Abstract
The gut mucosal immune system is considered to play an important role in counteracting potential adverse effects of food-derived antigens including nanovesicles. Whether nanovesicles naturally released from edible fruit work in a coordinated manner with gut immune cells to maintain the gut in a noninflammatory status is not known. Here, as proof of concept, we demonstrate that grapefruit-derived nanovesicles (GDNs) are selectively taken up by intestinal macrophages and ameliorate dextran sulfate sodium (DSS)-induced mouse colitis. These effects were mediated by upregulating the expression of heme oxygenase-1 (HO-1) and inhibiting the production of IL-1β and TNF-α in intestinal macrophages. The inherent biocompatibility and biodegradability, stability at wide ranges of pH values, and targeting of intestinal macrophages led us to further develop a novel GDN-based oral delivery system. Incorporating methotrexate (MTX), an anti-inflammatory drug, into GDNs and delivering the MTX-GDNs to mice significantly lowered the MTX toxicity when compared with free MTX, and remarkably increased its therapeutic effects in DSS-induced mouse colitis. These findings demonstrate that GDNs can serve as immune modulators in the intestine, maintain intestinal macrophage homeostasis, and can be developed for oral delivery of small molecule drugs to attenuate inflammatory responses in human disease.
Introduction
Conceptually, the gut immune system plays a critical role in preventing inflammation induced by food-derived antigens. However, the role the immune system plays in ensuring that plant-based materials are modified to be beneficial as opposed to being perceived as antigens is not clear. Numerous naturally occurring nanoparticles exist in our diet1,2,3,4,5 and are absorbed through the intestine daily. How they influence our mucosal and systemic immune system is poorly understood.
Inflammatory bowel diseases (IBD), including Crohn's disease (CD) and ulcerative colitis, are chronic, relapsing inflammatory disorders of gastrointestinal tract.6 Intestinal macrophages are critical in maintaining mucosal tolerance and suppressing inflammation to maintain the host's steady state. However, under pathophysiological conditions, such as IBD, intestinal macrophages lose their tolerogenic properties which results in uncontrolled intestinal inflammation. Therefore, manipulating the function of intestinal macrophages is considered a very important strategy in the treatment of IBD patients. Conventional therapies for IBD are steroidal drugs and immunosuppressants. However, these therapies generally fail to produce satisfactory results due to their lack of specific targeting and their toxicity to normal cells. Therefore, development of a new or an alternative delivery system to locate immunosuppressants selectively to intestinal inflammatory cells is essential for patients with IBD.
Through screening a variety of beneficial edible plants, recently we identified novel naturally occurring nanovesicles.7,8 In this study, we focused on grapefruit-derived nanovesicles (noted as grapefruit-derived nanovesicles (GDNs) henceforth). GDNs are enriched for phosphatidylethanolamine and phosphatidylcholine, both lipids have demonstrated antioxidant, anti-inflammatory, and anticolitic effect.9,10 Naringin constitutes the major flavonone found in grapefruit.11 It has been shown that intestinal microflora can hydrolyze naringin into the functional component naringenin. Naringenin has a wide range of pharmacological properties, including anticancer, anti-inflammation, antioxidant, and anticolitic effects.11,12 The uptake of GDNs by intestinal macrophages induces the expression of the antioxidant gene HO-1 and suppresses the production of proinflammatory cytokines. Mice prefed with GDNs had much less disease developed in dextran sulfate sodium (DSS)-induced mouse colitis, a widely used colitis model with many similarities to human IBD.13 We also demonstrate that the therapeutic effect of methotrexate (MTX), an anti-inflammatory drug, carried by GDNs was significantly enhanced whereas the side effects of MTX were remarkably reduced. These findings led us to develop a novel approach for the treatment of inflammatory gut diseases through oral drug delivery using GDNs.
Results
Characterization of GDNs
Grapefruit juice nanovesicles were isolated from the fruit pulp using a sucrose gradient centrifugation method.14 The majority of the nanovesicles accumulated at the 8/30% interface (band 1), and 30/45% interface (band 2) of the sucrose gradient. Vesicles from band 1 were excluded in the following studies because our preliminary data suggest that their role in preventing or ameliorating DSS-induced colitis in a mouse model could not be determined. Vesicles integrity and size was evaluated by electron microscopy (Figure 1a) and a nanozetasizer (Figure 1b). The results indicated that the vesicles were nanosize and that the size distribution range of isolated GDNs spanned a diameter from 105.7 to 396.1 nm and the average diameter of the vesicles population was 210.8 ± 48.62 nm. Zeta potential measurements indicated that GDNs had a negative zeta potential value ranging from −49.2 to −1.52 mV. Like the lipid profile of mammalian microvescicles,15 lipidomic data indicated that GDNs were enriched with phosphatidylethanolamine (45.52%) and phosphatidylcholine (28.53%) (Figure 1c). In particular, phosphatidylethanolamine (34:2) and phosphatidylcholine (34:2) are highly enriched (Supplementary Table S1). Of note, among the lipids, we analyzed naringin and its metabolite, the functional component naringenin were detected (Figure 1d). Similar to exosome-like nanoparticles identified in grapes,8 mass spectrometer analysis of the GDN protein profile indicates that a number of proteins identified in GDNs regulate carbohydrates/lipid metabolism (Supplementary Table S2).
Figure 1.
Characterization of grapefruit-derived nanoparticles (GDNs). (a) Sucrose-gradient band 2 indicated by the arrow (left) was collected for electron microscopy (EM) examination and an (EM) imaging of GDNs. The scale bar indicates 200 nm. (b) Particle size and surface charge were measured using a Zetasizer. (c) Pie chart of the lipid profile of GDNs, reported as percentage of total lipids in GDNs. PS, Phosphatidylserine; PI, Phosphatidylinositol; PE, Phosphatidylethanolamines; PC, Phosphatidylcholines; PG, Phosphatidylglycerol. (d) Representative high-performance liquid chromatography chromatograms of the standards naringin (NAR), naringenin (NE) and GDN extract and the quantification of the content of NAR and NE in GDNs. GDNs were incubated in either distilled water, a 0.5N HCl or a 0.5N NaOH solution at 37 °C for 30 minutes, then the change of particle size (e) and surface charge (f) were measured using a Zetasizer (n = 6).
To test the stability of GDNs under physiological conditions, we first mimicked in vivo conditions by suspending GDNs in water, a 0.5NHCl solution, or a 0.5N NaOH solution, incubating the GDNs at 37 °C for 30 minutes, and then analyzing the change in GDN size and surface charge. The results showed (Figure 1e) that GDNs were very stable at physiologic temperature (37 °C). Of note, compared with the size of GDNs in water, the heterogeneity of diameter of GDNs was reduced in an acidic solution, but no changes in an alkaline solution. The results suggest that the GDN surface at neutral pH or in an alkaline environment is negatively charged (Figure 1f); whereas, in an acid environment, GDNs are weakly positive charged. We then evaluated the stability of GNDs after serial digestion in gastric and intestinal enzymatic solutions. Strikingly, as shown in Supplementary Figure S1, GDNs were highly resistant to digestion by both gastric pepsin solution and intestinal pancreatin and bile extract solution.
Toxicity of orally administrated GDNs
To evaluate the potential systemic toxicity of orally administrated GDNs, mice were daily given 10 mg/kg GDNs (the dose was predetermined as the lowest dose with a protective effect in the colitis model) for 7 days and blood samples were collected 24 hours after the last dose. Oral gavage of GDNs did not change the serum levels of IFN-γ and the liver enzymes, aspartate aminotransferase, and alanine aminotransferase (Supplementary Figure S2a–c). No effects were detected on intestinal morphology or the number of alcian blue+ secretory goblet cells (Supplementary Figure S2d). The number of beneficial Lactobacillus bacteria tended to be higher in GDN-fed mice, yet not statistically significant when compared with the mice fed PBS (Supplementary Figure S3a). In addition, GDN treatment did not alter the immune cell composition in the lamina propria (Supplementary Figure S3b–e) or the percentage of TNF-α producing cell in mesenteric lymph nodes (Supplementary Figure S3f). Moreover, the potential cytotoxicity of GDNs was directly evaluated in an in vitro cultured mouse macrophage cell line. The Annexin V/PI assay, which indicates cell apoptosis/necrosis, revealed that GDN exposure to concentrations up to 60 µg/ml did not increase the percentage of treated cell death (Supplementary Figure S4). Together, these data suggest that oral administration of edible nanovesicles from grapefruit has not observed side effects at the local or systemic level.
