Abstract
Alzheimer's disease (AD) is a multifactorial neurological condition associated with a genetic profile that is still not completely understood. In this study, using a whole gene microarray approach, we investigated age-dependent gene expression profile changes occurring in the hippocampus of young and old transgenic AD (3xTg-AD) and wild-type (WT) mice. The aim of the study was to assess similarities between aging- and AD-related modifications of gene expression and investigate possible interactions between the two processes. Global gene expression profiles of hippocampal tissue obtained from 3xTg-AD and WT mice at 3 and 12 months of age (m.o.a.) were analyzed by hierarchical clustering. Interaction among transcripts was then studied with the Ingenuity Pathway Analysis (IPA) software, a tool that discloses functional networks and/or pathways associated with sets of specific genes of interest. Cluster analysis revealed the selective presence of hundreds of upregulated and downregulated transcripts. Functional analysis showed transcript involvement mainly in neuronal death and autophagy, mitochondrial functioning, intracellular calcium homeostasis, inflammatory response, dendritic spine formation, modulation of synaptic functioning, and cognitive decline. Thus, overexpression of AD-related genes (such as mutant APP, PS1, and hyperphosphorylated tau, the three genes that characterize our model) appears to favor modifications of additional genes that are involved in AD development and progression. The study also showed overlapping changes in 3xTg-AD at 3 m.o.a. and WT mice at 12 m.o.a., thereby suggesting altered expression of aging-related genes that occurs earlier in 3xTg-AD mice.
Keywords: Alzheimer's disease, aging, microarray, inflammation, synaptic plasticity, mitochondria
Alzheimer's disease (AD) is the most common form of dementia in the elderly population.1 According to the ‘amyloid cascade' hypothesis, amyloid dysmetabolism and formation of tau-containing neurofibrillary tangles (NFTs) are key steps in the disease.2 Aging is also a critical factor for sporadic AD and a lively debate on whether the disease is actually driven by aging is animating the field.3 The ‘age-based hypothesis' postulates that initiating injury factors like head trauma, infections, vascular alterations, diabetes, or even genetic mutations, when operating on the aging brain, promote neuroinflammation and initiate the AD-related pathogenic cascade.2, 3 Thus, brain aging along with neuroinflammation are crucial conditions on which pro-AD events must work to successfully initiate the disease.
Genetic aspects of AD have been intensively investigated for decades (see Tanzi4 for an extensive review on the topic); however, crucial information is still missing to successfully compose the puzzle. Microarray technology allows the simultaneous analysis of thousands of transcripts in a single experiment and is a useful approach for the investigation of a wide range of gene-related diseases. Gene expression studies in transgenic AD models have helped to unravel genetic factors influencing disease progression at well-defined stages as well as their relation with the development of cognitive deficits.5
Thus, in the present study we employed a whole genome microarray approach to investigate age-dependent gene expression profile changes in hippocampi obtained from young (3 months of age (m.o.a.)) and old (12 m.o.a.) 3xTg-AD mice. This model offers the selective advantage of combining both amyloid (Aβ)- and tau-dependent pathology and is a largely investigated and more comprehensive preclinical model of AD.6 To control for age-dependent transcript modifications, we also investigated changes occurring in age-matched wild-type (WT) mice.
In designing our experiment, gene expression of 12 m.o. 3xTg-AD mice versus 3 m.o. 3xTg-AD mice were not considered as such design would have produced results indicative of changes that can be indistinguishably depending on a mix of two factors (aging and the AD-like background).
The major aim of the study was to provide a better understanding of whether common genetic mechanisms are shared by aging and an AD-like background and whether overlapping pathogenic pathways can be identified in the two conditions.
Results
Cluster analysis of the investigated profiles revealed four different gene clusters (Supplementary Table 1), described in the following sections.
Cluster A
Cluster A consists of 35 transcripts that, compared with age-matched WT mice, were found downregulated in both 3 m.o. and 12 m.o. 3xTg-AD mice. The same gene set was found to be upregulated in 12 m.o. WT mice when compared with 3 m.o. WT (Figure 1a). The main biological functions associated with these genes are shown in Figure 2a and Table 1.
Figure 1.
Unsupervised hierarchical clustering analysis. Transcripts that are clustered according to their expression values (log ratios) are shown. Each row indicates a transcript. The nine columns depict three replicates for each of the three experimental conditions listed on the top of the figure. Quantitative changes in gene expression are shown in colors. Red and green indicate upregulated and downregulated transcripts, respectively. Black indicates no changes in expression. Missing data points are shown in gray. (a) Cluster of 35 transcripts that were found downregulated in 3 m.o. 3xTg-AD versus 3 m.o. WT mice and 12 m.o. 3xTg-AD versus 12 m.o. WT mice. The same genes were instead upregulated in 12 m.o. WT versus 3 m.o. WT mice. (b) Cluster of 59 transcripts that were found to be specifically upregulated in 3 m.o. 3xTg-AD versus 3 m.o. WT mice and in 12 m.o. WT versus 3 m.o. WT mice. The same genes were instead downregulated in 12 m.o. 3xTg-AD versus 12 m.o. WT mice. (c) Cluster of 193 transcripts that were upregulated in all the experimental conditions (3 m.o. 3xTg-AD versus 3 m.o. WT mice and 12 m.o. 3xTg-AD versus 12 m.o. WT mice as well as in 12 m.o. WT versus 3 m.o. WT mice). (d) Cluster of 76 transcripts that were found downregulated in 3 m.o. 3xTg-AD versus 3 m.o. WT mice and in 12 m.o. WT versus 3 m.o. WT mice. The same genes were instead upregulated in 12 m.o. 3xTg-AD versus 12 m.o. WT mice
Figure 2.
Biological functions as indicated by Ingenuity Pathway Analysis (IPA). Bar charts show results of IPA and indicate key biological functions modulated by genes selected in the four clusters described in Figure 1. (a) Cluster A, (b) cluster B, (c) cluster C, and (d) cluster D
Table 1. IPA functional analysis of cluster A genes.
| Category | P-value | Molecules |
|---|---|---|
| Cellular movement | 1,69E-03-4,63E-02 | GPR182, PEBP1, CPLX3, TMSB10/TMSB4X, HAS1 |
| Inflammatory response | 1,69E-03-1,69E-03 | TMSB10/TMSB4X |
| Cellular function and maintenance | 2,84E-03-3,66E-02 | PEBP1, CPLX3, TMSB10/TMSB4X, CDC42EP2, HAS1 |
| Cell cycle | 3,38E-03-8,43E-03 | CKAP2 |
| Lipid metabolism | 3,38E-03-3,38E-03 | PEBP1 |
| Molecular transport | 3,38E-03-3,66E-02 | PEBP1, CPLX3 |
| Cell death | 1,01E-02-3,84E-02 | TMSB10/TMSB4X, CKAP2, DYSF, null |
| Tissue morphology | 1,18E-02-1,18E-02 | HAS1 |
| Cell morphology | 1,51E-02-4,92E-02 | PEBP1, TMSB10/TMSB4X, ADCY6, VPS37C, HAS1 |
| Nervous system development and function | 3,66E-02-3,66E-02 | CPLX3 |
Cluster B
Cluster B consists of 59 transcripts that, compared with 3 m.o. WT mice, were upregulated in 3 m.o. 3xTg-AD mice and 12 m.o. WT mice. The same set was downregulated in 12 m.o. 3xTg-AD mice when compared with 12 m.o. WT mice (Figure 1b). These genes are implicated in key biological functions depicted in Figure 2b and Table 2.
