Abstract
Adeno-associated virus (AAV) vectors can move along axonal pathways after brain injection, resulting in transduction of distal brain regions. This can enhance the spread of therapeutic gene transfer and improve treatment of neurogenetic disorders that require global correction. To better understand the underlying cellular mechanisms that drive AAV trafficking in neurons, we investigated the axonal transport of dye-conjugated AAV9, utilizing microfluidic primary neuron cultures that isolate cell bodies from axon termini and permit independent analysis of retrograde and anterograde axonal transport. After entry, AAV was trafficked into nonmotile early and recycling endosomes, exocytic vesicles, and a retrograde-directed late endosome/lysosome compartment. Rab7-positive late endosomes/lysosomes that contained AAV were highly motile, exhibiting faster retrograde velocities and less pausing than Rab7-positive endosomes without virus. Inhibitor experiments indicated that the retrograde transport of AAV within these endosomes is driven by cytoplasmic dynein and requires Rab7 function, whereas anterograde transport of AAV is driven by kinesin-2 and exhibits unusually rapid velocities. Furthermore, increasing AAV9 uptake by neuraminidase treatment significantly enhanced virus transport in both directions. These findings provide novel insights into AAV trafficking within neurons, which should enhance progress toward the utilization of AAV for improved distribution of transgene delivery within the brain.
Introduction
Adeno-associated virus (AAV) is a nonenveloped parvovirus containing a single-stranded DNA genome of ˜4.7 kb.1,2 Recombinant AAV vectors, in which the endogenous rep and cap genes are replaced by a gene expression cassette, are replication-defective tools for clinical gene therapy and experimental gene delivery.3,4 AAV can achieve long-term transduction of postmitotic cells, with transgene expression observed beyond 1 year in rodents and 6 years in primates.5,6 Many AAV serotypes with unique cell tropism, transduction strength, and immunogenicity have been identified.7,8
AAV can induce gene expression in targeted brain regions in vivo6,9,10 and can also efficiently transduce primary cortical or hippocampal cultures in vitro.11 The use of AAV in the brain, however, presents a unique problem. AAV vectors are incapable of replication and proliferation, and thus, transgene expression is only observed within brain cells that receive the initial infusion of recombinant virions.3,6 As AAV diffuses a relatively short distance in brain tissue, transduction is often limited to a small region surrounding the injection site. Thus, widespread central nervous system transduction requires a prohibitively large number of injections,12,13 and AAV-mediated treatment of disorders that require global neurological correction remains challenging. Utilizing a secreted protein that undergoes axonal transport, such as β-glucuronidase or β-galactosidase, can further enhance the spread of therapeutic gene delivery from an AAV transduction site.14,15,16 For example, bilateral thalamic injection of AAV1 resulted in widespread transport of the therapeutic β-galactosidase protein and subsequent correction in a mouse model of GM1 gangliosidosis.14 Furthermore, AAV can be transported along some axonal pathways to transduce distal brain regions following injection. This property provides a way to further enhance the distribution of gene transfer, utilizing neuronal pathways to achieve more widespread AAV transduction.17 For example, a single injection of AAV9 into the ventral tegmental area, a region with widespread axonal projections, results in gene transfer to distal but axonally connected brain regions, leading to protein dissemination and correction of pathology throughout the brain in a mouse model of global disease.17
Previous studies of AAV trafficking have only been conducted in nonneuronal cells. AAV enters the cell through both clathrin-mediated and CLIC/GEEC endocytosis;18,19 traffics through early, late, and recycling endosomes;20 and accumulates in the Golgi.21 Furthermore, when AAV is injected directly into the cytosol, it does not move to the nucleus or induce transgene expression.22 More specifically, when trafficking of AAV into the late endosome is impaired or acidification and maturation of the late endosome is blocked, AAV transduction is substantially reduced.23 Thus, it appears that endosomal trafficking is required for efficient AAV transduction.
Although AAV vectors are widely used for gene delivery to the brain, their intracellular trafficking has never been investigated in primary neurons. Neurons are polarized cells with specialized mechanisms for long-distance retrograde and anterograde transport, and the study of AAV intracellular transport in cultured neurons is essential to establish a basis for the ongoing utilization of AAV in the brain.24 To examine AAV transport in neurons, we used a microfluidic culture platform that fluidically separates the cell bodies from the axon termini of cultured neurons. This allowed us to examine axonal uptake of AAV and retrograde transport to the cell body independent of other viral trafficking pathways, as well as cell body uptake and anterograde transport to the axon terminus, mirroring the axonal transport observed in vivo. We utilized AAV serotype 9, which consistently demonstrates strong transduction of neurons in the rodent brain.7,9,17 Understanding the intraneuronal trafficking of AAV is a critical step in its development as a vector for neurological gene therapy and will improve our ability to harness axonal transport as a tool for enhancing the spread of AAV-induced gene expression in the brain, thereby decreasing the number of injections required for global transduction.
Results
AAV9 undergoes fast retrograde and anterograde axonal transport
A microfluidic culture chamber was used to examine both the retrograde transport of AAV9 following entry at the axon terminus and anterograde transport following cell body entry.25 Microfluidic chambers consist of two independent channels, each of which links together a pair of large wells. The two channels are connected by an array of thin microgrooves. Rat E18 cortical neurons cultured in one channel extend axons through the grooves to the opposite channel (Figure 1a). A difference in media volume can then be used to induce a directional flow across the grooves. AAV that is applied to the channel connected to the wells with less media (termed the isolated channel) cannot penetrate this flow, and thus, it does not enter the grooves or reach the channel connected to wells with more media (termed the distal channel) (Figure 1b). This permits specific application of AAV9 to either the channel containing the cell bodies or the channel containing the axon termini. Specific application of AAV9 to the axon side of the chamber resulted in transduction and expression of eGFP transgene exclusively in neurons that extended axons across the grooves to the isolated channel (Figure 1c,d), indicating that retrogradely transported AAV9 is capable of transduction. By contrast, following specific application of AAV9 to the cell body side of the chamber, widespread eGFP expression was observed throughout the isolated wells and channel, consistent with previous reports that AAV9 can transduce rat embryonic cortical neuron cultures.11 Dye-labeled AAV9 was observed to move directionally along axons in the groove, both retrogradely after entry at the axon terminus and anterogradely after entry at the cell body (Figure 1e,f; Supplementary Movies S1 and S2).
Figure 1.
AAV9 particles undergo axonal transport and induce transgene expression following axon- or cell body–specific application. (a) Rat E18 cortical neurons cultured in the microfluidic chamber are healthy and extend axons through the grooves after 5 days in vitro (arrows). (b) An 80 µl difference in media volume across the grooves isolates 5 ng AF488-Dextran within the cell channel 4 hours after application. (c,d) Specific application of AAV9 to the axon termini of identical cultures for 2.5 hours resulted in retrograde transduction and expression of the eGFP transgene 13 days after application of virus. (c) Neurons that extended axons across the grooves to the isolated channel (arrow), to which AAV9 was applied, exhibited strong eGFP expression. (d) Cells that did not extend axons into the isolated channel were not transduced. Thresholds were adjusted to improve visibility of axonal eGFP. (e,f) AAV9 axonal transport was tracked in the groove by sequential image acquisition (3 seconds/frame, 63×). The leftmost panel of each sequence displays the imaged axon in phase. Thresholds were adjusted to improve visibility of Cy3-AAV9. (e) 1 hour after application of mCy3-AAV9 to the axon side of the chamber, an AAV9 punctum (arrows) makes a representative retrograde run of 124 µm at 1.01 µm/second. See also Supplementary Movie S1. (f) 1 hour after application of bCy3-AAV9 to the cell body side of the chamber, an AAV9 punctum (arrows) makes a representative anterograde run of 143 µm at 2.99 µm/second. See also Supplementary Movie S2. AAV, adeno-associated virus.
AAV9 axonal transport is mediated by dynein/dynactin and kinesin-2
As microfluidic live imaging of AAV9 demonstrated both retrograde and anterograde axonal transport, we sought to identify the molecular motors responsible for this directed movement. Rat E18 cortical neurons were electroporated with dominant negative inhibitors of endogenous motor function, plated as mass cultures, treated with 1 × 109 genome copies (GCs) mCy3- or bCy3-AAV9, and imaged from 1.5 to 4 hours. Because cells within the microfluidic channel cannot be specifically targeted by lipofection, and because electroporation of these dominant negative constructs can inhibit axon outgrowth, it was necessary to conduct this experiment in mass culture. In each condition, the frequency of anterograde- and retrograde-directed puncta was calculated and compared against mock-transfected neurons (Figure 2). To determine whether retrograde AAV9 transport was dependent on dynein, we transfected p150-CC1, a fragment of the p150Glued dynactin subunit that acts as a dominant negative inhibitor of the dynein/dynactin complex.26 Following CC1 transfection, a 59.1% decrease in retrograde-directed AAV9 puncta was observed (P = 0.0020). This ˜60% decrease is consistent with previous observations of the effect of CC1 transfection on other dynein/dynactin cargoes,27 indicating that the retrograde transport of AAV9 is mediated by dynein in complex with dynactin. Next, in order to determine whether kinesin-1 was required for the anterograde transport of AAV9, we transfected Kif5C Tail, a kinesin tail domain that acts as a dominant negative inhibitor of both the neuron-specific Kif5C isoform and the ubiquitous Kif5B isoform of kinesin-1.28,29 This plasmid had no effect, indicating that long-distance AAV9 transport is unlikely to involve kinesin-1. We also examined the transfection of Kif3A-HL, a headless Kif3A subunit that acts as a dominant negative inhibitor of kinesin-2.30 Kif3A-HL transfection almost completely abolished anterograde transport, decreasing anterograde-directed AAV9 puncta by 76.2% (P = 0.00040). A significant 26.2% decrease in retrograde-directed puncta was also observed (P = 0.041). As anterograde-directed AAV9-positive endosomes that moved into the axon were often observed to return retrogradely to the cell body, a concurrent effect on retrograde transport is not surprising. Thus, it appears that dynein/dynactin is necessary for retrograde transport of AAV9, that kinesin-2 is the primary mediator of AAV9 anterograde transport, and that kinesin-1 is not required for the long-distance axonal transport of AAV9.
Figure 2.
