Abstract
Adenoviruses are potent vectors for inducing and boosting cellular immunity to encoded recombinant antigens. However, the widespread seroprevalence of neutralizing antibodies to common human adenovirus serotypes limits their use. Simian adenoviruses do not suffer from the same drawbacks. We have constructed a replication-deficient chimpanzee adenovirus-vectored vaccine expressing the conserved influenza antigens, nucleoprotein (NP), and matrix protein 1 (M1). Here, we report safety and T-cell immunogenicity following vaccination with this novel recombinant simian adenovirus, ChAdOx1 NP+M1, in a first in human dose-escalation study using a 3+3 study design, followed by boosting with modified vaccinia virus Ankara expressing the same antigens in some volunteers. We demonstrate ChAdOx1 NP+M1 to be safe and immunogenic. ChAdOx1 is a promising vaccine vector that could be used to deliver vaccine antigens where strong cellular immune responses are required for protection.
Introduction
Annual influenza epidemics are associated with a nontrivial morbidity and mortality, up to one billion infections worldwide and ~250,000–500,000 associated deaths.1,2 Vaccination, the mainstay of preventative healthcare, currently represents the most effective intervention to reduce influenza-associated disease. Many countries have implemented stratified influenza vaccination programs targeting at-risk cohorts (such as the very young and elderly) each year. Unfortunately, the efficacy of currently licensed influenza vaccines is suboptimal in these targeted populations.3,4 In addition, currently licensed influenza vaccines induce strain-specific neutralizing antibodies (NAbs) primarily toward the surface proteins, hemagglutinin and neuraminidase, and hence confer limited immunity toward influenza viruses, which have undergone antigenic drift in these antigens, or toward viruses of a different subtype.
Internal proteins of influenza viruses, such as nucleoprotein (NP) and matrix protein 1 (M1), are highly conserved, and T-cell responses recognizing these antigens can protect against influenza disease.5,6,7,8,9 A vaccine against influenza that induces protective T-cell responses against conserved internal antigens could provide improved immunity not only against human seasonal influenza but also against other subtypes currently found in avian species or swine, which threaten to cause a new influenza pandemic.6,7 We have previously developed a T-cell–inducing influenza vaccine based on the internal proteins of the influenza A virus, modified vaccinia virus Ankara (MVA) expressing NP and M1 as a fusion protein, MVA NP+M1.10 Phase I and Phase IIa clinical trials of MVA NP+M1 have shown this vaccine to be safe and immunogenic.10,11,12 In a Phase IIa influenza challenge study, fewer vaccinated volunteers developed influenza than the unvaccinated volunteers, and there was a statistically significant reduction in duration of virus shedding in vaccinated volunteers.11
In addition to poxvirus vectors, adenoviral-vectored vaccines have been found to be potent vectors for inducing and boosting T-cell responses to recombinant transgene products.13,14,15 However, the widespread seroprevalence of antibodies to common human adenovirus serotype-5 (AdHu5)16 limits the utility of these viruses as vaccine vectors in humans and was implicated in the failure of an human immunodeficiency virus vaccine to demonstrate efficacy.17 Simian adenoviruses do not suffer from the same limitation, and we have constructed a novel replication-deficient chimpanzee adenovirus vector18 expressing conserved influenza antigens NP and M1 (ChAdOx1 NP+M1). The first clinical study of this novel vacine vector is described here. Safety and immunogencity were tested in a dose-escalation study starting at a dose of 5 × 108 viral particles (vp) and progressing through 5 × 109, 2.5 × 1010, and finally 5 × 1010 vp using a 3+3 study design.
Results
Safety
Volunteers were enrolled and vaccinated according to a 3+3 dose-escalation study plan19 as described in Materials and Methods section (Supplementary Table S1). For each dose of ChAdOx1 NP+M1 tested, initially one volunteer was vaccinated and reviewed after 48 hours. The study protocol allowed the second and third volunteers to be vaccinated, provided that there were no serious adverse reactions to vaccination in the first volunteer. Subsequent groups were then enrolled following a satisfactory review of the safety data collected from all three volunteers. This continued until six volunteers were vaccinated with the 5 × 1010 vp dose. A detailed breakdown of adverse reactions occurring after vaccination can be found in Figure 1 and Supplementary Table S1. At the highest dose, three of the six volunteers developed fevers (38.2–38.5 °C) and two of these three volunteers also developed severe local and systemic adverse reactions.
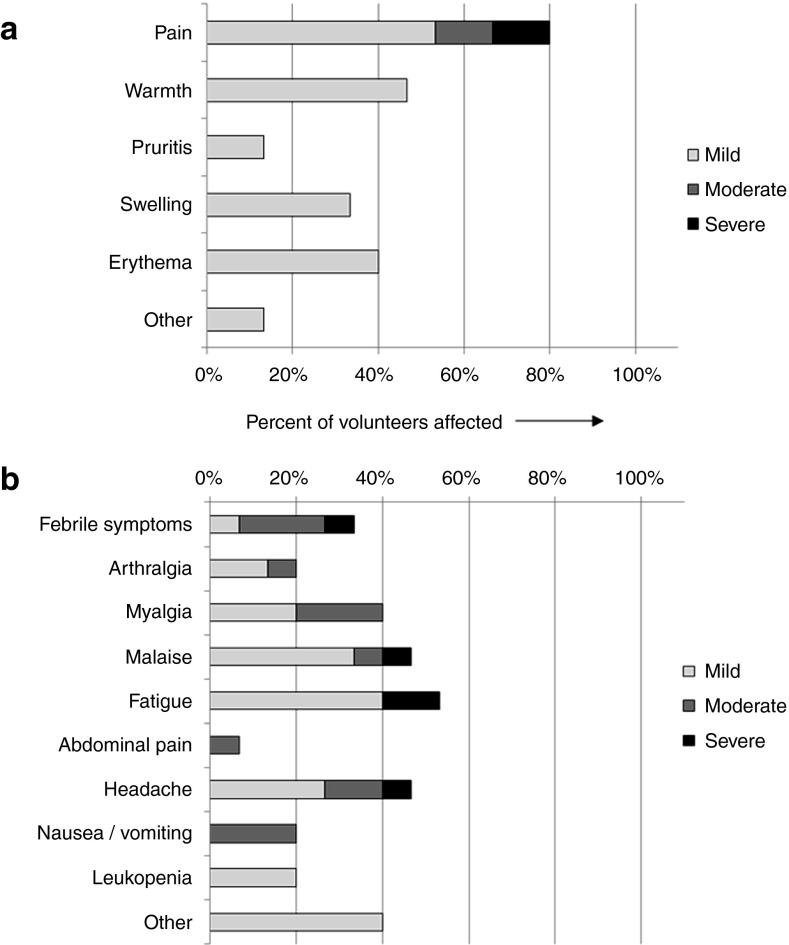
Figure 1.
