Abstract
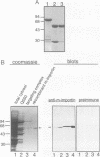
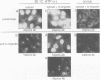
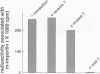
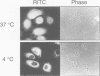
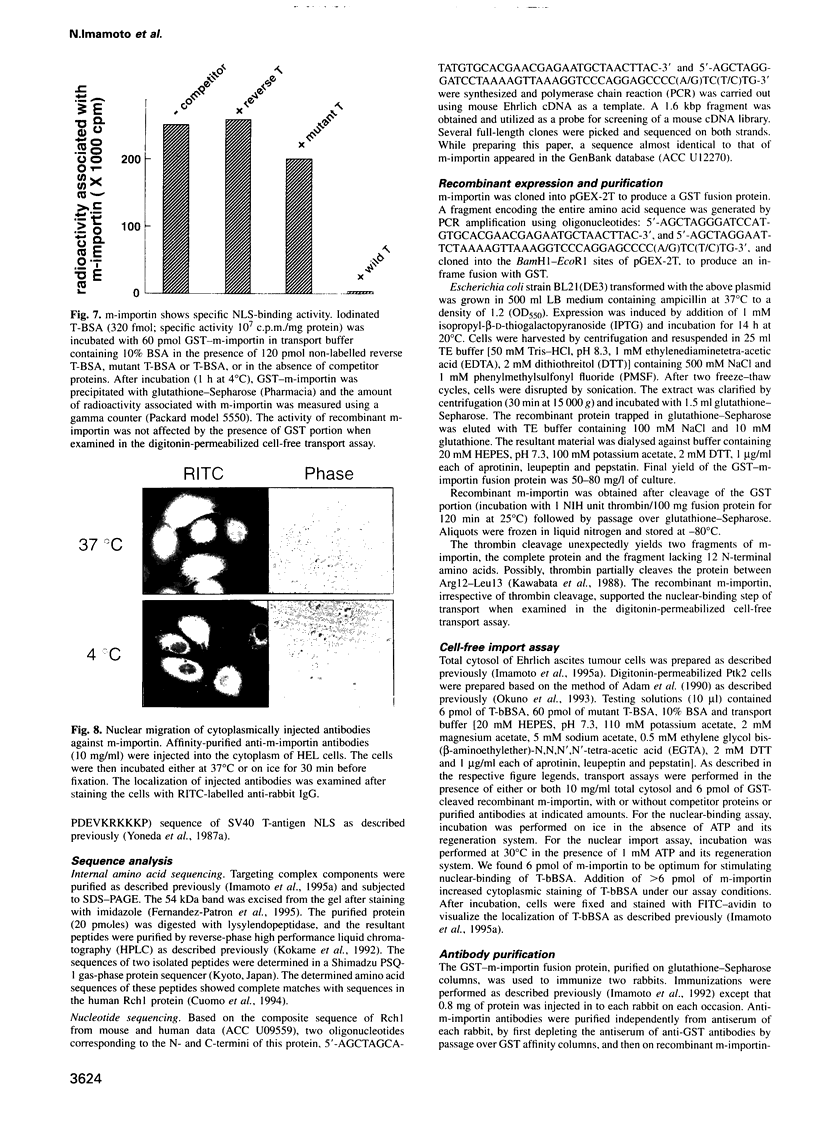
We recently showed that a nuclear location signal (NLS)-containing karyophile forms a stable complex with cytoplasmic components for nuclear pore-targeting The complex, termed nuclear pore-targeting complex (PTAC), contained two essential proteins of 54 and 90 kDa, respectively, as estimated by electrophoresis. In this study, we found that the 54 kDa component of PTAC is the mouse homologue of Xenopus importin (m-importin). Cytoplasmic injection of the antibodies raised against recombinant m-importin showed an inhibitory effect on nuclear import of a karyophile in living mammalian cells. A portion of cytoplasmically injected antibodies migrated rapidly into the nucleus, indicating dynamic movement of this protein across the nuclear envelope. Moreover, the injected antibodies co-precipitated the karyophile, in an NLS-dependent manner, with endogenous m-importin in the cytoplasm. These results provide in vivo evidence that m-importin is involved in nuclear protein import through association with a NLS in the cytoplasm before nuclear pore binding.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam E. J., Adam S. A. Identification of cytosolic factors required for nuclear location sequence-mediated binding to the nuclear envelope. J Cell Biol. 1994 May;125(3):547–555. doi: 10.1083/jcb.125.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam S. A., Gerace L. Cytosolic proteins that specifically bind nuclear location signals are receptors for nuclear import. Cell. 1991 Sep 6;66(5):837–847. doi: 10.1016/0092-8674(91)90431-w. [DOI] [PubMed] [Google Scholar]