AAV9 retrograde transport is mediated by dynein/dynactin, and anterograde transport is mediated by kinesin-2. In mass cultures of rat E18 cortical neurons treated with 1 × 109 mCy3- or bCy3-AAV9, the number of AAV9 puncta progressing more than 50 μm retrograde or anterograde was counted and then divided by the total length of axon imaged. Stars depict the statistical comparison of each plasmid transfection against mock transfection. **P < 0.01; ***P < 0.001. Error bars represent one standard deviation from the mean among n = 4 cultures for each condition. Transfection of CC1, a dominant negative inhibitor of dynein/dynactin, resulted in a 59.1% decrease in retrograde-directed puncta, indicating that the dynein/dynactin complex mediates retrograde AAV9 transport. Transfection of Kif5C Tail, a dominant negative inhibitor of kinesin-1, had no effect on AAV9 transport, whereas Kif3A-HL, a dominant negative inhibitor of kinesin-2, decreased anterograde-directed puncta by 76.2%. This indicates that kinesin-2, and not kinesin-1, mediates anterograde AAV9 transport. AAV, adeno-associated virus.
AAV9 distributes rapidly and widely throughout the endosomal system
Next, we sought to identify the endosomes that carry AAV9 in the axon and to determine whether the amount of AAV9 in these compartments changes based on the route of entry or time after entry. Colocalization cannot be calculated after GFP-Rab transfection (below), as AAV9 is present in both transfected and untransfected axons, which overlap in the grooves and cannot be discriminated. Thus, to calculate the percentage of AAV9 in the early endosome, the late endosome/lysosome, the recycling endosome, and the exocytic vesicle immunocytochemistry (ICC) was performed in microfluidic chambers using primary antibodies against Rab5, 7, 11, and 3, respectively (Figure 3a). Cells were fixed 1 and 4 hours after application of AAV9 to the axon side of the chamber and 4 hours after application to the cell body side (1 hour after cell body application was not examined, as very little AAV9 is present in the axon at this time point). Counting the percentage of colocalized AAV9 puncta in the axon and performing statistical analysis by logistic regression revealed few differences among time points or sites of entry (Figure 3b). Colocalization with the exocytic vesicle increased significantly over time, from 9.4% at 1 hour after axon channel application to ˜18% at 4 hours after axon or cell channel application (P = 9.60 × 10−6; P = 5.14 × 10−7). There was also significantly more AAV9 in the recycling endosome at 4 hours after axon terminus entry, 24.9%, than that after cell body entry, 17.9% (P = 0.017). Colocalization with the early and late endosomes was ˜15–20% for all conditions, with no significant differences detected.
Figure 3.

AAV9 distributes widely throughout the endosomal system regardless of the site of entry. (a) Images of a representative microfluidic immunocytochemistry (ICC) stain, in which mCy3-AAV9 was applied to the cell body side of the chamber for 4 hours, followed by fixation and staining with an anti-Rab11 primary antibody and AF488 secondary antibody. Colocalization of Rab11 and AAV9 can be observed in the grooves (arrow). All axons are stained with anti-Rab11 (green), although only a small percentage contain AAV9 (red). Similar punctate staining was observed with all other Rab antibodies. (b) ICC was conducted using antibodies against Rab5, 7, 11, or 3, 1 or 4 hours after application of AAV9 to the axon side of the chamber, or 4 hours after application to the cell body side. A minimum of n = 146 AAV9 puncta were counted for each time point, with a total n = 7828 counted. Stars depict the significance of a logistic regression among all time points for each antibody. ***P < 0.001; *P < 0.05. Error bars represent one standard deviation from the mean among a minimum of n = 3 cultures. Colocalization with the exocytic vesicle increased from 1 to 4 hours after AAV9 application, and colocalization with the recycling endosome was greater at 4 hours after axon terminus than after cell body application. (c) The colocalization of AAV9 with Lysotracker during microfluidic live imaging was calculated in 1 hour blocks. Each 1 hour block represents a minimum of n = 4 cultures and n = 172 puncta. Stars depict the statistical significance of a logistic regression among all three 1 hour blocks. ***P < 0.001. Error bars represent one standard deviation from the mean among cultures. For both application sites, colocalization with Lysotracker was observed to increase over time, peaking at ˜26% from 3 to 4 hours after AAV9 application. AAV, adeno-associated virus.
To determine the percentage of AAV9 in acidic endosomes (primarily lysosomal), Lysotracker live imaging (below) was counted and analyzed in the same manner as the ICC data. Colocalization was calculated in three 1 hour blocks after AAV9 application: 1–2, 2–3, and 3–4 hours. AAV9 was observed to accumulate over time in Lysotracker-stained endosomes (Figure 3c). Following both axon terminus and cell body entry, colocalization with the lysosome was low after 1–2 hours but increased to more than 25% of AAV9 by 3–4 hours (P = 1.69 × 10−9 and P = 2.00 × 10−16 for axon terminus and cell body, respectively). In summary, colocalization of AAV9 with the exocytic vesicle and the acidic lysosome is initially low, but it increases to ˜18 and 26%, respectively, by 4 hours after entry. No changes in colocalization were observed over time for the early, late, or recycling endosomes. As a whole, these data suggest that AAV9 is trafficked rapidly throughout the endosomal system regardless of the site of entry, quickly entering the early, late, and recycling endosomes, and accumulating in exocytic vesicles and lysosomes over time.
Finally, we examined whether enhancing AAV9 uptake at the plasma membrane would increase the amount of AAV in transported endosomes, and thus enhance directional transport along the axon. To accomplish this, we performed an identical experiment to those described above (Figure 2), comparing pretreatment of neurons with neuraminidase (NA) rather than transfection. NA cleaves sialic acid on the cell surface, exposing the more productive AAV9 receptor galactose, thereby increasing AAV9 entry.31 After NA treatment, a 47.7% increase in retrograde-directed puncta (P = 0.030) and a 117% increase in anterograde-directed puncta (P = 0.0038) was observed (Supplementary Figure S1). This indicates that enhancing AAV9 uptake can increase the amount of AAV9 in axonally transported endosomes, and therefore, it may be possible to increase AAV9 transport to distal brain regions in vivo by locally injecting a larger amount of AAV9 or coinjecting NA.
AAV9 is transported in the late endosome/lysosome but not in early or recycling endosomes
We next sought to identify the endosomal compartments that are involved in the long-distance axonal transport of AAV9. Cy3-AAV9 was live imaged in colocalization with GFP-Rab5 (early endosome) and GFP-Rab7 (late endosome/lysosome) following application to the axon side of the chamber, as well as with GFP-Rab11 (recycling endosome) and Lysotracker DND-26 (late endosome/lysosome, with bias for the more acidic lysosome)32 following application to either the axon or cell body side. From 1 to 5 hours after application to the axon side of the chamber, 20.7% of all AAV9 puncta progressed more than 10 µm retrograde and 2.63% more than 10 µm anterograde, with the remainder either stationary or moving bidirectionally and failing to progress more than 10 µm in either direction. By contrast, after application to the cell body side, 14.4% of all AAV9 puncta were retrograde directed and 14.3% were anterograde directed. When AAV9 colocalized with GFP-Rab5 or with GFP-Rab11, little movement was observed in either direction (Figure 4c, Supplementary Movies S3 and S4). Two out of 41 GFP-Rab5-colocalized puncta were retrograde directed, and none were anterograde directed. One retrograde- and one anterograde-directed punctum were observed out of 139 GFP-Rab11-colocalized puncta. No Rab5- or Rab11-colocalized AAV9 progressed more than 30 µm. By contrast, 70 out of 182 GFP-Rab7-colocalized puncta and 180 out of 463 Lysotracker-colocalized puncta were retrograde directed (Figure 4, Supplementary Movies S5 and S6). Thus, while AAV9 enters all four of these compartments, the late endosome/lysosome is the primary mediator of retrograde transport to the cell body. Because Rab5 and Rab7 specifically mediate endocytic sorting and retrograde axonal transport,33,34,35 GFP-Rab5 and GFP-Rab7 were not examined following application of AAV to cell bodies. As expected, anterograde-directed AAV particles observed after axon terminus application did not colocalize with either GFP-Rab5 or GFP-Rab7. Surprisingly, following cell body application, Lysotracker-colocalized AAV9 moved retrograde in the same manner as after axon terminus application (Figure 4c). This suggests that, regardless of whether the virus enters the axon anterogradely or retrogradely, AAV9 in the axon can be trafficked into the retrograde-directed late endosome/lysosome.
Figure 4.
AAV9 undergoes axonal transport in the late endosome/lysosome but not in early or recycling endosomes. (a,b) Colocalized movement was tracked by sequential image acquisition (4.5 seconds/frame, 63×). Displacement of the green punctum ahead of the red punctum occurs due to sequential acquisition (250 ms delay between channels). The leftmost panel of each sequence shows the imaged axon in phase. Thresholds were adjusted to improve visibility. Retrograde movement of AAV9 was observed in both GFP-Rab7- and Lysotracker-labeled endosomes. (a) 4 hours after application of mCy3-AAV9 (red) to the axon side of a chamber treated with Lysotracker (green), a colocalized punctum (arrows) makes a representative retrograde run of 33 µm at 0.42 µm/second, then pauses. See also Supplementary Movie S5. (b) 1 hour after application of bCy3-AAV9 (red) to the axon side of a chamber transfected with GFP-Rab7 (green), a colocalized punctum (arrows) makes a representative retrograde run of 86 µm at 1.1 µm/second. See also Supplementary Movie S6. (c) Colocalized puncta were counted from 1 to 4 hours after application of AAV9 and classified as retrograde-directed or anterograde-directed (progressing more than 10 µm in the respective direction over 3 minutes) or bidirectional/stationary (not progressing at least 10 µm in either direction). This graph summarizes categorical observations from all live imaging experiments that were performed, and thus the number of replicates is larger for groups that were statistically analyzed (Figure 7). Minimal movement was observed in colocalization with GFP-Rab5 after application of AAV9 to the axon channel (n = 41 puncta from three cultures), or with GFP-Rab11 after application to the axon (n = 45, 2) or cell body channel (n = 91, 6). By contrast, ˜40% of AAV9 puncta moved retrograde when colocalized with GFP-Rab7 (n = 182, 17), or with Lysotracker after application to the axon (n = 162, 4) or cell body channel (n = 301, 4). AAV, adeno-associated virus.
