Abstract
Gonadal steroids are important mediators of traits relevant to fitness, and thus may be targets of selection. However, more knowledge is needed about sources of variation along the endocrine axes that may contribute to functional variation in steroid levels. In a controlled captive environment, we studied males of two closely related subspecies of the dark-eyed junco (Junco hyemalis) that differ in testosterone-related phenotype, asking whether they also differ in testosterone (T), and assessing the contribution of the sequential links of the hypothalamo-pituitary-gonadal axis. When males of both subspecies were challenged with gonadotropin-releasing hormone (GnRH), they were similar in circulating luteinizing hormone (LH) and T responses. When challenged with exogenous LH, they again produced levels of T similar to one another, and to the levels produced in response to GnRH. However, the smaller, less ornamented, and less aggressive subspecies had greater abundance of mRNA for LH receptor in the testes and for androgen receptor in the rostral hypothalamus, suggesting potential differences in regulatory feedback. We suggest that circulating hormone levels may be less prone to evolutionary change than the responsiveness of individual hormone targets. Among individuals, T titers were highly repeatable whether males were challenged with GnRH or with LH, but LH produced in response to GnRH did not covary with T produced in response to LH. Testis mass, but not LH receptor transcript abundance, predicted individual variation in T responses. These data implicate the gonad, but not the pituitary, as an important source of individual variation in T production.
Keywords: Testosterone, luteinizing hormone, HPG axis, gonad, aromatase, individual variation, divergence, preoptic area, integration, independence
INTRODUCTION
The gonadal steroid testosterone (T) has profound effects on multiple morphological, physiological and behavioral traits throughout the brain and periphery, promoting traits such as sperm production, aggression, sexual behaviors and ornaments, sometimes at the expense of immunity and parental care (Folstad and Karter, 1992; Ketterson et al., 1992; Wingfield et al., 2001). Accordingly, gonad size and circulating concentrations of T often fluctuate seasonally along with these phenotypes. In temperate-breeding songbirds, circulating T is typically higher during territory establishment and mating, and lower during nesting and non-breeding (Goymann et al., 2007; Moore et al., 2002; Wingfield et al., 1990). T levels are also dynamic on finer time scales throughout the breeding season, associated with enhanced aggression or sexual behavior during male-male and male-female social interactions (Ball and Balthazart, 2004; Goymann et al., 2007; Wingfield et al., 1990). Individuals and species vary in T and related phenotype, and it has been demonstrated that individual variation in T has important consequences for fitness (e.g., McGlothlin et al., 2010; Veiga and Polo, 2008). Despite the potential role for hormonal systems in phenotypic evolution, relatively little research has addressed the mechanistic sources of variation in T titers and T signaling pathways upon which selection may act (Ball and Balthazart, 2008).
The neuroendocrine system responsible for T regulation is complex, and it is important to consider the multiple potential sources of variation in this system in order to understand the mechanistic underpinnings of T-mediated trait evolution (Hau and Wingfield, 2011). Most of the phenotypic changes associated with reproduction in birds are consequences of the regulation of the hypothalamo-pituitary-gonadal (HPG) axis. Environmental cues are relayed to the hypothalamus, eliciting an increase in the release of gonadotropin-releasing hormone (GnRH). GnRH triggers secretion of gonadotropins such as luteinizing hormone (LH) from the anterior pituitary, which in turn acts at the gonad via the G-protein coupled luteinizing hormone receptor (LHR), stimulating the synthesis and release of steroids including testosterone (T) from the gonads. Like LH, T must be transduced by receptors to have its effects (Hadley and Levine, 2007), and this process may be influenced by many variables including carrier proteins, conversion enzymes and co-factors, as well as receptor expression and affinity. T typically acts via intracellular receptors located at many targets throughout the brain and periphery, either by activating androgen receptors (ARs, binding directly or after conversion to 5α-dihydrotestosterone), or by being converted by the enzyme aromatase to 17β-estradiol (E2), which binds to estrogen receptors (ERs). When bound, these receptors function as transcription factors to regulate gene expression, affecting various physiological, morphological or behavioral processes (Ball and Balthazart, 2008; Wingfield, 2012).
Whereas the HPG axis is often visualized as acting top down, it is in fact a dynamic self-regulating system, sensitive to feedback and modulation at multiple levels. Early studies of the mechanisms underlying T-mediated trait expression focused on the gonad, while more recent research has emphasized mechanisms of steroid action in the brain (Adkins-Regan, 2005; Ball and Balthazart, 2008; Rosvall et al., 2012; Soma, 2006), including the many stimulatory and inhibitory afferents in the brain that act to integrate environmental responses and relay them to the level of the GnRH neuron, leading to downstream HPG activation (e.g., Bentley et al., 2009). Despite considerable advances in understanding the complexity of these constituent parts, little work has integrated multiple levels of the HPG axis simultaneously in an evolutionary framework. It has been theorized that the brain may be best suited to respond to selection on T-mediated traits via adjustments in the way environmental stimuli elicit GnRH release, while the responses of the downstream endocrine portions of the HPG axis may be less likely to be altered during evolution (Adkins-Regan, 2008). However, evidence that directly points to the sources of individual or population variation along the HPG axis is lacking.
GnRH challenges, in which individuals are administered a standardized dose of exogenous GnRH, essentially bypass the brain as a source of variation to measure downstream capacity to secrete androgens (see Goymann et al., 2007). Recent work in Zonotrichia has identified population differences in the ability to modulate T in response to social stimuli, despite similar propensity to elevate T following GnRH challenge (e.g., Addis et al., 2011), suggesting that divergence in HPG activity may occur at the level of the brain. However, studies comparing individual responses to GnRH injections have demonstrated functional variation in T that likely originates downstream of the brain: Variation in T response to GnRH challenge is repeatable among individuals (Jawor et al., 2006), maps onto phenotypic traits (McGlothlin et al., 2008; McGlothlin et al., 2007), and is under selection (McGlothlin et al., 2010). This suggests that variation in the pituitary-gonadal axis downstream of GnRH may contribute to differences among individuals and be an important target of selection.
In order to better understand the functional mechanisms by which variation in the HPG axis may be translated to phenotype, multiple levels of the endocrine system must be examined collectively as potential sources of variation in T. Any one or many of these components could vary among individuals, sexes, populations, or species, leading to variation in T and fitness. Importantly, it is unknown whether selection acts on different endocrine components as one integrated unit or separately as independently varying targets, which could have important implications for predicting responses to selection (Adkins-Regan, 2008; Hau, 2007; Hau and Wingfield, 2011; Ketterson et al., 2009). Comparing sources of variation in multiple endocrine components along multiple levels of the HPG axis across groups is a promising approach that has not yet been adequately explored.
Exceptions include work on alternative phenotypes. Research on white-throated sparrows (Zonotrichia albicollis), which have a chromosomal inversion resulting in a behavioral polymorphism, find morph differences in T but conflicting evidence as to whether differences are also reflected in upstream LH signal from the pituitary (Lake et al., 2008; Spinney et al., 2006). Research on the African cichlid Astatotilapia burtoni, which displays environment-induced dominant and subordinate phenotypes, has similarly looked at sources of variation along multiple levels of the HPG axis, and has suggested depression of the entire HPG axis in subordinates as compared to dominant individuals (Maruska and Fernald, 2010; Maruska et al., 2010). While these comparisons of genetic and environmentally induced morphs provide important insights into the mechanisms underlying phenotypic diversity, the relative lability and interconnectedness of endocrine components under selection remains unknown. From an evolutionary perspective, it is important to examine sources of variation in the HPG axis that may be shaped by selection. Artificial selection on white-footed mice (Peromyscus leucopus) for high and low reproductive suppression in winter has found genetic variation in the brain as well as in downstream pituitary LH release (Heideman and Pittman, 2009; Heideman et al., 2010). Recent work by Caro and colleagues (2006; 2009) has begun to identify mechanisms underlying population differences in the timing of breeding in Corsican blue tits (Cyanistes caeruleus). Still, studies like these are few, and those comparing individuals of naturally divergent populations are lacking.
Here, we contrast activity of the HPG axis of two subspecies of a songbird, the dark-eyed junco (Junco hyemalis). The post-glacial diversification of the junco is thought to be exceptionally extensive given the relatively short timescale and may represent incipient speciation (Mila et al., 2007). Thus this species provides an excellent system for the comparative study of endocrine mechanisms. Using captive male birds in breeding condition, we examined the degree to which individual and subspecies differences in T released in response to GnRH originate at the level of the pituitary, at the level of the gonad, or both. Using traditional measures of circulating hormones and modern molecular methods to assess measures of hormone sensitivity, we examined circulating LH and T responses to HPG axis stimulation, transcript abundance for LHR in the testes and testicular development, as well as both AR and AROM transcript abundance in the rostral hypothalamus, a potentially important site of negative feedback regulating T release. We explored the degree of interconnectedness (integration) among these endocrine parameters, as well as how they diverge between subspecies.