- Adam S. A., Marr R. S., Gerace L. Nuclear protein import in permeabilized mammalian cells requires soluble cytoplasmic factors. J Cell Biol. 1990 Sep;111(3):807–816. doi: 10.1083/jcb.111.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akey C. W., Goldfarb D. S. Protein import through the nuclear pore complex is a multistep process. J Cell Biol. 1989 Sep;109(3):971–982. doi: 10.1083/jcb.109.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger K. D., Kenna M. A., Wei S., Davis L. I. Genetic and physical interactions between Srp1p and nuclear pore complex proteins Nup1p and Nup2p. J Cell Biol. 1994 Aug;126(3):619–630. doi: 10.1083/jcb.126.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borer R. A., Lehner C. F., Eppenberger H. M., Nigg E. A. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell. 1989 Feb 10;56(3):379–390. doi: 10.1016/0092-8674(89)90241-9. [DOI] [PubMed] [Google Scholar]
- Bossie M. A., Silver P. A. Movement of macromolecules between the cytoplasm and the nucleus in yeast. Curr Opin Genet Dev. 1992 Oct;2(5):768–774. doi: 10.1016/s0959-437x(05)80137-6. [DOI] [PubMed] [Google Scholar]
- Breeuwer M., Goldfarb D. S. Facilitated nuclear transport of histone H1 and other small nucleophilic proteins. Cell. 1990 Mar 23;60(6):999–1008. doi: 10.1016/0092-8674(90)90348-i. [DOI] [PubMed] [Google Scholar]