Next, the localization of AAV9 in the cell body following retrograde transport was analyzed by way of ICC staining of the Golgi and nucleus (Figure 5). At 1 hour after application to the axon terminus, very little AAV9 was transported to the cell body, but most virions colocalized with the Golgi. A much greater percentage of AAV9 colocalized with the Golgi after retrograde transport than was observed after cell body entry (P < 0.0001). By 4 hours after axon application, ˜25% of AAV9 puncta in the cell body were associated with the nucleus, which was equivalent to either 1 or 4 hours after cell body application. This suggests that, following retrograde transport to the cell body within the late endosome/lysosome, AAV9 is efficiently delivered to the Golgi and nucleus, with an initial bias for the Golgi at early time points. By contrast, both 1 and 4 hours after cell body entry, a significantly greater percentage of AAV9 failed to colocalize with either the Golgi or the nucleus (P < 0.0001), reflecting the distribution of AAV9 into other compartments such as the early endosome, recycling endosome, and exocytic vesicle (Figure 3b).
Figure 5.
AAV9 is delivered to the Golgi and nucleus after retrograde transport to the cell body. Following specific application of mCy3-AAV9 for 1 or 4 hours to either the axon or the cell body side of microfluidic chambers, cells were fixed and stained with anti-Giantin (green, Golgi) and DAPI (blue, nucleus). (a) Representative images (63×) of cell bodies within the microfluidic channels demonstrate colocalization with both the Golgi and the nucleus. Thresholds were adjusted for improved visibility of colocalization. (b) The percentage of AAV9 puncta that colocalized with the Golgi, the nucleus, or neither (uncolocalized) was counted 1 hour after axon application (n = 38 cells from n = 2 cultures), 4 hours after axon application (n = 45, 2), 1 hour after cell body application (n = 45, 3), and 4 hour after cell body application (n = 45, 3). Stars depict post hoc significance following an ANOVA comparing all time points. ***P < 0.001; **P < 0.01; *P < 0.05. While all conditions demonstrated colocalization with both the Golgi and the nucleus, a greater percentage of cellular AAV9 was found in the Golgi following retrograde transport from the axon terminus, and less AAV9 was associated with the nucleus 1 hour after entry at the axon terminus. AAV, adeno-associated virus.
AAV9 is transported anterograde at high velocity and with infrequent pausing
In addition to AAV9-positive compartments, GFP-Rab5-, GFP-Rab7-, and Lysotracker-labeled endosomes were also tracked in microfluidic culture, in order to provide a baseline representation of intracellular trafficking in this system. The movement properties of these populations were quantified using a number of parameters (Supplementary Table S1a–d). The distribution of instantaneous velocities, the speed at which a punctum moves between two sequential images during runs in the forward direction, was unimodal for all populations except anterograde-directed AAV9 (Figure 6a, Supplementary Figure S2). Anterograde-directed AAV9 had a bimodal distribution and frequently moved at speeds beyond 2 µm/second (Figure 6a). Statistical analysis demonstrated that after cell body entry, anterograde-directed AAV9 moved at higher velocities, made runs of a longer distance, progressed further total distance, and spent less time paused than anterograde-directed Lysotracker-, GFP-Rab5-, or GFP-Rab7-labeled endosomes (Figure 6b–e; all P < 0.001). The duration of individual pauses did not differ among these four groups, with the exception of GFP-Rab5-labeled endosomes, which paused for slightly longer durations (Supplementary Table S1; P = 0.0044). These data indicate that after entry at the cell body, AAV9 moves into endosomal compartments that are specifically targeted for anterograde axonal transport, as virus puncta move anterograde along the axon in a significantly more directional manner than the general endosome population. However, anterograde AAV9 movement was not observed in colocalization with any of the above compartments (Figure 4c) or with a GFP-CAD transfection construct that was used to label all vesicles emerging from the Golgi, as previously described36 (provided by Jyoti Jaiswal, George Washington University, Washington, DC). This method labels all post-Golgi vesicles, including the exocytic vesicles stained by anti-Rab3 antibodies. Out of 34 anterograde-directed AAV9 puncta (progressing more than 50 µm) observed in transfected neurons, none colocalized with the labeled pool of post-Golgi vesicles. Thus, anterograde-directed AAV9 is not transported in the post-Golgi exocytic vesicle, the late endosome/lysosome, or the recycling endosome.
Figure 6.
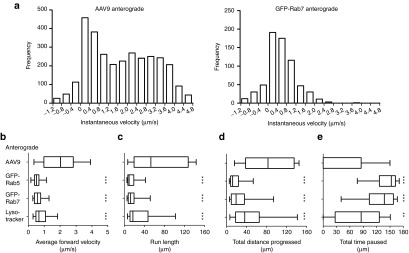
Comparison against endosomes indicates that AAV9 undergoes high-speed anterograde axonal transport. Anterograde-directed AAV9 puncta were tracked within the microfluidic groove from 1 to 5 h after specific application to the cell body side of the chamber. (a) Histograms of instantaneous velocity, defined as the speed (µm/second) of movement between two sequential images during a run, depict the speeds at which each population is capable of moving, as well as the relative frequency of each speed. Each value represents the velocity of a single step between two frames, with negative values representing steps in reverse of the overall direction of movement (e.g., a retrograde-directed punctum stepping backwards in the anterograde direction). Anterograde AAV9 movement is bimodal, making frequent high-velocity steps, whereas all other populations observed in this study were unimodal and showed little-to-no movement beyond 2.0 µm/second (see also Supplementary Figure S2). (b–e) Box plots portray the quantified axonal transport of anterograde-directed AAV9 and intracellular compartments. Stars depict the significance of the comparison between the anterograde AAV9 population and each of the GFP-Rab5, GFP-Rab7, or Lysotracker populations. ***P < 0.001; **P < 0.01. Whiskers represent the 5–95th percentile, with outliers omitted due to large sample sizes. (b) Anterograde-directed AAV9 puncta move at higher average velocities, (c) make runs of longer distance, (d) progress further total distance, and (e) spend less time paused than any of the examined intracellular compartments, suggesting that AAV9 is targeted for high speed anterograde axonal transport. AAV, adeno-associated virus.
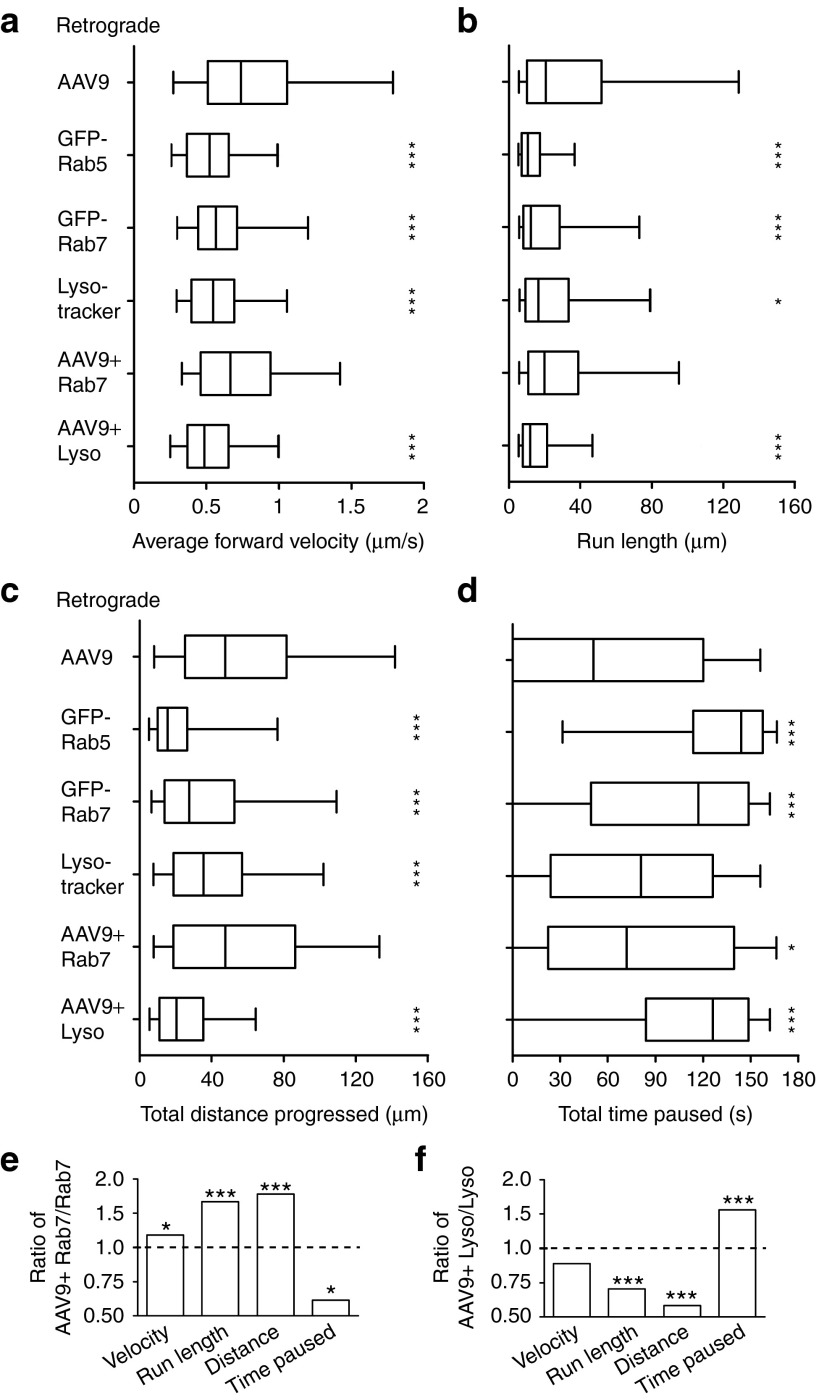
AAV9 undergoes high-speed retrograde transport in the Rab7-positive late endosome/lysosome
AAV9 was also found to move retrograde in a more directional manner than the general endosome population after entry at the axon terminus. Retrograde-directed AAV9 moved at higher velocities, made runs of a longer distance, and progressed further total distance than retrograde-directed Lysotracker-, GFP-Rab5-, or GFP-Rab7-labeled endosomes (Figure 7a–c; all P < 0.001). Retrograde-directed AAV9 also spent less time paused than GFP-Rab5- or GFP-Rab7-labeled endosomes, although no difference was detected with Lysotracker-labeled endosomes (Figure 7d; P < 0.0001). As was the case for anterograde-directed populations, the duration of individual pauses did not differ among these groups, with the exception of a small increase in pause duration for GFP-Rab5-labeled endosomes (Supplementary Table S1; P = 0.014). Thus, after entry at the axon terminus, AAV9 enters endosomal compartments that are targeted for retrograde transport to the cell body.
Figure 7.