We compared the Carolina subspecies of junco (J. h. carolinensis) from a population that breeds around Mountain Lake Biological Station near Pembroke, Virginia, USA (37°22′N, 80°32′W), and the white-winged junco (J. h. aikeni) from a population that breeds in the Black Hills National Forest near Custer, South Dakota, USA (43°46′N 103°36′W). The initiation of breeding differs by approximately one month in these subspecies (first eggs typically appear during mid- to late April in Carolina juncos, latter half of May in white-winged juncos), while the timing of the end of breeding is similar with first signs of molt appearing in both species in July (Nolan et al., 2002; Bergeon Burns and Ketterson, unpublished data)
Studies conducted on Carolina juncos have provided a wealth of information about T-mediated phenotypic traits and trade-offs (McGlothlin et al., 2010; Reed et al., 2006). Juncos vary in the degree to which they elevate T in response to a standardized injection of GnRH, and maximum T levels produced after GnRH challenges decline across the breeding season and are repeatable among individuals (Jawor et al., 2006). Further, T responses co-vary with phenotypic characters, such as ornamentation and body size, and predict reproductive success (McGlothlin et al., 2010; McGlothlin et al., 2007), presenting an ideal opportunity for examining the mechanisms underlying known individual variation in these fitness-relevant traits. The white-winged subspecies was chosen for comparison with the Carolina juncos because white-winged males have the largest body size and highest levels of ornamentation as compared to any other population of dark-eyed junco (Nolan et al., 2002), raising questions about the role for androgens in the population divergence.
We tested the hypothesis that T levels may contribute to population differences in body size and ornamentation in juncos, and we predicted greater T response to GnRH challenge in the larger and more ornamented white-winged junco subspecies. We hypothesized that selection acts on the HPG axis as one integrated unit, such that potential differences between these divergent populations in T production could be attributed to differences across each level of the HPG axis examined. Thus, we predicted greater activity at each level of the HPG axis in white-winged juncos, contributing to the greater predicted T response. We also tested the hypothesis that multiple levels of the HPG axis are tightly integrated among individuals, which are the targets of selection, such that males are consistently high- or low-responders at each level. Thus we predicted that variation in pituitary response to GnRH would positively correlate with gonadal response to LH among males. Similarly, we predicted that multiple characteristics of the gonad (transcript abundance of LHR in testes; gonad mass) would predict levels of T secretion.
METHODS
This study was approved by the Bloomington Institutional Animal Care and Use Committee under protocol #06-242 and 09-037.
Capture and housing
The experiment was executed in two cohorts: white-winged juncos were captured in 2009 and tested in 2010, and Carolina juncos were captured in 2010 and tested in 2011. Juvenile juncos of both sexes were captured in the field using baited mist-nets and potter traps at the end of the breeding season (N=33 white-winged juncos from South Dakota (SD); N=41 Carolina juncos from Virginia (VA)). Juveniles are those birds that hatched during the same breeding season, and are easily differentiated from adults by plumage. The birds were banded, measured and bled (~100 μl) from the wing vein upon capture and housed in a temporary aviary for the duration of the capture period. They were then transported back to Indiana University where they were housed in a single mixed-sex flock in a temperature-regulated indoor aviary. The experimental design provided individuals from each subspecies with the identical space, temperature and food environment for the eight months prior to, and throughout the duration of the experiment, although logistical constraints prevented both populations from being studied in the same year.
Photoperiod was adjusted every two weeks to match the naturally changing photoperiod of their respective capture sites. This allowed males to adjust to captivity, complete molt, and regain photosensitivity before the experiment began during the following spring. During the first week of March (of 2010 for SD; 2011 for VA), before natural photoperiod reached a stimulatory day length for gonadal growth, biweekly gradual photoperiod adjustments were discontinued and photoperiod was increased by 1 hour every two days until it reached the target 16L:8D. This rapid increase in photoperiod was intended to provide the stimulus needed to initiate gonadal recrudescence, while overriding any potential subspecies differences in stimulatory day length. This rapid light advancement ensured that individuals of the two subspecies were in equivalent reproductive stage at the onset of their respective experiments, allowing for comparison. During this time, birds were also moved from free-flying group housing into individual cages (60 × 60 × 52 cm) where they could see and hear other caged males, as well as females. The birds remained at this long day photoperiod for 21 days until the start of the experiment. Juncos of both subspecies had high survivorship and maintained excellent physical condition in captivity.
Hormone challenges
Beginning in April, juncos from each subspecies received a series of three hormone challenges to examine the sources of individual variation in T production. The three hormonal challenges were done consecutively but each only once, separated by five-day intervals. The order in which the birds received the 3 different hormone challenges was randomly assigned but counterbalanced with respect to subspecies, and having no effect (F=1.13, p=0.31), order was omitted from later analyses.
One of the challenges tested for concentrations of T produced in response to a GnRH challenge following methods that have proven to be effective in the junco (Jawor et al., 2006). Specifically, a pre-injection blood sample (100 μl) was collected to provide an initial measure of circulating T. GnRH (Chicken LH-RH; American Peptide, Sunnyvale, CA, USA) was injected intramuscularly at a dose of 1.25 μg GnRH per 50 μl phosphate-buffered saline, stimulating the birds to temporarily produce maximal levels of circulating T. Birds were placed in individual holding bags after injection, and bled again in the same manner for post-challenge T exactly 30 minutes after injection, when T levels are at their peak (Jawor et al., 2006).
The second type of hormone challenge consisted of a GnRH challenge preceded and followed by collection of a blood sample to determine circulating LH levels (with blood sampled prior to and 5 minutes following injection). Pilot work on free-living juncos indicated that five minutes was an appropriate time point for capturing elevated LH levels following intramuscular GnRH injection (Bergeon Burns, 2012); a similar timeframe has long been used following intravenous injections (e.g., Wingfield et al., 1979).
The third type of challenge consisted of an LH challenge preceded and followed by collection of a blood sample for circulating T levels (with blood sampled prior to and 30 minutes following injection). Ovine LH (oLH-26, Lot # AFP5551B) was obtained from NIDDK’s National Hormone & Peptide Program and A. F. Parlow, and was injected intramuscularly at a dose of 5 μg LH per 50 μl phosphate-buffered saline. Ovine LH has previously been shown to effectively elevate T in birds (e.g., Deviche et al., 2010), and pilot testing revealed that this intramuscular dose was sufficient for significant T elevation in juncos (see electronic supplementary material).
Pre-injection blood sampling began an average of 3.82±0.52 minutes after individual birds were removed from their cages. After blood was collected, samples were centrifuged and the plasma fraction stored at −20°C until the completion of the experiment. Circulating LH was measured in a single post-precipitation, double-antibody radioimmunoassay by TPH. Duplicate samples of 20 μl plasma were run in the assay, which employs radio-iodinated chicken LH and a goat anti-rabbit secondary antibody, as described previously (Follett et al., 1972; Follett et al., 1975; Sharp et al., 1987; Wingfield et al., 1991). The minimum detectable concentration was 0.078 ng/mL, and intra-assay variation was 12.1 ± 1.0%.
Testosterone was assayed at Indiana University using an EIA kit (Assay Designs, Inc., #901-065) as described previously (Clotfelter et al., 2004). Pre- and post-injection samples from each GnRH challenge were run in the same assay, the same standard was run three times on each plate for determination of assay variation. Approximately 2000 cpm of tritiated T was added to each sample, allowing determination of sample recovery after two rounds of diethyl ether extraction. Extracts were resuspended in 50 μl ethanol and diluted with 300 μl assay buffer. 100 μl quantities were run in duplicate in the EIA, and another 100 μl counted to determine individual 3H recovery. T concentrations were determined with a 4-parameter logistic curve-fitting program (Microplate Manager; Bio-Rad Laboratories, Inc.) Average recovery of 3H-labeled T after extraction was 90%, and individual T concentrations were corrected for incomplete recoveries. The intraassay coefficients of variation ranged from 4.6–11.0%, mean 7.4%. The interassay coefficient of variation was 19.9%, and correction factors were used to account for this plate variation (following Jawor et al., 2006).