Table 2. IPA functional analysis of cluster B genes.
| Category | P-value | Molecules |
|---|---|---|
| Neurological disease | 1,45E-04-4,55E-02 | CTR9, NEUROD1, CD200, YWHAE, HSPA1A/HSPA1B, EGR1, F7, SSR1, SCOC, SNAP25, MFAP2, CDH2, SOX9, RSPO2, PROCR, GABRA1 |
| Cell morphology | 1,61E-04-4,87E-02 | NEUROD1, LDB3, YWHAE, PAM, HSPA1A/HSPA1B, EGR1, F7, OPHN1, SOX9, CDH2, NDUFS1, RSPO2, PROCR, GABRA1 |
| Nervous system development and function | 1,14E-03-4,87E-02 | NEUROD1, CD200, YWHAE,RIT2, HSPA1A/HSPA1B, EGR1, ETV1, SNAP25, OPHN1, CDH2, SOX9, GABRA1, TACC1 |
| Cellular development | 1,6E-03-4,43E-02 | NEUROD1, SOX9, HSPA1A/HSPA1B, RIT2, EGR1, RSPO2, F7, JAK3, SPRED2 |
| Tissue development | 1,6E-03-4,75E-02 | NEUROD1, MFAP2, SOX9,CDH2, CD200, EGR1, F7, JAK3, CERCAM |
| Cell cycle | 3,32E-03-3,64E-02 | NEUROD1, SOX9, YWHAE, HSPA1A/HSPA1B, EGR1, JAK3 |
| Cell death | 3,32E-03-4,95E-02 | NEUROD1, CD200, YWHAE, HSPA1A/HSPA1B, EGR1, F7, DNAJB9, DSG1, NDUFS1, CDH2, SOX9, UBA7, PROCR, JAK3, TACC1 |
| Cell signaling | 3,32E-03-3,59E-02 | SOX9, CDH2, YWHAE, RIT2, IL10RB, F7, DOK3, SNAP25 |
| Lipid metabolism | 3,32E-03-2,62E-02 | Rdh1 (includes others), F7 |
| Inflammatory response | 1,65E-02-4,55E-02 | CD200, EGR1, F7, DOK3, YTHDF2 |
Cluster C
Cluster C consists of 193 transcripts that, compared with age-matched WT mice, were upregulated in 3 m.o. and 12 m.o. 3xTg-AD mice. The same set was upregulated in 12 m.o. WT mice compared with 3 m.o. WT mice (Figure 1c). Functional analysis revealed that the overexpressed transcripts are associated with functions depicted in Figure 2c and Table 3.
Table 3. IPA functional analysis of cluster C genes.
| Category | P-value | Molecules |
|---|---|---|
| Cell-to-cell signaling and interaction | 4,94E-05-2,9E-02 | B2M, APBA2, VCAM1, GABRA5, USP14, JAK1, NRP2, MAPK1, KIF1B, CACNB4, CRK, HES1 (includes EG:15205), VDAC3, App, IRF1 (includes EG:16362), SYBU, PMP22, DCC, RSU1 |
| Cellular assembly and organization | 4,94E-05-2,9E-02 | B2M, APBA2, FHL1 (includes EG:14199), YWHAH, GPR12, MAPK1, KIF1B, HAX1, LPPR4, BOK, SNX3, CRK, BECN1, App, WASL, MYRIP, STARD13, DCC, MAP1LC3B, NRN1, VCAM1, NRP2, MED1 (includes EG:19014), RYR2, SEPT7, SMARCA5, YWHAZ, STK38L, MAPK9, GDAP1, VDAC3, TSG101, SYBU, PMP22, CETN3, Hmg1l1, HPRT1, DNM1L, ENC1 |
| Cell morphology | 6,66E-05-3,45E-02 | B2M, APBA2, VCAM1, MAPK1, GPR12, NRP2, SEPT7, RYR2, STK38L, YWHAZ, CRK, GDAP1, HES1 (includes EG:15205), BECN1, VDAC3, App, TSG101, PMP22, Hmg1l1, STARD13, DCC, MAP1LC3B, DNM1L, NRN1 |
| Organismal development | 9,48E-05-2,9E-02 | PAFAH1B2, VCAM1, JAK1, MAPK1, NRP2, MED1 (includes EG:19014), PKD2 (includes EG:18764), MAPK9, BECN1, App, H2AFZ, TSG101, WASL, Hmg1l1,CST3,TIMP2 |
| Nervous system development and function | 1,75E-04-3,24E-02 | B2M, APBA2, USP14, GABRA5, GPR12, MAPK1, YWHAH, KIF1B, ITM2B, LPPR4, CRK, HES1 (includes EG:15205), App, SOX2, CST3, DCC, BPNT1, NRN1, HEY1, VCAM1, NRP2, MED1 (includes EG:19014), CACNB4, STK38L, YWHAZ, MAPK9, VDAC3, SYBU, PMP22, null, Hmg1l1, HPRT1, DNM1L, ENC1 |
| Neurological disease | 2,83E-04-3,34E-02 | GABRA5, YWHAH, MAPK1, ATP6V1C1, ATP2B1, KIF1B, LPPR4, SLMAP, SOX2, PDHA1, TTBK2, PGAM1, GABRB1, PKIA, PGRMC1, G3BP2, IMPA1, HIAT1, YWHAZ, SPARCL1, HPRT1, CYC1, TMEM66, RSU1, ORC4, ENC1, B2M, APBA2, CA2, FHL1 (includes EG:14199), ITM2B, PPP1CB, BECN1, App, UNC79, TBCEL, FARSB, HADHB, TMEFF1, PPP3CB, CST3, STARD13, DCC, null, JKAMP, VCAM1, NRP2, SLC2A13, CACNB4, RYR2, MAPK9, GDAP1, KIFAP3 (includes EG:16579), HSPA12A, PMP22, AK5, null, DOCK8, PPIA, GLT25D2, DNM1L |
| Cell signaling | 1,23E-03-3,34E-02 | G3BP2, JAK1, MAPK1, ATP2B1, PKD2 (includes EG:18764), RYR2, CACNB4, STK38L, MAPK9, CRK, KIFAP3 (includes EG:16579), App, IRF1 (includes EG:16362), SOX2, CLK1, TRIM13, TTBK2, PPM1A, RSU1, TMEM9B, TIMP2 |
| Cellular compromise | 1,23E-03-3E-02 | VCAM1, MAPK1, SEPT7, PGAM1, CRK, GDAP1, DNM1L, TSG101, App, IRF1 (includes EG:16362) |
| Cell death | 1,93E-03-3,45E-02 | PAFAH1B2, CA2, VCAM1, JAK1, MAPK1, KIF1B, MED1 (includes EG:19014), CUL1, YWHAZ, SMARCA5, MAPK9, BOK, HES1 (includes EG:15205), BECN1, KIFAP3 (includes EG:16579), TSG101, App, IRF1 (includes EG:16362), GNPTG, NT5C3, PMP22, AK5, Hmg1l1, DCC, PPM1A, TIMP2 |
| Inflammatory response | 5,57E-03-3E-02 | VCAM1, JAK1, Hmg1l1, PPIA, PGAM1, App, IRF1 (includes EG:16362), TIMP2 |
Cluster D
Cluster D consists of 76 transcripts that, compared with 3 m.o. WT mice, were downregulated in 3 m.o. 3xTg-AD mice and 12 m.o. WT mice. These transcripts were upregulated in 12 m.o. 3xTg-AD mice when compared with 3 m.o. WT mice (Figure 1d). The main biological functions associated with these genes are provided in Figure 2d and Table 4.