GDN pretreatment increases the resistance of mice to DSS-induced colitis
It is well documented that fruit is beneficial for human health and considered as a preventive medicine supplement. Pretreatment with GDNs significantly increased the resistance of C57BL/6 mice to DSS-induced colitis, as evidenced by less weight loss and less colon shortening when compared with PBS-treated mice (Figure 2a,b). Histological analysis revealed a reduced severity of colitis in GDN-treated mice (Figure 2c) and the colitis score was generally lower than that of PBS-treated mice (P = 0.052; Figure 2d). These data demonstrate a protective role of GDNs in DSS-induced colonic injury.
Figure 2.
Grapefruit-derived nanoparticles (GDN) pretreatment ameliorates DSS-induced colitis in mice. C57/B6 mice were treated with either PBS/DSS or GDN/DSS. (a) Body weight (n = 15). (b) Colon length, values are represented as percentage of untreated control mice (n = 15). On day 7 of DSS treatment, colons were harvested. (c) Histological analysis (n = 15). (d) Histological scoring was evaluated by the combined score of epithelial damage and extension of leukocyte infiltration (n = 15). (e) Immunofluorescent staining for E-cadherin of representative inflamed areas of colon (n = 6). Dotted line indicates basement membrane. (f) Distal colons were harvested for qPCR analysis of inflammatory cytokines and chemokines. Values are shown relative to the mRNA levels of naive mice (n = 15). Alternatively, distal colons isolated from the indicated mice were cultured overnight and then IL-6, TNF-α and IL-1β in the supernatants were measured by ELISA (n = 15). On day 7 of DSS treatment, colons were harvested and digested. Doublets were excluded from colonic digests on the basis of FSC-Aand FSC-H, hematopoietic cells were gated on (g) CD45.2 and myeloid-derived cells were selected as CD11b+. The resulting cells were then analyzed for (h) Ly6C or (i) Ly6G (n = 15).
E-cadherin is a major component of adherent junctions. Impaired expression of E-cadherin has been linked to a disturbed intestinal barrier function and homeostasis. Immunofluorescence analysis revealed that in PBS/DSS-treated mice, the expression of E-cadherin on colonic epithelial cells was dramatically reduced when compared with GDN/DSS-treated mice (Figure 2e). Prostaglandin E2 (PGE2) plays a critical role in the regeneration of the epithelial crypts after DSS-induced colitis.16 GDN treatment did not induce significant change in COX-2 expression (Figure 2f) or PGE2 production (Supplementary Figure S5). The induction of proinflammatory cytokines and chemokines plays a causative role in DSS-induced colitis. The expression of IL-6 and IL-1β were reduced significantly in the colon of GDN/DSS-treated mice compared with those in the PBS/DSS group (Figure 2f). The ELISA analysis results further confirmed that colons from GDN/DSS mice secreted significantly less IL-6 and IL-1β than PBS/DSS mice (IL-6: 641.99 ± 75.36 versus 1,116.73 ± 85.45 pg/ml, P < 0.05; IL-1β: 41.15 ± 6.15 versus 82.77 ± 7.75 pg/ml, P < 0.05). Furthermore, the administration of GDNs resulted in significantly reduced mRNA levels of the chemokines MCP-1, CXCL9, and CXCL10, which are all known for playing an important role in the recruitment of inflammatory monocytes and T cells. FACS analysis of the infiltrating immune cells in lamina propria of colonic tissues revealed significant reduction of CD45.2 cells in GDN-treated mice (50.72 ± 4.20%) compared with the control group (67.03 ± 3.27%) (Figure 2g). Infiltration of CD11b+Ly6Chigh inflammatory neutrophils after GDN treatment were both reduced significantly (Figure 2h,i). The fact that daily administration of GDNs attenuates DSS-induced colon inflammation suggests that GDNs may function as immunomodulators and enhance the anti-inflammation capacity of intestinal immune cells.
The majority of orally delivered GDNs are taken up by intestinal macrophages
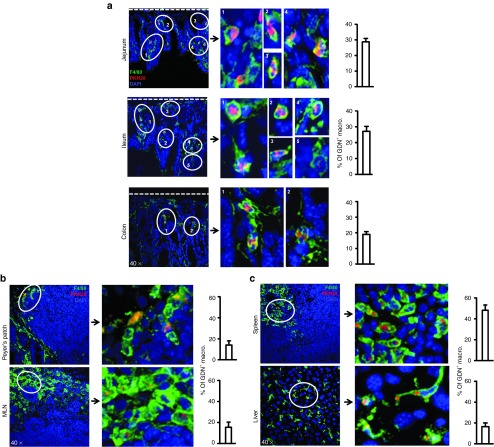
We determined the GDNs biodistribution and cellular target after oral administration. Near-infrared imaging showed that DiR-labeled GDNs rapidly passed through the stomach, proximal small intestine and accumulated at the middle and distal part of small intestine, cecum, and colon. By 4 hours after oral administration, large amounts of DiR-labeled GDNs were still visible in the large intestine (Supplementary Figure S6). Confocal analysis of tissue sections revealed that the majority of GNDs were colocalized with F4/80+macrophages in the lamina propria of both small and large intestine (Figure 3a). The 3D image constructed from z-series stacks indicate that GDNs were internalized by macrophages (Supplementary Video S1). Intestinal macrophages were further confirmed by FACS analysis as the major target of orally given GDNs (Supplementary Figure S7). The difference in percentage of PKH26+ macrophages detected by confocal microscopy compared with FACS may be due to the difference in tissue processing, staining, and sensitivity. Surprisingly, PKH26+ macrophages were also readily detected in Peyer's patches, mesenteric lymph node, spleen, and liver (Figure 3b,c).
Figure 3.
The majority of GDNs are taken up by intestinal and systemic macrophages. Sections of (a) small intestine and colon, (b) Peyer's patches and mesenteric lymph node and spleen and (c) liver revealed uptake of PKH-26 (red) labeled GDNs specifically by F4/80+ (green) macrophages. Nuclei were labeled with DAPI. Dotted line indicates basement membrane. Original magnification was ×40 (left panel) with enlargement of the indicated area shown in the right panels. Percentage of macrophages that phagocytized GDNs is shown (n = 15).