- Cuomo C. A., Kirch S. A., Gyuris J., Brent R., Oettinger M. A. Rch1, a protein that specifically interacts with the RAG-1 recombination-activating protein. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6156–6160. doi: 10.1073/pnas.91.13.6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre E., Hurt E. C. Nuclear transport. Curr Opin Cell Biol. 1994 Jun;6(3):335–342. doi: 10.1016/0955-0674(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Featherstone C., Darby M. K., Gerace L. A monoclonal antibody against the nuclear pore complex inhibits nucleocytoplasmic transport of protein and RNA in vivo. J Cell Biol. 1988 Oct;107(4):1289–1297. doi: 10.1083/jcb.107.4.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Patron C., Hardy E., Sosa A., Seoane J., Castellanos L. Double staining of coomassie blue-stained polyacrylamide gels by imidazole-sodium dodecyl sulfate-zinc reverse staining: sensitive detection of coomassie blue-undetected proteins. Anal Biochem. 1995 Jan 1;224(1):263–269. doi: 10.1006/abio.1995.1039. [DOI] [PubMed] [Google Scholar]
- Finlay D. R., Newmeyer D. D., Price T. M., Forbes D. J. Inhibition of in vitro nuclear transport by a lectin that binds to nuclear pores. J Cell Biol. 1987 Feb;104(2):189–200. doi: 10.1083/jcb.104.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes D. J. Structure and function of the nuclear pore complex. Annu Rev Cell Biol. 1992;8:495–527. doi: 10.1146/annurev.cb.08.110192.002431. [DOI] [PubMed] [Google Scholar]
- Garcia-Bustos J., Heitman J., Hall M. N. Nuclear protein localization. Biochim Biophys Acta. 1991 Mar 7;1071(1):83–101. doi: 10.1016/0304-4157(91)90013-m. [DOI] [PubMed] [Google Scholar]