Table 4. IPA functional analysis of cluster D genes.
| Category | P-value | Molecules |
|---|---|---|
| Cell-to-cell signaling and interaction | 9,74E-05-4,89E-02 | ST5, EPHB1, ARID1A, CCND3, BAIAP2, RGS4, STAT3 |
| Nervous system development and function | 9,74E-05-4,99E-02 | ISLR2, NEUROD2, EPHB1, PRPF19, CCND3, ACTB, S100B, BAIAP2, PDE4A, RGS4, STAT3, DIO2, KLK8, RNF6, HIP1R |
| Organ development | 3,38E-04-4,59E-02 | EPHB1, CCND3, STAT3, DIO2 |
| Cellular development | 4,87E-04-4,78E-02 | PRPF19, PA2G4, ELAVL3, PDE4A, RGS4, STAT3, DIO2, BIN1, GPC1, NEUROD2, LSMD1, CCND3, S100B, BAIAP2, BRD4, UBTF, TRRAP, SCAND1 |
| Cell cycle | 8,76E-04-4,78E-02 | GPC1, MAP4, ARID1A, null, CCND3, PA2G4, EHMT2 (includes EG:10919), LAS1L, BRD4, STAT3, TRRAP |
| Inflammatory disease | 2,39E-03-3,47E-02 | CCND3, PDE4A, STAT3 |
| Lipid metabolism | 3,14E-03-3,6E-02 | GPC1, MAP4, null, COTL1, HIP1R |
| Cell death | 4,07E-03-3,21E-02 | PRPF19, ARID1A, PA2G4, ACTB, PDE4A, RGS4, STAT3, CAPN10, KLK8, BIN1, ITPK1, GPC1, MAP4, LSMD1, CCND3, S100B, VCP, UBTF, TEX261 |
| Cell morphology | 4,07E-03-4,39E-02 | ISLR2, MAP4, ACTB, BAIAP2, VCP, S100B, PDE4A, BRD4, RGS4, STAT3, TRRAP, RNF6 |
| Cellular growth and proliferation | 4,07E-03-4,99E-02 | PRPF19, PA2G4, ACTB, RGS4, STAT3, NAA10, BIN1, GPC1, LSMD1, CCND3, EIF4A1, S100B, BRD4, PTPRS, LAS1L, UBTF, TRRAP |
| Neurological disease | 8,12E-03-3,21E-02 | S100B, STAT3 |
TaqMan qRT-PCR: microarray data validation
In order to validate the microarray results, quantitative real-time PCR (qRT-PCR) analysis was performed on RNA extracted from the same hippocampal samples employed for microarray experiments. Analysis was performed on three upregulated genes (BECN1, CST3, and GABRA5) belonging to cluster C. In all cases, qRT-PCR confirmed the microarray results (Figure 3). Moreover, expression of the employed housekeeping gene (GAPDH) was stable among all the samples.
Figure 3.
Validation of microarray gene expression data by qRT-PCR. Bar graph shows mRNA levels of CST3, GABRA5, and BECN1 measured by real-time PCR in young and old WT mice and 3xTg-AD mice as well as in old WT mice and 3xTg-AD mice. Data are expressed as mean values of relative fold changes±S.D. of three independent experiments performed in triplicate. The symbol ‘*' indicates that CST3, GABRA5, and BECN1 are significantly upregulated when compared with gene expression in 3 m.o. WT mice (P<0.05). The symbol ‘#' indicates that CST3, GABRA5, and BECN1 are significantly upregulated when compared with expression of these genes in 12 m.o. WT mice (P<0.01)
Interestingly, with the limitation and interpretative caution dictated by the low number of genes investigated by qRT-PCR (n=3), we observed that young 3xTg-AD animals did already reach maximal expression that was not further changed, in a statistically significant manner, by aging (see 12 m.o. 3xTg-AD animals). On the contrary, in WT mice, these changes were occurring in an age-dependent way. Interestingly, young 3xTg-AD mice actually showed expression values that were higher compared with old WT mice.
Discussion
The main purpose of the study was to compare gene effects promoted by the pro-AD environment offered by the 3xTg-AD mice with those triggered, in WT mice, by senescence.
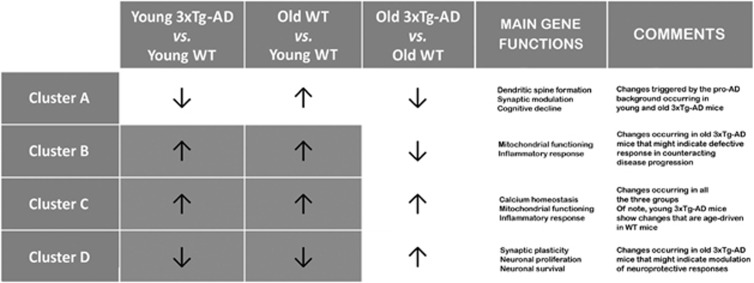
The most relevant finding is the identification of profile modifications detected in young 3xTg-AD mice that closely resemble those occurring upon physiological aging in WT animals, thereby suggesting that 3 m.o. 3xTg-AD mice undergo premature modulation of aging-related genes (Figure 4). For clarity and to improve readability of the section, we are discussing results of the three experimental conditions as follows:
3 m.o. 3xTg-AD versus 3 m.o. WT mice;
12 m.o. 3xTg-AD versus 12 m.o. WT mice;
12 m.o. WT versus 3 m.o. WT mice.
Figure 4.
Graphical synopsis of cluster analysis results. Synoptic view of all the transcriptome profiles that were found altered in the three experimental conditions. The main findings associated with observed gene expression changes are summarized. Note that, as depicted by the dark gray boxes, 3 m.o. 3xTg-AD versus 3 m.o. WT mice as well as 12 m.o. WT versus 3 m.o. WT mice show a similar pattern of expression changes in clusters B, C, and D. Also note that, only in the case of genes of cluster C, a common pattern of expression profiles is observed across all the three experimental conditions
3 m.o. 3xTg-AD versus 3 m.o. WT mice
Comparisons between expression profile changes in young 3xTg-AD mice versus 3 m.o. WT mice revealed overexpression of several genes that are depicted in clusters B and C (Figures 1 and 4). These genes modulate several mechanisms (discussed below) that are serving a pathogenic role in AD.