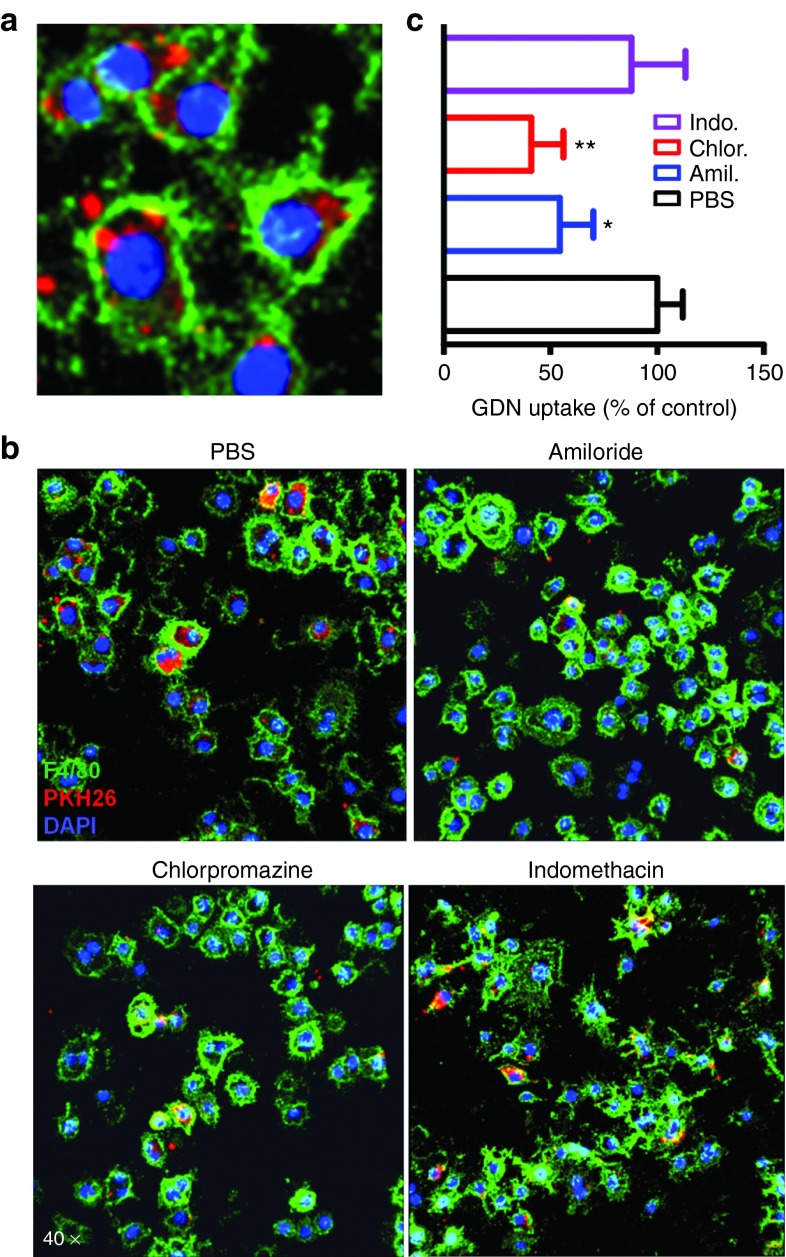
There are multiple pathways for internalization of particles, including phagocytosis, macropinocytosis, clathrin-mediated, and caveolae-mediated endocytosis.17 To delineate the role of specific endocytosis pathways involved in GDN cellular internalization, a series of GDN uptake assays were performed in the presence of biochemical inhibitors to block specific pathways.18,19 Confocal analysis showed that the vesicles accumulated in the perinuclear region of the cells (Figure 4a). Amiloride inhibits the process of macropinocytosis, GDN uptake was inhibited by 45.48 ± 15.64% (Figure 4b,c). Chlorpromazine inhibits clathrin-mediated endocytosis, which reduced the GDN uptake by 58.98 ± 15.21%. Treatment of cells with indomethacin, an inhibitor of caveolae-mediated endocytosis did not significantly alter GDN uptake (Figure 4b,c). These results indicated that GDNs were internalized via both macropinocytosis and clathrin-dependent pathways.
Figure 4.
GDNs utilize both micropinocytosis and clathrin-dependent uptake mechanism for entry into macrophages. (a) The uptake of GDNs by Raw264.7 macrophages. (b) Raw 264.7 macrophages were pretreated with 50 µmol/l amiloride (Amil.), 12.5 µmol/l chlorpromazine (Chlor.) or 100 µmol/l of indomethacin (Indo.) for 30 minutes and then incubated with 2 µg/ml PKH26-labeled GDNs for 3 hours. To exclude membrane contamination, we stained the cell surface with F4/80 antibody. (c) The percentage of GDN uptake relative to control (n = 6). Nuclei were labeled with DAPI; original magnification was ×40.
GDNs enhance the anti-inflammatory capacity of intestinal macrophages
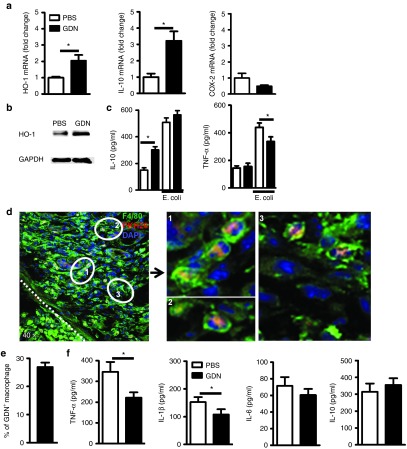
HO-1 and IL-10, expressed in macrophages, play a crucial role in preventing colitis with their potent anti-inflammatory capacity.20,21 Colonic macrophages (CD11b+F4/80+ lamina propria macrophages (LPMs)) isolated from mice prefed with GDNs for 1 week showed significantly enhanced HO-1 (2.06 ± 0.35; P < 0.05) and IL-10 expression (3.22 ± 0.58; P < 0.05) (Figure 5a), and the augmented expression of HO-1 and IL-10 was further confirmed by western blot and ELISA, respectively (Figure 5b,c).
To determine whether this enhanced expression of HO-1 and Il-10 inhibited the sequential activation of LPMs, we isolated LPMs from mice prefed with GNDs, stimulated with heat-killed Escherichia coli for 24 hours, and then measured the secretion of IL-10 and TNF-α in culture supernatants. In response to stimulation with Escherichia coli, LPMs produced large amounts of IL-10 and the addition of GDNs did not further increase IL-10 secretion. However, GDN pretreatment significantly inhibited the production of the proinflammatory cytokine TNF-α (Figure 5c). Next, we tested whether reduction of TNF-α also took place in the DSS-induced colitis mouse model. On day 5 after DSS treatments, the number of F4/80+ cells was increased dramatically (Figure 5d) and GDNs were taken up by around 30% of the colonic F4/80+ cells (Figure 5e). Colonic macrophages were isolated and the production of proinflammatory cytokines was measured by ELISA. Macrophages isolated from inflamed colon produced large amounts of TNF-α (346.45 ± 36.66 pg/ml), IL-1β (152.48 ± 13.62 pg/ml), IL-10 (314.67 ± 37.94 pg/ml), and to a lesser degree IL-6 (Figure 5f). By contrast, colonic macrophages from GDN-treated mice secreted significantly less TNF-α (221.63 ± 19.80 pg/ml; P = 0.045) and IL-1β (107.95 ± 14.67 pg/ml; P = 0.021). These results suggest that the protective effect of GDNs in experimental colitis may be through the induction of HO-1 and IL-10 expression in colonic macrophages, which in turn inhibits the secretion of the inflammatory cytokines IL-1β and TNF-α.
Methotrexate carried by GDNs (GMTX) selectively targets lamina propria macrophages
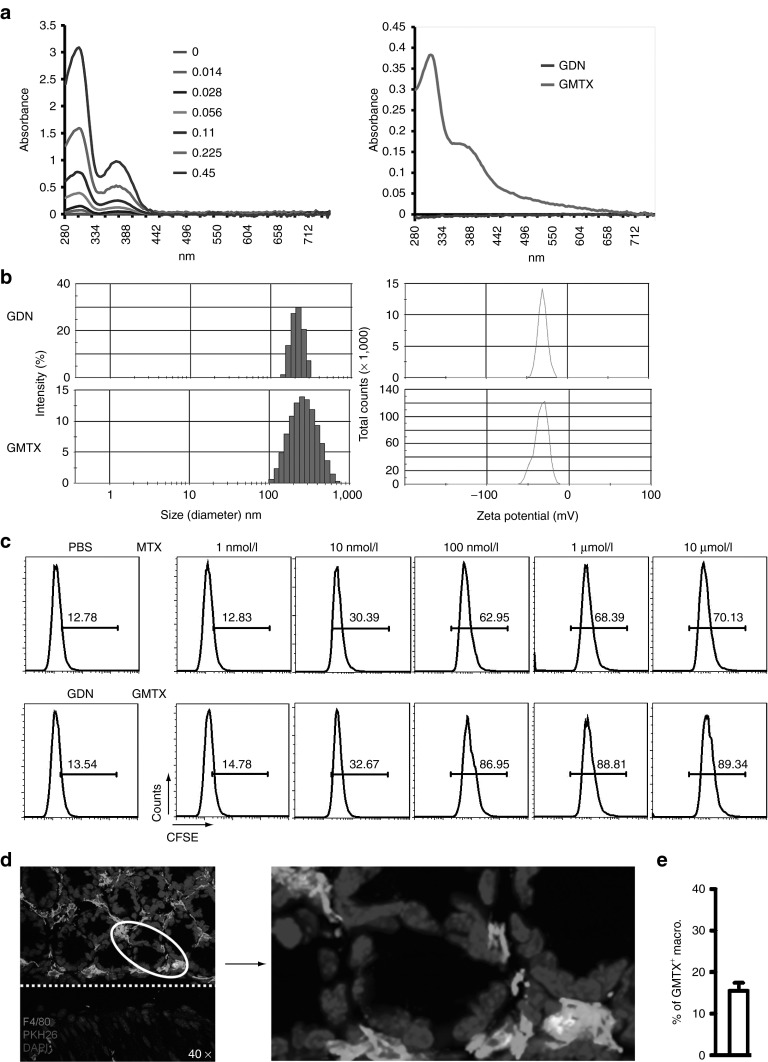
When comparing commercially available liposomes with GDNs, liposomes were much less efficient at transfecting intestinal macrophages after oral delivery, and uptake of liposomes by intestinal macrophages was hardly visible in both small and large intestine (Supplementary Figure S8). Since intestinal macrophages are one of the major immune cells in the intestine and targeted by GDNs, we wanted to know whether GDNs can be used as an oral drug delivery vehicle. We conjugated GDNs with methotrexate (MTX), an immunosuppressant and anti-inflammatoryagent.22 To evaluate the success of this conjugation, we first separated GMTX from free MTX through discontinued sucrose gradient centrifugation and extensive washing with PBS. The quantity of MTX in GMTX (Figure 6a) and the stability of GMTX (Supplementary Figure S9) were measured by spectrometry analysis and the influence of conjugation on particle size and surface charge was determined using a nanozetasizer (Figure 6b). To confirm the preservation of MTX function in GMTX, we compared the antiproliferation effect of GMTX to free MTX.23 As determined by FACS analysis, GMTX showed a dose-dependent inhibition of cell proliferation similar to the effect of free MTX (Figure 6c). Next, we observed the cellular targeting of GMTX in vivo after oral administration. As shown in Figure 6d, orally delivered GMTX targeted to F4/80+ macrophages localized in the lamina propria. Collectively, these results suggest that the conjugation of MTX to GDNs preserved the MTX function and successfully targets the majority of MTX to lamina propria macrophages.