Target tissue sensitivity to hormones
Five days after the third and final hormone challenge, birds were euthanized by overdose of isoflurane and each individual was weighed prior to dissection. Brains and testes were then dissected, frozen on powdered dry ice, and stored at −80°C. Rostral hypothalamus was later microdissected from brain following anatomical markers (Soma et al., 1999). The Trizol method (Invitrogen, Carlsbad, CA) was used to extract total RNA from both the rostral hypothalamus and one testis (left) for each individual. Following spectrophotometry to quantify total RNA, 1 μg was treated with DNAse (Promega, Madison, WI) and underwent reverse-transcription PCR using oligo dT primers and Superscript III reverse transcriptase (Invitrogen, Carlsbad, CA).
The resulting cDNA was used as a template for quantitative real-time PCR (qPCR) to measure abundance of mRNA expression of AR and AROM in the rostral hypothalamus, and LHR in the testis. We also examined expression of GAPDH, for normalization of the expression of each gene of interest in each sample. Gene-specific primers for AR, AROM and GAPDH were based on the zebra finch (Taeniopygia guttata): AR forward: ATGAGTACCGCATGCACAAA, reverse: AACTCCTGGGGTGTGATCTG (100 bp amplicon); AROM forward: GGATGAGCACATGGATTTTGC, reverse: GCAGTCAGATCCCCTCTGTTCT (63 bp amplicon); ERα forward: CTGCCAGGCCTGCCGACTGAGAAA, reverse: TGCGGTCTTTCCGGATTCCGCCT (71 bp amplicon). GAPDH forward: TGACCTGCCGTCTGGAAAA, reverse: CCATCAGCAGCAGCCTTCA (70 bp amplicon). LH receptor primers are based on white-throated sparrow (Zonotrichia albicollis) predicted LHR cDNA sequence: LH forward: TCTCAGAGCGACTCCCTG, reverse: TCCGTCCTCAATGTGCAAC (111 bp amplicon). We later confirmed with sequences from the junco transcriptome (Peterson et al., 2012; 95–98% identity with zebra finch in these genes).
Quantitative PCR (qPCR) reactions (25 μl) were run in duplicate in a Stratagene MX3000p thermocycler (Agilent) using Perfecta SYBR green low ROX, with 2.5 μl cDNA diluted 1:10 and primers at concentration of 0.3uM. Thermocycling conditions were the same for all reactions, as follows: 10 min at 95°C, 40 cycles of 95°C for 30s, 60°C for 1 min, and 70°C for 30s. A final melting phase of 95°C for 1 min, 55°C for 30s, and 95°C for 1 min was run to confirm single-product specificity of each sample.
Standard curves were created from known cDNA dilutions. MxPro software (v.4.10, Agilent) was used to set thresholds for each reaction based on background fluorescence and to correct amplification data for imperfect reaction efficiencies (ranging from 93% to 116%). An arbitrary cDNA sample derived from junco neural tissue was run on every qPCR plate, serving as a calibrator to which each individual sample was compared. We used the 2−ΔΔCt method (Livak and Schmittgen, 2001), which reports relative abundance of transcript for each gene of interest relative to the calibrator, while controlling for the abundance of a reference gene (GAPDH).
Testes development
For each individual, the testis used for qPCR was first measured for an assessment of gonadal development. Testes were weighed and measured while frozen. Testis volume was calculated using the formula for an ellipsoid: V=4/3πa2b; where a is half its width and b is half its length (long axis).
Statistical methods
All data were analyzed with SPSS 19 and are reported as means ±1 standard error of the mean. Data were transformed where necessary to meet assumptions of normality: LH and T were ln-transformed, and transcript abundance was measured as log2-fold change relative to calibrator.
Body mass at euthanization was compared across subspecies using unpaired t-tests. To compare reproductive physiology between the subspecies at multiple levels of the HPG axis, we ran a single one-way MANOVA with subspecies as a between-subjects variable and the following as dependent variables: LH (post-GnRH-injection), gonad mass and volume, transcript abundance of gonadal LHR, testosterone (post-LH-injection), and transcript abundance of anterior hypothalamic AR and AROM. Post-hoc pairwise comparisons with Bonferroni correction were used to look for subspecies differences in each of the dependent variables.
Next, repeated measures ANOVAs were used to compare subspecies for pre- and post-challenge LH levels in response to GnRH challenge, and for pre- and post-challenge T levels measured in response to both LH and GnRH challenges. We tested for effects of population, time point (pre- versus post- challenge) and injection type (LH or GnRH). Pearson correlations were used to examine the relationship between individuals’ T responses to LH and GnRH challenges (both prior to and following injection). Pearson correlations were also used to ask whether LH response to GnRH injection was predictive of T response to either LH or GnRH challenge. For each of the three hormone challenges, relationships between pre- and post-challenge hormone levels were also examined with Pearson correlations.
We used repeated measures linear mixed models (LMM) to explore whether characteristics of the gonad were related to T response. We used post-challenge T as the dependent variable, and injection type (LH or GnRH challenge) was entered as a fixed effect with individual as a random repeated factor. Population, gonad mass and transcript abundance of gonadal LHR were also included as fixed effects in the model, along with all of their interactions. Gonad mass and volume were highly correlated (Pearson correlation, R2=0.77, p<0.001), so gonad volume was omitted from this analysis to avoid issues of colinearity.
RESULTS
Population differences
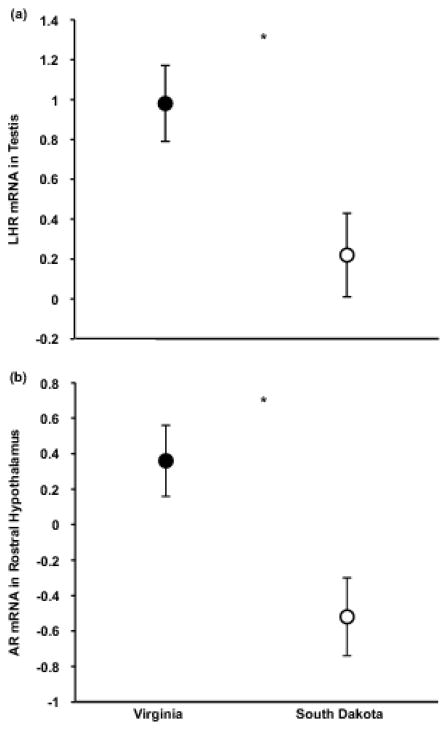
Body mass was significantly different across subspecies (SD: 24.58±0.60 g, VA: 20.92±0.26 g; Mann-Whitney U Test, p<0.001). A MANOVA including the 7 dependent variables revealed a significant overall effect of subspecies (Wilks’ λ=0.612, F(7,35)=3.18, p=0.01). The results of the post-hoc pairwise comparisons revealed significant differences between subspecies (Table 1): LHR mRNA and hypothalamic AR mRNA were more abundant in VA than in SD males (Figure 1), but AROM mRNA did not differ, and the subspecies did not significantly differ in testes size or circulating hormone levels.
Table 1.
Results of MANOVA comparing HPG axis variables between males of white-winged (South Dakota, N=19) and Carolina (Virginia, N=24) junco subspecies. Significant differences are indicated in bold.
| Variable | Pop | Mean | F | p |
|---|---|---|---|---|
| LH (Post-GnRH ng/mL, ln-transformed) | Virginia | 1.65±0.07 | .085 | .772 |
| S. Dakota | 1.62±0.08 | |||
| Testis Mass (g) | Virginia | 0.16±0.007 | .440 | .511 |
| S. Dakota | 0.15±0.007 | |||
| Testis Volume (mm3) | Virginia | 175.9±6.7 | 2.730 | .106 |
| S. Dakota | 159.2±7.5 | |||
| Testicular LHR mRNA abundance | Virginia | 0.98±0.19 | 7.401 | .010 |
| S. Dakota | 0.22±0.21 | |||
| T (Post-LH ng/mL, ln-transformed) | Virginia | 2.06±0.06 | .146 | .704 |
| S. Dakota | 2.02±0.07 | |||
| Ant. Hypo. AR mRNA abundance | Virginia | 0.36±0.20 | 8.645 | .005 |
| S. Dakota | −0.52±0.22 | |||
| Ant. Hypo. AROM mRNA abundance | Virginia | 0.90±0.18 | 1.922 | .173 |
| S. Dakota | 0.53±0.20 |
Figure 1.