Safety data for ChAdOx1 NP+M1: the frequency of adverse reactions following vaccination with ChAdOx1 NP+M1 is shown, with severity indicated by shading. (a) Local adverse reactions and (b) systemic adverse reactions. Data represent adverse reactions from all 15 volunteers across all four doses. A breakdown of adverse reactions by dose is provided in the supplementary information. M1, matrix protein 1; NP, nucleoprotein.
There were no serious adverse reactions following vaccination with ChAdOx1 NP+M1, at any dose, and the majority of adverse events were mild in nature. Of those adverse events considered related to vaccination, 35 were local and 77 were systemic. Local adverse reactions included pain, redness, swelling, itching, and warmth. The most common systemic adverse reactions solicited using diary cards were fatigue (67% of volunteers), malaise (58%), and headache (58%). In total, 55% of systemic adverse reactions were reported on either the day of vaccination or the first day after vaccination, and 71% of systemic adverse reactions resolved within 48 hours. With the exception of fevers, which were only reported in the highest dose group, there was no clear relationship between dose and the adverse events.
Immunogencity
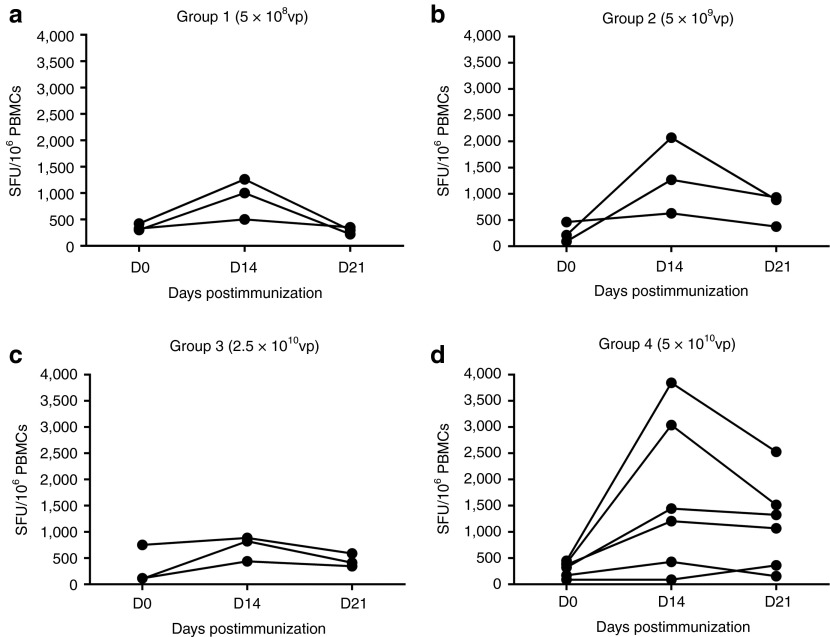
Interferon γ (IFN-γ) enzyme-linked immunospot (ELISpot) responses to the vaccine antigens, NP and M1, are shown in Figure 2a–d. Unless stated otherwise, values reported represent the median of total summed responses to peptide pools spanning the NP and M1 vaccine insert. Prior to vaccination, these responses did not differ significantly between groups (group 1: 326.7; group 2: 211.7; group 3; 115; and group 4: 345.8 spot forming units per 106 peripheral blood mononuclear cells (SFUs/106 PBMCs)). Individual peak immune responses occurred at 14 days post-ChAdOx1 NP+M1 vaccination. NP+M1-specific T-cell responses in group 1 (5 × 108 vp) increased threefold on day (D)14 over baseline (326.7 versus 1,002 SFUs/106 PBMCs) and returned to baseline levels (298.3 SFUs/106 PBMCs) by D21 (Figure 2a). Group 2 (5 × 109 vp) responses were increased sixfold on D14 over baseline (211.7 versus 1,268 SFUs/106 PBMCs) with a median response of 885 SFUs/106 PBMCs on D21 (Figure 2b). Group 3 (2.5 × 1010 vp) had the greatest fold-change in antigen-specific T-cell responses at D14, with a sevenfold increase over baseline (115 versus 823.3 SFUs/106 PBMCs). Again, responses remained elevated (115 versus 411.7 SFUs/106 PBMCs) over baseline on D21 (Figure 2c). Group 4 (5 × 1010 vp) responses were increased fourfold on D14 over baseline (345.8 versus 1,325 SFUs/106 PBMCs) with a median response of 1,196.67 SFUs/106 PBMCs on D21 (Figure 2d). At D21 post-ChAdOx1 NP+M1 vaccination, there was a nonsignificant trend toward higher responses in vaccinees who received the highest dose (5 × 1010 vp) of ChAdOx1 NP+M1 (1,197 SFUs/106 PBMCs), when compared with the lowest dose group (5 × 108 vp; 298.3 SFUs/106 PBMCs).