Figure 6.
Preparation and characterization of GDN–MTX conjugates (GMTX). (a) UV spectra of standard-free MTX (mg/ml) and GMTX (n = 15). (b) The size and surface charge of GMTX were measured using a Zetasizer. (c) Comparative antiproliferative effect of GMTX versus free MTX on mouse macrophage cell line. The bold numbers within each histogram represent the percentage of cells containing CFSE (n = 6). (d) Confocal images show the uptake of PKH26 (red)-labeled GMTX by F4/80+ (green) macrophages. Nuclei were labeled with DAPI. Original magnification was ×40 (left panel) with enlargement of the indicated area shown in the right panel. Dotted line indicates basement membrane. (e) Percentage of macrophages that phagocytized GMTXs is shown, n = 6.
The therapeutic effects of GMTX in the DSS-induced mouse colitis model
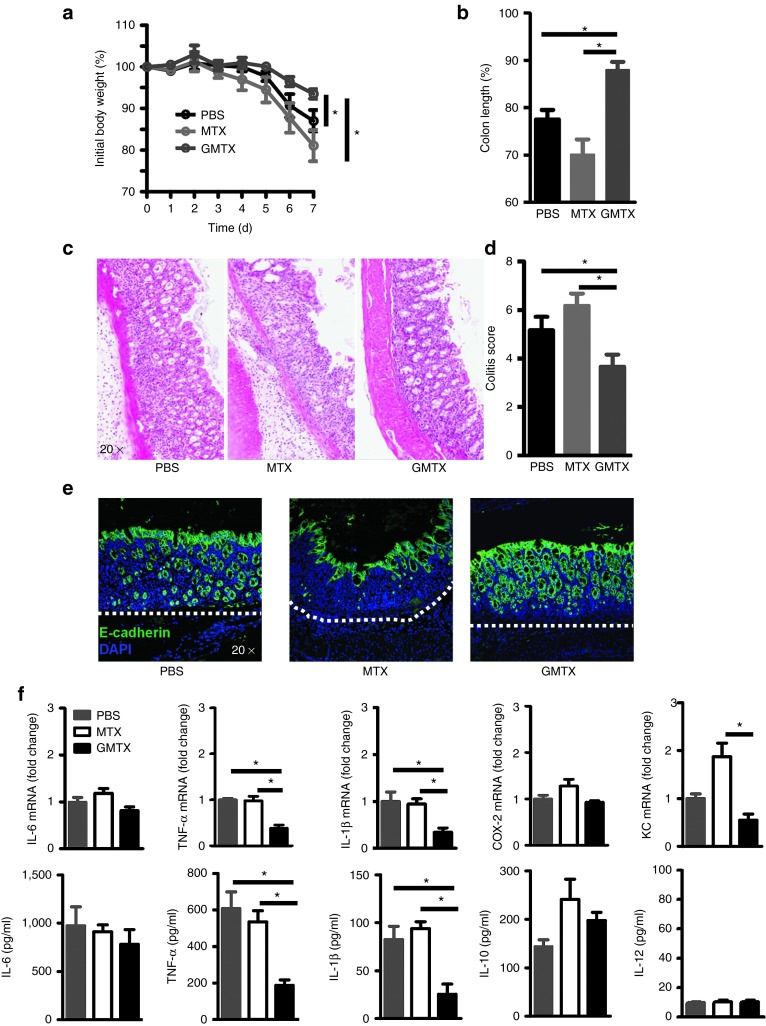
Next, we examined the therapeutic effects of GMTX in acute colitis. Our results showed that GMTX-treated mice had a significant reduction in DSS-induced body weight loss and colon length shortening (Figure 7a,b). These results were further supported by histological analysis. The results from H&E-stained colon tissue indicated that colon tissue damage and inflammatory cell infiltration (Figure 7c,d) of mice treated with GMTX were much less than those treated with free MTX or PBS. Noticeably and reproducibly, mice treated with free MTX even had aggravated symptoms of colitis in comparison with PBS-treated mice in terms of the degree of colon tissue damage, colon length reduction, and decreased expression of E-cadherin (Figure 7e). The result of having a reduced inflammatory cell infiltration in the colon was consistent with a significant reduction of specific inflammatory cytokines which included TNF-α and IL-1β at the mRNA and protein level and a reduction of the expression of the neutrophil chemokine KC (Figure 7f).
Figure 7.
The therapeutic effect of GMTX on active colitis. The therapeutic effects of GMTX were evaluated by (a) body weight, (b) colon length, (c) pathology changes, (d) colitis score, (e) the epithelial integrity and (f) cytokine expression and secretion by inflamed colon. Nuclei were labeled with DAPI. Dotted line indicates basement membrane. Data show means ± SEM of five independent experiments with five mice per group.
The effects of MTX on intestinal macrophages in the inflamed colon are unknown. First, we tested the effect of MTX in the induction of apoptosis on colonic macrophages. As shown in Figure 8a,b, the percentage of TUNEL+ macrophages did not show significant difference among treatments. Then, the effect of GMTX on colonic macrophage function was analyzed. As shown in Figure 8c, GMTX treatment significantly reduced the production of proinflammatory cytokines TNF-α, IL-1β, and IL-6 by colonic macrophages. Collectively, our results suggest that GMTX treatment is superior to MTX in terms of anti-inflammatory effect with fewer side effects in the DSS mouse colitis model. As proof of concept, these data demonstrate that GDNs are suitable as an oral delivery vehicle to target intestinal macrophages for the treatment of intestinal inflammatory-related diseases.
Figure 8.
Immunomodulation but not cytotoxic effect of GMTX on activated intestinal macrophages. Colitis was induced in mice with 2% dextran sulfate sodium and then mice were given orally either PBS, MTX, or GMTX on day 3, 5, and 6 of dextran sulfate sodium treatment. (a) Colons were collected on day 7 and stained with F4/80 (red) and TUNEL (green). Nuclei were labeled with DAPI, n = 9. Dotted line indicates basement membrane. (b) Percentage of TUNEL+ macrophages is shown. (c) Colonic macrophages were isolated and cultured in 96-well plate for 36 hours. The amounts of TNF-α, IL-1β, IL-6 and IL-10 in the culture supernatants were measured by ELISA. Data show means ± SEM of five independent experiments from pooled macrophages of five mice per group.