[Ca2+]i homeostasis
Deregulation of [Ca2+]i is a key contributing factor for AD development and progression.1, 7 Perturbations in Ca2+ handling by the endoplasmic reticulum (ER) and mitochondria as well as impaired intra/extracellular cation exchange contribute to the neuronal dysfunction and degeneration occurring in brain aging and AD.7 In cluster C, we found five genes involved in [Ca2+]i homeostasis (ATP2B1, CACNB4, RYR2, PKD2, and NDUFA9). ATP2B1 encodes for the plasma membrane Ca2+-ATPase, a key system controlling high-capacitance/low-affinity Ca2+ extrusion, whereas CACNB4 encodes for the voltage-dependent L-type calcium channel subunit β-4, a critical route for Ca2+ influx. Altered expression and functionality of these proteins correlate with deregulation of [Ca2+]i as well as with development of a seizure-prone phenotype.8 CACNB4 is also associated with neuroprotective mechanisms in animal models of ischemic brain injury.9 We also found changes in the expression of RYR2. RYR2 encodes for the ryanodine receptor 2, a major system controlling Ca2+ release from the ER. RYR2 overexpression found in 3xTg-AD mice is in line with previous findings supporting an important role for the ryanodine receptor type 2 that is promoting altered ER-Ca2+ release in presymptomatic 3xTg-AD mice.10 This data set suggests a complex scenario in which enhanced [Ca2+]i extrusion, mediated by ATP2B1, may compensate for impaired [Ca2+]i buffering and excessive ER-Ca2+ release operated by RYR2.
Mitochondrial functioning
Alterations of mitochondrial functioning are crucial in physiological aging as well as in neurodegenerative conditions.11 In clusters B and C, we found transcripts involved in the modulation of mitochondrial morphology and metabolism (TRAK1 and Ndufs1 (cluster B); Hax1, NDUFA9, GDAP1, KIF1B, HADBH, PGAM1, Ppia, and DNM1L (cluster C)). GDAP1 and DNM1L encode for proteins that promote mitochondrial fission; PGAM1 encodes for an enzyme of the glycolytic pathway that is involved in mitochondrial-mediated protective effects against Aβ-induced cell death; TRAK1 plays a critical role in axonal transport of mitochondria.12, 13, 14, 15 Finally, Ndufs1 encodes for the NADH-ubiquinone oxidoreductase 75 kD, a subunit that regulates the activity of mitochondrial complex I. The early and sustained overexpression of transcripts involved in mitochondrial fission (GDAP1, and DNML1) and neuroprotection (PGAM1) is in line with recent findings indicating that mitochondrial fission is neuroprotective through reduction of oxidative damages.15
Inflammatory response
Neuroinflammation is detrimental in brain aging and age-related cognitive decline.16 The AD brain shows chronic, discrete, and microlocalized areas of inflammation as well as the appearance of activated microglia and reactive astrocytes in the vicinity of senile plaques.1 Moreover, inflammatory-related genes and proteins (i.e., CD33, NLRP3) play a substantial role in Aβ-driven neurodegeneration and AD progression.17, 18 Cluster C revealed the presence of some transcripts (APP, IRF1, PPIA, VCAM1, TIMP2, HMGB1L1, PGAM1, and JAK1) that encode for proteins involved in the inflammatory response. It is noteworthy that in analogy with what was found for mitochondrial functioning, 3xTg-AD mice showed an early activation of upstream (IRF1) and downstream (JAK1) proinflammatory transcripts.
Additional genes involved in the inflammatory response (Hsp70, IL10 receptor, and CD200) were also found (cluster D). However, these transcripts were downregulated (instead of upregulated when comparing 3 m.o. 3xTg-AD mice with 3 m.o. WT mice). Among these, Hsp70 and CD200 are known to play an anti-inflammatory role. Thus, the overexpression of inflammatory genes appears to be coupled with simultaneous decrease in the expression of anti-inflammatory transcripts, thereby suggesting an overall proinflammatory status.
Dendrite formation, synaptic plasticity, and memory
Age- and AD-related cognitive decline is associated with synaptic dysfunction.19 In that respect, we found overexpression of several transcripts (CD200, Neurod1, SOX9, SNAP25, EGR1, OPHN1, ETV1, CDH2, GABRA1, and Spond1 (cluster B); APBA2, APP, GABRA5, MAPK1, PMP22, SYBU, USP14, VDAC3, CST3, DCC, HES1, HEY1, HPRT1, SOX2, YWHAH, CRK, GPR12, HMGB1L1, STK38L, and VCAM1 (cluster C)) that are implicated in dendritic spine formation, modulation of synaptic functioning, and cognitive decline. Overexpression of transcripts encoding for proteins involved in synaptic activity and neurotransmission may be interpreted as a protective event against AD-related synaptic dysfunctions.
Overexpression of transcripts present in cluster C suggests a bimodal set of activities. One set of actions may counteract neuronal damage and promote protective effects aimed at maintaining cognitive abilities. For instance, APBA2 is known to bind to APP and reduce Aβ production, thereby counteracting memory deficits and promoting long-term potentiation (LTP) in Tg AD mice.20 Similarly, CST3 upregulation is protective against the neurotoxic effects of Aβ oligomers and oxidative stress damage. This phenomenon has been reported in Tg AD models, in the brains of AD patients, and in those of elderly individuals.21 The GABRA5 transcript increase found in our 3xTg-AD mice lends support to the compensatory hypothesis as GABRA5 has been reported to be downregulated with aging and this process has been linked to defective spatial memory performance.22
These protective actions are counteracted by the concurrent upregulation of transcripts that, on the contrary, are associated with neuronal dysfunction. This is the case of APP and ERK2 transcripts. Given the aminoacidic composition of murine Aβ, murine APP is marginally involved in the formation of toxic Aβ species, but some studies have also proposed that the protein can favor ERK1,2 activation, tau phosphorylation, and the formation of NFTs.23 Chronic ERK2 activation by Aβ oligomers can be detrimental as early and sustained ERK2 stimulation has been shown to promote cognitive decline in AD mice24 and induce neuronal toxicity through caspase-3 activation.25
It is interesting to note that in clusters A and D, we found downregulation of transcripts (PEBP1 and Complexin III (cluster A) and BIN, ACTB, BAIAP2, EphB, KLK8, ARD1, PDE4, Ehmt2, Neurod2, STAT3, S100B, and CCND3 (cluster D)) that are involved in dendritic spine formation, modulation of synaptic functioning, and cognitive decline. In this respect, PEBP1 and Complexin III downregulation is associated with impaired cholinergic neurotransmission (PEBP1)26 and synaptic transmission deficits (Complexin III),27 the two mechanisms largely impaired in AD and in mild cognitive impairment.28
BAIAP2, EphB1, BIN1, and KLK8 are involved in synaptic plasticity. BAIAP2 (also known as IRSp53) modulates NMDA receptor-related synaptic transmission, LTP, as well as learning and memory. BAIAP2 knockout mice show impaired spatial learning and novel object recognition.29 Thus, BAIAP2 downregulation might be involved in the development of cognitive deficits affecting this model that has been also shown to suffer from significant decrease in BDNF levels.30 NMDAR deregulation has been associated with EphB (a family of ligand proteins) receptor deficits and linked to the appearance of neurological disorders including AD.31 Still, in the set of synaptic plasticity-related genes that we found underexpressed, KLK8 seems important as the gene is involved in brain development, neuronal plasticity, and stress response.32 Thus, KLK8 downregulation can favor aberrant CNS development. S100B is a calcium-binding protein released by astroglial cells. Compelling evidence indicates that transgenic animals, overexpressing S100B, show neuronal loss and increased expression of the pro-apoptotic protein clusterin, a protein that is increased in hippocampi and frontal cortices of AD patients.33
In summary, our findings suggest that 3xTg-AD mice not only show early modulation of transcripts that exert detrimental effects on synaptic functioning but also develop changes that counteract this impairment. The process appears to go on chronically as the same pattern is present in 12 m.o. 3xTg-AD mice.