Male Carolina (Virginia) juncos showed greater transcript abundance for LHR in the gonad (a) and for AR in rostral hypothalamus (b) than white-winged (South Dakota) juncos. Measures of transcript abundance are log2-fold change relative to arbitrary calibrator (unitless). Figures show means ± 1 standard error. Significant differences denoted by asterisk (*).
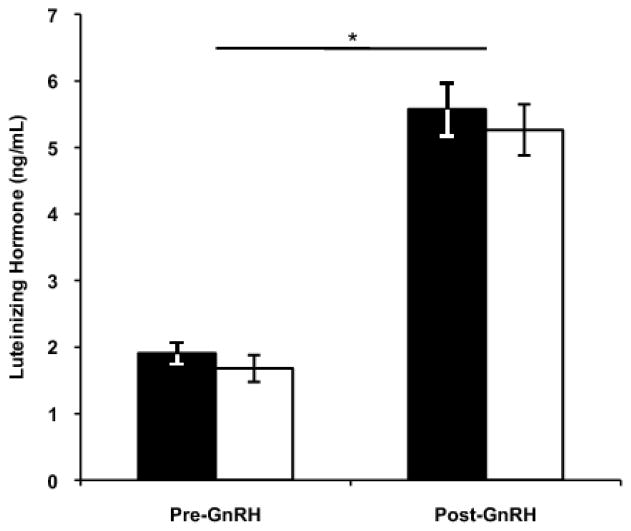
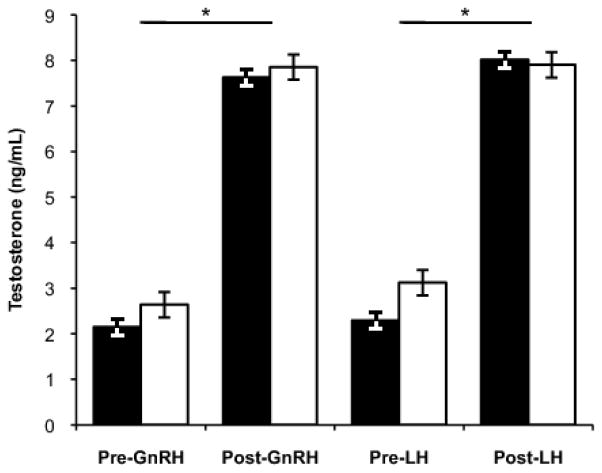
Males significantly elevated circulating LH in response to GnRH challenge (repeated measures ANOVA, effect of time point [pre- or post-injection]: F(1,43)=216.59, p<0.001). There was no subspecies difference in the degree of LH elevation in response to GnRH (effect of population: F(1,43)=0.54, p=0.47 and no significant interaction, Table 2, Figure 2). Testosterone concentration was significantly elevated following injection with LH and GnRH (repeated measures ANOVA, effect of time point: F(1,42)=333.27, p<0.001). Testosterone was similar whether the challenge was LH or GnRH (effect of challenge: F(1,42)=0.08, p=0.77). There was no subspecies difference in these patterns (effect of population: F(1,42)= 1.01, p=0.32) and no significant interactions. In other words, males of both populations significantly elevated T in response to HPG axis stimulation, but magnitude of response did not differ between subspecies, nor if challenged at the level of the pituitary, with GnRH, or at the level of the gonad, with LH (Table 2, Figure 3).
Table 2.
Results of repeated-measures ANOVAs examining predictors of circulating luteinizing hormone or testosterone in male dark-eyed juncos. Hormones were sampled prior to or following injection (time point) in Carolina or white-winged juncos (population). LH was sampled following GnRH challenge, while T was sampled following either LH or GnRH challenge (injection type). Significant predictors are indicated in bold.
| Factor | F | df | p |
|---|---|---|---|
| Luteinizing Hormone | |||
| Time point | 216.6 | 1, 43 | .000 |
| Population | 0.54 | 1, 43 | .468 |
| Time point * Population | 0.03 | 1, 43 | .865 |
| Testosterone | |||
| Time point | 333.27 | 1, 42 | .000 |
| Population | 1.01 | 1, 42 | .320 |
| Injection type | 0.08 | 1, 42 | .773 |
| Time point * Population | 2.26 | 1, 42 | .140 |
| Time point * Injection type | 0.75 | 1, 42 | .391 |
| Population * Injection type | 0.21 | 1, 42 | .650 |
| Time point * Population * Injection type | 0.09 | 1, 42 | .767 |
Figure 2.
Luteinizing hormone levels prior to and following GnRH challenge in Carolina juncos from Virginia (filled bars) and white-winged juncos from South Dakota (open bars). Males significantly elevated circulating LH in response to GnRH challenge, but subspecies did not differ in LH levels. Figures show means ± 1 standard error. Significant differences denoted by asterisk (*).
Figure 3.
Testosterone levels prior to and following HPG stimulation in Carolina (filled bars) and white-winged (open bars) juncos. Males significantly elevated circulating T following injection, but the degree of T elevation did not differ between subspecies, and did not differ if males were challenged at the level of the pituitary, with GnRH, or at the level of the gonad, with LH. Figures show means ± 1 standard error. Significant differences denoted by asterisk (*).
Individual variation
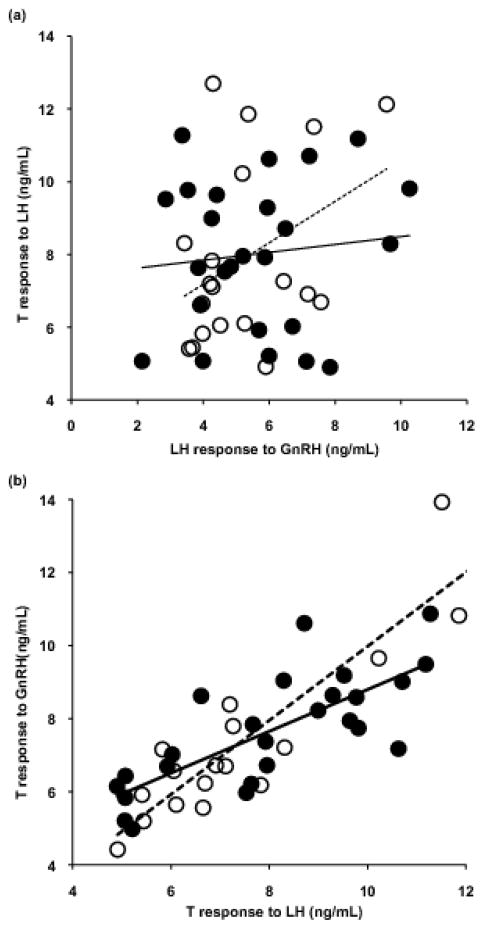
Pre-injection T was not correlated with post-injection T among individuals (Pearson correlation, GnRH: r=0.23, p=0.13; LH: r=0.16, p=0.30), but pre-injection LH was significantly correlated with post-injection LH (Pearson r=0.534, p<0.001). Individual birds were highly consistent in T levels, whether challenged with LH or GnRH (Pearson correlation, pre-injection: r=0.48, p=0.001; post-injection: r=0.82, p<0.001). However, pituitary LH (in response to GnRH) was not predictive of this consistent individual gonadal T release (T in response to LH: Pearson’s r=0.19, p=0.21; Figure 4). Repeated measures LMM analysis implicated gonad mass as a significant predictor of gonadal T in response to HPG activation. For visualization of this relationship, individual testis mass is plotted against the average T level following GnRH and LH challenge for males from both subspecies (Figure 5). T response was not predicted by population, injection type, or any interactions (Table 3).
Figure 4.
(a) Individual variation in circulating LH in response to a GnRH challenge shows no relationship with circulating T following a LH challenge. (b) T in response to a LH challenge co-varies with T in response to a GnRH challenge. Points represent individual male Carolina juncos from Virginia (filled circles, solid lines) and white-winged juncos from South Dakota (open circles, dashed lines).
Figure 5.