Discussion
In this study, nanovesicles isolated from an edible plant, i.e., grapefruit, were characterized and demonstrated to target intestinal macrophages. Our results suggest that nanovesicles from beneficial grapefruit do not adversely affect intestinal immune homeostasis, but can strengthen host anti-inflammatory capacity. The biological effect of grapefruit nanovesicles on macrophages was demonstrated in their protective effect in a DSS-induced mouse colitis model. More significantly, our work provided additional insight on developing a grapefruit nanovesicle-based oral drug delivery vehicle for the treatment of inflammation-related diseases. As proof of concept, using MTX carried by grapefruit nanovesicles (GMTX) as an example, GMTX treatment significantly enhanced the anti-inflammatory effect of MTX whereas MTX-induced side effects were decreased remarkably in the DSS-induced mouse colitis model. In addition, unlike nanovesicles synthesized artificially, oral administration of GDNs was found to cause no hepatotoxicity. Therefore, oral drug delivery by GDNs could be a novel means to transport small molecule drugs to intestinal macrophages in a noncytotoxic manner. Obviously, producing large quantities of nanovesicles from fruit is an additional advantage for accelerating the incorporation of this technology into clinical settings.24
Maintaining oral tolerance to food antigens results from interaction between the intestinal immune system and food we eat daily.25 The results of this study favor the hitherto unrecognized hypothesis that nanovesicles from grapefruit and perhaps other edible plants do not act as potential immune stimuli as they were thought to, but actively sustain/enhance anti-inflammatory responses against inflammatory insults by suppressing induction of inflammatory cytokines of intestinal macrophages. The unique propensity of these edible nanovesicles to enhance anti-inflammatory responses of intestinal macrophages offers attractive strategies to prevent or treat autoimmune diseases and colon cancer. Such approaches would exploit effective yet selective natural immunosuppressive mechanisms, thereby avoiding unwanted side effects caused by long-term treatment with immunomodulatory drugs.
Furthermore, a GDN-based delivery system could be further manipulated for achieving targeted oral delivery in general. Delivery of therapeutic agents via oral administration has many advantages over other administration routes,26,27 the most evidence being reduction of systemic exposure. Various delivery systems including nanotechnology and viral and nonviral delivery systems have been employed.28 Each of these approaches has advantages. However, potential toxicity, tissue specific targeting, hazardous effects on the environment, and large scale economical production are challenging issues confronting these technologies. Our approach using edible nanovesicles has the advantage of inherent biocompatibility and biodegradability, the potential of being used as personalized oral delivery vehicles because grape exosome-like nanovesicles target intestinal stem cells8 and grapefruit nanovesicles target macrophages. Therefore, using edible nanovesicles as a delivery vehicle might allow personalize modulation of the function of recipient cells according to therapeutic aims.
Although our data suggest that grapefruit GDNs are selectively taken up by intestinal macrophages and ameliorate DSS-induced mouse colitis, the detailed molecular mechanism(s) responsible for the regulation of macrophage function remains to be determined. A number of potential GDN-derived molecules could contribute to its anti-inflammatory response. Further analysis of the roles of GND-derived proteins (enzymes involved in lipid/carbohydrates metabolic pathways), lipids (phosphatidylethanolamine and phosphatidylcholine are dominated), and other unidentified substances, such as GND-derived glycosylated proteins in the regulation of macrophage function are challenging. Identifying those factors derived from GNDs will have potential impacts on developing preventive/therapeutic agents. For example, naringin is the most abundant flavonoid in grapefruit.29 Our HPLC data show that not only naringin but also its metabolite, the functional component-naringenin, are detected in the GDNs. Another potent anti-inflammatory molecule, ω 3-serie fatty acid (22:6; ω 3-serie), might be present in the GDNs,9 however, our initial attempts failed to detect ω 3-serie fatty acid in GDNs. Further research is needed to determine which bioactive components from GDNs play a causative role(s) in the GDN-mediated anti-inflammatory effects.
The results from this study suggest that the effects of GDNs could be altered by conjugation with a therapeutic agent. Here, we show that GDNs significantly inhibited the expression and secretion of IL-6 and IL-1β but did not show a significant effect on TNF-α in DSS-induced acute intestinal inflammation. By contrast, GMTX induces a significant suppression of the production of IL-1β and TNF-α, but does not have a significant effect on IL-6. The mechanism underlying the differential inhibition of these proinflamatory cytokines by GDNs versus GDNs plus MTX requires further investigation.
It is known that maintaining a stable level of PGE2 in the gut is very important for keeping homeostasis of gut resident cells. In normal colon, PGE2 is mainly derived from COX-1-expressing epithelial cells16 and COX-2-expressing stromal cells.30,31 Our data indicate that intestinal macrophages are the major targets of orally given GDNs. Therefore, a lack of significant change in the level of gut PGE2 due to GDNs treatment is expected. During inflammation, although COX-2 mRNA is induced, the induction of PGE2 regulated by COX-2 would be expected. However, levels of PGE2 are regulated by the local balance between the COX-2-driven synthesis and 15-hydroxyprostaglandin dehydrogenase (15-PGDH)-mediated degradation of PGE2.32 GDN treatment may affect both enzymes' activity as well as multiple factors that affect the stability of PGE2 in the colon tissue.
From this study, we also know that GDNs are very heterogenic in size; we speculate that the effect of each subpopulation of GDNs on macrophages may be different. In addition, some subpopulations of GDNs may be taken up through the endocytosis pathway; and others through micro/macropinocytosis. Blocking one pathway may lead to an alteration of the expression profiles of GDN-targeted macrophages which could contribute to one subset population of GDNs or the same size GDNs being packed with different functional molecules. Demonstration of either assumption has to wait for the technology that allows separating the subsets of nanovesicles based on the size of GDNs, followed by mass spectrometer analysis.
The heterogeneity in size of GDNs is also affected by pH value. Under acid conditions like those found in the stomach, we observed that the heterogeneity in size of GDNs is decreased. A number of reasons may lead to the reduction of heterogeneity. One of them could be the increase in the affinity of interaction between certain GDN proteins or lipids or carbohydrates.
Materials and Methods
Preparation and characterization of GDNs. Grapefruit skin was removed and the fruit pulp homogenized in a high-speed blender for 1 minute at 4 °C. The collected juice was sequentially centrifuged at 2,000g for 20 minutes and then 10,000g for 1 hour to exclude debris. The nanovesicles were pelleted at 150,000g for 1.5 hours, washed once with PBS, and then purified and separated using sucrose gradients (8, 30, 45, and 60%, respectively).33 Band 1 at the 8/30% interface and band 2 at the 30/45% interface were separately harvested. The concentration of samples was represented as protein concentration using the Bio-Rad protein quantification assay kit (Bio-Rad, Hercules, CA).
Particle size and surface charge analysis. The particle size and zeta potential were measured using a Zetasizer Nano ZS (Malvern Instrument, UK) with the following settings: 11 measurements per samples, 25 °C and baseline viscosity for water established at 0.8872 cP.
Electron microscopy. Particle pellets were fixed and processed for electron microscopy using a conventional procedure34 and observed with a JEOL model 1200EX electron microscope (JEOL, Tokyo, Japan). Digital images were acquired using an AMT Advantage HR (Advanced Microscopy Techniques, MA) high definition CCD, 1.3 megapixel TEM camera.
Lipid extraction and lipidomic analysis. Total lipid extraction of GDNs was performed according to the method of Bligh and Dyer,35 and the lipids were dissolved in chloroform for analysis. The lipid composition was analyzed on a triple quadrupole tandem mass spectrometer (API 4000; Applied Biosystems, Carlsbad, CA) as previously described.36 The data were reported as percentage of total signal of the molecular species after normalization of the signals to internal standards of the same lipid class.
Proteomic analysis. Total proteins were extracted from GDNs using Trizol (Invitrogen, Grand Island, NY) following the manufacturer's instruction and resuspended in 1% SDS with 0.8 mol/l urea. Proteins were electrophoresed on 10% SDS-polyacrylamide gels, stained with Coomassie blue and then the individual protein bands were cut and sent for proteomics analysis as described previously.8
Extraction of naringin from GDNs. The extraction procedure was performed as previously described.29,37 In brief, 5 mg of GDNs (based on the protein concentration) were resuspended in 800 µl of 50% ethanol, heated to 90 °C immediately, and allowed to stand for 2 hours. The extracts were cooled to 22 °C and filtered for HPLC analysis.