Neuronal death, cell cycle, and autophagy
Aberrant expression of cell cycle- and autophagy-related molecules plays a pivotal role in the neuronal loss associated with AD and brain aging.34, 35 Our analysis revealed overexpression of a group of transcripts (SPIN1, JAK3, Neurod1, SOX9, YWHAE, EGR1, and HSPA1A/HSPA1B (cluster B); Beclin-1, MAP1LC3-B, HADHB, AK5, CyC1, SIRT7 and ENC1 (cluster C)) involved in autophagy, cell cycle, and cell death, thereby suggesting the potential activation of a complex network of neuronal death-related responses occurring in young 3xTg-AD mice.
Early EGR1 upregulation has been reported in AD patients36 and can contribute, along with other cell cycle-related genes, to NFT formation, a phenomenon that is also mediated by the increased expression of cell cycle proteins.34, 37 Furthermore, overexpression of SPIN1 (a gene involved in cell cycle impairment) promotes chromosome instability, a process leading to cellular senescence and apoptosis.38 Sirt7, a less investigated member of the sirtuin family, is a nuclear protein expressed in the brain with a cellular distribution and functions that are still unknown.39 Experiments employing Sirt7 knockout mice or Sirt7-overexpressing cells have indicated the antiproliferative role of the protein and suggested that Sirt7 activity can improve tissue integrity in aging animals.40
Beclin-1 and MAP1LC3-B are two autophagy-related genes whose overexpression might counteract pathology development in 3 m.o. 3xTg-AD mice. This hypothesis is consistent with recent findings indicating that early induction of autophagy reduces cognitive decline in 3xTg-AD mice.41, 42 Autophagy, in fact, clears out damaged proteins that are prone to aggregation,43 and the process can be neuroprotective by mediating the degradation of misfolded proteins.44 Intriguingly, in AD, autophagy can play a dual role. On one hand, it is protective by promoting the removal of Aβ and tau aggregates and, on the other hand, autophagosomes are nevertheless sites of choice for accumulation and production of Aβ.45, 46, 47
Analysis of networks of genes involved in neuronal death also showed several transcripts that, compared with young WT mice, were found downregulated in young 3xTg-AD mice (ARID1A, BRD4, CCND3, EHMT2, GPC1, LAS1L, MAP4, PA2G4, PTGES3, STAT3, and TRRAP (cluster D)). Interestingly, aberrant expression of CCND3 has been reported to be closely associated with AD-related neuronal death occurring in specific hippocampal regions.48
12 m.o. 3xTg-AD versus 12 m.o. WT mice
In order to evaluate how AD-like progression affects gene expression, we investigated profiles of 12 m.o. 3xTg-AD mice compared with 12 m.o. WT mice and matched this set of data with results of 3 m.o. 3xTg-AD mice that were compared with age-matched WT mice. The analysis revealed a similar overexpression of all transcripts of cluster C and downexpression of those of cluster A. Interesting differences appeared when analyzing transcripts belonging to clusters B and D (Figures 1 and 4).
Cluster B transcripts, overexpressed in young 3xTg-AD mice, were downregulated in 12 m.o. 3xTg-AD mice when compared with age-matched WT mice, a finding that supports the hypothesis that aged 3xTg-AD mice may show deficits in counteracting disease progression. Underexpression of many transcripts found in cluster B has been previously reported in several models of aging and AD as well as in AD patients. This is the case for SNAP25 (encoding for a key protein involved in synaptic vesicle cycle and neurotransmission modulation),49 GABRA1 (encoding for a γ-aminobutyric acid (GABA) receptor subunit downregulated in AD brains),50 CDH2 (encoding for N-cadherin, whose deregulation triggers apoptotic pathway activation),51 and OPHN1 (encoding for a Rho-GTPase involved in controlling excitatory synapses maturation and plasticity).52
Cluster D transcripts, downexpressed in 3 m.o. 3xTg-AD, were instead overexpressed in aged 3xTg-AD. The upregulation of transcripts that are involved in the modulation of synaptic plasticity, neuronal proliferation, survival, differentiation, and regulation of cell division process might reflect a compensatory neuroprotective response (BAIAP2), although the net result suggests a more complex scenario (see, for instance, the role of KLK8). BAIAP2 downregulation is related to altered synaptic plasticity as observed in BAIAP2-deficient mice,29 whereas its overexpression increases dendritic spine density and postsynaptic potentiation, ultimately enhancing cognition.53 KLK8 deficiency promotes decreased synaptic plasticity, whereas, on the contrary, gain in KLK8 mRNA levels has been observed in AD brains and equally associated with detrimental effects on hippocampal functioning.54 Inhibition of S100B has positive effects on the Aβ load and gliosis observed in AD patients,55 whereas S100B overexpression has been associated with exacerbation of AD clinical signs.56, 57 Finally, altered expression of cell cycle proteins (i.e., CCND3) in postmitotic neurons has been observed both in aging and in AD.
Collectively, over- and underexpression of the aforementioned transcripts confirm a pattern of responses in which some genes exert detrimental effects that are in line with the development of AD-like pathology. At the same time, other transcripts appear to counteract these effects. Interestingly, this process appears to progress chronically as a similar pattern has been reported in 3 m.o. and in 12 m.o. 3xTg-AD mice.
12 m.o. WT versus 3 m.o. WT mice
Finally, we compared expression profiles of 12 m.o. WT mice with 3 m.o. WT mice to analyze the effects of physiological aging.
Interestingly, changes driven by aging were largely overlapping with those observed in 3 m.o. 3xTg-AD mice when compared with 3 m.o. WT mice. We, in fact, observed overexpression of several transcripts belonging to clusters B and C and downregulation of a gene data set present in cluster D (Figures 1 and 4). The functional correlates of these changes have been discussed above.
Overall, these data support the hypothesis that the pro-AD environment offered by the 3xTg-AD mice accelerates and promotes early modifications of genes that are otherwise changed by aging in old WT mice. Moreover, comparison between 12 m.o. WT mice and age-matched 3xTg-AD mice showed expression changes that were similar only for genes belonging to cluster C.
Interestingly, Ingenuity Pathway Analysis (IPA) analysis of this cluster revealed transcripts (HSPA12A, HIAT1, KIAA1409, NRP2, STARD13, UBB, and PKIA) that genome-wide association studies have recently shown to be involved in AD-related single-nucleotide polimorphisms (SNPs) as well as transcripts (APP, ATP6V1C1, BECN1, CST3, DCC, DNM1L, GABRA5, GABRB1, HIAT1, HSPA12A, KIAA1409, LPPR4, MAPK9, NRP2, PKIA, RYR2, STARD13, UBB, and YWHAZ) that are closely related to AD development.
Finally, cluster A is the only one showing a unique behavior in 3xTg-AD mice, indicating changes that are specifically triggered by the pro-AD genetic background of the mice. It is intriguing to hypothesize that these genes have a functional role in AD progression and represent potential AD biomarkers. Among these, we observed a downregulation of Homer3 and Dysferlin. Homer3 interacts with APP and inhibits Aβ production. Thus, Homer3 downregulation can lead to enhanced Aβ production.58 Accumulation in senile plaques of Dysferlin, a protein involved in membrane repair, leads to defective neuronal repair.59 Thus, it is conceivable that the Dysferlin downregulation that we found in the 3xTg-AD mice can exert a similar detrimental effect.