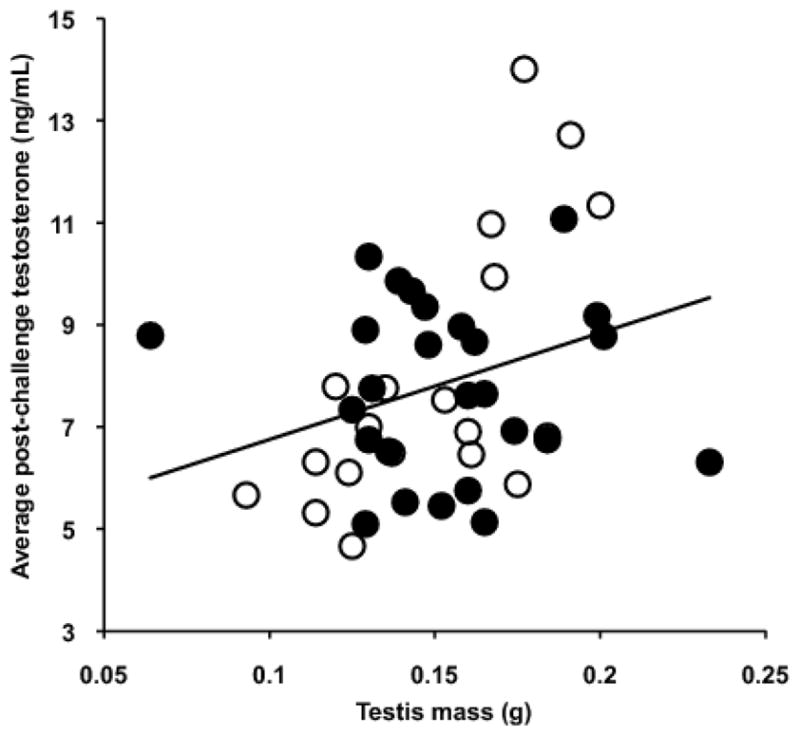
Relationship between testis mass and individual mean testosterone response to GnRH and LH challenges. Points represent male dark-eyed juncos of the Carolina (filled circles) and white-winged (open circles) subspecies.
Table 3.
Results of repeated-measures LMM examining predictors of average testosterone response to LH or GnRH challenge (injection type) in male Carolina and white-winged juncos (population). Significant predictor is indicated in bold.
| Factor | F | df | p |
|---|---|---|---|
| Injection Type | 1.061 | 1, 43.4 | .309 |
| Population | 1.514 | 1, 37.6 | .226 |
| Testis Mass | 10.928 | 1, 37.4 | .002 |
| Testicular LHR mRNA abundance | 2.700 | 1, 37.3 | .109 |
| Population * Testis Mass | 1.712 | 1, 37.4 | .199 |
| Population * Testicular LHR mRNA abundance | .224 | 1, 37.3 | .638 |
| Gonad Mass * Testicular LHR mRNA abundance | 2.307 | 1, 37.2 | .137 |
| Population * Testis Mass * Testicular LHR mRNA abundance | .433 | 1, 37.2 | .514 |
DISCUSSION
To understand the mechanistic underpinnings of T-mediated trait evolution, variation in multiple levels of the HPG axis must be examined simultaneously, both among individuals and between divergent taxa. In this study, we demonstrated that two closely related subspecies of the dark-eyed junco, when held under the same conditions in captivity, did not differ in circulating levels of LH or T released in response to a GnRH challenge. However, following the completion of the experiment, we found that they differed significantly in abundance of transcript for LHR in the testes, and AR in the rostral hypothalamus (Figure 6). Together these data suggest a pattern of population-level variation that resides not at the level of circulating hormones (measures of organismal responsiveness to GnRH challenge), but rather in measures of target tissue sensitivity to hormones in at least two levels within the HPG axis, raising important questions about divergence in the function and feedback regulation of the HPG axis.
Figure 6.
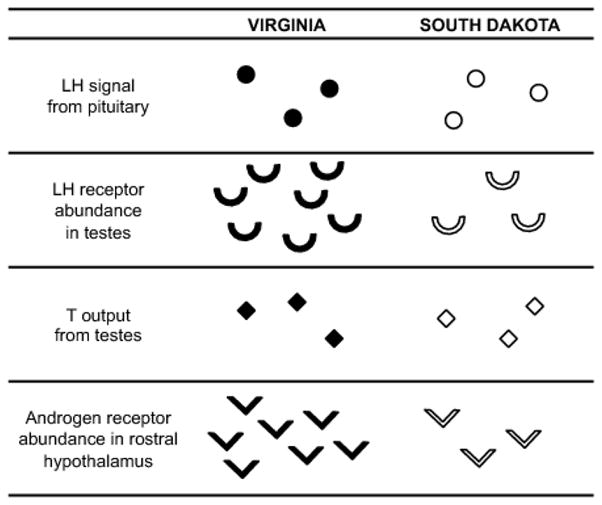
Schematic table of patterns identified in multiple, related components of the HPG axis between Carolina (Virginia, left) and white-winged (South Dakota, right) subspecies of the dark-eyed junco. Responsiveness of individual hormone targets varied between closely related species, but circulating hormone levels did not.
With respect to individuals, variation in LH response to GnRH was unrelated to T in response to LH. This suggests that the source of among-individual variation in T response to GnRH resides at the level of the gonad and not the pituitary, and indeed post-challenge T responses were consistent among individuals whether the gonad was stimulated with a standardized dose of exogenous LH, or with endogenous LH released following an exogenous GnRH challenge. Our subsequent examination of gonadal variables indicated that gonad mass, but not LHR, was positively related to T responses among individuals. These results provide insights into the potential sources of variation upon which selection may act and reveal the degree to which components of the HPG axis co-evolve or change independently.
Population differences
The series of hormone challenges was designed to deconstruct the GnRH challenge as a measure of capacity to secrete androgens, by examining LH response to GnRH and T response to LH, as they relate to the more commonly measured T response to GnRH (e.g., Tpotential per Goymann et al. 2007). We predicted higher T in the white-winged (SD) subspecies, which has larger body size, more white ornamentation, and greater territorial aggression than the Carolina (VA) subspecies (Bergeon Burns et al., 2013; Nolan et al., 2002), based on the hypothesis that T levels may contribute to population differences in body size and ornamentation. We hypothesized that selection acts on the HPG axis as one integrated unit, and thus predicted that possible sources of this variation included subspecies differences in LH signal that were relayed to the gonad, as well as differential sensitivity to LH signal as measured by LHR mRNA expression. However, the subspecies did not differ in mean T responses. Alternative explanations, such as environmental or organizational effects, will need to be explored to explain why the two subspecies continue to vary in multiple T-mediated phenotypes, despite no detectable differences in T (see also Bergeon Burns et al., 2013).
The lack of subspecies difference in mean T response to GnRH or LH in this study was also surprising from a mechanistic perspective, given that male Carolina juncos demonstrated greater abundance for LHR transcript in the gonad than white-winged juncos, suggesting greater sensitivity to the upstream LH signal. This increased sensitivity, combined with similar LH levels across subspecies, might be expected to lead to a greater T response in Carolina juncos, yet no subspecies difference in T was observed. One possible explanation for this apparent paradox is that LHR transcript abundance may not impact actual sensitivity to LH. Transcript (mRNA) abundance cannot always be assumed to reflect actual protein abundance, and sensitivity can also be affected by variables other than receptor density (e.g., receptor affinity, coactivators). Notably, even if these subspecies differ in this measure of sensitivity to LH, they may also vary in other steroidogenic machinery in the gonad. The binding of LH to its receptor is just the first step towards steroid production in the gonad, but other aspects of the testosterone production pathway, such as key steroidogenic enzymes, could also influence T output and vary between subspecies, in potentially opposing ways. Ongoing work is examining this interesting possibility.
Glucocorticoids can also affect the HPG axis and testosterone release through a variety of mechanisms (Wingfield and Sapolsky, 2003), including a direct inhibition of gonadal T release without apparent impact on upstream LH levels (Deviche et al., 2010; Wingfield et al., 1982). Thus it is possible that the subspecies may have differential sensitivity to the suppressive effects of stress on reproduction at the level of the testes, resulting in similar T levels. Gonadotropin inhibitory hormone receptors are also present in the gonad (McGuire and Bentley, 2010), providing another possible mechanism by which variation at the level of the gonad might regulate output of testosterone. Ecological differences between these subspecies (described below) may warrant further research in these areas.
Alternatively, a broader look at multiple levels of the HPG axis across subspecies could provide a promising functional explanation for the lack of population difference in T (Figure 6). In addition to greater LHR expression, male Carolina juncos also showed greater abundance for AR transcript in the rostral hypothalamus, which includes the preoptic area. Increased steroid receptor density in this area could result in dampened GnRH release and a turning down of the activity of the HPG axis via negative feedback. Thus, it is possible that the testes of Carolina juncos breeding in VA are indeed more sensitive to LH signal, and thus produce a faster (or easier or greater) rise in circulating T following stimulation, but this difference may be balanced by increased hypothalamic sensitivity to feedback, resulting in a faster return of T to baseline. In contrast, the white-winged junco testes may be less prone to activation by LH (lower gonadal LHR mRNA abundance), but also less readily suppressed by negative feedback (lower hypothalamic AR mRNA abundance). While speculative, future mechanistic work should explore the hypothesis that populations might vary not just in the magnitude of HPG axis activation, but also the time course or ease of activation, any of which may present potential evolutionary implications, as discussed below.