- Imamoto N., Matsuoka Y., Kurihara T., Kohno K., Miyagi M., Sakiyama F., Okada Y., Tsunasawa S., Yoneda Y. Antibodies against 70-kD heat shock cognate protein inhibit mediated nuclear import of karyophilic proteins. J Cell Biol. 1992 Dec;119(5):1047–1061. doi: 10.1083/jcb.119.5.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
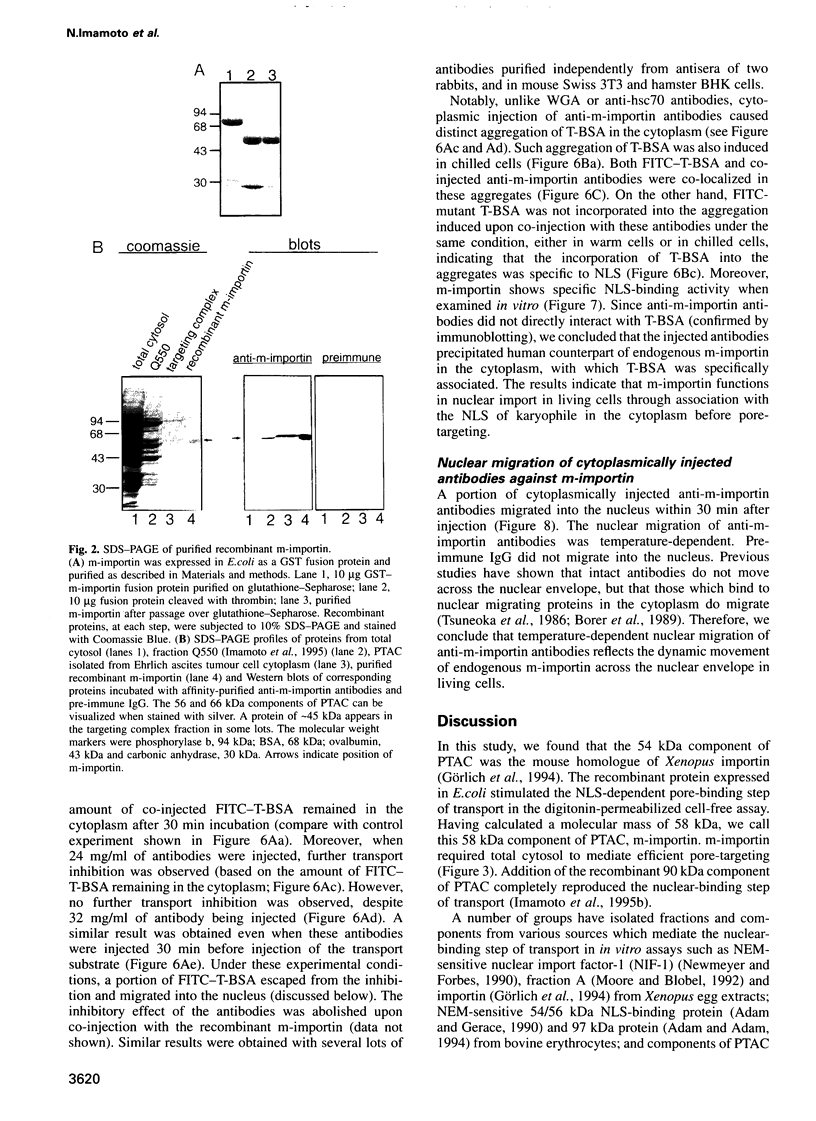
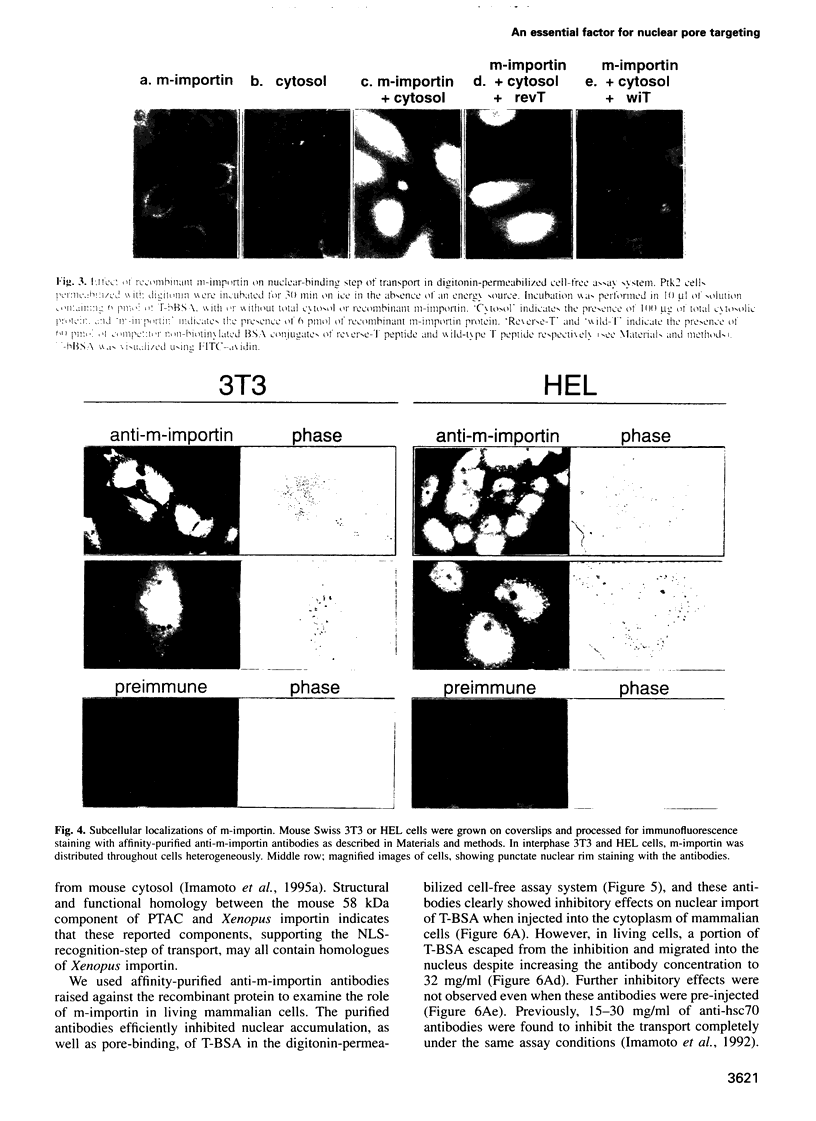
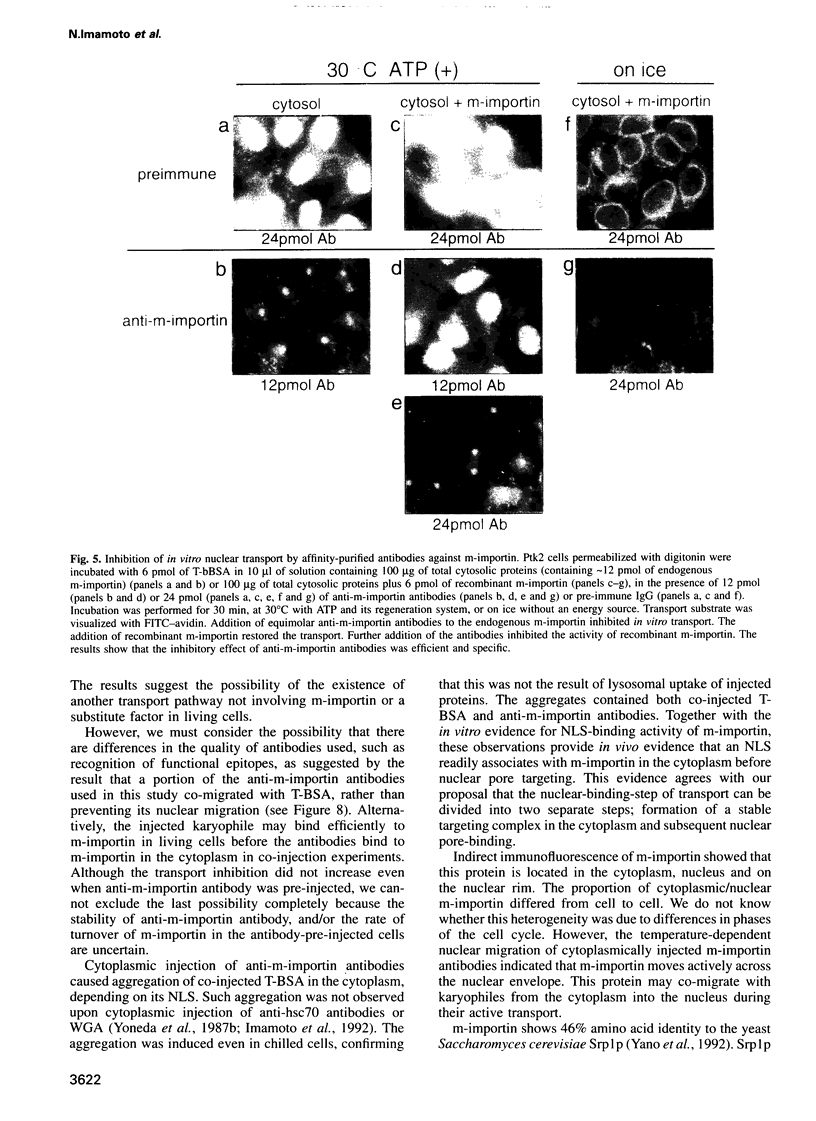
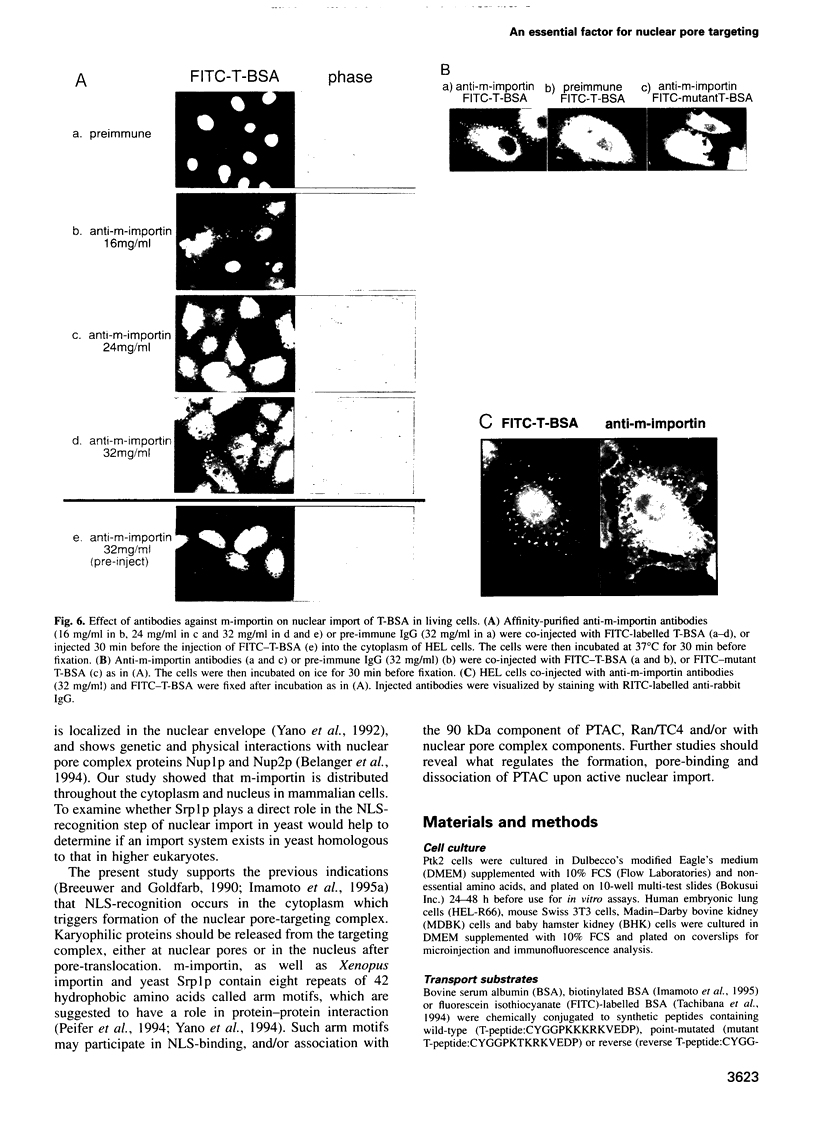
- Imamoto N., Tachibana T., Matsubae M., Yoneda Y. A karyophilic protein forms a stable complex with cytoplasmic components prior to nuclear pore binding. J Biol Chem. 1995 Apr 14;270(15):8559–8565. doi: 10.1074/jbc.270.15.8559. [DOI] [PubMed] [Google Scholar]
- Kawabata S., Miura T., Morita T., Kato H., Fujikawa K., Iwanaga S., Takada K., Kimura T., Sakakibara S. Highly sensitive peptide-4-methylcoumaryl-7-amide substrates for blood-clotting proteases and trypsin. Eur J Biochem. 1988 Feb 15;172(1):17–25. doi: 10.1111/j.1432-1033.1988.tb13849.x. [DOI] [PubMed] [Google Scholar]
- Kokame K., Fukada Y., Yoshizawa T., Takao T., Shimonishi Y. Lipid modification at the N terminus of photoreceptor G-protein alpha-subunit. Nature. 1992 Oct 22;359(6397):749–752. doi: 10.1038/359749a0. [DOI] [PubMed] [Google Scholar]
- Melchior F., Paschal B., Evans J., Gerace L. Inhibition of nuclear protein import by nonhydrolyzable analogues of GTP and identification of the small GTPase Ran/TC4 as an essential transport factor. J Cell Biol. 1993 Dec;123(6 Pt 2):1649–1659. doi: 10.1083/jcb.123.