Summary
Exposure to radiation and some chemotherapeutic agents is associated with an increased risk of developing second cancers. Short telomeres are almost universally associated with malignant cancer progression. An unanswered question is whether inherited short telomeres or therapy related telomere shortening is a biomarker of development of second malignant neoplasms.
In this issue of Clinical Cancer Research, Gramatges and colleagues (1) analyzed telomere content between childhood cancer survivors with and without second malignant neoplasms. They observed that patients with short telomeres at first cancer occurence increased the risk of developing a second cancer. By way of background, human telomeres are repetitive TTAGGG repeats of DNA at the ends of chromosomes that are essential to preserve the integrity of the genome. Telomeres progressively shorten in all dividing human cells leading to cellular senescence (with increases in inflammatory signaling and reduced immune responses). In combination with oncogenic changes, chromosomal instability occurs and almost all human cancers have short telomeres and express the ribonucleoprotein enzyme, telomerase (2). There is an inverse relationship between telomere length in human leukocytes and both cancer incidence and mortality (3). Also, short telomeres and high levels of telomerase in tumors at the time of diagnosis are predictors of poor outcomes in a cancer-specific manner (4). In association studies in individuals without cancer it remains unclear if short telomeres correlate with increased risk for cancer (5, 6). In pediatric cancer patients that undergo spontaneous remissions such as neuroblastoma 4s (7) and low-grade gliomas (8) there is a lack of activation of telomerase. These studies demonstrate that in the absence of telomerase short telomeres are initially protective against cancer. These studies also illustrate that telomerase is not required for initiation of tumorigenesis but tumor cells must engage a mechanism to maintain telomeres to continue dividing. Thus, expression of telomerase is a critical event in the progression to advanced tumors and its inhibition could be a potent, almost universal, anticancer therapeutic target. Telomere length may also be an enrichment biomarker for clinical trial enrollment as well as an independent prognostic indicator of second cancers as is suggested in the current study (1).
Gramatges and colleagues (1) obtained specimens from the Childhood Cancer Survivor Study and performed a matched case-control analysis of telomere contents between childhood survivors with and without second malignant neoplasms. These patients were treated for their primary cancer from 1970-1986 and contains more than 14,000 survivors, including 4,000 siblings without cancer, that have been followed for long-term health outcomes. Gramatges (1) is the first to analyze telomere content in this specific group of cancer survivors. An association was observed between shortened telomeres in buccal (mouthwash) samples at first cancer and the incidence of second cancers that was primarily driven by thyroid cancers, even though breast cancers and sarcomas were also associated with secondary cancer incidence (1). By matching controls at diagnosis and by treatments, a statistically significant increase in thyroid cancer occurred in patients with less telomere content (1). What is not known is whether these patients with a second cancer had short telomeres prior to first cancer therapy or afterwards. Knowledge of this could separate a genetic susceptibility to second cancers versus an environmental effect of the treatment protocol. Exposure to chemotherapy (9) and ionizing radiation (10) is known to increase the risk of thyroid tumors. In addition, radiation exposure (with or without chemotherapy) in the same Childhood Cancer Survivor Study cohort led to an 83% incidence of skin basal cell carcinoma (11) but telomere content was not examined. Previously it was shown that there was a significant telomere length decrease in Chernobyl clean-up workers compared to healthy age-matched donors 20 years after radiation exposure (12). These studies document that radiation-induced telomere loss is prolonged.