Having studied the subspecies in two different years, it is possible that the population-level findings reported here could relate to year effects. However, every effort was made to ensure that the conditions were identical for the two subspecies, including capturing the individuals early in their independence, providing many months of acclimation to the same rooms and cages for both subspecies, even using the same personnel for animal care and experimentation. Thus the potential for a year effect was recognized a priori, minimized insofar as possible and seems unlikely. Further, our findings are supported by a recent field study comparing these same two subspecies in the same year, which reported population level variation in aggressive behavior and in neural sensitivity to steroids, but similarly found no differences in average T levels (Bergeon Burns et al., 2013).
Individual variation
Much recent research in behavioral endocrinology has focused on the top level of the HPG axis, examining regulation of GnRH synthesis and secretion from the hypothalamus as a source of variation in T responses (e.g., Bentley et al., 2009; Cheng et al., 2010). In contrast, our data exploring variation among individual males in T response to a standardized dose of GnRH confirms that meaningful individual variation can also lie in the periphery, and point to the gonad in particular as an important source of repeatable variation in T responses. We found that individual male T responses were strikingly similar whether testes were stimulated with a standardized dose of exogenous LH, or with endogenously produced LH in response to GnRH. Further, individual variation in LH response to GnRH challenge did not predict T responses. Our data suggest that individual variation in gonadal T release is not simply a reflection of upstream variation in the amount of LH, but appears to be independent of this signal. Thus, the male pituitary LH signal appears to operate more as a step function (Adkins-Regan, 2005) than a signal bearing quantitative information. This idea is not new, as LH is typically described as a surge with respect to triggering ovulation in females (Johnson, 2000). Taken together with previous findings that have linked T elevation with phenotype and fitness (Jawor et al., 2006; McGlothlin et al., 2010), these data suggest that the gonad, or modulators of gonad function, may be a more important target for selection on T responses than the pituitary in the dark-eyed junco.
The findings raise the question of what it is about the testes that generates repeatable individual differences in elevation of circulating T? Abundance of LHR transcript did not predict T among individuals. Interestingly, we found a significant pattern of covariation among males between testicular mass and circulating T responses. Testosterone is required for spermatogenesis, so one possible explanation for this relationship is that those testes with greater T output were also undergoing relatively more spermatogenesis, which could contribute to increased gonad mass. Additional work examining individuals at multiple time points or stages of breeding would aid in interpreting and testing the generalizability of this finding. Likewise, examination of the expression of enzymes involved in gonadal steroidogenesis may further elucidate the mechanisms underlying meaningful variation in T responses to GnRH.
Evolutionary implications of population level and individual variation
The HPG axis consists of many levels of regulation and integration. The question of how this axis responds to selection has received some theoretical attention (Adkins-Regan, 2008), but little empirical study, particularly between closely related taxa. At one extreme, the endocrine cascade that gives rise to T may be tightly integrated, such that co-variation between multiple components of the HPG axis would be expected. This is an extension of the evolutionary constraint hypothesis of Hau (2007) or phenotypic integration as described by Ketterson et al. (2009) which predict linkage between circulating T and multiple T-mediated traits. For example, many temperate-zone birds increase their aromatase activity in behaviorally-relevant brain areas concurrently with elevated circulating T levels during the breeding season (Foidart et al., 1998; Riters et al., 2000), and variation among both individuals and species in these two endocrine parameters have been shown to be positively correlated (Silverin et al., 2000). In line with this thinking, we tested the hypothesis that multiple components of the HPG axis would vary in tandem across individuals, such that subspecies differences were reflected in both hormone release and response.
Contrary to expectation, however, findings reported here are more in keeping with predictions of the evolutionary potential (Hau, 2007) or phenotypic independence (Ketterson et al., 2009) hypotheses. That is, we found that the populations differed in transcript abundance for hormone receptors, but not in the corresponding circulating hormone levels (Figure 6). We suspect that to the extent that differences within individuals are repeatable, the differences detected between subspecies in receptor transcript are more likely to represent genetic divergence than plasticity or early developmental effects because the birds in this study were captured prior to maturity and were transported, housed and handled identically prior to these measurements. Future studies that compare wild and captive birds are needed to tease apart the degree of plasticity in these endocrine parameters (e.g., Cheviron et al., 2008), but our findings to date point to a relative ease with which components of the HPG diverge independently. Further, our data suggest that circulating hormone levels may be less prone to evolutionary change than the responsiveness of individual hormone targets, supporting previous findings (Bergeon Burns et al., 2013; Gahr et al., 1993; Shaw and Kennedy, 2002; Young and Crews, 1995). Functionally, this finding may relate to the role of circulating hormones as important systemic regulators of many varied phenotypic traits, providing constraints to expression (e.g. many traits would be affected by selection on circulating T levels, McGlothlin and Ketterson, 2008). Receptors, in contrast, are localized at specific target tissues and may therefore be freer to diverge (e.g., Canoine et al., 2007; Voigt and Goymann, 2007). Mechanistically, receptor transcripts are direct gene products, providing a straightforward target of divergence, unlike steroids that are derived from cholesterol (Adkins-Regan, 2005).
The subspecies of junco studied here are thought to represent recent and rapid post-glacial divergence (Mila et al., 2007), providing an interesting model for understanding how the HPG axis is likely to evolve. Physiological differences between these subspecies could be attributable to a potential founder effect during divergence (Mila et al., 2007). Alternatively, some population differences may be adaptive, including our findings that white-winged (SD) males exhibit less apparent gonadal sensitivity to LH and less apparent hypothalamic sensitivity to androgen-mediated feedback. For example, South Dakota is a more extreme environment, where juncos often face late snow storms and intense dry heat within the same breeding season, as compared to the relatively milder and longer breeding season in Virginia (Bergeon Burns and Ketterson, unpublished data). We suspect that territory sizes are also larger, and poorer quality, and brood parasitism much higher, in the vastly different South Dakota habitat. Thus for the white-winged subspecies, fewer breeding opportunities may exist and the costs of a failed attempt may be higher. Ecological variables like these have been thoroughly discussed as they relate to whether or not divergent taxa elevate T, and the extent of T elevation (Goymann et al., 2007; Wingfield et al., 2007; Wingfield et al., 2001). However, studies addressing potential differences in the mechanisms underlying T elevation, particularly among closely related taxa, are lacking (Lynn, 2008). We suggest that the relatively more extreme habitat facing white-winged juncos may be reflected by divergence within the reproductive axis (e.g. less sensitive to suppressive effects of stress in the testes, and/or less reactive HPG axis as a whole) even where differences in T response to GnRH challenge are absent.
Supplementary Material
HIGHLIGHTS.
We examined multiple levels of the HPG axis in an evolutionary framework
We predicted integration: consistently high or low responses at each level
Males of two subspecies differed in hormone receptor mRNA, but not hormone levels
LH levels and LH-receptor mRNA did not predict repeatable male testosterone levels
Gonad mass positively covaried with individual variation in testosterone production
Acknowledgments
We are grateful to the University of Virginia’s Mountain Lake Biological Station, Black Hills National Forest, and Jim and Connie Gorsuch for access to field sites; to M Boser, R Hanauer, EM Schultz, and C Wood for assistance in the field, to S Jayaratna, R Kiley, MP Peterson, K Roth, R Stewart, E Swanger, C Taylor, S Wanamaker and E Zeller for assistance in the aviary and lab; and to DL Maney and D Abebe for LH primer sequences. The authors have no conflicts of interest to disclose. The authors were supported by: NIH NRSA fellowship (F32HD068222) to KAR; NSF predoctoral fellowship and NSF DDIG (IOS-0909834) to CMBB; NSF IOS-0820055 to EDK, including an REU supplement; and NIH training grant (T32HD049336; “Common Themes in Reproductive Diversity”) to KAR, CMBB, and EDK; NSF IOS-0744705 to TPH. The funding sources had no involvement in any aspect of the study design, execution or publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kimberly A. Rosvall, Email: krosvall@indiana.edu.
Thomas P. Hahn, Email: tphahn@ucdavis.edu.
Gregory E. Demas, Email: gdemas@indiana.edu.
Ellen D. Ketterson, Email: ketterso@indiana.edu.