Conclusions
With the limitation of any Tg-AD model, our data support the hypothesis that aging and AD share some mechanistic similarities. In our Tg-AD mouse, the pro-AD environment promotes and accelerates changes that, upon aging, are otherwise occurring in WT animals. Although the 3xTg-AD mice harbor three mutations that do not occur, at the same time, in AD human brains, this cumulative pro-AD background modulates in Tg mice the early on expression of genes, the so-called BioAge genes (associated with neuronal loss, glial activation, and lipid dysmetabolism) that are in humans biomarkers for brain aging and found prematurely expressed in AD patients.60 The same accelerated expression of aging-related genes in AD has been recently reported in a study that analyzed AD brains,61, 62 thereby providing support for the heuristic value of 3xTg-AD mice as preclinical model of AD.
Our data support and integrate the age-based hypothesis for AD, a concept that postulates that aging is required to set in motion the AD-related pathogenic cascade. Our findings suggest the presence of a feed-forward mechanism by which pro-AD factors can promote aging-related gene profile changes, thereby boosting and accelerating the disease process. Furthermore, this is the first study that offers an extensive analysis of the transcriptomic profile of the 3xTg-AD model, one of the most comprehensive preclinical models of the disease. The novel set of AD-related genes found in our analysis will, likely, further studies investigating the molecular roadmap of the disease and its modulation by aging.
Materials and Methods
Animal models
All the procedures involving animals were approved by the institutional ethics committee (CeSI protocol no.: AD-301) and performed according to institutional guidelines and in compliance with national (DL no. 116, GU, Suppl. 40, 18 February 1992) and international laws and policies. The 3xTg-AD (harboring AD- related human mutation APP (Swe), PS1 (M146V), and tau (P301L) transgenes) female mice (n=2 at 3 m.o.a.; n=2 at 12 m.o.a.) and age-matched female control WT mice (129SV × C57BL/6, n=2 at 3 m.o.a.; n=2 at 12 m.o.a.) were kept in a temperature-controlled room at 25°C under a 12-h light/dark cycle and fed ad libitum with tap water and commercial chow. Mice at 3 or 12 m.o.a. were anesthetized and killed by decapitation, hippocampi excised, tissue transferred into RNA-later solution, and stored at −80°C for RNA processing.
Microarray analysis
Hippocampi were homogenized using a hand glass potter and total RNA extracted using the SVtotal RNA Isolation System kit following the manufacturer's instructions (Promega, Madison, WI, USA). RNA purity and quantity were estimated using an Agilent 8453 spectrophotometer (Agilent, Santa Clara, CA, USA). RNA samples were not pooled. Using the Amino Allyl MessageAmp II aRNA Amplification Kit (Ambion, Grand Island, NY, USA) RNA was amplified and fluorescently labeled with Cy3-Cy5 cyanins following the manufacturer's instructions. Labeled RNA was hybridized on high-density array containing 31 802 mouse transcripts (Mouse OneArray Whole Genome DNA microarray v1, Biosense, Milan, Italy). In order to increase the experimental homogeneity, we performed three replicates with a dye swap and each experiment contained RNA from hippocampi of young (3 m.o.a.) and old (12 m.o.a.) 3xTg-AD mice versus age-matched WT hippocampal RNA as control for a total of nine experiments. After hybridization, Cy3–Cy5 fluorescent signals were detected with a confocal laser scanner ‘ScanArray Express' (Packard BioScience, Waltham, MA, USA) and analyzed with the ‘ScanArray Express–MicroArray Analysis System 3.0' software (Perkin Elmer, Waltham, MA, USA). Raw data are stored in the GEO public database (accession number: GSE35210). Raw data were normalized using locally weighted scatterplot smoothing (LOWESS) algorithm. A transcript was considered differentially expressed when showing an absolute log-ratio value of ≥±0.5, that is, 1.4 fold-change in transcript quantity. The transcript data set was analyzed with gene clustering analysis using Cluster 3.0 (Stanford University Labs, Stanford, CA, USA). We evaluated only spots showing significant expression in at least 80% of the experiments. Data were centered to the mean of each mRNA and unsupervised average linkage hierarchical clustering of both the mRNAs and samples was performed using uncentered correlation metrics. Dendrograms and selected transcripts were visualized by employing TreeView software (Stanford University Labs). Gene biological functions were inferred using IPA software (Ingenuity Systems, Redwood City, CA, USA).
Real-time PCR
Microarray results were validated by performing qRT-PCR analysis on RNA extracted from hippocampi obtained from the same samples used for microarray analysis. Gene expression of three genes found upregulated by microarray (BECN1, CST3, and GABRA5) was evaluated. The GAPDH housekeeping gene was employed as internal control. qRT-PCR was performed in a total volume of 50 μl containing: 1 × TaqMan Universal PCR Master Mix, no AmpErase UNG, and 2 μl of cDNA using TaqMan assay on an Abi 7900HT Sequencing Detection System (Applied Biosystems, Paisley, UK). Transcript primers and probe sets employed were: GAPDH: primers FW: CTTTGTCAAGCTCATTTCCTGG, RW: TCTTGCTCAGTGTCCTTGC, probe CACCCTGTTGCTGTAGCCGTATTCA; BECN1: primers FW: GTACCGACTTGTTCCCTATGG, RW: ACACAGTCCAGAAAAGCTACC, probe: CCCCAGAACAGTATAACGGCAACTCC; CST3: primers FW: CTGACTGTCCTTTCCATGACC, RW: TCCTTCTAGACTCAGCCCTTAG, probe: CCTTCCAGATCTACAGCGTGCCC; GABRA5: primers FW: GGGAATGGACAATGGAATGC, RW: TCTCATTGGTCTCGTCTTGTAC, probe: CATTTGCGAAAAGCCAAAGTGACCTGGA (Integrated DNA Technologies, Coralville, IA, USA). Real-time amplifications included 10 min at 95°C followed by 48 cycles of 15 s at 95°C and 1 min at 60°C. For each transcript, relative expression levels were normalized against the GAPDH gene. The ΔΔCt method was used to compare relative fold expression differences. One-way ANOVA was performed to assess statistically significant expression differences among the analyzed transcripts.
Glossary
- AD
Alzheimer's disease
- m.o.a.