AAV9 undergoes retrograde transport within highly motile Rab7-positive late endosomes/lysosomes. Retrograde-directed AAV9 puncta were tracked within the microfluidic groove from 1 to 5 hours after specific application to the axon side of the chamber. (a–d) Box plots portray quantified axonal transport parameters. Stars depict the statistical comparison of retrograde-directed AAV9 against each intracellular compartment, as well as against GFP-Rab7- or Lysotracker-colocalized AAV9 subpopulations. ***P < 0.001; **P < 0.01; *P < 0.05. Whiskers represent the 5–95th percentile, with outliers omitted due to large sample sizes. Retrograde-directed AAV9 puncta (a) move at higher average velocities, (b) make longer runs, and (c) progress further total distance than any of the examined endosomal compartments, and also (d) spend less time paused than GFP-Rab5- and GFP-Rab7-labeled endosomes. No significant differences were detected between GFP-Rab7-colocalized AAV9 and AAV9 alone, except for a small increase in time spent paused when colocalized with GFP-Rab7. Lysotracker-colocalized AAV9 moved more slowly, made shorter runs, progressed less total distance, and spent more time paused than the AAV9 retrograde population as a whole. (e–f) Ratio comparisons of data from a–d indicate that late endosomes/lysosomes move differently when carrying AAV9. Stars represent a statistical comparison between those labeled endosomes which colocalized with AAV9 and the labeled endosome population as a whole (i.e., the two populations compared within each bar). ***P < 0.001; *P < 0.05. (e) GFP-Rab7-labeled late endosomes/lysosomes moved faster, made longer runs, progressed further distance, and spent less time paused when colocalized with AAV9. (f) By contrast, Lysotracker-labeled late endosomes/lysosomes made shorter runs, progressed less total distance, and spent more time paused when colocalized with AAV9. AAV, adeno-associated virus.
To further characterize the retrograde transport of AAV9, we tracked all retrograde-directed GFP-Rab7- or Lysotracker-colocalized AAV9 puncta that were acquired during live imaging (Figure 4). These tracking data were analyzed using the same parameters as above (Supplementary Table S1e–f), facilitating the direct comparison of these compartmentalized subpopulations to the AAV9 retrograde population as a whole. Although both Rab7- and Lysotracker-colocalized AAV9 had a unimodal distribution of instantaneous velocities, the Lysotracker-colocalized distribution was more narrow, rarely moving faster than 1.2 µm/second (Supplementary Figure S2). Statistical comparison indicated that Lysotracker-colocalized AAV9 moved at a slower average velocity, made shorter runs, traveled less total distance, and spent more time paused than the AAV9 retrograde-directed population as a whole (all P < 0.0001). By contrast, no significant differences were observed between Rab7-colocalized AAV9 and the overall retrograde AAV9 population in terms of movement speed, run length, or distance progressed (Figure 7a–d). Thus, GFP-Rab7-colocalized AAV9 is a faster and more directional component of AAV9 retrograde transport, although Lysotracker-labeled acidic late endosomes/lysosomes appear to play an important role as well.
In addition, endosomes labeled with either GFP-Rab7 or Lysotracker were found to move differently when colocalized with AAV9. Lysotracker-labeled endosomes made shorter runs, progressed less total distance, and spent more time paused when carrying AAV9, although no difference in the average velocity of movement was detected (Figure 7f; all P < 0.0001). By contrast, when carrying AAV9, GFP-Rab7-labeled endosomes moved at higher average velocities, made longer runs, progressed further total distance, and spent less time paused than GFP-Rab7 endosomes alone (Figure 7e; P = 0.0226; P < 0.0001; P = 0.0005; P = 0.0226). This could result from the transport of AAV9 within a specific subpopulation of fast-moving Rab7-positive endosomes, or from a cargo-induced modification of the late endosome/lysosome. If the former is true, one possible subpopulation would be autophagosomes, which are Rab7 positive and have been shown to make long retrograde runs in the axon.32 To label autophagosomes, we transfected microfluidic cultures with GFP-LC3 (provided by Tamotsu Yoshimori, Osaka University, Osaka, Japan) in experiments identical to those described in Figure 4. GFP-LC3 diffusely stains the neuronal cytosol,32 allowing AAV9 in transfected axons to be counted. We counted 493 GFP-LC3-labeled autophagosomes and 128 AAV9 puncta in transfected axons following application of mCy3-AAV9 to the axon channel. Surprisingly, only one colocalized punctum was observed. This punctum did not progress more than 10 µm in either direction. Thus, it is unlikely that the increased retrograde speed of Rab7-labeled endosomes that are associated with AAV9 results from the selective targeting of AAV9 to autophagosomes.
The difference in retrograde movement between Rab7- and Lysotracker-colocalized AAV9 was striking, as both label the late endosome/lysosome compartment. Although there is substantial overlap between these two labels, GFP-Rab7 and Lysotracker are known to target different populations of endosomes.37 GFP-Rab7 labels all late endosomes and a fraction of the acidic lysosome population. By contrast, Lysotracker labels all acidic compartments, which includes all lysosomes and an acidic fraction of late endosomes. Thus, if late, endosomes/lysosomes are considered as a continuum of compartments with progressively decreasing pH, these labels overlap within a region of moderate acidity. This is an intrinsic property of both reagents, and alternative markers that specifically label the late endosome or the lysosome do not exist, likely due to the close relation of these compartments.37 Of note, distributions of average retrograde velocity and total retrograde distance for Lysotracker-colocalized AAV9 are well defined and clearly unimodal, reflecting a single population of slow acidic carriers that most frequently move at speeds between 0.4 and 0.5 µm/second and progress between 0 and 20 µm during 3 minutes of imaging (Supplementary Figure S3). By contrast, these distributions appear bimodal for Rab7-colocalized AAV9, representing multiple populations of retrograde-directed AAV9. The first population closely mirrors Lysotracker-colocalized AAV9, most frequently moving at speeds between 0.4 and 0.5 µm/second and progressing 0–20 µm (Supplementary Figure S3). However, a second population most frequently moves at speeds between 0.7 and 0.8 µm/second and progresses 60–90 µm (Supplementary Figure S3). This fast-moving, Rab7-colocalized population is not transported within an acidic compartment, as Lysotracker-colocalized AAV9 does not commonly move at these speeds or travel these distances (Supplementary Figure S3 and Table S1). Thus, AAV9 is initially transported retrograde at high speed within a nonacidic Rab7-specific compartment, as evidenced by the high-speed population of Rab7-colocalized AAV9. As this late endosome/lysosomal compartment matures and acidifies, it continues to transport AAV9 retrograde, but at much lower speeds, as evidenced by the nearly identical movement of the acidic Lysotracker-colocalized AAV9 population and the low-speed Rab7-colocalized population. The nonacidic late endosome/lysosome thus appears to be the primary mediator of fast retrograde AAV9 transport, although this compartment continues to move retrograde at slower speeds after it has acidified.
Finally, to confirm that the late endosome/lysosome is essential for the axonal transport of AAV and retrograde delivery to the nucleus, the frequency of AAV9 transport was examined after dominant negative inhibition of Rab7 function or after pharmacological inhibition of endosome acidification. Mass cultured neurons transfected with Rab7:T22N, which inhibits Rab7 function by reducing its affinity for GTP,38 demonstrated an 80.4% reduction in retrograde (P = 0.000126) and a 70.3% reduction in anterograde (P = 0.00153) AAV9 transport compared with mock-transfected cells (Supplementary Figure S1). Similarly, neurons treated with bafilomycin A1 (BFA), which inhibits acidification of the late endosome/lysosome,19 demonstrated a 78.0% reduction in retrograde (P = 0.000649) and an 85.3% reduction in anterograde (P = 0.00229) AAV9 transport compared with untreated cells (Supplementary Figure S1). All neurons exhibited robust bidirectional movement of AAV9, indicating that cell health was not affected and normal uptake of AAV was retained. The concurrent effect on anterograde transport is not surprising, as we observed minimal anterograde movement of AAV9 after application to the axon terminus despite frequent retrograde transport, suggesting that anterograde transport is downstream of retrograde transport and occurs only after sufficient levels of AAV9 have accumulated in the cell body. Thus, reducing retrograde delivery of AAV9 to the cell body is also expected to reduce anterograde transport. These data indicate that both Rab7 function and acidification of the late endosome/lysosome are required for the retrograde axonal transport of AAV9.
Discussion
This study investigated the intracellular trafficking of AAV9, describing how it moves within the endosomal system, as well as the compartments and motors that are engaged for directional transport along the axon. These findings are illustrated as a model in Figure 8. AAV9 accumulated in the exocytic vesicle and the lysosome over time, while colocalization with the early, late, and recycling endosomes remained constant. The Rab7-positive late endosome/lysosome was found to transport AAV9 retrograde at high velocity by the dynein/dynactin complex. By contrast, the more acidified Lysotracker-labeled late endosome/lysosome made shorter runs and paused more frequently when carrying AAV9, but retained the retrograde directionality characteristic of AAV9 in the late endosome/lysosome. Both dominant negative inhibition of Rab7 and blocking acidification of the late endosome/lysosome significantly reduced AAV9 axonal transport, indicating that Rab7-mediated trafficking within the late endosome/lysosome is the primary mechanism by which AAV is transported retrogradely to the perinuclear area. Following retrograde transport to the cell body within the late endosome/lysosome, AAV9 was efficiently delivered to the Golgi and nucleus. AAV9 also moved anterograde at much higher velocities than any of the intracellular compartments that were examined. This transport was mediated by the motor kinesin-2; kinesin-1 was not required. Although the compartment carrying anterograde-directed AAV9 is still unknown, we tested and excluded the most likely candidates, demonstrating that AAV9 does not move anterograde in the recycling endosome, the late endosome/lysosome, or the post-Golgi exocytic vesicle.
Figure 8.