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. S., Blobel G. Purification of a Ran-interacting protein that is required for protein import into the nucleus. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10212–10216. doi: 10.1073/pnas.91.21.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. S., Blobel G. The GTP-binding protein Ran/TC4 is required for protein import into the nucleus. Nature. 1993 Oct 14;365(6447):661–663. doi: 10.1038/365661a0. [DOI] [PubMed] [Google Scholar]
- Moore M. S., Blobel G. The two steps of nuclear import, targeting to the nuclear envelope and translocation through the nuclear pore, require different cytosolic factors. Cell. 1992 Jun 12;69(6):939–950. doi: 10.1016/0092-8674(92)90613-h. [DOI] [PubMed] [Google Scholar]
- Newmeyer D. D., Forbes D. J. An N-ethylmaleimide-sensitive cytosolic factor necessary for nuclear protein import: requirement in signal-mediated binding to the nuclear pore. J Cell Biol. 1990 Mar;110(3):547–557. doi: 10.1083/jcb.110.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmeyer D. D., Forbes D. J. Nuclear import can be separated into distinct steps in vitro: nuclear pore binding and translocation. Cell. 1988 Mar 11;52(5):641–653. doi: 10.1016/0092-8674(88)90402-3. [DOI] [PubMed] [Google Scholar]
- Okuno Y., Imamoto N., Yoneda Y. 70-kDa heat-shock cognate protein colocalizes with karyophilic proteins into the nucleus during their transport in vitro. Exp Cell Res. 1993 May;206(1):134–142. doi: 10.1006/excr.1993.1129. [DOI] [PubMed] [Google Scholar]