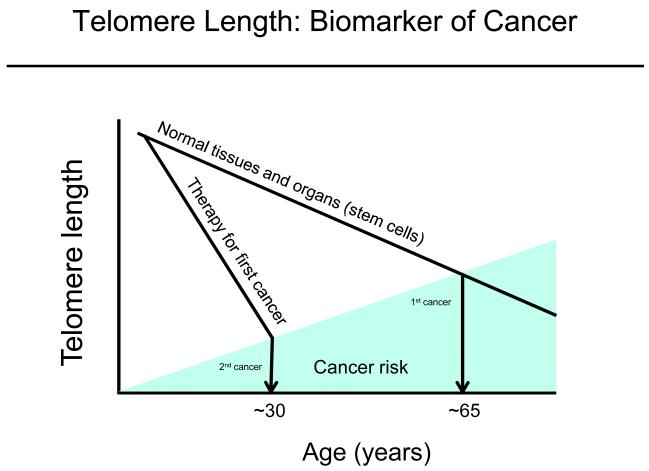
Is telomere length a molecular biomarker of recurring cancers (Figure)? Approximately two-thirds of all first cancers occur in the general population over the age of 65, while in patients treated for primary cancers during childhood, the incidence of second cancer increases much more quickly. While intuitively this suggests the treatment is leading to the increased incidence of second cancers, it is not proven, and these individuals may have a pre-existing inherited susceptibility to cancer.
Figure.
Are short telomeres hallmarks of cancer recurrence? It is well established that the incidence of first cancers in the general population increases with age with almost 70% of all cancers occurring in the 65 and older segment of the population. However, the risk of second cancers in patients treated for pediatric primary tumors is an ongoing area of research.This figure illustrates that children treated for first cancers with short telomeres have second cancers well before the typical age of cancer in the general population. The speculation is that the therapy or perhaps a germline inheritance of short telomeres predisposes to second cancers.
So what are the limitations/implications of this study and others going forward? First, these pediatric cancer patients were treated with significantly different regimens ~30 years ago, so it is not certain if the same results would occur today. Also, these studies were conducted at a specific time point post initial cancer therapy with some of the cohort having additional rounds of therapy. It is not known if more or fewer second cancers will occur during the next decade(s) making the association potentially more or less dramatic. Another concern is the use of buccal (mouthwash) samples from which DNA is subject to degradation and contamination artifacts. Finally, the analyses were done on average and not the shortest telomere lengths. There is increasing evidence that telomeres are heritable and mutations in telomere regulatory genes have a causal role in human diseases such as bone marrow failure and idiopathic pulmonary fibrosis. These have been referred to as telomeropathies or telomere syndromes. It is becoming recognized that these telomere maintenance disorders are a spectrum of diseases and thus it is too early to draw general conclusions about the causative versus correlative role of telomere biology in most human diseases. However, there are several issues that should be addressed in future studies.
Importantly, the methods for measuring and quantifying telomere length are not standardized, and the quality of the analyses and interpretations are often not robust. There are several methods to quantitate telomere length and most determine average lengths when it is actually the shortest telomeres that lead to DNA damage signaling and an increased risk for disease. Quantitating the length of chromosome ends can be done by quantitative telomere-FISH with a labeled telomere specific probe. This can be done on interphase cells, analyzed by flow cytometry or fluorescence microscopy, providing precise lengths (with about a 5-10% variability). While only a single or a few dysfunctional telomeres are sufficient to trigger a senescence-like response, in general these assays have only recently become amenable to high throughput reproducible analyses. Most, but not all, reported studies use telomere length measurements techniques such as the TRF (Telomere Restriction Fragment analysis) or PCR-based methods. While many samples can be analyzed by these methods, determining average telomere lengths in leukocytes/buccal washings is unlikely to be as valuable as measuring individual chromosome ends. Finally, showing differences of a few percent changes in average telomere length should be viewed with skepticism.
This study (1) and others reaffirm that radiation and chemotherapy pose significant future risks to patients. With increases in cancer survivors due to improved therapies and overall increases in human longevity, the risk of second or even third cancers is likely to increase. One possibility would be to identify non-toxic radioprotectors for normal cells that do not also protect cancer cells. There are national efforts to identify radioprotectors for first responders to nuclear disasters and to protect astronauts on long-term missions into space. Perhaps some of these identified agents can be re-purposed for pre-treating patients with first cancers before therapy thus reducing the probability of second cancers. In conclusion, the study by Gramatges and colleagues (1) is important because it found an association between telomere contents and second malignant neoplasms in childhood cancer survivors using an invaluable resource provided by the cohort. In summary, measuring telomeric DNA may be a useful metric/biomarker of predicting cancer risk, recurrence and outcomes.