Figure 2.
Ex vivo interferon-γ (IFN-γ) enzyme-linked immunospot (ELISpot) responses to influenza vaccine antigen NP+M1 following vaccination with increasing doses of ChAdOx1 NP+M1. Individual ex vivo IFN-γ ELISpot responses from vaccinated volunteers at baseline day (D) 0, D14, and D21. Volunteers were vaccinated with a single dose of ChAdOx1 NP+M1 intramuscularly at a dose of (a) 5 × 108, (b) 5 × 109, (c) 2.5 × 1010, or (d) 5 × 1010 viral particles (vp). Controls included cells stimulated with PHA/SEB, PPD, or irrelevant peptide TRAP33 (data not shown). Negative control was cells stimulated with media alone (data not shown). M1, matrix protein 1; NP, nucleoprotein; PBMC, peripheral blood mononuclear cell; PHA, phytohaemagglutinin; PPD, tuberculin purified protein derivative; SEB, staphylococcal enterotoxin B; SFU, spot forming unit.
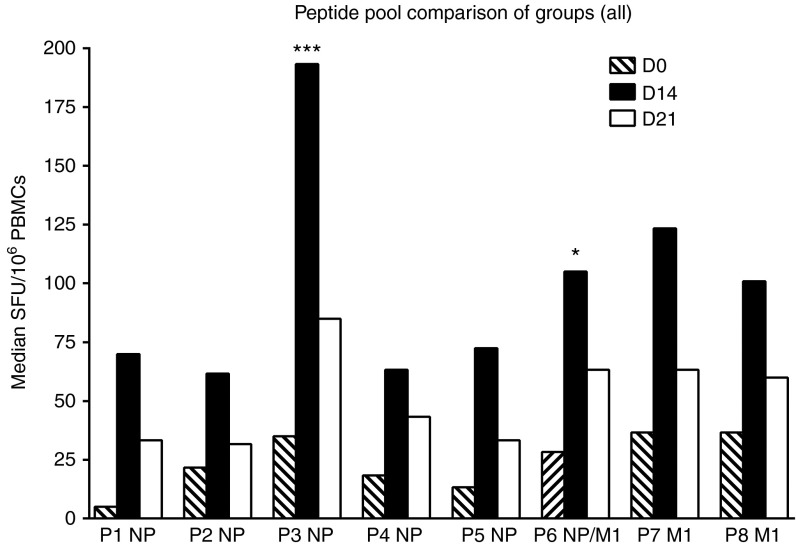
To assess the breadth of the NP+M1-specific T-cell response in vaccinees, we assessed ELISpot responses for eight individual peptide pools, which comprised of ten 15-20mer peptides overlapping by 10 amino acids, spanning the NP+M1 insert (Figure 3). Considering the small number of samples for each dose group, we pooled data from each group as previously described.13 The T-cell response to the NP+M1 antigen was spread over all eight pools both before and after vaccination with ChAdOx1 NP+M1 (Figure 3). Compared with D0, median responses to individual pools at D14 were increased ~12-fold (pool 1), 2.8-fold (pool 2), ~5.5-fold (pool 3; ***P < 0.05), ~3.4-fold (pool 4), ~5.5-fold (pool 5), ~3.7-fold (pool 6; *P < 0.05), ~3.4-fold (pool 7), and ~2.7-fold (pool 8). Responses decreased by D21 but remained elevated over baseline. The ELISpot response toward the eight individual peptide pools for each volunteer is shown in Supplementary Figure S1.
Figure 3.
Breadth of ex vivo interferon-γ (IFN-γ) enzyme-linked immunospot (ELISpot) responses to NP+M1 peptide pools following vaccination with ChAdOx1NP+M1. Median and ex vivo IFN-γ ELISpot responses from all vaccinated volunteers at day (D) 0, D14, and D21. P1–8 indicate NP+M1 peptide pools 1–8, composed of 10 peptides per pool. M1, matrix protein 1; NP, nucleoprotein.
The HLA-A*02-restricted epitope in M1 (GILGFVFTL, M1 amino acid residues 58–66, included in pool 6 here) has been found to be immunodominant.20 Seven of the 15 volunteers in this study were HLA A*02 positive, and these volunteers largely displayed the greatest increase in T-cell responses to peptides within pool 6. However, the increase in T-cell responses toward NP+M1 peptides was not limited to pool 6 in these HLA A*02 volunteers.