months of age
- Aβ
β-amyloid
- WT
wild type
- qRT-PCR
quantitative real-time PCR
- ER
endoplasmic reticulum
- LTP
long-term potentiation
- NFT
neurofibrillary tangle
- GABA
γ-aminobutyric acid
- IPA
Ingenuity Pathway Analysis
- SNP
single-nucleotide polimorphism
- LOWESS
locally weighted scatterplot smoothing
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by A Verkhratsky
Supplementary Material
References
- Querfurth HW, LaFerla FM. Alzheimer's disease. New Eng J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Krstic D, Knuesel I. Deciphering the mechanism underlying late-onset Alzheimer disease. Nat Rev Neurol. 2013;9:25–34. doi: 10.1038/nrneurol.2012.236. [DOI] [PubMed] [Google Scholar]
- Herrup K. Reimagining Alzheimer's disease—an age-based hypothesis. J Neurosci. 2010;30:16755–16762. doi: 10.1523/JNEUROSCI.4521-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi RE. The genetics of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:1–10. [Google Scholar]
- Reddy PH, McWeeney S. Mapping cellular transcriptosomes in autopsied Alzheimer's disease subjects and relevant animal models. Neurobiol Aging. 2006;27:1060–1077. doi: 10.1016/j.neurobiolaging.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Corona C, Pensalfini A, Frazzini V, Sensi SL. New therapeutic targets in Alzheimer's disease: brain deregulation of calcium and zinc. Cell Death Dis. 2011;2:e176. doi: 10.1038/cddis.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketelaars SO, Gorter JA, Aronica E, Wadman WJ. Calcium extrusion protein expression in the hippocampal formation of chronic epileptic rats after kainate-induced status epilepticus. Epilepsia. 2004;45:1189–1201. doi: 10.1111/j.0013-9580.2004.03304.x. [DOI] [PubMed] [Google Scholar]
- Jin K, Mao XO, Eshoo MW, Nagayama T, Minami M, Simon RP, et al. Microarray analysis of hippocampal gene expression in global cerebral ischemia. Ann Neurol. 2001;50:93–103. doi: 10.1002/ana.1073. [DOI] [PubMed] [Google Scholar]
- Chakroborty S, Kim J, Schneider C, Jacobson C, Molgo J, Stutzmann GE. Early presynaptic and postsynaptic calcium signaling abnormalities mask underlying synaptic depression in presymptomatic Alzheimer's disease mice. J Neurosci. 2012;32:8341–8353. doi: 10.1523/JNEUROSCI.0936-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Pedrola L, Espert A, Wu X, Claramunt R, Shy ME, Palau F. GDAP1, the protein causing Charcot-Marie-Tooth disease type 4A, is expressed in neurons and is associated with mitochondria. Hum Mol Genet. 2005;14:1087–1094. doi: 10.1093/hmg/ddi121. [DOI] [PubMed] [Google Scholar]
- Miyamae Y, Han J, Sasaki K, Terakawa M, Isoda H, Shigemori H. 3,4,5-tri-O-caffeoylquinic acid inhibits amyloid beta-mediated cellular toxicity on SH-SY5Y cells through the upregulation of PGAM1 and G3PDH. Cytotechnology. 2011;63:191–200. doi: 10.1007/s10616-011-9341-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley K, Stephenson FA. Trafficking kinesin protein (TRAK)-mediated transport of mitochondria in axons of hippocampal neurons. J Biol Chem. 2011;286:18079–18092. doi: 10.1074/jbc.M111.236018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Kageyama Y, Sesaki H. Mitochondrial division prevents neurodegeneration. Autophagy. 2012;8:1531–1533. doi: 10.4161/auto.21213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ownby RL. Neuroinflammation and cognitive aging. Curr Psychiatry Rep. 2010;12:39–45. doi: 10.1007/s11920-009-0082-1. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, et al. NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griciuc A, Serrano-Pozo A, Parrado AR, Lesinski AN, Asselin CN, Mullin K, et al. Alzheimer's disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron. 2013;78:631–643. doi: 10.1016/j.neuron.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Mitchell JC, Ariff BB, Yates DM, Lau KF, Perkinton MS, Rogelj B, et al. X11beta rescues memory and long-term potentiation deficits in Alzheimer's disease APPswe Tg2576 mice. Hum Mol Genet. 2009;18:4492–4500. doi: 10.1093/hmg/ddp408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G, Levy E. Cystatin C in Alzheimer's disease. Front Mol Neurosci. 2012;5:79. doi: 10.3389/fnmol.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberman RP, Quigley CK, Gallagher M. Characterization of CpG island DNA methylation of impairment-related genes in a rat model of cognitive aging. Epigenetics. 2012;7:1008–1019. doi: 10.4161/epi.21291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizzari M, Venezia V, Repetto E, Caorsi V, Magrassi R, Gagliani MC, et al. Amyloid precursor protein and Presenilin1 interact with the adaptor GRB2 and modulate ERK 1,2 signaling. J Biol Chem. 2007;282:13833–13844. doi: 10.1074/jbc.M610146200. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Cerbai F, Bellucci A, Melani C, Grossi C, Bartolozzi C, et al. Differential activation of mitogen-activated protein kinase signalling pathways in the hippocampus of CRND8 transgenic mouse, a model of Alzheimer's disease. Neuroscience. 2008;153:618–633. doi: 10.1016/j.neuroscience.2008.02.061. [DOI] [PubMed] [Google Scholar]
- Chong YH, Shin YJ, Lee EO, Kayed R, Glabe CG, Tenner AJ. ERK1/2 activation mediates Abeta oligomer-induced neurotoxicity via caspase-3 activation and tau cleavage in rat organotypic hippocampal slice cultures. J Biol Chem. 2006;281:20315–20325. doi: 10.1074/jbc.M601016200. [DOI] [PubMed] [Google Scholar]
- Maki M, Matsukawa N, Yuasa H, Otsuka Y, Yamamoto T, Akatsu H, et al. Decreased expression of hippocampal cholinergic neurostimulating peptide precursor protein mRNA in the hippocampus in Alzheimer disease. J Neuropathol Exp Neurol. 2002;61:176–185. doi: 10.1093/jnen/61.2.176. [DOI] [PubMed] [Google Scholar]
- Landgraf I, Muhlhans J, Dedek K, Reim K, Brandstatter JH, Ammermuller J. The absence of Complexin 3 and Complexin 4 differentially impacts the ON and OFF pathways in mouse retina. Eur J Neurosci. 2012;36:2470–2481. doi: 10.1111/j.1460-9568.2012.08149.x. [DOI] [PubMed] [Google Scholar]
- Haense C, Kalbe E, Herholz K, Hohmann C, Neumaier B, Krais R, et al. Cholinergic system function and cognition in mild cognitive impairment. Neurobiol Aging. 2012;33:867–877. doi: 10.1016/j.neurobiolaging.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Kim MH, Choi J, Yang J, Chung W, Kim JH, Paik SK, et al. Enhanced NMDA receptor-mediated synaptic transmission, enhanced long-term potentiation, and impaired learning and memory in mice lacking IRSp53. J Neurosci. 2009;29:1586–1595. doi: 10.1523/JNEUROSCI.4306-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona C, Masciopinto F, Silvestri E, Viscovo AD, Lattanzio R, Sorda RL, et al. Dietary zinc supplementation of 3xTg-AD mice increases BDNF levels and prevents cognitive deficits as well as mitochondrial dysfunction. Cell Death Dis. 2010;1:e91. doi: 10.1038/cddis.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffler-Collins SI, Dalva MB. EphBs: an integral link between synaptic function and synaptopathies. Trends Neurosci. 2012;35:293–304. doi: 10.1016/j.tins.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef GM, Kishi T, Diamandis EP. Role of kallikrein enzymes in the central nervous system. Clin Chim Acta. 2003;329:1–8. doi: 10.1016/s0009-8981(03)00004-4. [DOI] [PubMed] [Google Scholar]