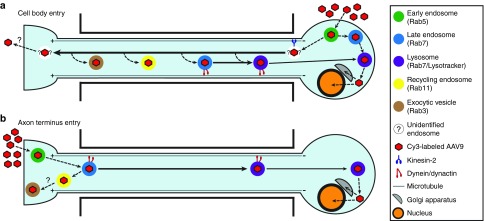
AAV9 axonal transport and endosomal trafficking after entry at the cell body or axon terminus. (a) After endocytosis at the plasma membrane of the cell body, AAV9 moves by the late endosome/lysosome to the perinuclear region, where it is released and trafficked into the Golgi and the nucleus. AAV9 is also trafficked into an unknown compartment that moves anterograde at high velocity by the motor kinesin-2. AAV9 in the axon can be transferred from this unknown compartment into recycling endosomes, exocytic vesicles, and late endosomes/lysosomes, which move retrogradely to the cell body by the motor dynein in complex with dynactin. In vivo, AAV9 is released at the synapse and endocytosed by second-order neurons. (b) After endocytosis at the plasma membrane of the axon terminus, AAV9 is trafficked into the early endosome, the late endosome, the lysosome, the recycling endosome, and the exocytic vesicle. AAV9 in the late endosome/lysosome is transported retrogradely to the cell body by the motor dynein in complex with dynactin. AAV9 in the Rab7-labeled late endosome/lysosome moves at higher velocities and spends less time paused than AAV9 in the later, more acidic Lysotracker-labeled late endosome/lysosome, indicating that movement slows as this compartment matures. Retrograde transport of AAV9 by the late endosome/lysosome requires both Rab7 function and endosomal acidification. Once the acidified late endosome/lysosome reaches the cell body, AAV9 is efficiently delivered to the Golgi and the nucleus. AAV, adeno-associated virus.
These data expand on past observations of AAV in endosomes and the Golgi of nonneuronal cells20,21 by quantifying the distribution of AAV9 within the endosomal system at different time points and by identifying the endosomes and motors engaged for directional movement in the axon. The extent and speed with which AAV9 distributed throughout the endosomal system was notable, with virus present in the exocytic vesicle, the lysosome, and the early, late, and recycling endosomes only 1 hour after application to the culture. Furthermore, following entry at the cell body and anterograde transport into the axon, AAV9 distributed almost identically, suggesting that route of entry does not affect trafficking among these compartments. These novel observations suggest that AAV disseminates rapidly and nonspecifically through the endosomal system, with a percentage accumulating in compartments that are directed for axonal transport.
Although the spread of AAV-induced gene transfer to distal regions that are axonally connected to the injection site has been frequently observed in vivo,9,10,39,40 most studies measured the presence of gene product, and not the AAV particle or genome, leading to questions regarding the underlying mechanism. In this study, we demonstrated that the AAV9 capsid is indeed targeted for axonal transport in vitro. AAV9 moves both retrograde and anterograde at higher speeds than the early endosome, late endosome, or lysosome (Figures 6 and 7), indicating that it enters endosomal compartments that are specifically directed for transport along the axon. This transport is driven by the microtubule motors dynein and kinesin-2 (Figure 2), consistent with the observation that a functional microtubule network is required for efficient AAV trafficking to the nucleus in nonneuronal cells.41 However, AAV transduction is delayed but persists in the presence of microtubule-destabilizing drugs, possibly due to a slower, microtubule-independent trafficking pathway.42
Our data indicate that movement to the nucleus within, and subsequent escape from, the late endosome/lysosome is an essential step in AAV transduction. Consistent with this hypothesis, studies have shown that AAV2 transduction is blocked by the lysomotropic drug ammonium chloride or the proton pump inhibitor BFA, both of which raise the pH of intracellular organelles.19,23 We observed a strong decrease in both retrograde and anterograde AAV9 movement following BFA treatment (Supplementary Figure S1), suggesting that acidification and maturation of the late endosome/lysosome is also required for the axonal transport of AAV9. Furthermore, transfection of dominant negative Rab7:T22N almost completely eliminated AAV9 axonal transport (Supplementary Figure S1), providing evidence that Rab7 function is essential for transport of AAV9 within the late endosome/lysosome. AAV associates strongly with Rab7-specific late endosomes/lysosomes at both the very low multiplicity of infection (MOI) of 100 GCs per cell and the moderate MOI of 10,000 GCs per cell,20 providing further evidence that this compartment is conserved as the primary mediator of retrograde transport and delivery to the perinuclear area. Our data also support past studies of axonal transport, which indicated that Rab5 regulates early sorting after endocytosis, but is not axonally transported, while Rab7 is essential for retrograde axonal transport.34,43 We did not observe directed movement of AAV9 in colocalization with GFP-Rab5 or GFP-Rab11 (Figure 4c, Supplementary Movies S3 and S4), indicating that AAV9 retrograde transport occurs specifically in the late endosome/lysosome.
Of note, both adenovirus CAV-2 and tetanus toxin (TeNT) also undergo retrograde axonal transport in Rab7-labeled carriers, although CAV-2 transport, unlike AAV9, occurs exclusively in nonacidic endosomes.34,44 Furthermore, we observed an ˜30 minute delay between AAV9 endocytosis and the onset of retrograde transport, similar to that reported for CAV-2 and TeNT,34,44 likely reflecting the time required for endosomal maturation and sorting of AAV9 into the late endosome/lysosome. The speed of AAV9 retrograde transport was similar to CAV-2, herpes simplex virus (HSV), and pseudorabies virus, which were observed to move retrograde at ˜1 µm/second.44,45,46 Unlike other viruses such as CAV-2, HSV, vaccinia virus, ectromelia virus, and baculovirus, which have been shown to associate with kinesin-1,44,47,48,49,50 AAV9 appears to rely exclusively on kinesin-2 for anterograde transport. To our knowledge, AAV9 is only the second example of a virus that primarily utilizes kinesin-2 for long-distance anterograde movement, with Kaposi's sarcoma-associated herpesvirus also requiring kinesin-2 for anterograde egress.51 HSV-1 nucleocapsids also bind kinesin-248 but do not require its function for anterograde movement, as kinesin-2 inhibition does not decrease HSV-1 egress.51
The exceptionally high velocity of anterograde-directed AAV9, which frequently made instantaneous velocity steps from 2.0 to 4.0 µm/second, was unexpected. These speeds are similar to those reported for HSV,46 an enveloped virus that interacts directly with kinesin.48 Anterograde-directed AAV9 did not associate with any expected compartments, including the post-Golgi exocytic vesicle. It is unlikely that cytoplasmic AAV9 interacts directly with kinesin-2 for movement, as anterograde-directed puncta were observed to be brighter than retrograde-directed puncta, almost certainly representing a greater number of virus particles. Thus, it appears that AAV9 is trafficked anterograde in one or more unidentified vesicles, organelles, or endosomes. The bimodal distribution of anterograde instantaneous velocities could indicate the presence of multiple anterograde carriers. However, the strong inhibition of anterograde movement by Kif3A-HL suggests that kinesin-2 is the primary anterograde motor.
We also found that endosomes that were positive for both Rab7 and AAV9 moved at higher speeds and spent less time paused than endosomes that were positive for only Rab7 (Figure 7). This could be due to trafficking of AAV9 into a specific subpopulation of fast-moving Rab7-positive endosomes. One obvious candidate is autophagosomes or autolysosomes, which are Rab7 positive and make long retrograde runs along the axon.32 However, the lack of colocalization of AAV9 with the well-characterized autophagosomal marker LC3 excludes this compartment. Alternatively, our observations suggest that the increased speed of the late endosome/lysosome when carrying AAV9 may be influenced by the viral cargo itself. This is an interesting possibility that will require further investigation. When compared with AAV9 (Supplementary Table S1 and Figure S2), both CAV-2 and TeNT within Rab7-specific endosomes exhibit similar distributions of retrograde velocities between 0.2 and 2.0 µm/second, as well as slightly greater average velocities,34,44 suggesting that this enhancement of Rab7-specific retrograde movement is not specific to AAV9 and may be a general mechanism for the retrograde transport of endocytic cargo.
Our data suggest that AAV9 undergoes fast retrograde transport as the late endosome/lysosome matures, and that AAV9, upon reaching the nucleus, escapes from the slow-moving acidified lysosome. AAV is known to undergo structural modification within acidified endosomes, extending from the capsid surface an N-terminal VP1u domain that possesses catalytic phosopholipase A2 activity and two nuclear localization signals.52 Externalization of this domain is required for transduction.22 However, AAV2 particles with heat-induced VP1u N-terminal extensions do not enter the nucleus or induce gene expression after cytoplasmic injection,22 indicating that this conformational change alone is not sufficient for transduction. Furthermore, it has been shown that AAV2 accumulates in the perinuclear cytosol before nuclear import and transduction and that this accumulation is facilitated by microtubule-based transport.42 Thus, the phospholipase A2 activity of the VP1u domain most likely promotes endosomal escape of AAV into the perinuclear cytosol following retrograde transport to the nucleus. It is possible that AAV has evolved to synchronize endosomal escape with retrograde delivery to the perinuclear area, fully extending the catalytic VP1u domain only after the late endosome has undergone retrograde transport to the nucleus and matured into an acidic lysosome. This mechanism would allow a percentage of endocytosed AAV particles to traffic directly to the perinuclear cytosol for subsequent import into the nucleus, despite our observation that AAV does not appear to be directly targeted to transported compartments, but rather distributes nonspecifically throughout the endosome system (Figure 3). In rat E18 cortical neurons, ˜25% of AAV9 that is endocytosed at the cell body plasma membrane reaches the nucleus within 4 hours (Figure 5), although this may vary with the MOI and cell type. Furthermore, although our data suggest that at a given time ˜20% of AAV is transported retrograde by the late endosome/lysosome, it is unlikely that the remaining 80% of untransported AAV will be released into the cytoplasm due to the pH specificity of VP1u extension. Thus, AAV that is not initially transported retrograde may linger in the endosome system until it reaches a late endosome/lysosome, resulting in the efficient but gradual delivery to the perinuclear area that is observed over a prolonged time period in nonneuronal cells.53
Together, our observations regarding the axonal transport of AAV in neurons demonstrate that AAV9 disseminates widely throughout the endosome system but is capable of undergoing fast retrograde transport by trafficking within late endosomes/lysosomes. This compartment initially carries AAV9 retrograde at high velocity but slows as it matures and acidifies. The late endosome/lysosomal compartment efficiently delivers AAV9 to the Golgi and nucleus after retrograde transport to the cell body; this transport is driven by dynein and requires both Rab7 function and acidification of the late endosome/lysosome. When moving anterograde, AAV9 utilizes kinesin-2, only the second known virus to do so, and travels at exceptionally high velocity. Furthermore, increasing AAV entry greatly enhanced both retrograde and anterograde axonal transport in vitro, suggesting a potential means to enhance the spread of AAV9 gene transfer in vivo. This improved understanding of AAV axonal transport will advance progress toward utilizing this transport as a tool for better distribution of transgene delivery to the brain.
