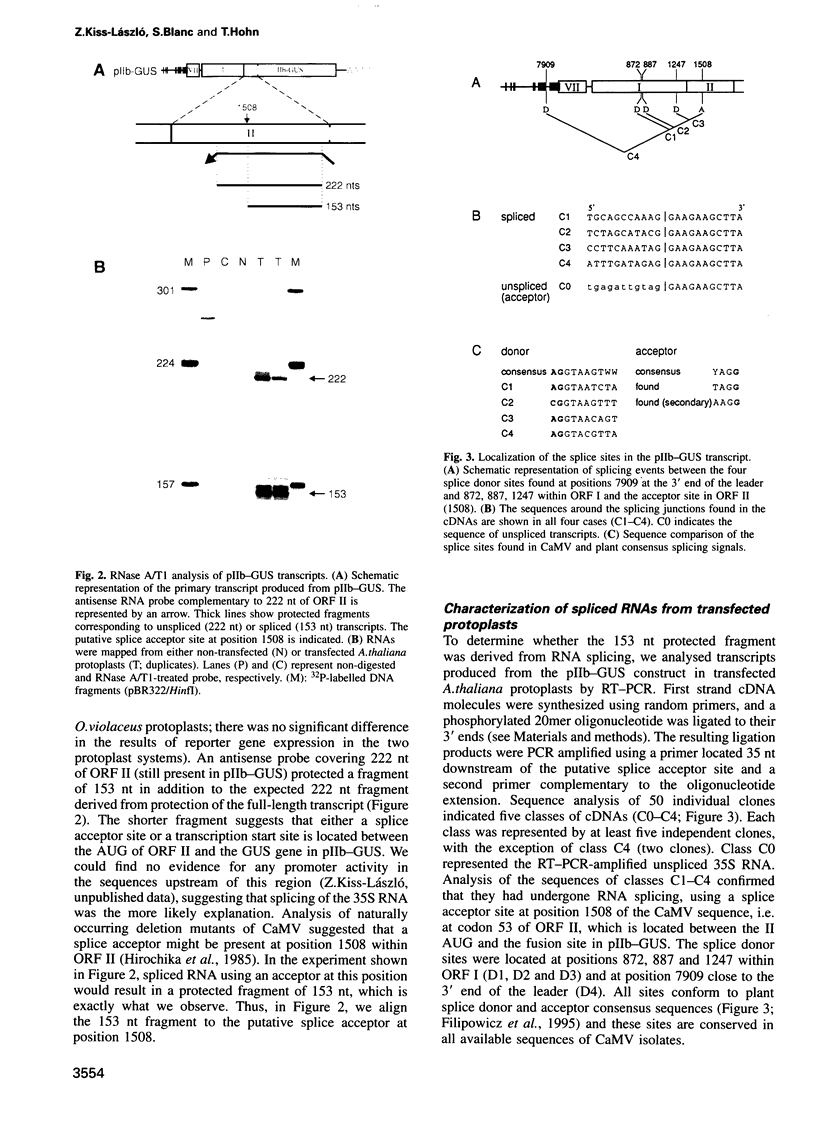
- Hirochika H., Takatsuji H., Ubasawa A., Ikeda J. E. Site-specific deletion in cauliflower mosaic virus DNA: possible involvement of RNA splicing and reverse transcription. EMBO J. 1985 Jul;4(7):1673–1680. doi: 10.1002/j.1460-2075.1985.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn T., Fütterer J. Transcriptional and translational control of gene expression in cauliflower mosaic virus. Curr Opin Genet Dev. 1992 Feb;2(1):90–96. doi: 10.1016/s0959-437x(05)80328-4. [DOI] [PubMed] [Google Scholar]
- Jang S. K., Wimmer E. Cap-independent translation of encephalomyocarditis virus RNA: structural elements of the internal ribosomal entry site and involvement of a cellular 57-kD RNA-binding protein. Genes Dev. 1990 Sep;4(9):1560–1572. doi: 10.1101/gad.4.9.1560. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeurier G., Hirth L., Hohn T., Hohn B. Infectivities of native and cloned DNA of cauliflower mosaic virus. Gene. 1980 Dec;12(1-2):139–146. doi: 10.1016/0378-1119(80)90024-4. [DOI] [PubMed] [Google Scholar]
- Luehrsen K. R., Walbot V. Addition of A- and U-rich sequence increases the splicing efficiency of a deleted form of a maize intron. Plant Mol Biol. 1994 Feb;24(3):449–463. doi: 10.1007/BF00024113. [DOI] [PubMed] [Google Scholar]
- Mougeot J. L., Guidasci T., Wurch T., Lebeurier G., Mesnard J. M. Identification of C-terminal amino acid residues of cauliflower mosaic virus open reading frame III protein responsible for its DNA binding activity. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1470–1473. doi: 10.1073/pnas.90.4.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R., Siliciano P. G. Evidence for an essential non-Watson-Crick interaction between the first and last nucleotides of a nuclear pre-mRNA intron. Nature. 1993 Feb 18;361(6413):660–662. doi: 10.1038/361660a0. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988 Jul 28;334(6180):320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- Plant A. L., Covey S. N., Grierson D. Detection of a subgenomic mRNA for gene V, the putative reverse transcriptase gene of cauliflower mosaic virus. Nucleic Acids Res. 1985 Dec 9;13(23):8305–8321. doi: 10.1093/nar/13.23.8305. [DOI] [PMC free article] [PubMed] [Google Scholar]
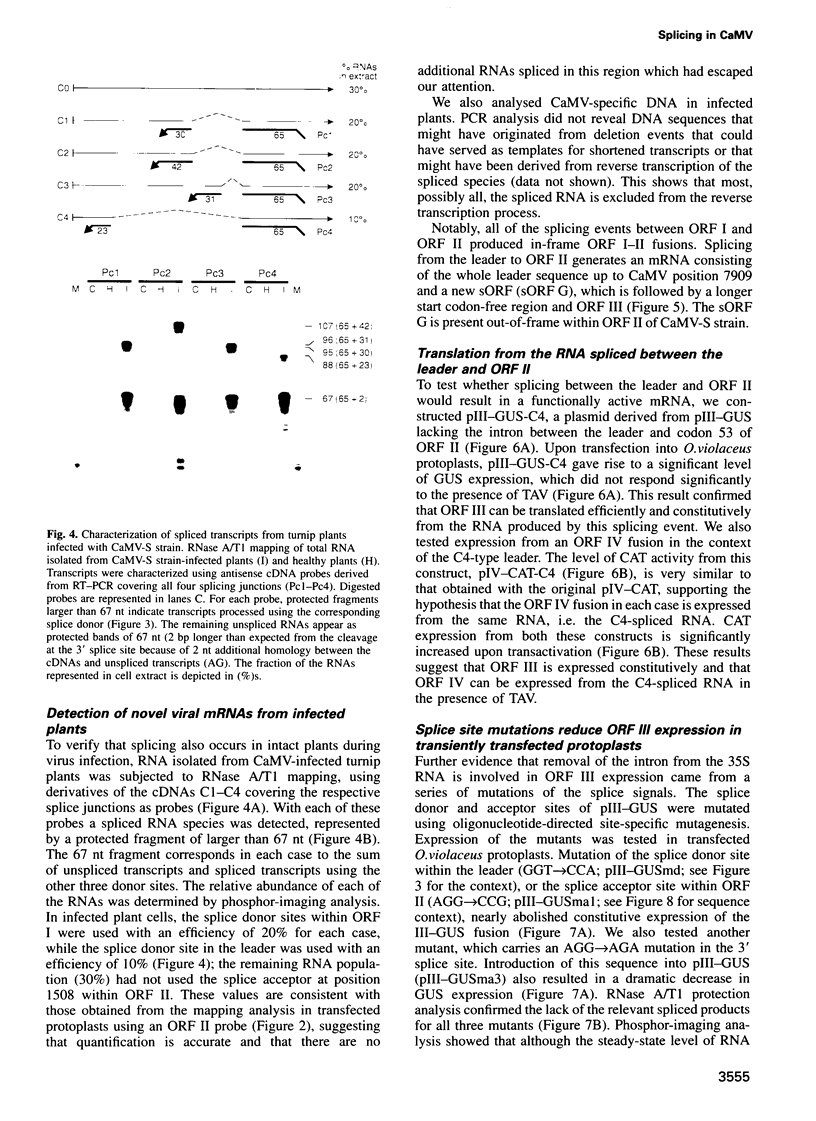