- Shapiro LA, Bialowas-McGoey LA, Whitaker-Azmitia PM. Effects of S100B on serotonergic plasticity and neuroinflammation in the hippocampus in Down syndrome and Alzheimer's disease: studies in an S100B overexpressing mouse model. Cardiovasc Psychiatry Neurol. 2010;2010:1–13. doi: 10.1155/2010/153657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowser R, Smith MA. Cell cycle proteins in Alzheimer's disease: plenty of wheels but no cycle. J Alzheimers Dis. 2002;4:249–254. doi: 10.3233/jad-2002-4316. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Yang DS. Autophagy and neuronal cell death in neurological disorders. Cold Spring Harb Perspect Biol. 2012;4:10. doi: 10.1101/cshperspect.a008839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez Ravetti M, Rosso OA, Berretta R, Moscato P. Uncovering molecular biomarkers that correlate cognitive decline with the changes of hippocampus' gene expression profiles in Alzheimer's disease. PLoS One. 2010;5:e10153. doi: 10.1371/journal.pone.0010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Li T, Qureshi HY, Han D, Paudel HK. Early growth response 1 (Egr-1) regulates phosphorylation of microtubule-associated protein tau in mammalian brain. J Biol Chem. 2011;286:20569–20581. doi: 10.1074/jbc.M111.220962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Zhang P, Qin L, Chen L, Shi S, Lu Y, et al. Overexpression of SPINDLIN1 induces cellular senescence, multinucleation and apoptosis. Gene. 2008;410:67–74. doi: 10.1016/j.gene.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Schwer B, Schumacher B, Lombard DB, Xiao C, Kurtev MV, Gao J, et al. Neural sirtuin 6 (Sirt6) ablation attenuates somatic growth and causes obesity. Proc Natl Acad Sci USA. 2010;107:21790–21794. doi: 10.1073/pnas.1016306107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakhrusheva O, Braeuer D, Liu Z, Braun T, Bober E. Sirt7-dependent inhibition of cell growth and proliferation might be instrumental to mediate tissue integrity during aging. J Physiol Pharmacol. 2008;59 (Suppl 9:201–212. [PubMed] [Google Scholar]
- Majumder S, Richardson A, Strong R, Oddo S. Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PLoS One. 2011;6:e25416. doi: 10.1371/journal.pone.0025416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal PJ, Herman AM, Hoe HS, Rebeck GW, Moussa CE. Parkin mediates beclin-dependent autophagic clearance of defective mitochondria and ubiquitinated Abeta in AD models. Hum Mol Genet. 2011;20:2091–2102. doi: 10.1093/hmg/ddr091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Qiang J, Gu P, Wang Y, Geng Y, Wang M. Age-related autophagy alterations in the brain of senescence accelerated mouse prone 8 (SAMP8) mice. Exp Gerontol. 2011;46:533–541. doi: 10.1016/j.exger.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Metcalf DJ, Garcia-Arencibia M, Hochfeld WE, Rubinsztein DC. Autophagy and misfolded proteins in neurodegeneration. Exp Neurol. 2012;238:22–28. doi: 10.1016/j.expneurol.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WH, Cuervo AM, Kumar A, Peterhoff CM, Schmidt SD, Lee JH, et al. Macroautophagy—a novel Beta-amyloid peptide-generating pathway activated in Alzheimer's disease. J Cell Biol. 2005;171:87–98. doi: 10.1083/jcb.200505082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung YT, Wang BJ, Hu MK, Hsu WM, Lee H, Huang WP, et al. Autophagy: a double-edged sword in Alzheimer's disease. J Biosci. 2012;37:157–165. doi: 10.1007/s12038-011-9176-0. [DOI] [PubMed] [Google Scholar]
- Busser J, Geldmacher DS, Herrup K. Ectopic cell cycle proteins predict the sites of neuronal cell death in Alzheimer's disease brain. J Neurosci. 1998;18:2801–2807. doi: 10.1523/JNEUROSCI.18-08-02801.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanGuilder HD, Yan H, Farley JA, Sonntag WE, Freeman WM. Aging alters the expression of neurotransmission-regulating proteins in the hippocampal synaptoproteome. J Neurochem. 2010;113:1577–1588. doi: 10.1111/j.1471-4159.2010.06719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limon A, Reyes-Ruiz JM, Miledi R. Loss of functional GABA(A) receptors in the Alzheimer diseased brain. Proc Natl Acad Sci USA. 2012;109:10071–10076. doi: 10.1073/pnas.1204606109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando K, Uemura K, Kuzuya A, Maesako M, Asada-Utsugi M, Kubota M, et al. N-cadherin regulates p38 MAPK signaling via association with JNK-associated leucine zipper protein: implications for neurodegeneration in Alzheimer disease. J Biol Chem. 2011;286:7619–7628. doi: 10.1074/jbc.M110.158477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadif Kasri N, Nakano-Kobayashi A, Malinow R, Li B, Van Aelst L. The Rho-linked mental retardation protein oligophrenin-1 controls synapse maturation and plasticity by stabilizing AMPA receptors. Genes Dev. 2009;23:1289–1302. doi: 10.1101/gad.1783809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Ko J, Racz B, Burette A, Lee JR, Kim S, et al. Regulation of dendritic spine morphogenesis by insulin receptor substrate 53, a downstream effector of Rac1 and Cdc42 small GTPases. J Neurosci. 2005;25:869–879. doi: 10.1523/JNEUROSCI.3212-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu-Okabe C, Yousef GM, Diamandis EP, Yoshida S, Shiosaka S, Fahnestock M. Expression of the kallikrein gene family in normal and Alzheimer's disease brain. Neuroreport. 2001;12:2747–2751. doi: 10.1097/00001756-200108280-00031. [DOI] [PubMed] [Google Scholar]
- Mori T, Koyama N, Arendash GW, Horikoshi-Sakuraba Y, Tan J, Town T. Overexpression of human S100B exacerbates cerebral amyloidosis and gliosis in the Tg2576 mouse model of Alzheimer's disease. Glia. 2010;58:300–314. doi: 10.1002/glia.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialowas-McGoey LA, Lesicka A, Whitaker-Azmitia PM. Vitamin E increases S100B-mediated microglial activation in an S100B-overexpressing mouse model of pathological aging. Glia. 2008;56:1780–1790. doi: 10.1002/glia.20727. [DOI] [PubMed] [Google Scholar]
- Michetti F, Corvino V, Geloso MC, Lattanzi W, Bernardini C, Serpero L, et al. The S100B protein in biological fluids: more than a lifelong biomarker of brain distress. J Neurochem. 2012;120:644–659. doi: 10.1111/j.1471-4159.2011.07612.x. [DOI] [PubMed] [Google Scholar]
- Parisiadou L, Bethani I, Michaki V, Krousti K, Rapti G, Efthimiopoulos S. Homer2 and Homer3 interact with amyloid precursor protein and inhibit Abeta production. Neurobiol Dis. 2008;30:353–364. doi: 10.1016/j.nbd.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Palamand D, Strider J, Milone M, Pestronk A. The muscle protein dysferlin accumulates in the Alzheimer brain. Acta Neuropathol. 2006;112:665–671. doi: 10.1007/s00401-006-0147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podtelezhnikov AA, Tanis KQ, Nebozhyn M, Ray WJ, Stone DJ, Loboda AP. Molecular insights into the pathogenesis of Alzheimer's disease and its relationship to normal aging. PLoS One. 2011;6:e29610. doi: 10.1371/journal.pone.0029610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saetre P, Jazin E, Emilsson L. Age-related changes in gene expression are accelerated in Alzheimer's disease. Synapse. 2011;65:971–974. doi: 10.1002/syn.20933. [DOI] [PubMed] [Google Scholar]
- Cooper-Knock J, Kirby J, Ferraiuolo L, Heath PR, Rattray M, Shaw PJ. Gene expression profiling in human neurodegenerative disease. Nat Rev Neurol. 2012;8:518–530. doi: 10.1038/nrneurol.2012.156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.