Anti-vector NAbs
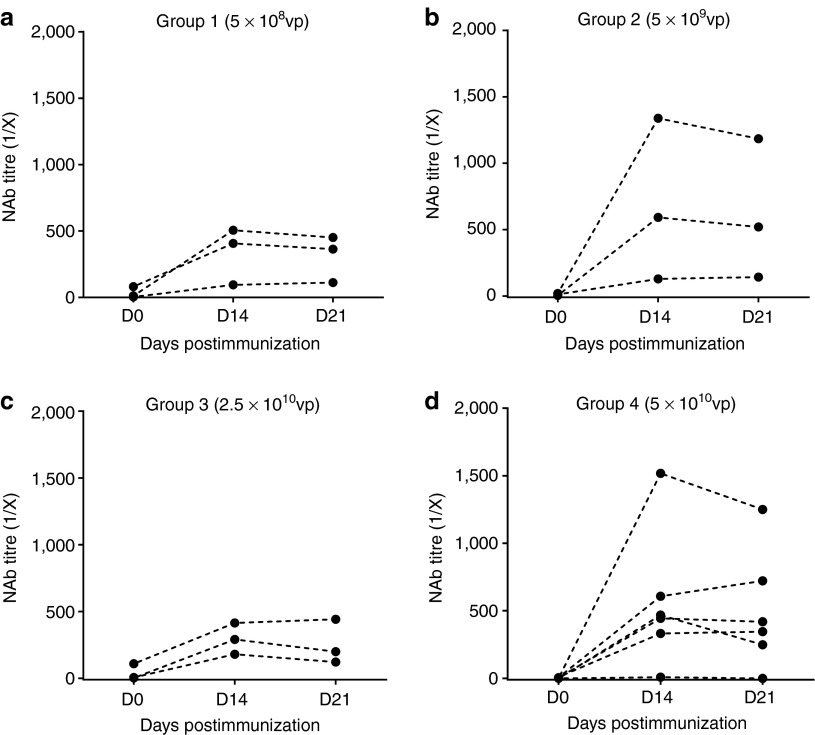
We wished to establish whether volunteers enrolled in the study had preexisting NAbs to ChAdOx1 or AdHu5 and also whether the levels of NAbs were affected by vaccination with a ChAdOx1-vectored vaccine. Titers of NAbs in volunteer sera were determined using a previously described assay,18 which measures the reciprocal of the serum dilution required to reduce in vitro expression of vector-expressed secreted alkaline phosphatase (SEAP) by 50%, 24 hours posttransduction (Figure 4). Volunteers with neutralizing titers <1:18 in this assay were classified as negative, and titers of 1:18–200 were considered to be low, in accordance with other published clinical studies.21 Two participants (of 15 tested) had low levels of preexisting NAbs to ChAdOx1 (titers 1:81 or 1:112) on D0, whereas all other volunteers were seronegative for anti-ChAdOx1 NAbs prior to vaccination (titers <1:18). Following vaccination with ChAdOx1 NP+M1, 11 out of the 15 subjects (73%) seroconverted, with NAb titers to ChAdOx1 ranging from >1:200 to 1:1,600. One volunteer did not seroconvert (<1:18) and 3/15 responded with low-level NAb titers (1:18–1:200). We did not observe any trend toward increased NAb responses with increasing vaccination dose. In this small study, there was no correlation between the magnitude of the IFN-γ response and the NAb titer.
Figure 4.
Neutralizing antibody (NAb) responses to adenoviral vectors in ChAdOx1 NP+M1 vaccinated cohorts. NAb titers in volunteer sera against ChAdOx1 NP+M1 (a–d) were determined pre- and postvaccination (day (D) 0, D14, and D21) for each group in the dose-escalation vaccination study. NAb titers were expressed as the reciprocal of the serum dilution required to reduce secreted alkaline phosphatase expression by 50% 24 hours posttransduction. 1/X, 1/dilution; M1, matrix protein 1; NP, nucleoprotein.
A total of 12/15 vaccinees (80%) were seronegative for AdHu5 NAbs (titers <1:18), 1/15 subjects had low-level NAb titers (1:105) and 2/15 had high-level (>1:1,350) preexisting immunity to AdHu5 (data not shown). NAb responses to Ad5 were unaffected by vaccination with the ChAdOx1 vector for the duration of the trial. Volunteers who had high preexisting NAb titers to AdHu5 were not the same as volunteers with low anti-ChAdOx1 NAbs.
ChAdOx1-MVA heterologous prime-boost regimen
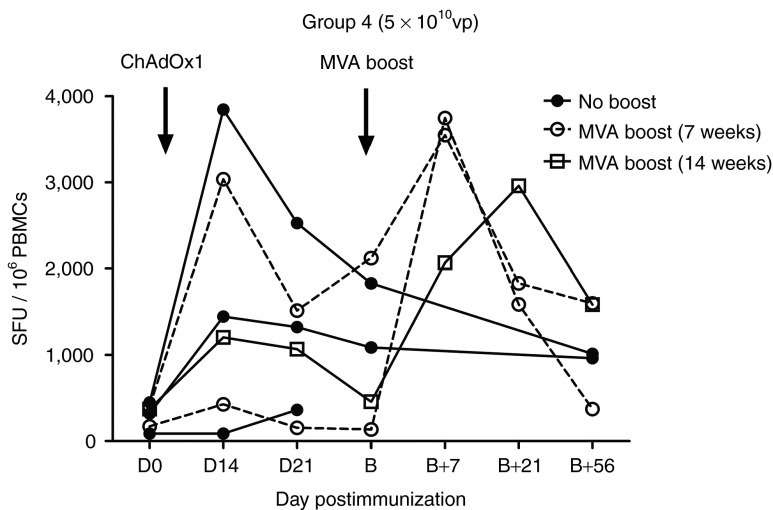
Half of the volunteers (3/6) in group 4 were boosted with a vaccination of 1.5 × 108 PFUs MVA-NP+M1 7 weeks (n = 2 volunteers) or 14 weeks (n = 1 volunteer) after ChAdOx1 NP+M1 vaccination (Figure 5). Compared with the pre-boost timepoint (B), median ELISpot responses were elevated approximately threefold (1,087 versus 3,548 SFUs; B versus B+7) at 7 days following the MVA-NP+M1 boost (B+7). At time points B+21 and B+56, median responses were 1,832 and 1,582 SFUs, respectively. The MVA-NP+M1 boost at B+7 resulted in a statistically significant increase in antigen-specific immunogenicity when compared with D0 (345.8 versus 3,548; P = 0.039; Krustkal–Wallis test). Two further subjects in group 4 (2/6) who did not receive an MVA-NP+M1 boost were also followed up at time points corresponding to B (1,086.7 and 1,831.7 SFUs) and B+56 (963.3 and 1,013.3 SFUs). The ELISpot response toward the eight individual peptide pools for each of the volunteers who received the MVA-NP+M1 boost is shown in Supplementary Figure 2.
Figure 5.