Acknowledgements
The author of this CCR Translations was supported by NASA Grants # NNX11AC15G, NNJ05HD36G, NNX09AU95G and the Simmons Cancer Center Support Grant 5P30 CA 142543. The author holds the Southland Financial Corporation Distinguished Chair in Geriatric Research. This work was performed in space constructed with support from National Institute of Health grant C06 RR30414.
Footnotes
Conflicts of interest: the author has no conflicts of interest
References
- 1.Gramatages MM, Liu Q, Yasui Y, Okcu MF, Neglia JP, Strong LC, et al. Telomere content and risk of second malignant neoplasm in survivors of childhood cancers: a report from the Childhood Survivor Study. Clin Can Res. :XXXXX. doi: 10.1158/1078-0432.CCR-13-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shay JW, Wright WE. The reactivation of telomerase activity in cancer progression. Trends Genet. 1996;12:129–131. doi: 10.1016/0168-9525(96)30018-8. [DOI] [PubMed] [Google Scholar]
- 3.Willeit P, Willeit J, Mayr A, Weger S, Oberhollenzer F, Brandstätter A, et al. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010;304(1):69–75. doi: 10.1001/jama.2010.897. [DOI] [PubMed] [Google Scholar]
- 4.Avigad S, Naumov I, Ohali A, Jeison M, Berco GH, Mardoukh J, et al. Short telomeres: a novel potential predictor of relapse in Ewing sarcoma. Clin Cancer Res. 2007;13(19):5777–83. doi: 10.1158/1078-0432.CCR-07-0308. [DOI] [PubMed] [Google Scholar]
- 5.Weischer M, Nordestgaard BG, Cawthon RM, Freiberg JJ, Tybjaerg-Hansen A, Bojesen SW. Short telomere length, cancer survival, and cancer risk in 47102 individuals. J Natl Cancer Inst. 2013;105:459–468. doi: 10.1093/jnci/djt016. [DOI] [PubMed] [Google Scholar]
- 6.Gu J, Wu X. Short Telomere Length, Cancer Survival, and Cancer Risk in 47 102 Individuals. J Natl Cancer Inst. 2013;105(15):1157. doi: 10.1093/jnci/djt154. [DOI] [PubMed] [Google Scholar]
- 7.Hiyama E, Hiyama K, Yokoyama T, Matsuura Y, Piatyszek MA, Shay JW. Correlating telomerase activity levels with human neuroblastoma outcomes. Nature Med. 1995;1:249–257. doi: 10.1038/nm0395-249. [DOI] [PubMed] [Google Scholar]
- 8.Tabori U, Vukovic B, Zielenska M, Hawkins C, Braude I, Rutka J, et al. The role of telomere maintenance in the spontaneous growth arrest of pediatric low-grade gliomas. Neoplasia. 2006;8(2):136–42. doi: 10.1593/neo.05715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veiga LH, Bhatti P, Ronckers CM, Sigurdson AJ, Stovall M, Smith SA, et al. Chemotherapy and thyroid cancer risk: a report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2012;21(1):92–101. doi: 10.1158/1055-9965.EPI-11-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatti P, Veiga LHS, Ronckers CM, Sigurdson AJ, Stovall M, Smith SA, et al. Risk of second primary thyroid cancer after radiotherapy for childhood cancer in a large cohort study: An update from the Childhood Cancer Survivor Study. Radiat Res. 2010;174:741–52. doi: 10.1667/RR2240.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watt TC, Inskip PD, Stratton K, Smith SA, Kry SF, Sigurdson AJ, et al. Radiation-related risk of basal cell carcinoma: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2012;104(16):1240–50. doi: 10.1093/jnci/djs298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ilyenko I, Lyaskivska O, Bazyka D. Analysis of relative telomere length and apoptosis in humans exposed to ionising radiation. Exp Oncol. 2011;33(4):235–8. [PubMed] [Google Scholar]