Materials and Methods
Fabrication and use of microfluidic chambers. Master molds were designed in AutoCAD software, based on the model of Park et al.,25 with the following dimensions: channels of 80 µm height, 1.5 mm width, and 8.25 mm length and grooves of 5 µm height, 15 µm width, and 400 µm length. Two-layer photoresist molds were manufactured by the Stanford Microfluidic Foundry (Stanford, CA). Polydimethylsiloxane (Sylgard 184, Dow Corning, Midland, MI) microfluidic chambers were fabricated from these molds as described.25 For primary neuron culture, microfluidic chambers were reversibly bonded to a 50 mm glass fluorodish (World Precision Instruments, Sarasota, FL) coated overnight at 4 °C with 1 mg/ml Poly-L-Lysine MW 70K–150K (Sigma-Aldrich, St. Louis, MO). Chambers were filled with Neurobasal media (Invitrogen, Grand Island, NY) containing 1× B27 supplement (Invitrogen), 2 mmol/l Glutamax (Invitrogen), and 25 µmol/l 2-mercaptoethanol (Sigma-Aldrich), henceforth NB, and incubated at 37 °C for a minimum of 24 hours before plating cells. Primary E18 rat cortical neurons were obtained as described,11,54 and these were provided courtesy of Marc Dichter (University of Pennsylvania, Philadelphia, PA) as tissue byproducts of hippocampal dissection. All NB was removed from the chamber, and 300,000 cells were added to a single well at a concentration of 6,000 cells per µl. After incubation for 2 hours at 37 °C, 150 µl NB was added back to each well.
Half of the NB in each well of the chamber was replaced with fresh NB every 24 hours. All experiments were performed 5–6 days after plating cells. Fluidic isolation was established by removing all media and replacing with 120 µl NB in both wells connected to the channel to be isolated and 80 µl NB in both wells connected to the channel receiving AAV. For live imaging experiments, Hibernate E Low Fluoresence media (Brainbits, Springfield, IL) containing 1× B27 supplement and 2 mmol/l Glutamax, henceforth HibE, was used instead of NB. Fluidic isolation under these conditions was confirmed out to 8 hours by live imaging of Alexa Fluor 488-labeled Dextran (Invitrogen), as well as by quantitative polymerase chain reaction, with no AAV genomes detected on the fluidically isolated side 8 hours after application of mCy3-AAV9 to the axotomized opposite channel.
Preparation and validation of dye-labeled AAV9. Psuedotyped AAV2/9 vectors were produced by the Penn Vector Core as described.55 AAV2/9.CMV.eGFP was labeled with the red dyes mono-functional Cy3 (mCy3, GE Healthcare Biosciences, Pittsburgh, PA) and bis-functional Cy3 (bCy3, GE Healthcare Biosciences), and AAV2/9.CMV.turboRFP was labeled with the green dye DyLight 488 (DL488, Thermo Fisher, Rockford, IL). Before labeling, 40 µg AAV9 was concentrated to 1 µg/µl using a Microcon YM-100 centrifugal filter unit (Millipore, Billerica, MA). The AAV solution was then brought to 0.1 mol/l sodium carbonate, pH 9.3 (for Cy3 labeling) or 0.5 mol/l sodium borate, pH 8.5 (for DL488 labeling). This solution was added to a single vial of dye containing 150 µg Cy3 or 50 µg DL488, then incubated 1.5 hours at room temperature (RT) under gentle agitation. To remove unbound dye, the AAV solution was dialyzed against 1 l phosphate-buffered saline (PBS) for 2 hours, 2 hours, and overnight at 4 °C using a 20K MWCO Slide-a-Lyzer MINI unit (Thermo Fisher, Rockford, IL), then washed four times with 400 µl PBS in a Microcon YM-100 centrifugal filter unit. No free dye was detected in the flow-through of the final wash. Stocks were stored in PBS with 10% glycerol at −80 °C. DNAse-resistant particles were titered by quantitative polymerase chain reaction as described.55 To confirm purity of preps, a Coomassie stain was performed, followed by a Western blot using anti-AAV capsid antibody B1 (1:2500, Meridian Life Science, Memphis, TN), as described.55 The expected VP1/2/3 bands were observed for all preps, and all bands colocalized with B1 antibody, indicating that no contaminating proteins were present.
Finally, SYBR quantitative polymerase chain reaction (Invitrogen; manufacturer's protocols) confirmed that axonally transported Cy3-AAV consists of genome-containing virus particles, and not dye-labeled empty capsids or capsid fragments. After specific application of 1 × 1010 GCs mCy3-AAV9 to the axon side of the chamber, the isolated cell bodies were obtained and lysed and were found to contain an average of 217,500 AAV9 GCs per chamber. No AAV DNA was detected in cell bodies from negative control chambers axotomized before AAV9 application.
Immunocytochemistry. For ICC experiments, chambers were prepared as above and 3 × 109 GCs bCy3- or mCy3-AAV9 were applied to the axon side of the chamber or 1 × 1010 GCs to the cell body side, in a volume of 5 μl. This equates to an estimated maximum titer of 9 × 107 GCs applied to the axon termini or 3 × 108 GCs to the cell bodies (see Microfluidic Live Imaging and Analysis). 1 or 4 hours after axon application, or 4 hours after cell body application, chambers were incubated for 30 minutes at RT in 4% paraformaldehyde in PBS, with 150 µl in each cell well and 100 µl in each axon well. Chambers were then gently detached from the dish, and standard ICC was performed. Briefly, dishes were permeabilized for 5 minutes at RT in 0.3% Triton X-100 in PBS and blocked for 1 hour at RT in 10% goat serum in PBS. Each was then incubated overnight at 4 °C in a solution of 10% goat serum in PBS containing one of the following primary antibodies: anti-Rab3 (1:1000, Synaptic Systems, Goettingen, Germany), anti-Rab5 (1:250, Abcam, Cambridge, MA), anti-Rab7 (1:1000, Abcam), anti-Rab11 (1:200, Abcam), or anti-Giantin (1:250, Abcam). Finally, dishes were incubated 2 hours at RT in a solution of 10% goat serum in PBS containing an Alexa Fluor 488 secondary antibody (1:500, Invitrogen), then coverslipped in Vectashield (Vector Labs, Burlingame, CA). Dishes were imaged using the microscope described below, with all grooves that contained intact axons photographed. For Giantin stains, cell bodies proximal to the grooves were photographed by z-stack (40 images over 10 µm). Axon ICC images (Figure 3) were analyzed in Metamorph by drawing a line along the axon bisecting each AAV9 punctum. The intensity of both red and green channels were plotted at each point along this line, with each peak in the red channel with intensity greater than one standard deviation above the mean counted as an AAV punctum. If this AAV peak was accompanied by an identical peak in the green channel, it was scored as colocalized; if not, it was scored as uncolocalized. Cell body ICC z-stacks (Figure 5) were analyzed by counting AAV9 puncta across the stack and scoring each as colocalized with the Golgi, the nucleus, or neither.
For axon ICC, data were collected as a large number of binomial observations (colocalized or uncolocalized), and logistic regressions performed using R software (The R Foundation, Vienna, Austria). One logistic regression was performed for each antibody (four total), with each regression comparing among all three conditions, using the null hypothesis that all conditions were identical. For each pair of conditions, a Wald test was performed to determine a χ2 value and to test for statistical significance. In addition, colocalized and uncolocalized AAV9 particles were counted during live imaging with Lysotracker (see below), and these data were analyzed identically. Two logistic regressions (followed by Wald tests) were conducted, one each for axon and cell body application of AAV9, comparing among all three 1 hour blocks. For the above analyses, standard deviation was calculated using the binomial formula sqrt[P × (1−P) / N]. For cell body ICC, three one-way ANOVAs were performed, comparing the percentage of puncta colocalized with the Golgi, the nucleus, or neither among all four conditions, with the null hypothesis that colocalization would be identical among conditions. Multiple comparisons were performed by Tukey's post hoc test.
Labeling of intracellular compartments for live imaging. To label acidic endosomes, 120 µl HibE containing 1 µmol/l Lysotracker DND-26 (Invitrogen) was added to each well on the axon side of the chamber and 80 µl to each well on the cell side. Chambers were incubated at 37 °C for 5 minutes, washed three times with HibE, and prepared for imaging as described below. To label endosomes with GFP-Rab transfection contructs, primary neurons were transfected before plating with an Amaxa electroporator and the Rat Neuron Nucleofector kit (Lonza, Basel, Switzerland), using 2 µg GFP-Rab5 (provided by Marino Zerial, Max Planck Institute of Molecular Cell Biology and Genetics, Dresden, Germany), GFP-Rab7 (Addgene, Cambridge, MD), or GFP-Rab11 (Addgene) plasmids and the manufacturer's protocols.
Microfluidic live imaging and analysis. For microfluidic live imaging, 1–3 × 109 GCs bCy3-, mCy3-, or DL488-AAV9 were specifically applied to the axon side of the chamber or 1 × 1010 GCs specifically to the cell body side, in a volume of 5 µl. Equilibration of this 5 µl media difference between the two isolated wells induced flow of 3% of the infected well's media and AAV through the isolated channel, resulting in a maximum titer of 3–9 × 107 GCs applied to the axon termini in the channel, or 3 × 108 GCs to the cell bodies. Although the density of cells and axon termini in the channel varied among chambers, we estimate the maximum MOI to be ˜5,000 GCs per cell for both cell bodies and axon termini, based on an estimated cell density of 60,000 and an estimated axon density of 6,000–18,000 within the channels. Imaging was performed using a Leica (Leica, Wetzlar, Germany) DMI6000B inverted microscope with high-speed filter wheel, 63× plan apo oil-immersion objective, climate controlled chamber, CTR 7000 HS control box, and Hamamatsu Photonics (Hamamatsu, Japan) C10600 Orca-R2 camera. Chambers were imaged from 1 to 5 hours following application of virus. When imaging AAV9 alone, all image series were acquired for 3 minutes, 3 seconds/frame. When imaging AAV9 colocalization with Lysotracker or a GFP-Rab construct, all series were acquired for 3 minutes, 4.5 seconds/frame. These parameters were chosen based on real-time monitoring of AAV9, which was observed to make long, continuous runs that were infrequently interrupted by prolonged pauses. Thus, an interval of seconds between frames provides an accurate measure of AAV9 movement while allowing for long-term imaging without bleaching. Only AAV9 puncta within the groove were imaged.