- Peifer M., Berg S., Reynolds A. B. A repeating amino acid motif shared by proteins with diverse cellular roles. Cell. 1994 Mar 11;76(5):789–791. doi: 10.1016/0092-8674(94)90353-0. [DOI] [PubMed] [Google Scholar]
- Powers M. A., Forbes D. J. Cytosolic factors in nuclear transport: what's importin? Cell. 1994 Dec 16;79(6):931–934. doi: 10.1016/0092-8674(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Richardson W. D., Mills A. D., Dilworth S. M., Laskey R. A., Dingwall C. Nuclear protein migration involves two steps: rapid binding at the nuclear envelope followed by slower translocation through nuclear pores. Cell. 1988 Mar 11;52(5):655–664. doi: 10.1016/0092-8674(88)90403-5. [DOI] [PubMed] [Google Scholar]
- Rout M. P., Wente S. R. Pores for thought: nuclear pore complex proteins. Trends Cell Biol. 1994 Oct;4(10):357–365. doi: 10.1016/0962-8924(94)90085-x. [DOI] [PubMed] [Google Scholar]
- Shi Y., Thomas J. O. The transport of proteins into the nucleus requires the 70-kilodalton heat shock protein or its cytosolic cognate. Mol Cell Biol. 1992 May;12(5):2186–2192. doi: 10.1128/mcb.12.5.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana T., Imamoto N., Seino H., Nishimoto T., Yoneda Y. Loss of RCC1 leads to suppression of nuclear protein import in living cells. J Biol Chem. 1994 Oct 7;269(40):24542–24545. [PubMed] [Google Scholar]
- Tsuneoka M., Imamoto N. S., Uchida T. Monoclonal antibody against non-histone chromosomal protein high mobility group 1 Co-migrates with high mobility group 1 into the nucleus. J Biol Chem. 1986 Feb 5;261(4):1829–1834. [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. Has the breast cancer gene been found? Cell. 1994 Oct 7;79(1):1–3. doi: 10.1016/0092-8674(94)90393-x. [DOI] [PubMed] [Google Scholar]