High-performance liquid chromatography. Chromatography was performed on an Agilent 1120 system. An Eclipse Plus C18 column was employed. The mobile phase consisted of 20 mmol/l HCl (A)/Acetonitrile (B). The separation was performed as previously described.38 The UV detector was set at 280 nm. The analyses were performed at 25 °C with a 1 ml/min flow rate and the injection volume was 100 µl.
In vitro digestion. In vitro digestion conditions were based on a previous description.39 1 ml of GDNs in a water solution were incubated with slow rotation at 37 °C for 30 minutes after the addition of 1.34 µl of 18.5% w/v HCl (pH 2.0) and 24 µl of a pepsin solution (80 mg/ml in 0.1 N of HCl, pH 2.0, Sigma, St Louis, MO). Then, 80 µl of a mixture containing 24 mg/ml of bile extract and 4 mg/ml of pancreatin (Sigma) in 0.1 N of NaHCO3 was added. The pH value of the bulk solution was adjusted to 6.5 with 1 N NaHCO3 and GDNs were incubated for another 30 minutes under the same conditions. The stability of GDNs was evaluated by the changes of particle size and surface charge.
Preparation of GDN-MTX conjugates (GMTX). GMTX was prepared as previously described.22,40 In brief, MTX (2 mg, Sigma) with EDC (1.5 mg, Thermo Scientific, Waltham, MA) was dissolved in DMSO (0.125 ml). The solution was incubated at 50 °C for 15 minutes, cooled to 22 °C, and then added to GDN solution (25 mg in 3 ml PBS pH 7.4). After 2 hours of reaction, GMTX conjugates were purified using a sucrose gradient. The concentration of MTX in the conjugates was determined by using UV-spectrophotometry at a wavelength of 307 nm (Nanodrop 8000, Thermo Scientific) with unconjugated GDNs as blanks. The GDNs content in the conjugates was represented by their protein concentration.
In vitro release of MTX. Free MTX (0.5 mg) and GMTX (MTX equaled to 0.5 mg) were each dissolved in 0.5 ml of PBS. The solutions were dispensed into a dialysis cassette (cutoff 10 kDa, Pierce, Rockford, IL). The cassettes were placed into capped dishes containing 1.5 ml of PBS as a release medium. These dishes were placed on a shaker (15 rpm/min) at 37 °C. Every 1 hour, 0.1 ml of release medium was removed from the incubated dishes, and replaced with 0.1 ml fresh PBS. Collected samples were assayed spectrophotometrically for MTX at 307 nm.
Animal studies Experimental animals. Six-to-eight-week-old C57BL/6J male mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All experiments were approved by the Institutional Animal Care and Use Committee at the University of Louisville.
Biodistribution and cellular target of orally administrated GDNs. Mice fasted overnight were orally given 30 mg/kg of either near-infrared lipophilic carbocyanine dye (1,1′-dioctadecyl-3,3,3′3′-tetramethyl-indotricarbocyanine-iodide, DiR, Invitrogen, Carlsbad, CA) or PKH26 fluorescent dye (Sigma)-labeled GDNs twice, i.e., at 2 and 4 hours before tissues were harvested. Mice were killed and small intestine, colon, Peyer's patch, mesenteric lymph node, spleen, and liver tissues were used for immunofluorescence staining and imaging.
DSS-induced colitis. Colitis was induced in mice by the addition of 2% (wt/vol) DSS (Mw 36-50KD molecular weight, MP Biomedicals, OH) in their drinking water for the duration of the study. DSS solution was freshly prepared every other day. Body weight and physical activity were monitored daily.
To test the protective effect of GDNs, mice fasted for 4 hours were orally given either PBS or 10 mg/kg of GDNs daily for 7 days and then treated with 2% DSS in drinking water for 7 days to induce colitis with continued administration of GDNs.
To test the therapeutic effect of GMTX, colitis was first induced in mice by providing 2% DSS in their drinking water. On day 3, 5, and 6 of DSS-induced colitis, mice were treated with free MTX (5 mg/kg body weight) or GMTX (MTX equaled to 5 mg/kg) by oral administration. 5 mg/kg was predetermined to have optimal therapeutic effect.
AST and ALT measurement. To test for hepatotoxicity, levels of ALT and AST activity in serum were measured using the Infinity Enzymatic Assay Kit (Themo Scientific).
Detection of mouse commensal bacteria. DNA from mouse feces was extracted using the QIAgen stool kit for stool pathogen detection. qPCR assays were performed on a CFX96 Real-Time System (BioRad, Hercules, CA) using SYBR Green Master Mix (Bio-Rad laboratories) and bacteria or phyla-specific primers.41
Histology. For histopathology, tissues were fixed in 10% neutral formalin and then embedded in paraffin. Tissue samples were cut at 5 µm thicknesses and stained with either hematoxylin and eosin or Alcian blue and nuclear fast red.42 Histological examination was performed in a blinded fashion and the severity of colitis was scored as described previously.43 For immunofluorescence analysis, tissue sections were first blocked at 22 °C for 1 hour with 5% BSA in PBS. Slides were incubated at 4 °C overnight with the primary antibodies: E-cadherine (1:1,000, BD Bioscience, San Jose, CA) and F4/80 (1:200, Biolegend, San Diego, CA). E-cadherin and F4/80 binding was detected by Alexa Fluor 488 conjugated goat anti–mouse IgG or goat anti–rat IgG (1:600, Invitrogen), respectively. Apoptosis in colon tissues was identified by TUNEL assay using the in situ Cell Death Detection kit from Roche Applied Science (Indianapolis, IN). Tissues were counterstained with DAPI and images were captured on a Zeiss LSM 510 confocal microscope equipped with a digital image analysis system (Pixera).
Internalization assay. Macrophages were plated on Lab-Tek chamber slide (Sigma) and incubated for 24 hours in growth medium. Before the uptake assay, GDNs were labeled with PKH26, sucrose gradient purified, and diluted in PBS. Uptake was performed by incubating cell cultures with 2 µg/ml of GDNs for 3 hours in a humid chamber (37 °C, 5% CO2). For inhibition experiments, cell cultures were preincubated with individual inhibitors for 30 minutes before performing the uptake experiments.
Isolation of intestinal epithelial cells, intestinal leukocytes, and flow cytometry. Intestinal epithelial cells, intraepithelial leukocytes (IEL), and lamina propria leukocytes were isolated as described previously.44,45,46 Briefly, intestines were flushed with PBS, everted, and rinsed in cold PBS three times. For epithelial isolation, tissues were treated with 0.5 mmol/l dithiothreitol for 30 minutes at 37 °C with slow rotation, followed by vortexing for 1 minute. The liberated intestinal epithelial cells were collected and subjected to Percoll (GE Healthcare) discontinuous gradient centrifugation. Intestinal epithelial cells were recovered at the interface of the 20 and 40% Percoll solutions. For lymphocyte isolation, tissues were incubated twice in 25 ml of IEL medium (RPMI 1640 containing penicillin/streptomycin, 0.02 mol/l Hepes, and 2% FBS) for 20 minutes at 37 °C with slow rotation (150 rpm/minutes). IELs were removed by vigorous vortexing and filtering through a 100 µm cell strainer and new IEL solution was added. After a second round incubation, the tissues were washed in HBSS, cut into small pieces, and placed in 5 ml of digestion solution (IEL medium with 1 mg/ml Collagenase type VIII and 50 µg/ml DNase I (Sigma)) at 37 °C for two rounds of incubation at 25 minutes each with slow rotation. Cells were filtered through a cell strainer and subjected to Percoll discontinuous gradient centrifugation. IELs and lamina propria leukocytes were recovered at the interface of the 40 and 80% Percoll solutions. IELs and lamina propria leukocytes were suspended in PBS/0.5% BSA and stained for 45 minutes with anti-CD3 Ab (145 2C11; eBioscience), anti-TCRγδAb(eBioGL3, eBioscience), anti-F4/80 (Cl:A3-1; Serotec), anti-CD4 Ab (GK1.5), anti-CD8 Ab (53-6.7), anti-CD11b Ab (M1/70), and Gr1 Ab (RB6-8C5) (all from Biolegend unless otherwise noted). In some experiments, stained cells were sorted to purify indicated populations on a BD FACS Aria III.