Each series was analyzed using Metamorph Software (Leica, Wetzlar, Germany). First, all AAV9 puncta in the frame were counted and classified as bidirectional/stationary (not progressing more than 10 µm in either direction), or as retrograde- or anterograde-directed (progressing more than 10 µm toward the cell body or toward the axon terminus, respectively). AAV9 puncta in GFP-Rab or Lysotracker chambers were also classified as either colocalized or uncolocalized. Next, all directed AAV9 puncta were tracked using the Track Objects module within Metamorph. All directed Lysotracker, GFP-Rab5, or GFP-Rab7 puncta were also tracked. Tracking data were analyzed in Excel software (Microsoft, Redmond, WA). Any sequence of frames during which the punctum moved below 0.2 µm/second in each frame was classified as a pause (with a minimum pause duration of 9 seconds). Any sequence of frames in which the punctum moved further than 10 µm was classified as a run (if it progressed in the overall direction of movement) or as a reversal (if it progressed in reverse of the overall direction of movement, e.g., a retrograde-directed punctum stepping backwards in the anterograde direction). Any movement less than 10 µm between two pauses, or between a pause and the beginning or end of the series, was not counted as a run and thus was incorporated into the adjacent pause(s). Puncta that entered or exited the frame during the series and progressed less than 20 µm were not counted or tracked. Using these criteria, the length of each run, the duration of each pause, and the average velocity of each punctum when making runs in the forward direction was quantified, as well as the total distance traveled and the total time spent paused for each punctum.
Statistical analyses were performed using Prism software (Graphpad, San Diego, CA). Sample sizes are found in Supplementary Table S1. First, to determine whether differences in the five transport parameters described above existed among the uncolocalized retrograde-directed populations (AAV9, Lysotracker, Rab5, and Rab7), a Kruskal–Wallis test (Dunn's posttests) was performed for each of the five parameters, with a null hypothesis that all four conditions were identical. As each of these parameters has a nonparametric distribution, the Kruskal–Wallis test was used. Second, to determine whether differences existed among the uncolocalized anterograde-directed populations, an identical set of five Kruskal–Wallis tests was performed. However, as the distribution of some parameters was bimodal for anterograde-directed populations, all datasets underwent log10 transformation to obtain unimodal distributions appropriate for analysis with the Kruskal–Wallis test. Third, to determine whether a difference existed between AAV9 colocalized with Lysotracker and the AAV9 population as a whole for each of the five movement parameters, a set of five unpaired two-tailed Mann–Whitney tests was performed, each with a null hypothesis that the two groups were identical. Again, the Mann–Whitney test was selected due to the nonparametric distribution of the data. Fourth, to determine whether a difference existed between AAV9 colocalized with GFP-Rab7 and the AAV9 population as a whole, an identical set of five unpaired two-tailed Mann–Whitney tests was performed. Finally, to determine whether uptake of AAV9 cargo altered the movement of GFP-Rab7- or Lysotracker-labeled endosomes, two more sets of five Mann–Whitney tests were conducted. Each set of tests compared the movement of AAV9-colocalized GFP-Rab7 or Lysotracker to uncolocalized GFP-Rab7 or Lysotracker, respectively, for each of the five movement parameters, with null hypotheses that AAV9 cargo would not alter endosomal movement.
Dominant negative motor protein transfection and drug treatment of mass cultures. Rat E18 cortical neurons were mock-transfected by Amaxa electroporation or transfected with 3 µg GFP-CC1, GFP-KIF5C Tail (provided by Yoshiyuki Konishi, Hamamatsu University, Hamamatsu, Japan), GFP-KIF3A-HL (provided by Kozo Kaibuchi, Nagoya University, Nagoya, Japan), or GFP-Rab7:T22N (Addgene, Cambridge, MD). A total of 100,000 transfected neurons were plated on 35 mm fluorodishes (World Precision Instruments, Sarasota, FL) coated overnight at 4 °C with 0.1 mg/ml Poly-L-Lysine MW 70K–150K. Efficient overexpression of dominant negative constructs was confirmed by examining the disruption of a known cargo. Briefly, 3 µg GFP-CC1 and 2 µg GPP130-mCherry (provided by Adam Linstedt, Carnegie Mellon University, Pittsburgh, PA) were cotransfected, and disruption of the Golgi was confirmed by live imaging comparison against GPP130-mCherry alone.26 In addition, either 3 µg GFP-KIF5C Tail or 3 µg GFP-KIF3A-HL was cotransfected with 2 µg LAMP1-RFP (Addgene, Cambridge, MD). Both dominant negative plasmids induced a more centralized localization of LAMP1-labeled lysosomes than LAMP1-RFP alone, with a greatly reduced number of lysosomes observed within the axons of dominant negative-transfected cells.
Three to 4 days after plating cells, 1 × 109 GCs mCy3- or bCy3-AAV9 were applied to the transfected culture, and live imaging was performed. All series were acquired for 10 minutes, 3 seconds/frame. In Metamorph software, the number of puncta that progressed more than 50 µm in either direction along the axon was counted and then divided by the total length of axon imaged. In addition, mock-transfected neurons were incubated for 2 hours at 37 °C in 50 mU/ml NA (Sigma-Aldrich) or for 1 hour at 37 °C in 2.5 µg/ml BFA (Sigma-Aldrich), washed three times with HibE, and then imaged and analyzed as above. N = 4 cultures were analyzed for each condition. A total of n = 79 retrograde and n = 55 anterograde puncta were counted for mock transfection, n = 32 and 51 for CC1, n = 77 and 72 for Kif5C Tail, n = 58 and 13 for Kif3A-HL, n= 17 and 16 for Rab7:T22N, n = 12 and 6 for BFA, and n = 139 and 142 for NA. Statistical analysis was performed using Prism software. Twelve unpaired two-tailed Student's t-tests were conducted to compare both retrograde and anterograde counts from each treatment (CC1, KIF5C Tail, KIF3A-HL, Rab7:T22N, BFA, or NA) against the mock-transfected baseline, each with the null hypothesis that the two conditions would be identical.
SUPPLEMENTARY MATERIAL Figure S1. Rab7 function and endosomal acidification are required for the axonal transport of AAV9. Figure S2. Instantaneous velocity histograms of retrograde-directed populations. Figure S3. Rab7- and Lysotracker-colocalized AAV9 populations exhibit distinct distributions of retrograde velocity and distance progressed. Movie S1. Retrograde axonal transport of AAV9. Movie S2. Anterograde axonal transport of AAV9. Movie S3. Representative movement of AAV9 within a GFP-Rab5-labeled endosome. Movie S4. Representative movement of AAV9 within a GFP-Rab11-labeled endosome. Movie S5. Representative movement of AAV9 within a Lysotracker-labeled endosome. Movie S6. Representative movement of AAV9 within a GFP-Rab7-labeled endosome. Table S1. Quantified movement properties of AAV9 and endosomal compartments
Acknowledgments
We thank Margaret Maronski and Marc Dichter (University of Pennsylvania) for providing dissociated rat cortical neurons, Rui Xiao (University of Pennsylvania) for assistance with statistical analysis, Luk Vandenberghe (Harvard University) for AAV-related technical advice and discussion, and Jessica Melin (Stanford Microfluidics Foundry) for microfluidic mold fabrication and design assistance. All research was conducted in Philadelphia, PA, USA. This work was supported by NINDS grants T32NS007413, R01NS038690, and R01NS060698.
Supplementary Material
References
- Atchison RW, Casto BC, Hammon WM. Adenovirus-associated defective virus particles. Science. 1965;149:754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- Hoggan MD, Blacklow NR, Rowe WP. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc Natl Acad Sci USA. 1966;55:1467–1474. doi: 10.1073/pnas.55.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samulski RJ, Chang LS, Shenk T. A recombinant plasmid from which an infectious adeno-associated virus genome can be excised in vitro and its use to study viral replication. J Virol. 1987;61:3096–3101. doi: 10.1128/jvi.61.10.3096-3101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieger JC, Choi VW, Samulski RJ. Production and characterization of adeno-associated viral vectors. Nat Protoc. 2006;1:1412–1428. doi: 10.1038/nprot.2006.207. [DOI] [PubMed] [Google Scholar]
- Rivera VM, Gao GP, Grant RL, Schnell MA, Zoltick PW, Rozamus LW, et al. Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood. 2005;105:1424–1430. doi: 10.1182/blood-2004-06-2501. [DOI] [PubMed] [Google Scholar]
- Xiao X, Li J, McCown TJ, Samulski RJ. Gene transfer by adeno-associated virus vectors into the central nervous system. Exp Neurol. 1997;144:113–124. doi: 10.1006/exnr.1996.6396. [DOI] [PubMed] [Google Scholar]
- Cearley CN, Vandenberghe LH, Parente MK, Carnish ER, Wilson JM, Wolfe JH. Expanded repertoire of AAV vector serotypes mediate unique patterns of transduction in mouse brain. Mol Ther. 2008;16:1710–1718. doi: 10.1038/mt.2008.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH, Wilson JM. New recombinant serotypes of AAV vectors. Curr Gene Ther. 2005;5:285–297. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- Cearley CN, Wolfe JH. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol Ther. 2006;13:528–537. doi: 10.1016/j.ymthe.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, et al. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10:302–317. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Royo NC, Vandenberghe LH, Ma JY, Hauspurg A, Yu L, Maronski M, et al. Specific AAV serotypes stably transduce primary hippocampal and cortical cultures with high efficiency and low toxicity. Brain Res. 2008;1190:15–22. doi: 10.1016/j.brainres.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vite CH, McGowan JC, Niogi SN, Passini MA, Drobatz KJ, Haskins ME, et al. Effective gene therapy for an inherited CNS disease in a large animal model. Ann Neurol. 2005;57:355–364. doi: 10.1002/ana.20392. [DOI] [PubMed] [Google Scholar]
- Worgall S, Sondhi D, Hackett NR, Kosofsky B, Kekatpure MV, Neyzi N, et al. Treatment of late infantile neuronal ceroid lipofuscinosis by CNS administration of a serotype 2 adeno-associated virus expressing CLN2 cDNA. Hum Gene Ther. 2008;19:463–474. doi: 10.1089/hum.2008.022. [DOI] [PubMed] [Google Scholar]