Median ex vivo interferon-γ (IFN-γ) enzyme-linked immunospot (ELISpot) responses to influenza vaccine antigen NP+M1 in a ChAdOx1 NPM1 prime, MVA-NP+M1 boost regime. Individual ex vivo summed IFN-γ ELISpot responses to NP+M1 at baseline day (D) 0, D14, and D21 in vaccinated volunteers receiving a single dose of 5 × 1010 viral particles (vp) of ChAdOx1 NP+M1. Arrows indicate the time points for prime (D0) and MVA-NP+M1 boost (B) vaccinations. Three volunteers were boosted by intramuscular injection of 1.5 × 108 PFUs MVA-NP+M1 at 7 or 14 weeks (B) post-ChAdOx1 NP+M1 prime and followed up on D7, D21, and D56 post-boost (B+7, B+21, and B+56). Two volunteers who did not receive the MVA-NP+M1 boost were followed at B and B+56 time points. Vector names are abbreviated to ChAdOx1 and MVA on the graph. M1, matrix protein 1; MVA, modified vaccinia virus Ankara; NP, nucleoprotein.
Discussion
Replication-deficient adenoviral vectors such as AdHu5 are known to be potent inducers of T-cell and humoral responses in preclinical studies. The existence of NAb to AdHu5 in humans has prompted the investigation of rare species adenoviral vectors,22,23,24,25 hexon chimeric vectors,26,27 as well as those derived from chimpanzees13 in an effort to circumvent preexisting immunity.27 A number of simian adenoviruses have also been found to exhibit comparable immunogenicity in preclinical studies,28 including demonstrating efficacy against Plasmodium berghei infection in mice.29 These studies on simian adenoviruses have been extended to demonstrate good immunogenicity in non-human primates30 and in a number of clinical studies.13 It is therefore desirable to increase the range of adenovirus vectors available for clinical use, and we now report on the first clinical use of ChAdOx1, a novel replication-deficient simian adenovirus vector. In this clinical study, we found no evidence of preexisting NAb toward ChAdOx1 in 13 of the 15 volunteers and low titers of NAb in the remaining two volunteers. As expected, vaccination with ChAdOx1 had no effect on anti-AdHu5 NAb titers.
For this first-in-human clinical study, we used a 3+3 dose-escalation study design,19 allowing us to ensure the safety of the trial participants while also efficiently testing a range of doses. There were no serious adverse events, and the vaccine was well tolerated by the first three volunteers receiving the vaccine at each dose, so we then proceeded to test a further three volunteers at the highest dose, 5 × 1010 vp. However, of these three further vaccines, two experienced severe local and systemic reactions, which were deemed unacceptable for prophylactic vaccination, so the dose of 2.5 × 1010 vp has now been chosen for further studies of ChAdOx1 NP+M1.
The magnitude of the T-cell response following vaccination with ChAdOx1 NP+M1 was in the same range as described following vaccination with MVA-NP+M1,10,12 and indeed, the levels of peak immunogenicity were comparable with volunteers vaccinated with the simian adenovirus vector ChAd63 encoding malaria antigens.31,32,33,34 These data suggest that simian adenovirus-vectored vaccines can induce high levels of antigen-specific T cells with no trend toward increased NAb responses with increasing vaccination dose.
Heterologous prime-boost regimens in which the same antigen is delivered by different vaccine vectors, in antigen-naive or experienced recipients, have previously been demonstrated to induce the highest level of immunogenicity.5,13,34 However, in a recent study using one human and one simian adenovirus vector in a heterologous prime-boost regimen, no boosting of antigen-specific T cells was demonstrated.35 When ChAd63 is used to prime either naive or antigen-experienced individuals and followed with an MVA boost (encoding the same antigen), immunogenicity is substantially boosted.13,34 In this study, responses in each of the three individuals tested were all increased (Figure 5, peak after ChAdOx1 compared with peak after MVA). Indeed, these responses were well maintained to 8 weeks post-boost. It is possible that the T-cell response toward NP+M1 may have been naturally boosted upon exposure to subclinical levels of circulating influenza virus; however, it is unlikely that the immune responses were significantly boosted as the incidence of general practitioner (GP)-reported “influenza-like illness” remained relatively low throughout the 2012/2013 influenza season (http://www.hpa.org.uk). Now, the safety and immunogenicity profiles of ChAdOx1 NP+M1 have been demonstrated, and a further clinical study will test heterologous prime-boost regimens in larger numbers of individuals.
Influenza-specific T-cell responses play an important role during viral infection, and human challenge studies have demonstrated a negative correlation between T-cell responses to viral antigens and influenza disease and viral shedding.6,7 Cellular responses, particularly toward well-conserved internal influenza antigens, can provide protection against viruses of different subtypes, including both seasonal and pandemic influenza viruses.5,8,9 Although human cytotoxic T-lymphocyte immunity declines over time, these cells are still detectable after 5 years, with an estimated half-life of 2–3 years,25 making cellular immunity an ideal target for vaccination-induced boosting. A strain-independent influenza vaccine could potentially circumvent the need to produce different vaccines for each influenza A subtype and clade, and could either be stockpiled in readiness for use at the start of a pandemic, or used routinely to protect against seasonal influenza and “stockpile immunity” in the population.
MVA-NP+M1 boosts preexisting T-cell responses to NP and M112 but did not prevent disease in all volunteers in an influenza challenge study.11 In a series of malaria vaccine studies using the same antigen in different viral vectors, a simian adenovirus vector induced considerably higher responses than MVA,13,36 with the highest responses induced by simian adenovirus priming followed by MVA boosting.13 The greater ability of simian adenovirus vectors to prime responses may be particularly relevant for vaccination of children, a cohort of the population particularly susceptible to influenza infection. ChAdOx1 NP+M1 could be used in a prime-boost regimen with MVA-NP+M1 to induce high levels of antigen-specific IFN-γ producing T cells in influenza-naive individuals. ChAdOx1 NP+M1 could also be used to boost influenza-restricted responses in influenza-experienced adults.