References
- Addis EA, Clark AD, Wingfield JC. Modulation of androgens in southern hemisphere temperate breeding sparrows (Zonotrichia capensis): An altitudinal comparison. Hormones And Behavior. 2011;60:195–201. doi: 10.1016/j.yhbeh.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E. Hormones and Animal Social Behavior. Princeton University Press; 2005. [Google Scholar]
- Adkins-Regan E. Do hormonal control systems produce evolutionary inertia? Philosophical Transactions of the Royal Society B-Biological Sciences. 2008;363:1599–1609. doi: 10.1098/rstb.2007.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball G, Balthazart J. Hormonal regulation of brain circuits mediating male sexual behavior in birds. Physiology & Behavior. 2004;83:329–346. doi: 10.1016/j.physbeh.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Ball G, Balthazart J. Individual variation and the endocrine regulation of behaviour and physiology in birds: a cellular/molecular perspective. Philosophical Transactions Of The Royal Society B-Biological Sciences. 2008;363:1699–1710. doi: 10.1098/rstb.2007.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley GE, Ubuka T, McGuire NL, Calisi R, Perfito N, Kriegsfeld LJ, Wingfield JC, Tsutsui K. Gonadotrophin-Inhibitory Hormone: A Multifunctional Neuropeptide. Journal Of Neuroendocrinology. 2009;21:276–281. doi: 10.1111/j.1365-2826.2009.01851.x. [DOI] [PubMed] [Google Scholar]
- Bergeon Burns CM. Dissertation: Coordination of testosterone-mediated phenotypes and underlying endocrine mechanisms across divergent populations of the dark-eyed junco. Indiana University; Ann Arbor: 2012. p. 214. [Google Scholar]
- Bergeon Burns CM, Rosvall KA, Ketterson ED. Neural steroid sensitivity and aggression: comparing individuals of two songbird subspecies. Journal Of Evolutionary Biology. 2013;26:820–831. doi: 10.1111/jeb.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canoine V, Fusani L, Schlinger B, Hau M. Low sex steroids, high steroid receptors: Increasing the sensitivity of the nonreproductive brain. Developmental Neurobiology. 2007;67:57–67. doi: 10.1002/dneu.20296. [DOI] [PubMed] [Google Scholar]
- Caro S, Lambrechts M, Chastel O, Sharp P, Thomas D, Balthazart J. Simultaneous pituitary–gonadal recrudescence in two Corsican populations of male blue tits with asynchronous breeding dates. Hormones And Behavior. 2006;50:347–360. doi: 10.1016/j.yhbeh.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Caro SP, Charmantier A, Lambrechts MM, Blondel J, Balthazart J, Williams TD. Local adaptation of timing of reproduction: females are in the driver’s seat. Functional Ecology. 2009;23:172–179. [Google Scholar]
- Cheng G, Coolen L, Padmanabhan V, Goodman R, Lehman M. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology. 2010;151:301. doi: 10.1210/en.2009-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheviron ZA, Whitehead A, Brumfield RT. Transcriptomic variation and plasticity in rufous- collared sparrows (Zonotrichia capensis) along an altitudinal gradient. Molecular Ecology. 2008;17:4556–4569. doi: 10.1111/j.1365-294X.2008.03942.x. [DOI] [PubMed] [Google Scholar]
- Clotfelter E, O’Neal D, Gaudioso J, Casto J, Parker-Renga I, Snajdr E, Duffy D, Nolan V, Ketterson E. Consequences of elevating plasma testosterone in females of a socially monogamous songbird: evidence of constraints on male evolution? Hormones and Behavior. 2004;46:171–178. doi: 10.1016/j.yhbeh.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Deviche PJ, Hurley LL, Fokidis HB, Lerbour B, Silverin B, Silverin B, Sabo J, Sharp PJ. Acute stress rapidly decreases plasma testosterone in a free-ranging male songbird: potential site of action and mechanism. General and Comparative Endocrinology. 2010;169:82–90. doi: 10.1016/j.ygcen.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Foidart A, Silverin B, Baillien M, Harada N, Balthazart J. Neuroanatomical distribution and variations across the reproductive cycle of aromatase activity and aromatase-immunoreactive cells in the pied flycatcher (Ficedula hypoleuca) Hormones and Behavior. 1998;33:180–196. doi: 10.1006/hbeh.1998.1448. [DOI] [PubMed] [Google Scholar]
- Follett B, Scanes C, Cunningham F. Radioimmunoassay for avian luteinizing hormone. Journal Of Endocrinology. 1972;52:359–378. [PubMed] [Google Scholar]
- Follett BK, Farner DS, Mattocks PW., Jr Luteinizing hormone in the plasma of white-crowned sparrows (Zonotrichia leucophrys gambelii) during artificial photostimulation. General and Comparative Endocrinology. 1975;26:126–134. doi: 10.1016/0016-6480(75)90223-3. [DOI] [PubMed] [Google Scholar]
- Folstad I, Karter A. Parasites, bright males, and the immunocompetence handicap. The American Naturalist. 1992;139:603. [Google Scholar]
- Gahr M, Güttinger HR, Kroodsma DE. Estrogen receptors in the avian brain: survey reveals general distribution and forebrain areas unique to songbirds. The Journal of Comparative Neurology. 1993;327:112–122. doi: 10.1002/cne.903270109. [DOI] [PubMed] [Google Scholar]
- Goymann W, Landys MM, Wingfield JC. Distinguishing seasonal androgen responses from male-male androgen responsiveness - Revisiting the Challenge Hypothesis. Hormones and Behavior. 2007;51:463–476. doi: 10.1016/j.yhbeh.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Hadley ME, Levine JE. Endocrinology. 6. Pearson Prentice Hall; Upper Saddle River, N.J: 2007. [Google Scholar]
- Hau M. Regulation of male traits by testosterone: implications for the evolution of vertebrate life histories. Bioessays. 2007;29:133–144. doi: 10.1002/bies.20524. [DOI] [PubMed] [Google Scholar]
- Hau M, Wingfield J. Hormonally-regulated trade-offs: Evolutionary variability and phenotypic plasticity in testosterone signaling pathways. In: Flatt A, Heyland T, editors. Mechanisms of Life History Evolution. Oxford University Press; 2011. [Google Scholar]
- Heideman PD, Pittman JT. Microevolution of neuroendocrine mechanisms regulating reproductive timing in Peromyscus leucopus. Integrative and Comparative Biology. 2009;49:550–562. doi: 10.1093/icb/icp014. [DOI] [PubMed] [Google Scholar]
- Heideman PD, Pittman JT, Schubert KA, Dubois CMR, Bowles J, Lowe SM, Price MR. Variation in levels of luteinizing hormone and reproductive photoresponsiveness in a population of white-footed mice (Peromyscus leucopus) AJP: Regulatory, Integrative and Comparative Physiology. 2010;298:R1543–R1548. doi: 10.1152/ajpregu.00686.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawor JM, McGlothlin JW, Casto JM, Greives TJ, Snajdr EA, Bentley GE, Ketterson ED. Seasonal and individual variation in response to GnRH challenge in male dark-eyed juncos (Junco hyemalis) General and Comparative Endocrinology. 2006;149:182–189. doi: 10.1016/j.ygcen.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Johnson AL. Chapter 22 - Reproduction in the Female. In: Whittow GC, editor. Sturkie’s Avian Physiology. 5. Academic Press; San Diego: 2000. pp. 569–596. [Google Scholar]
- Ketterson E, Nolan V, Wolf L, Ziegenfus C. Testosterone and avian life histories: Effects of experimentally elevated testosterone on behavior and correlates of fitness in the dark-eyed junco (Junco hyemalis) The American Naturalist. 1992;140:980–999. [Google Scholar]