For intracellular staining, cells were stimulated with 20 ng/ml PMA (phorbol 12-myristate 13-acetate, Sigma) and 0.5 µg/ml ionomycin (Sigma) for 5 hours in the presence of Golgistop (BD Bioscience) in the media. After stimulation, cells were first stained with surface Ab and fixed, then permeabilized, and stained for intercellular TNF-α. The relevant isotype Abs were used as controls.
For FACS analysis of cell proliferation, RAW264.7 cells labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; invitrogen) were plated in a 24-well plate and incubated for 72 hours with MTX or GMTX at the indicated concentration. Proliferation was measured by CFSE dilution.
Flow cytometry analysis was performed using a FACSCalibur (BD Bioscience) and analyzed using FlowJo software (Tree Star, Ashland, OR).
Colon organ culture. To assess the local levels of IL-6, IL-1β, TNF-α, we generated organ cultures from naive, GDN-, PBS/DSS-, and GDN/DSS-challenged mice.45,47 In brief, the distal most 2 cm of colon was washed with PBS-containing penicillin/streptomycin and then further cut into 1 cm2 sections. Colon sections were cultured in serum-free RPMI 1640 medium supplemented with penicillin/streptomycin. After 24 hours, cell-free supernatants were harvested and assayed for cytokine secretion by ELISA kits (eBioscience). The local levels of PGE2 were determined by a PGE2-specific EIA (Cayman Chemical, Ann Arbor, MI) as described previously.48
Real-time PCR. Total RNA was extracted from either distal colon or GDN-treated intestinal macrophages using Trizol (Invitrogen) and reverse transcribed with random primers (Invitrogen Superscript III). qPCR analysis was done using the SYBR Green Master Mix (Bio-Rad laboratories) and specific primers (Supplementary Table S3). Signals were normalized to GPDH and β-actin levels within each sample and normalized data were used to calculate ΔΔCt and determine the relative levels of gene expression.
ELISA. To test the potential immunomodulation effect of GDNs on LPMs, Freshly isolated LPMs (106/ml) were stimulated by heat-killed Escherichia coli bacteria (MOI = 50)49 for 24 hours at 37 °C. Culture supernatants were collected and the production of IL-10 and TNF-α were assessed using ELISA kits (eBioscience, San Diego, CA).
Statistical analysis. Data are represented as mean ± SEM. Statistical significance was calculated using either the student's t-test for two samples with unequal variances or one-way ANOVA with Holm's post hoc test for three variables with *P < 0.05 and **P < 0.01 as levels of significance.
SUPPLEMENTARY MATERIAL Figure S1. GDNs are resistant to gastric and intestinal enzymatic digestion. Figure S2. The oral administration of GDNs does not induce systemic toxicity and change intestinal morphology. Figure S3. Oral administration of GDNs does not alter the intestinal microenvironment during steady-state. Figure S4. Cytotoxicity effect of GDNs on RAW264.7 mouse macrophages after 24 hours incubation. Figure S5. PGE2 production in colonic mucosa during both steady-state (a) and on day 7 of DSS induced colitis (b). Figure S6. Tissue biodistribution of orally administrated GDNs. Figure S7. Intestinal macrophages are the major targets of orally administrated GDNs. Figure S8. The uptake of liposome in small and large intestines. Figure S9. In vitro release curve of free MTX and GMTX in PBS at 37°C. Video S1. The internalization of GDNs by intestinal macrophages. Table S1. Composition of phosphocholine (PC) and phosphoethanolamine (PE) found in GDN. Table S2. Identities of proteins found in GDNs. Table S3. Primer sets used in PCR analysis.
Figure 5.
Five GDNs enhance the anti-inflammatory capacity of resident intestinal macrophages. Intestinal resident macrophages were isolated from colons of B6 mice treated with PBS or GDNs for 7 days. (a) The expression of HO-1, IL-10, and COX-2 were analyzed by real-time RT-PCR. Values are shown relative to the mRNA levels of PBS group. (b) The upregulation of HO-1 expression by GDNs was confirmed by western blot. (c) Isolated macrophages were stimulated with heat-killed E. coli. (MOI = 50) for 24 hours with 2 µg/ml of GDNs in the GDN-treated group. The amounts of IL-10 and TNF-α in the culture supernatants were measured by ELISA. Data show means ± SEM of five independent experiments from pooled macrophages of 15 mice per group. (d) Mice were orally given 2% DSS for 5 days, fasted overnight, and then gavaged twice with 30 mg/kg of PKH26-labeled GDNs 2 and 4 hours before harvesting the colon. Confocal images showed the uptake of PKH26 (red)-labeled GDNs by F4/80+ (green) macrophages. Nuclei were labeled with DAPI. Original magnification was ×40 (left panel) with enlargement of the indicated area shown in the right panels. Dotted line indicates basement membrane. (e) Percentage of macrophages that phagocytized GDNs is shown, n = 6. (f) Mice were pretreated with GDNs for 7 days and then given 2% DSS for 5 days with continued GDN administration. Colonic macrophages were isolated and incubated with GDNs for 36 hours. The amounts of TNF-α, IL-1β, IL-6, and IL-10 in the culture supernatants were measured by ELISA. Data show means ± SEM of five independent experiments from pooled macrophages of five mice per group.
Acknowledgments
We thank Dr Jerald Ainsworth for editorial assistance. This work was supported by grants from the National Institutes of Health (RO1AT004294) and the Louisville Veterans Administration Medical Center (VAMC) Merit Review Grants (H.-G.Z.).
Supplementary Material
The internalization of GDNs by intestinal macrophages.
References
- Powell JJ, Faria N, Thomas-McKay E, Pele LC. Origin and fate of dietary nanoparticles and microparticles in the gastrointestinal tract. J Autoimmun. 2010;34:J226–J233. doi: 10.1016/j.jaut.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Powell JJ, Thoree V, Pele LC. Dietary microparticles and their impact on tolerance and immune responsiveness of the gastrointestinal tract. Br J Nutr. 2007;98 suppl. 1:S59–S63. doi: 10.1017/S0007114507832922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JC. Can microparticles contribute to inflammatory bowel disease: innocuous or inflammatory. Exp Biol Med (Maywood) 2007;232:1–2. [PubMed] [Google Scholar]
- Setser CS, Racette WL. Macromolecule replacers in food products. Crit Rev Food Sci Nutr. 1992;32:275–297. doi: 10.1080/10408399209527600. [DOI] [PubMed] [Google Scholar]
- Badens E, Magnan C, Charbit G. Microparticles of soy lecithin formed by supercritical processes. Biotechnol Bioeng. 2001;72:194–204. doi: 10.1002/1097-0290(20000120)72:2<194::aid-bit8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M, et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59:1192–1199. doi: 10.1136/gut.2009.197822. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhuang X, Mu J, Deng ZB, Jiang H, Xiang X, et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat Commun. 2013;4:1867. doi: 10.1038/ncomms2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju S, Mu J, Dokland T, Zhuang X, Wang Q, Jiang H, et al. Grape Exosome-like Nanoparticles Induce Intestinal Stem Cells and Protect Mice From DSS-Induced Colitis. Mol Ther. 2013;21:1345–1357. doi: 10.1038/mt.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JY, Chi SG, Chun HS. Oral administration of docosahexaenoic acid attenuates colitis induced by dextran sulfate sodium in mice. Mol Nutr Food Res. 2011;55:239–246. doi: 10.1002/mnfr.201000070. [DOI] [PubMed] [Google Scholar]
- Stremmel W, Merle U, Zahn A, Autschbach F, Hinz U, Ehehalt R. Retarded release phosphatidylcholine benefits patients with chronic active ulcerative colitis. Gut. 2005;54:966–971. doi: 10.1136/gut.2004.052316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inês Amaro M, Rocha J, Vila-Real H, Eduardo-Figueira M, Mota-Filipe H, Sepodes B, et al. Anti-inflammatory activity of naringin and the biosynthesised naringenin by naringinase immobilized in microstructured materials in a model of DSS-induced colitis in mice. Food Research International. 2009;42:1010–1017. [Google Scholar]
- Dou W, Zhang J, Sun A, Zhang E, Ding L, Mukherjee S, et al. Protective effect of naringenin against experimental colitis via suppression of Toll-like receptor 4/NF-?B signalling. Br J Nutr. 2013;110:599–608. doi: 10.1017/S0007114512005594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F, Cao H, Cover TL, Washington MK, Shi Y, Liu L, et al. Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. J Clin Invest. 2011;121:2242–2253. doi: 10.1172/JCI44031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Yu S, Zinn K, Wang J, Zhang L, Jia Y, et al. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J Immunol. 2006;176:1375–1385. doi: 10.4049/jimmunol.176.3.1375. [DOI] [PubMed] [Google Scholar]
- Laulagnier K, Motta C, Hamdi S, Roy S, Fauvelle F, Pageaux JF, et al. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem J. 2004;380 Pt 1:161–171. doi: 10.1042/BJ20031594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morteau O, Morham SG, Sellon R, Dieleman LA, Langenbach R, Smithies O, et al. Impaired mucosal defense to acute colonic injury in mice lacking cyclooxygenase-1 or cyclooxygenase-2. J Clin Invest. 2000;105:469–478. doi: 10.1172/JCI6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- Ghigo E, Kartenbeck J, Lien P, Pelkmans L, Capo C, Mege JL, et al. Ameobal pathogen mimivirus infects macrophages through phagocytosis. PLoS Pathog. 2008;4:e1000087. doi: 10.1371/journal.ppat.1000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock S, Antrobus R, Newton L, Kampa B, Rossa J, Latham S, et al. Uptake and trafficking of liposomes to the endoplasmic reticulum. FASEB J. 2010;24:1866–1878. doi: 10.1096/fj.09-145755. [DOI] [PubMed] [Google Scholar]
- Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8:240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- Sheikh SZ, Hegazi RA, Kobayashi T, Onyiah JC, Russo SM, Matsuoka K, et al. An anti-inflammatory role for carbon monoxide and heme oxygenase-1 in chronic Th2-mediated murine colitis. J Immunol. 2011;186:5506–5513. doi: 10.4049/jimmunol.1002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri A, Atyabi F, Salman Nouri F, Ahadi F, Derakhshan M, Amini M, et al. 2011Nanoparticles of conjugated methotrexate-human serum albumin: preparation and cytotoxicity evaluations. J Nanomatepub ahead of print).