- Baek RC, Broekman ML, Leroy SG, Tierney LA, Sandberg MA, d'Azzo A, et al. AAV-mediated gene delivery in adult GM1-gangliosidosis mice corrects lysosomal storage in CNS and improves survival. PLoS ONE. 2010;5:e13468. doi: 10.1371/journal.pone.0013468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passini MA, Lee EB, Heuer GG, Wolfe JH. Distribution of a lysosomal enzyme in the adult brain by axonal transport and by cells of the rostral migratory stream. J Neurosci. 2002;22:6437–6446. doi: 10.1523/JNEUROSCI.22-15-06437.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig AK, Levy B, Ogilvie JM, Vogler CA, Galvin N, Bassnett S, et al. Intravitreal gene therapy reduces lysosomal storage in specific areas of the CNS in mucopolysaccharidosis VII mice. J Neurosci. 2003;23:3302–3307. doi: 10.1523/JNEUROSCI.23-08-03302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cearley CN, Wolfe JH. A single injection of an adeno-associated virus vector into nuclei with divergent connections results in widespread vector distribution in the brain and global correction of a neurogenetic disease. J Neurosci. 2007;27:9928–9940. doi: 10.1523/JNEUROSCI.2185-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonnenmacher M, Weber T. Adeno-associated virus 2 infection requires endocytosis through the CLIC/GEEC pathway. Cell Host Microbe. 2011;10:563–576. doi: 10.1016/j.chom.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett JS, Wilcher R, Samulski RJ. Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors. J Virol. 2000;74:2777–2785. doi: 10.1128/jvi.74.6.2777-2785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W, Zhang LN, Yeaman C, Engelhardt JF. rAAV2 traffics through both the late and the recycling endosomes in a dose-dependent fashion. Mol Ther. 2006;13:671–682. doi: 10.1016/j.ymthe.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantel-Schaal U, Hub B, Kartenbeck J. Endocytosis of adeno-associated virus type 5 leads to accumulation of virus particles in the Golgi compartment. J Virol. 2002;76:2340–2349. doi: 10.1128/jvi.76.5.2340-2349.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag F, Bleker S, Leuchs B, Fischer R, Kleinschmidt JA. Adeno-associated virus type 2 capsids with externalized VP1/VP2 trafficking domains are generated prior to passage through the cytoplasm and are maintained until uncoating occurs in the nucleus. J Virol. 2006;80:11040–11054. doi: 10.1128/JVI.01056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Qing K, Srivastava A. Adeno-associated virus type 2-mediated gene transfer: altered endocytic processing enhances transduction efficiency in murine fibroblasts. J Virol. 2001;75:4080–4090. doi: 10.1128/JVI.75.9.4080-4090.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist LW. Five questions about viral trafficking in neurons. PLoS Pathog. 2012;8:e1002472. doi: 10.1371/journal.ppat.1002472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Vahidi B, Taylor AM, Rhee SW, Jeon NL. Microfluidic culture platform for neuroscience research. Nat Protoc. 2006;1:2128–2136. doi: 10.1038/nprot.2006.316. [DOI] [PubMed] [Google Scholar]
- King SJ, Brown CL, Maier KC, Quintyne NJ, Schroer TA. Analysis of the dynein-dynactin interaction in vitro and in vivo. Mol Biol Cell. 2003;14:5089–5097. doi: 10.1091/mbc.E03-01-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwinter DM, Lo K, Mafi P, Silverman MA. Dynactin regulates bidirectional transport of dense-core vesicles in the axon and dendrites of cultured hippocampal neurons. Neuroscience. 2009;162:1001–1010. doi: 10.1016/j.neuroscience.2009.05.038. [DOI] [PubMed] [Google Scholar]
- Konishi Y, Setou M. Tubulin tyrosination navigates the kinesin-1 motor domain to axons. Nat Neurosci. 2009;12:559–567. doi: 10.1038/nn.2314. [DOI] [PubMed] [Google Scholar]
- Wilson MH, Holzbaur EL. Opposing microtubule motors drive robust nuclear dynamics in developing muscle cells. J Cell Sci. 2012;125 Pt 17:4158–4169. doi: 10.1242/jcs.108688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Kato K, Yamaguchi T, Fukata Y, Ohno S, Kaibuchi K. Role of the PAR-3-KIF3 complex in the establishment of neuronal polarity. Nat Cell Biol. 2004;6:328–334. doi: 10.1038/ncb1118. [DOI] [PubMed] [Google Scholar]
- Bell CL, Vandenberghe LH, Bell P, Limberis MP, Gao GP, Van Vliet K, et al. The AAV9 receptor and its modification to improve in vivo lung gene transfer in mice. J Clin Invest. 2011;121:2427–2435. doi: 10.1172/JCI57367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maday S, Wallace KE, Holzbaur EL. Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. J Cell Biol. 2012;196:407–417. doi: 10.1083/jcb.201106120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng EL, Tang BL. Rab GTPases and their roles in brain neurons and glia. Brain Res Rev. 2008;58:236–246. doi: 10.1016/j.brainresrev.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Deinhardt K, Salinas S, Verastegui C, Watson R, Worth D, Hanrahan S, et al. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron. 2006;52:293–305. doi: 10.1016/j.neuron.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Zhang K, Fishel Ben Kenan R, Osakada Y, Xu W, Sinit RS, Chen L, et al. Defective axonal transport of Rab7 GTPase results in dysregulated trophic signaling. J Neurosci. 2013;33:7451–7462. doi: 10.1523/JNEUROSCI.4322-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal JK, Rivera VM, Simon SM. Exocytosis of post-Golgi vesicles is regulated by components of the endocytic machinery. Cell. 2009;137:1308–1319. doi: 10.1016/j.cell.2009.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B. Rab7: a key to lysosome biogenesis. Mol Biol Cell. 2000;11:467–480. doi: 10.1091/mbc.11.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitelli R, Santillo M, Lattero D, Chiariello M, Bifulco M, Bruni CB, et al. Role of the small GTPase Rab7 in the late endocytic pathway. J Biol Chem. 1997;272:4391–4397. doi: 10.1074/jbc.272.7.4391. [DOI] [PubMed] [Google Scholar]
- Reimsnider S, Manfredsson FP, Muzyczka N, Mandel RJ. Time course of transgene expression after intrastriatal pseudotyped rAAV2/1, rAAV2/2, rAAV2/5, and rAAV2/8 transduction in the rat. Mol Ther. 2007;15:1504–1511. doi: 10.1038/sj.mt.6300227. [DOI] [PubMed] [Google Scholar]
- Sondhi D, Hackett NR, Peterson DA, Stratton J, Baad M, Travis KM, et al. Enhanced survival of the LINCL mouse following CLN2 gene transfer using the rh.10 rhesus macaque-derived adeno-associated virus vector. Mol Ther. 2007;15:481–491. doi: 10.1038/sj.mt.6300049. [DOI] [PubMed] [Google Scholar]
- Sanlioglu S, Benson PK, Yang J, Atkinson EM, Reynolds T, Engelhardt JF. Endocytosis and nuclear trafficking of adeno-associated virus type 2 are controlled by rac1 and phosphatidylinositol-3 kinase activation. J Virol. 2000;74:9184–9196. doi: 10.1128/jvi.74.19.9184-9196.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao PJ, Samulski RJ. Cytoplasmic trafficking, endosomal escape, and perinuclear accumulation of adeno-associated virus type 2 particles are facilitated by microtubule network. J Virol. 2012;86:10462–10473. doi: 10.1128/JVI.00935-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XQ, Wang B, Wu C, Pan J, Yuan B, Su YY, et al. Endosome-mediated retrograde axonal transport of P2X3 receptor signals in primary sensory neurons. Cell Res. 2012;22:677–696. doi: 10.1038/cr.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas S, Bilsland LG, Henaff D, Weston AE, Keriel A, Schiavo G, et al. CAR-associated vesicular transport of an adenovirus in motor neuron axons. PLoS Pathog. 2009;5:e1000442. doi: 10.1371/journal.ppat.1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curanovic D, Lyman MG, Bou-Abboud C, Card JP, Enquist LW. Repair of the UL21 locus in pseudorabies virus Bartha enhances the kinetics of retrograde, transneuronal infection in vitro and in vivo. J Virol. 2009;83:1173–1183. doi: 10.1128/JVI.02102-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GA, Pomeranz L, Gross SP, Enquist LW. Local modulation of plus-end transport targets herpesvirus entry and egress in sensory axons. Proc Natl Acad Sci USA. 2004;101:16034–16039. doi: 10.1073/pnas.0404686101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepis A, Stauber T, Krijnse Locker J. Kinesin-1 plays multiple roles during the vaccinia virus life cycle. Cell Microbiol. 2007;9:1960–1973. doi: 10.1111/j.1462-5822.2007.00927.x. [DOI] [PubMed] [Google Scholar]
- Radtke K, Kieneke D, Wolfstein A, Michael K, Steffen W, Scholz T, et al. Plus- and minus-end directed microtubule motors bind simultaneously to herpes simplex virus capsids using different inner tegument structures. PLoS Pathog. 2010;6:e1000991. doi: 10.1371/journal.ppat.1000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn H, Horsington J, Ter LK, Han S, Chew YL, Diefenbach RJ, et al. Loss of cytoskeletal transport during egress critically attenuates ectromelia virus infection in vivo. J Virol. 2012;86:7427–7443. doi: 10.1128/JVI.06636-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danquah JO, Botchway S, Jeshtadi A, King LA. Direct interaction of baculovirus capsid proteins VP39 and EXON0 with kinesin-1 in insect cells determined by fluorescence resonance energy transfer-fluorescence lifetime imaging microscopy. J Virol. 2012;86:844–853. doi: 10.1128/JVI.06109-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathish N, Zhu FX, Yuan Y. Kaposi's sarcoma-associated herpesvirus ORF45 interacts with kinesin-2 transporting viral capsid-tegument complexes along microtubules. PLoS Pathog. 2009;5:e1000332. doi: 10.1371/journal.ppat.1000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatakrishnan B, Yarbrough J, Domsic J, Bennett A, Bothner B, Kozyreva OG, et al. Structure and dynamics of adeno-associated virus serotype 1 VP1-unique N-terminal domain and its role in capsid trafficking. J Virol. 2013;87:4974–4984. doi: 10.1128/JVI.02524-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao PJ, Li C, Neumann A, Samulski RJ. Quantitative 3D tracing of gene-delivery viral vectors in human cells and animal tissues. Mol Ther. 2012;20:317–328. doi: 10.1038/mt.2011.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox KS, Dichter MA. Paired pulse depression in cultured hippocampal neurons is due to a presynaptic mechanism independent of GABAB autoreceptor activation. J Neurosci. 1994;14 3 Pt 2:1775–1788. doi: 10.1523/JNEUROSCI.14-03-01775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Qu G, Burnham MS, Huang J, Chirmule N, Joshi B, et al. Purification of recombinant adeno-associated virus vectors by column chromatography and its performance in vivo. Hum Gene Ther. 2000;11:2079–2091. doi: 10.1089/104303400750001390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.