- Restrepo M. A., Freed D. D., Carrington J. C. Nuclear transport of plant potyviral proteins. Plant Cell. 1990 Oct;2(10):987–998. doi: 10.1105/tpc.2.10.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothnie H. M., Chapdelaine Y., Hohn T. Pararetroviruses and retroviruses: a comparative review of viral structure and gene expression strategies. Adv Virus Res. 1994;44:1–67. doi: 10.1016/s0065-3527(08)60327-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholthof H. B., Gowda S., Wu F. C., Shepherd R. J. The full-length transcript of a caulimovirus is a polycistronic mRNA whose genes are trans activated by the product of gene VI. J Virol. 1992 May;66(5):3131–3139. doi: 10.1128/jvi.66.5.3131-3139.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholthof H. B., Wu F. C., Richins R. D., Shepherd R. J. A naturally occurring deletion mutant of figwort mosaic virus (caulimovirus) is generated by RNA splicing. Virology. 1991 Sep;184(1):290–298. doi: 10.1016/0042-6822(91)90845-3. [DOI] [PubMed] [Google Scholar]
- Schultze M., Hohn T., Jiricny J. The reverse transcriptase gene of cauliflower mosaic virus is translated separately from the capsid gene. EMBO J. 1990 Apr;9(4):1177–1185. doi: 10.1002/j.1460-2075.1990.tb08225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezzini G. F., Horrichs A., Somssich I. E. Isolation of putative defense-related genes from Arabidopsis thaliana and expression in fungal elicitor-treated cells. Plant Mol Biol. 1993 Jan;21(2):385–389. doi: 10.1007/BF00019954. [DOI] [PubMed] [Google Scholar]
- Vaden V. R., Melcher U. Recombination sites in cauliflower mosaic virus DNAs: implications for mechanisms of recombination. Virology. 1990 Aug;177(2):717–726. doi: 10.1016/0042-6822(90)90538-3. [DOI] [PubMed] [Google Scholar]
- Varagona M. J., Schmidt R. J., Raikhel N. V. Nuclear localization signal(s) required for nuclear targeting of the maize regulatory protein Opaque-2. Plant Cell. 1992 Oct;4(10):1213–1227. doi: 10.1105/tpc.4.10.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra C., Hohn T. Cauliflower Mosaic Virus Gene VI Controls Translation from Dicistronic Expression Units in Transgenic Arabidopsis Plants. Plant Cell. 1992 Dec;4(12):1471–1484. doi: 10.1105/tpc.4.12.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]