- Majumdar S, Aggarwal BB. Methotrexate suppresses NF-κB activation through inhibition of IκBα phosphorylation and degradation. J Immunol. 2001;167:2911–2920. doi: 10.4049/jimmunol.167.5.2911. [DOI] [PubMed] [Google Scholar]
- Desai N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012;14:282–295. doi: 10.1208/s12248-012-9339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst O, Mowat AM. Oral tolerance to food protein. Mucosal Immunol. 2012;5:232–239. doi: 10.1038/mi.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka YJ, Leong KW. Engineering strategies to enhance nanoparticle-mediated oral delivery. J Biomater Sci Polym Ed. 2008;19:1549–1570. doi: 10.1163/156856208786440479. [DOI] [PubMed] [Google Scholar]
- Wilson DS, Dalmasso G, Wang L, Sitaraman SV, Merlin D, Murthy N. Orally delivered thioketal nanoparticles loaded with TNF-a-siRNA target inflammation and inhibit gene expression in the intestines. Nat Mater. 2010;9:923–928. doi: 10.1038/nmat2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend DR. New oral delivery systems for treatment of inflammatory bowel disease. Adv Drug Deliv Rev. 2005;57:247–265. doi: 10.1016/j.addr.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Jourdan PS, McIntosh CA, Mansell RL. Naringin Levels in Citrus Tissues: II. Quantitative Distribution of Naringin in Citrus paradisi MacFad. Plant Physiol. 1985;77:903–908. doi: 10.1104/pp.77.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry RD, Stenson WF, Lorenz RG. Cyclooxygenase-2-dependent arachidonic acid metabolites are essential modulators of the intestinal immune response to dietary antigen. Nat Med. 1999;5:900–906. doi: 10.1038/11341. [DOI] [PubMed] [Google Scholar]
- Newberry RD, McDonough JS, Stenson WF, Lorenz RG. Spontaneous and continuous cyclooxygenase-2-dependent prostaglandin E2 production by stromal cells in the murine small intestine lamina propria: directing the tone of the intestinal immune response. J Immunol. 2001;166:4465–4472. doi: 10.4049/jimmunol.166.7.4465. [DOI] [PubMed] [Google Scholar]
- Tai HH, Ensor CM, Tong M, Zhou H, Yan F. Prostaglandin catabolizing enzymes. Prostaglandins Other Lipid Mediat. 2002;68-69:483–493. doi: 10.1016/s0090-6980(02)00050-3. [DOI] [PubMed] [Google Scholar]
- Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18:1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng ZB, Zhuang X, Ju S, Xiang X, Mu J, Wang Q, et al. Intestinal mucus-derived nanoparticle-mediated activation of Wnt/ß-catenin signaling plays a role in induction of liver natural killer T cell anergy in mice. Hepatology. 2013;57:1250–1261. doi: 10.1002/hep.26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Wanjie SW, Welti R, Moreau RA, Chapman KD. Identification and quantification of glycerolipids in cotton fibers: reconciliation with metabolic pathway predictions from DNA databases. Lipids. 2005;40:773–785. doi: 10.1007/s11745-005-1439-4. [DOI] [PubMed] [Google Scholar]
- Li X-H, Xiong Z-L, Lu S, Zhang Y, Li F-M. Pharmacokinetics of naringin and its metabolite naringenin in rats after oral administration of rhizoma drynariae extract assayed by UPLC-MS/MS. Chinese J Nat Med. 2010;8:40–46. [Google Scholar]
- Ribeiro IA, Ribeiro MHL. Naringin and naringenin determination and control in grapefruit juice by a validated HPLC method. Food Control. 2008;19:432–438. [Google Scholar]
- Hermida LG, Sabés-Xamaní M, Barnadas-Rodríguez R. Combined strategies for liposome characterization during in vitro digestion. J Liposome Res. 2009;19:207–219. doi: 10.1080/08982100902740847. [DOI] [PubMed] [Google Scholar]
- Majoros IJ, Thomas TP, Mehta CB, Baker JR., Jr Poly(amidoamine) dendrimer-based multifunctional engineered nanodevice for cancer therapy. J Med Chem. 2005;48:5892–5899. doi: 10.1021/jm0401863. [DOI] [PubMed] [Google Scholar]
- Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JP, Perreault N, Goldstein BG, Lee CS, Labosky PA, Yang VW, et al. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development. 2002;129:2619–2628. doi: 10.1242/dev.129.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrer A, Sprenger N, Kurakevich E, Borsig L, Chassard C, Hennet T. Milk sialyllactose influences colitis in mice through selective intestinal bacterial colonization. J Exp Med. 2010;207:2843–2854. doi: 10.1084/jem.20101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- Ismail AS, Seversona KM, Vaishnavaa S, Behrendta CL, Yua X, Benjamina JL, et al. γδ intraepithelial lymphocytes are essential mediators of host–microbial homeostasis at the intestinal mucosal surface. Proceedings of the National Academy of Sciences. 2011;108:8743–8748. doi: 10.1073/pnas.1019574108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Koroleva EP, Kruglov AA, Kuprash DV, Nedospasov SA, Fu YX, et al. Lymphotoxin beta receptor signaling in intestinal epithelial cells orchestrates innate immune responses against mucosal bacterial infection. Immunity. 2010;32:403–413. doi: 10.1016/j.immuni.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen IC, TeKippe EM, Woodford RM, Uronis JM, Holl EK, Rogers AB, et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med. 2010;207:1045–1056. doi: 10.1084/jem.20100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehl TE, George RJ, Sturmoski MA, May R, Dieckgraefe B, Anant S, et al. Azoxymethane protects intestinal stem cells and reduces crypt epithelial mitosis through a COX-1-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1062–G1070. doi: 10.1152/ajpgi.00129.2006. [DOI] [PubMed] [Google Scholar]
- Kamada N, Hisamatsu T, Okamoto S, Sato T, Matsuoka K, Arai K, et al. Abnormally differentiated subsets of intestinal macrophage play a key role in Th1-dominant chronic colitis through excess production of IL-12 and IL-23 in response to bacteria. J Immunol. 2005;175:6900–6908. doi: 10.4049/jimmunol.175.10.6900. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The internalization of GDNs by intestinal macrophages.