Replication-deficient simian adenovirus vectors are ideal for infectious disease vaccines where cellular immunity is required as they induce broad, potent, and well-maintained immune responses after a single vaccination, so it could be used to confer broad immunity rapidly. The ChAdOx1 adenovirus vector is safe and immunogenic in adult humans, providing a viable alternative to human adenoviruses as a vaccine vector for clinical use.
Materials and Methods
Study design and participants. Volunteers were recruited and the study was progressed according to a protocol approved within the UK by the Medicines and Healthcare products Regulatory Agency and the Regional Ethics Committee (http://www.clinicaltrials.gov, identifier: NCT01623518). All volunteers were healthy adults, residents in Oxford, with negative prevaccination tests for human immunodeficiency virus antibodies, hepatitis B surface antigen, and hepatitis C antibodies. Written informed consent was obtained in all cases, and the trial was performed according to the principals of the Declaration of Helsinki.
Fifteen volunteers aged 18–50 years were enrolled into four groups (Supplementary Table S1). Volunteers were enrolled and doses escalated according to a 3+3 study plan as follows. The first volunteer was vaccinated with 5 × 108 vp of ChAdOx1 NP+M1. No other volunteers were vaccinated until 48 hours had elapsed following this first vaccination. No severe or serious adverse reactions occurred and, therefore, a further two volunteers were vaccinated with the 5 × 108 vp dose. Following review of the safety data, the profile of adverse reactions was found to be acceptable and the next group was enrolled.
The first volunteer in each group was vaccinated ahead of the other volunteers and the safety profile, in the ensuing 48 hours, examined. Because the safety profile was found to be acceptable, a further two volunteers were vaccinated at that dose.
However, if one of these three volunteers had experienced an unacceptable adverse reaction, then a further three volunteers would have been vaccinated at the same dose (hence 3+3 design). Because none of the volunteers experienced an unacceptable adverse reaction, subsequent volunteers were vaccinated at the next highest incremental dose (outlined in Supplementary Table S1).
If more than two volunteers had experienced unacceptable adverse reactions at a given dose, then no further volunteers would have been vaccinated at that dose or a higher dose.
On the day of enrollment, blood samples were taken before vaccination. In all cases, the vaccine was administered in the deltoid muscle and observations were taken up to 2 hours after vaccination. Volunteers were seen in clinic 2 days later to collect information regarding adverse events. Volunteers completed diary sheets for 2 weeks following vaccination.
Design and construction of ChAdOx1 NP+M1 vaccine. The construction of replication-defective E1/E3 deleted chimpanzee adenovirus vector ChAdOx1 from wild-type isolate Y25 (species human adenovirus E) has been described previously.18 The NP+M1 vaccine antigen construct contains complete NP and M1 sequences from Influenza A/Panama/2007/99 joined by a 7 amino acid linker sequence.10 Expression of NP+M1 is driven by the human cytomegalovirus immediate early promoter, and the construct was inserted at the E1 locus of the ChAdOx1 genome by GalK recombineering.37 ChAdOx1 NP+M1 was manufactured to clinical good manufacturing practice (cGMP) by the Clinical Biomanufacturing Facility (University of Oxford, Oxford, UK) in the human embryonic kidney 293 cell line. The vectored vaccine was purified by cesium chloride isopycnic centrifugation and sterile filtered to generate a clinical lot at a concentration of 1.1 × 1011 vps per ml.
MVA-NP+M1 vaccine. The vaccine was described previously10 and consists of MVA expressing the NP and M1 antigens from influenza A/Panama/2007/99 as a single fusion protein.
IFN-γ ELISpot. Ex vivo IFN-γ ELISpot assays were performed to determine volunteer responses to the NP+M1 vaccine antigen pre- and postvaccination. Fresh PBMCs were isolated from 60 ml of whole blood and stimulated with peptides corresponding to the influenza NP+M1 vaccine insert, as described previously.26,38,39 Samples were assessed in triplicate using 2 × 105 PBMCs in a final volume of 100 μl per well, plates were incubated for 18–20 hours at 37 °C, and SFUs were detected using an AID ELISpot reader (AID Diagnostika, Strassberg, Germany). Results were expressed as SFUs per million PBMCs, calculated by subtracting the mean negative control response from the mean peptide pool response and summing the net response for the eight peptide pools. Responses in negative control wells were consistently <60 SFUs/106 PBMCs and positive control wells displayed >500 SFUs/106 PBMCs.
NAb assay. NAb titers to ChAdOx1 or AdHu5 vectors were measured using a SEAP assay, as previously described.18 SEAP-expressing ChAdOx1/AdHu5 was preincubated with an equal volume of serially diluted, heat-inactivated (56 °C for 90 minutes) human sera (dilutions of 1:18, 1:72. 1:288, 1:1,152, and 1:4,608) for 1 hour at 37 °C. Selected sera which with known high ChAdOx1 or Adhu5 neutralizing titers (titers >1,000) were used as positive controls. Following incubation, 200 µl serum:virus dilutions were used to transduce GripTite 293 Macrophage Scavenger receptor (MSR) cells (Invitrogen, Carlsbad, CA), seeded in 96-well plates at 3 × 104 cells/well. Cell supernatants were collected 24 hours posttransduction, and SEAP concentration was quantified using the Tropix PhosphaLite Chemiluminescent Assay Kit (Applied Biosystems, Warrington, UK). Luminescence was measured using a Varioskan flash luminometer (Thermo Scientific, Loughborough, UK). Neutralizing titers were expressed as the reciprocal of the serum dilution required to reduce SEAP expression by 50%, 24 hours posttransduction.