- Yang J., DeFranco D. B. Differential roles of heat shock protein 70 in the in vitro nuclear import of glucocorticoid receptor and simian virus 40 large tumor antigen. Mol Cell Biol. 1994 Aug;14(8):5088–5098. doi: 10.1128/mcb.14.8.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano R., Oakes M. L., Tabb M. M., Nomura M. Yeast Srp1p has homology to armadillo/plakoglobin/beta-catenin and participates in apparently multiple nuclear functions including the maintenance of the nucleolar structure. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):6880–6884. doi: 10.1073/pnas.91.15.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano R., Oakes M., Yamaghishi M., Dodd J. A., Nomura M. Cloning and characterization of SRP1, a suppressor of temperature-sensitive RNA polymerase I mutations, in Saccharomyces cerevisiae. Mol Cell Biol. 1992 Dec;12(12):5640–5651. doi: 10.1128/mcb.12.12.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda Y., Arioka T., Imamoto-Sonobe N., Sugawa H., Shimonishi Y., Uchida T. Synthetic peptides containing a region of SV 40 large T-antigen involved in nuclear localization direct the transport of proteins into the nucleus. Exp Cell Res. 1987 Jun;170(2):439–452. doi: 10.1016/0014-4827(87)90319-3. [DOI] [PubMed] [Google Scholar]
- Yoneda Y., Imamoto-Sonobe N., Yamaizumi M., Uchida T. Reversible inhibition of protein import into the nucleus by wheat germ agglutinin injected into cultured cells. Exp Cell Res. 1987 Dec;173(2):586–595. doi: 10.1016/0014-4827(87)90297-7. [DOI] [PubMed] [Google Scholar]