- Ketterson ED, Atwell JW, McGlothlin JW. Phenotypic integration and independence: Hormones, performance, and response to environmental change. Integrative and Comparative Biology. 2009;49:365–379. doi: 10.1093/icb/icp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake J, Lange H, O’Brien S, Sanford S, Maney D. Activity of the hypothalamic–pituitary–gonadal axis differs between behavioral phenotypes in female white-throated sparrows (Zonotrichia albicollis) General and Comparative Endocrinology. 2008;156:426–433. doi: 10.1016/j.ygcen.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Livak K, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2-[Delta][Delta] CT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lynn SE. Behavioral insensitivity to testosterone: Why and how does testosterone alter paternal and aggressive behavior in some avian species but not others? General and Comparative Endocrinology. 2008;157:233–240. doi: 10.1016/j.ygcen.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Maruska KP, Fernald RD. Plasticity of the Reproductive Axis Caused by Social Status Change in an African Cichlid Fish: II. Testicular Gene Expression and Spermatogenesis. Endocrinology. 2010;152:291–302. doi: 10.1210/en.2010-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruska KP, Levavi-Sivan B, Biran J, Fernald RD. Plasticity of the Reproductive Axis Caused by Social Status Change in an African Cichlid Fish: I. Pituitary Gonadotropins. Endocrinology. 2010;152:281–290. doi: 10.1210/en.2010-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlothlin J, Whittaker D, Schrock S, Gerlach N, Jawor J, Snajdr E, Ketterson E. Natural selection on testosterone production in a wild songbird population. The American Naturalist. 2010;175:687–701. doi: 10.1086/652469. [DOI] [PubMed] [Google Scholar]
- McGlothlin JW, Jawor JM, Greives TJ, Casto JM, Phillips JL, Ketterson ED. Hormones and honest signals: males with larger ornaments elevate testosterone more when challenged. Journal of Evolutionary Biology. 2008;21:39–48. doi: 10.1111/j.1420-9101.2007.01471.x. [DOI] [PubMed] [Google Scholar]
- McGlothlin JW, Jawor JM, Ketterson ED. Natural variation in a testosterone-mediated trade-off between mating effort and parental effort. American Naturalist. 2007;170:864–875. doi: 10.1086/522838. [DOI] [PubMed] [Google Scholar]
- McGlothlin JW, Ketterson ED. Hormone-mediated suites as adaptations and evolutionary constraints. Philosophical Transactions of the Royal Society B-Biological Sciences. 2008;363:1611–1620. doi: 10.1098/rstb.2007.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire NL, Bentley GE. A functional neuropeptide system in vertebrate gonads: Gonadotropin-inhibitory hormone and its receptor in testes of field-caught house sparrow (Passer domesticus) General and Comparative Endocrinology. 2010;166:565–572. doi: 10.1016/j.ygcen.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Mila B, McCormack JE, Castaneda G, Wayne RK, Smith TB. Recent postglacial range expansion drives the rapid diversification of a songbird lineage in the genus Junco. Proceedings of the Royal Society B-Biological Sciences. 2007;274:2653–2660. doi: 10.1098/rspb.2007.0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore I, Perfito N, Wada H, Sperry T, Wingfield J. Latitudinal variation in plasma testosterone levels in birds of the genus Zonotrichia. General and Comparative Endocrinology. 2002;129:13–19. doi: 10.1016/s0016-6480(02)00563-4. [DOI] [PubMed] [Google Scholar]
- Nolan V, Ketterson ED, Cristol DA, Rogers CM, Clotfelter ED, Titus RC, Schoech SJ, Snajdr E. In: Dark-eyed Junco (Junco hyemalis) Poole A, editor. Cornell Lab of Ornithology; Ithaca: 2002. [Google Scholar]
- Peterson MP, Whittaker DJ, Ambreth S, Sureshchandra S, Buechlein A, Podicheti R, Choi JH, Lai Z, Mockatis K, Colbourne J, Tang H, Ketterson ED. De novo transcriptome sequencing in a songbird, the dark-eyed junco (Junco hyemalis): genomic tools for an ecological model system. BMC Genomics. 2012;13:305. doi: 10.1186/1471-2164-13-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed WL, Clark ME, Parker PG, Raouf SA, Arguedas N, Monk DS, Snajdr E, Nolan V, Ketterson ED. Physiological effects on demography: A long-term experimental study of testosterone’s effects on fitness. American Naturalist. 2006;167:667–683. doi: 10.1086/503054. [DOI] [PubMed] [Google Scholar]
- Riters LV, Eens M, Pinxten R, Duffy DL, Balthazart J, Ball GF. Seasonal changes in courtship song and the medial preoptic area in male European starlings (Sturnus vulgaris) Hormones and Behavior. 2000;38:250–261. doi: 10.1006/hbeh.2000.1623. [DOI] [PubMed] [Google Scholar]
- Rosvall KA, Bergeon Burns CM, Barske JJ, Goodson JL, Schlinger BA, Sengelaub DR, Ketterson ED. Neural sensitivity to sex steroids predicts individual differences in aggression: implications for behavioural evolution. Proceedings of the Royal Society B. 2012;279:3547–3555. doi: 10.1098/rspb.2012.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PJ, Dunn IC, Talbot RT. Sex differences in the LH responses to chicken LHRH-I and -II in the domestic fowl. Journal of Endocrinology. 1987;115:323–331. doi: 10.1677/joe.0.1150323. [DOI] [PubMed] [Google Scholar]
- Shaw BK, Kennedy GG. Evidence for species differences in the pattern of androgen receptor distribution in relation to species differences in an androgen-dependent behavior. Journal of Neurobiology. 2002;52:203–220. doi: 10.1002/neu.10079. [DOI] [PubMed] [Google Scholar]
- Silverin B, Baillien M, Foidart A, Balthazart J. Distribution of aromatase activity in the brain and peripheral tissues of passerine and nonpasserine avian species. General and Comparative Endocrinology. 2000;117:34–53. doi: 10.1006/gcen.1999.7383. [DOI] [PubMed] [Google Scholar]
- Soma K. Testosterone and Aggression: Berthold, Birds and Beyond. Journal Of Neuroendocrinology. 2006;18:543. doi: 10.1111/j.1365-2826.2006.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma KK, Bindra RK, Gee J, Wingfield JC, Schlinger BA. Androgen-metabolizing enzymes show region-specific changes across the breeding season in the brain of a wild songbird. Journal of Neurobiology. 1999;41:176–188. [PubMed] [Google Scholar]
- Spinney L, Bentley G, Hau M. Endocrine correlates of alternative phenotypes in the white-throated sparrow (Zonotrichia albicollis) Hormones And Behavior. 2006;50:762–771. doi: 10.1016/j.yhbeh.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Veiga JP, Polo V. Fitness consequences of increased testosterone levels in female spotless starlings. The American Naturalist. 2008;172:42–53. doi: 10.1086/587850. [DOI] [PubMed] [Google Scholar]
- Voigt C, Goymann W. Sex-role reversal is reflected in the brain of African black coucals (Centropus grillii) Developmental Neurobiology. 2007;67:1560–1573. doi: 10.1002/dneu.20528. [DOI] [PubMed] [Google Scholar]
- Wingfield J, CRIM J, MATFOCKS P, JR, FARNER D. Responses of photosensitive and photorefractory male white-crowned sparrows (Zonotrichia leucophrys gambelii) to synthetic mammalian luteinizing hormone releasing hormone (Syn-LHRH) Biology of Reproduction. 1979;21:801. doi: 10.1095/biolreprod21.4.801. [DOI] [PubMed] [Google Scholar]
- Wingfield J, Meddle S, Moore I, Busch S, Wacker D, Lynn S, Clark A, Vasquez R, Addis E. Endocrine responsiveness to social challenges in northern and southern hemisphere populations of Zonotrichia. J Ornithol. 2007;148:435–441. [Google Scholar]
- Wingfield JC. Regulatory mechanisms that underlie phenology, behavior, and coping with environmental perturbations: An alternative look at biodiversity. The Auk. 2012;129:1–7. [Google Scholar]
- Wingfield JC, Hegner RE, Dufty AM, Ball GF. The Challenge Hypothesis - Theoretical Implications for Patterns of Testosterone Secretion, Mating Systems, and Breeding Strategies. American Naturalist. 1990;136:829–846. [Google Scholar]
- Wingfield JC, Hegner RE, Lewis DM. Circulating levels of luteinizing hormone and steroid hormones in relation to social status in the cooperatively breeding white-browed sparrow weaver, Plocepasser mahali. Journal of Zoology. 1991;225:43–58. [Google Scholar]
- Wingfield JC, Lynn SE, Soma KK. Avoiding the ‘costs’ of testosterone: Ecological bases of hormone-behavior interactions. Brain Behavior and Evolution. 2001;57:239–251. doi: 10.1159/000047243. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Sapolsky RM. Reproduction and resistance to stress: when and how. Journal Of Neuroendocrinology. 2003;15:711–724. doi: 10.1046/j.1365-2826.2003.01033.x. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Smith JP, Farner DS. Endocrine responses of white-crowned sparrows to environmental stress. Condor. 1982:399–409. [Google Scholar]
- Young LJ, Crews D. Comparative neuroendocrinology of steroid receptor gene expression and regulation: Relationship to physiology and behavior. Trends in endocrinology and metabolism: TEM. 1995;6:317–323. doi: 10.1016/1043-2760(95)00175-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.