Statistical analyses. Statistical analyses were carried out using GraphPad Prism software version 5.04 (GraphPad Software, La Jolla, CA). Area under the curve values were determined for the response of each volunteer within each group and analyzed using Kruskal–Wallis test with post-hoc Dunn's multiple comparison test. For comparison of the breadth of responses to peptide pools, significance was determined by analysis of variance using one-way analysis of variance and a Bonferroni posttest to compare pairs of columns (D14 versus D0). To compare between time points within the same group of volunteers, data were analyzed by one-way analysis of variance using Kruskal–Wallis test with post-hoc Dunn's multiple comparison test. *P < 0.05, ***P < 0.001, NS = P >0.05.
SUPPLEMENTARY MATERIAL Figure S1. Breadth of ex vivo IFN-γ ELISPOT responses to NPM1 peptide pools following dose-escalation vaccination with ChAdOx1-NPM1. Figure S2. Breadth of ex vivo IFN-γ ELISPOT responses to NPM1 peptide pools following MVA-NP+M1 boost. Table S1. Study design.
Acknowledgments
We acknowledge Carly Bliss, Nick Edwards, and Pooja Mange (The Jenner Institute, Oxford, UK) for their assistance with data collection. R.D.A. is supported by the NIHR Biomedical Research Centre, Oxford. T.L is an Oxford Martin fellow. S.C.G and A.V.S.H. are Jenner Investigators. S.C.G, M.D.D, and A.V.S.H are named inventors on a patent application describing the ChAdOx1 vector (GB Patent Application No. 1108879.6). The study was funded by grants from the UK MRC, the NIHR through the Oxford Biomedical Research Centre, and the Oxford Martin school.
Supplementary Material
References
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25:5086–5096. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- Lambert LC, Fauci AS. Influenza vaccines for the future. N Engl J Med. 2010;363:2036–2044. doi: 10.1056/NEJMra1002842. [DOI] [PubMed] [Google Scholar]
- Jefferson T, Di Pietrantonj C, Al-Ansary LA, Ferroni E, Thorning S, Thomas RE. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2010. p. CD004876. [DOI] [PubMed]
- Jefferson T, Rivetti A, Harnden A, Di Pietrantonj C, Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev. 2008. p. CD004879. [DOI] [PubMed]
- Lambe T, Carey JB, Li Y, Spencer AJ, van Laarhoven A, Mullarkey CE, et al. Immunity against heterosubtypic influenza virus induced by adenovirus and MVA expressing nucleoprotein and matrix protein-1. Sci Rep. 2013;3:1443. doi: 10.1038/srep01443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael AJ, Gotch FM, Noble GR, Beare PA. Cytotoxic T-cell immunity to influenza. N Engl J Med. 1983;309:13–17. doi: 10.1056/NEJM198307073090103. [DOI] [PubMed] [Google Scholar]
- Wilkinson TM, Li CK, Chui CS, Huang AK, Perkins M, Liebner JC, et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med. 2012;18:274–280. doi: 10.1038/nm.2612. [DOI] [PubMed] [Google Scholar]
- Epstein SL, Kong WP, Misplon JA, Lo CY, Tumpey TM, Xu L, et al. Protection against multiple influenza A subtypes by vaccination with highly conserved nucleoprotein. Vaccine. 2005;23:5404–5410. doi: 10.1016/j.vaccine.2005.04.047. [DOI] [PubMed] [Google Scholar]
- Sridhar S, Begom S, Bermingham A, Hoschler K, Adamson W, Carman W, et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med. 2013;19:1305–1312. doi: 10.1038/nm.3350. [DOI] [PubMed] [Google Scholar]
- Berthoud TK, Hamill M, Lillie PJ, Hwenda L, Collins KA, Ewer KJ, et al. Potent CD8+ T-cell immunogenicity in humans of a novel heterosubtypic influenza A vaccine, MVA-NP+M1. Clin Infect Dis. 2011;52:1–7. doi: 10.1093/cid/ciq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie PJ, Berthoud TK, Powell TJ, Lambe T, Mullarkey C, Spencer AJ, et al. Preliminary assessment of the efficacy of a T-cell-based influenza vaccine, MVA-NP+M1, in humans. Clin Infect Dis. 2012;55:19–25. doi: 10.1093/cid/cis327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antrobus RD, Lillie PJ, Berthoud TK, Spencer AJ, McLaren JE, Ladell K, et al. A T cell-inducing influenza vaccine for the elderly: safety and immunogenicity of MVA-NP+M1 in adults aged over 50 years. PLoS ONE. 2012;7:e48322. doi: 10.1371/journal.pone.0048322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara GA, Duncan CJ, Ewer KJ, Collins KA, Elias SC, Halstead FD, et al. Clinical assessment of a recombinant simian adenovirus ChAd63: a potent new vaccine vector. J Infect Dis. 2012;205:772–781. doi: 10.1093/infdis/jir850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldt NJ, Aldhamen YA, Godbehere-Roosa S, Seregin SS, Kousa YA, Amalfitano A. Immunogenicity when utilizing adenovirus serotype 4 and 5 vaccines expressing circumsporozoite protein in naïve and adenovirus (Ad5) immune mice. Malar J. 2012;11:209. doi: 10.1186/1475-2875-11-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsis N, Fitzgerald JC, Reyes-Sandoval A, Harris-McCoy KC, Hensley SE, Zhou D, et al. Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: implications for their use as vaccines. Blood. 2007;110:1916–1923. doi: 10.1182/blood-2007-02-062117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch DH, Kik SV, Weverling GJ, Dilan R, King SL, Maxfield LF, et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine. 2011;29:5203–5209. doi: 10.1016/j.vaccine.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, et al. Step Study Protocol Team Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicks MD, Spencer AJ, Edwards NJ, Wadell G, Bojang K, Gilbert SC, et al. A novel chimpanzee adenovirus vector with low human seroprevalence: improved systems for vector derivation and comparative immunogenicity. PLoS ONE. 2012;7:e40385. doi: 10.1371/journal.pone.0040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer BE. Design and analysis of phase I clinical trials. Biometrics. 1989;45:925–937. [PubMed] [Google Scholar]
- Boon AC, de Mutsert G, Graus YM, Fouchier RA, Sintnicolaas K, Osterhaus AD, et al. The magnitude and specificity of influenza A virus-specific cytotoxic T-lymphocyte responses in humans is related to HLA-A and -B phenotype. J Virol. 2002;76:582–590. doi: 10.1128/JVI.76.2.582-590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, et al. Step Study Protocol Team HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol. 2007;81:4654–4663. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penaloza-MacMaster P, Provine NM, Ra J, Borducchi EN, McNally A, Simmons NL, et al. Alternative serotype adenovirus vaccine vectors elicit memory T cells with enhanced anamnestic capacity compared to Ad5 vectors. J Virol. 2013;87:1373–1384. doi: 10.1128/JVI.02058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden LR, Walsh SR, Seaman MS, Tucker RP, Krause KH, Patel A, et al. First-in-human evaluation of the safety and immunogenicity of a recombinant adenovirus serotype 26 HIV-1 Env vaccine (IPCAVD 001). J Infect Dis. 2013;207:240–247. doi: 10.1093/infdis/jis670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Xiang ZQ, Li Y, Kurupati RK, Jia B, Bian A, et al. Adenovirus-based vaccines: comparison of vectors from three species of adenoviridae. J Virol. 2010;84:10522–10532. doi: 10.1128/JVI.00450-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlan L, Bradshaw AC, Parker AL, Robinson H, White K, Custers J, et al. Ad5:Ad48 hexon hypervariable region substitutions lead to toxicity and increased inflammatory responses following intravenous delivery. Mol Ther. 2012;20:2268–2281. doi: 10.1038/mt.2012.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DM, Nanda A, Havenga MJ, Abbink P, Lynch DM, Ewald BA, et al. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature. 2006;441:239–243. doi: 10.1038/nature04721. [DOI] [PubMed] [Google Scholar]
- Colloca S, Barnes E, Folgori A, Ammendola V, Capone S, Cirillo A, et al. Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Sci Transl Med. 2012;4:115ra2. doi: 10.1126/scitranslmed.3002925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Sandoval A, Sridhar S, Berthoud T, Moore AC, Harty JT, Gilbert SC, et al. Single-dose immunogenicity and protective efficacy of simian adenoviral vectors against Plasmodium berghei. Eur J Immunol. 2008;38:732–741. doi: 10.1002/eji.200737672. [DOI] [PubMed] [Google Scholar]
- Capone S, Reyes-Sandoval A, Naddeo M, Siani L, Ammendola V, Rollier CS, et al. Immune responses against a liver-stage malaria antigen induced by simian adenoviral vector AdCh63 and MVA prime-boost immunisation in non-human primates. Vaccine. 2010;29:256–265. doi: 10.1016/j.vaccine.2010.10.041. [DOI] [PubMed] [Google Scholar]
- Sheehy SH, Duncan CJ, Elias SC, Biswas S, Collins KA, O'Hara GA, et al. Phase Ia clinical evaluation of the safety and immunogenicity of the Plasmodium falciparum blood-stage antigen AMA1 in ChAd63 and MVA vaccine vectors. PLoS ONE. 2012;7:e31208. doi: 10.1371/journal.pone.0031208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy SH, Duncan CJ, Elias SC, Collins KA, Ewer KJ, Spencer AJ, et al. Phase Ia clinical evaluation of the Plasmodium falciparum blood-stage antigen MSP1 in ChAd63 and MVA vaccine vectors. Mol Ther. 2011;19:2269–2276. doi: 10.1038/mt.2011.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy SH, Duncan CJ, Elias SC, Choudhary P, Biswas S, Halstead FD, et al. ChAd63-MVA-vectored blood-stage malaria vaccines targeting MSP1 and AMA1: assessment of efficacy against mosquito bite challenge in humans. Mol Ther. 2012;20:2355–2368. doi: 10.1038/mt.2012.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogwang C, Afolabi M, Kimani D, Jagne YJ, Sheehy SH, Bliss CM, et al. Safety and immunogenicity of heterologous prime-boost immunisation with Plasmodium falciparum malaria candidate vaccines, ChAd63 ME-TRAP and MVA ME-TRAP, in healthy Gambian and Kenyan adults. PLoS ONE. 2013;8:e57726. doi: 10.1371/journal.pone.0057726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes E, Folgori A, Capone S, Swadling L, Aston S, Kurioka A, et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci Transl Med. 2012;4:115ra1. doi: 10.1126/scitranslmed.3003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkey SJ, Reece WH, Moorthy VS, Webster D, Dunachie S, Butcher G, et al. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat Med. 2003;9:729–735. doi: 10.1038/nm881. [DOI] [PubMed] [Google Scholar]
- Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 2005;33:e36. doi: 10.1093/nar/gni035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud TK, Fletcher H, Porter D, Thompson F, Hill AV, Todryk SM. Comparing human T cell and NK cell responses in viral-based malaria vaccine trials. Vaccine. 2009;28:21–27. doi: 10.1016/j.vaccine.2009.09.132. [DOI] [PubMed] [Google Scholar]
- Antrobus RD, Berthoud TK, Mullarkey CE, Hoschler K, Coughlan L, Zambon M, et al. Coadministration of seasonal influenza vaccine and MVA-NP+m1 simultaneously achieves potent humoral and cell-mediated responses. Mol Ther. 2014;22:233–238. doi: 10.1038/mt.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.