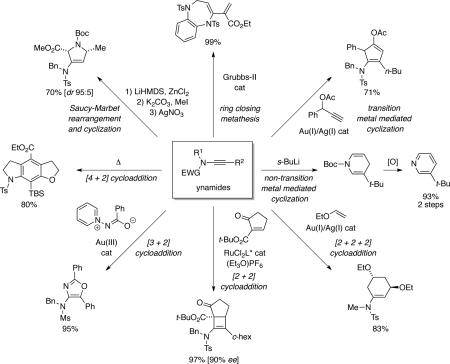
Conspectus
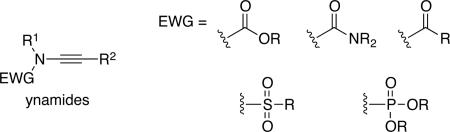
The ynamide functional group activates carbon-carbon triple bonds through an attached nitrogen atom that bears an electron-withdrawing group. As a result, the alkyne has both electrophilic and nucleophilic properties. Through the selection of the electron-withdrawing group attached to nitrogen chemists can modulate the electronic properties and reactivity of ynamides, making these groups versatile synthetic building blocks. The reactions of ynamides also lead directly to nitrogen-containing products, which provides access to important structural motifs found in natural products and molecules of medicinal interest. Therefore, researchers have invested increasing time and research in the chemistry of ynamides in recent years.
This Account surveys and assesses new organic transformations involving ynamides developed in our laboratory and in others around the world. We showcase the synthetic power of ynamides for rapid assembly of complex molecular structures. Among the recent reports of ynamide transformations, ring-forming reactions provide a powerful tool for generating molecular complexity quickly. In addition to their synthetic utility, such reactions are mechanistically interesting. Therefore, we focus primarily on the cyclization chemistry of ynamides.
This Account highlights ynamide reactions that are useful in the rapid synthesis of cyclic and polycyclic structural manifolds. We discuss the mechanisms active in the ring formations and describe representative examples that demonstrate the scope of these reactions and provide mechanistic insights. In this discussion we feature examples of ynamide reactions involving radical cyclizations, ring-closing metathesis, transition metal and non-transition metal mediated cyclizations, cycloaddition reactions, and rearrangements. The transformations presented rapidly introduce structural complexity and include nitrogen within, or in close proximity to, a newly formed ring (or rings). Thus, ynamides have emerged as powerful synthons for nitrogen-containing heterocycles and nitrogen-substituted rings, and we hope this Account will promote continued interest in the chemistry of ynamides.
1. Introduction
Over the past 15 years, ynamides have become a modern functional group that has been prominently featured in a variety of synthetic transformations including natural product total syntheses.1-3 Fueled by preparative access that is efficient and atom economical,4,5 the field of ynamide chemistry has rapidly expanded. Ynamides provide a means of activating carbon-carbon triple bonds, giving them both electrophilic and nucleophilic properties. The electronic properties of ynamides are tunable based on selection of the electron-withdrawing group attached to nitrogen, thereby rendering them highly versatile synthons. Furthermore, among all heteroatom-substituted alkynes, ynamides are special because nitrogen is one of the most privileged elements in nature. Consequently, many transformations involving ynamides offer a diverse array of novel structural entities that are not only powerful platforms for further transformations, but also prevalent among important pharmacophores. These properties have contributed to a continued and dramatic increase in the number of publications in the last few years.
This Account aims to examine the literature from late 2009 through early 2013 related to the use of ynamides in synthetic transformations that form cyclic and polycyclic manifolds. Consequently, the Account is organized by reaction types used in the ring formation, and representative examples are selected to showcase scope and mechanistic insight of each transformation. The intention here is not to comprehensively review the ynamide chemistry that appeared during this period, but to highlight major advancements in ynamide chemistry through revelation of its utility in rapid assembly of structural complexity. As a result, some beautiful recent works are not presented here. This includes improved ynamide preparations and syntheses of de novo structural analogs of ynamides. Both topics have been the subject of a thorough review published recently by Evano.4 In addition, new advances related to in situ generation of ketenimines or metallo-ynamides via Huisgen's azide-[3 + 2] cycloaddition/retro-[3 + 2] are not covered here.6
2. Radical Cyclizations
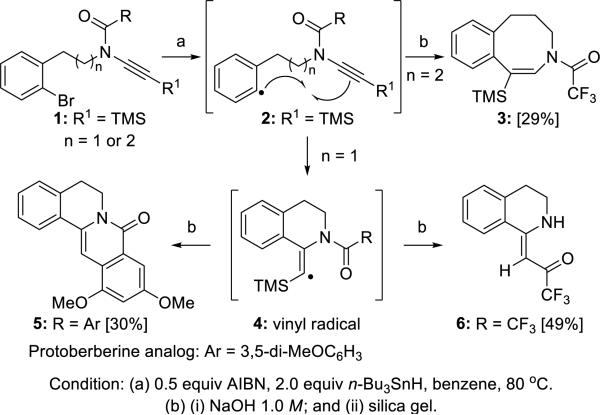
Balieu and Courillon7 reported the formation of sixmembered rings 5 and 6, and the eight-membered ring 3 via the radical cyclization of ynamides 1 (Scheme 1). A tandem radical cyclization also took place when R was 3,5-dimethoxyphenyl through vinyl radical 4.
Scheme 1.
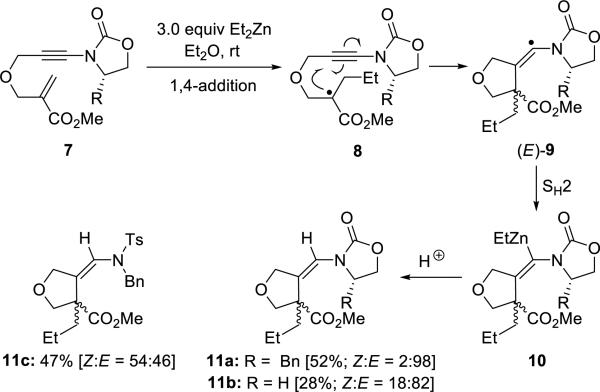
Chemla and Perez-Luna8 reported synthesis of 3-alkylidenetetrahydrofurans 11 from ynamides 7 through a 1,4-addition/alkyne carbozincation sequence based on a radical zinc-atom transfer process (Scheme 2). The addition of ethyl radical gave enoxy radical 8, which underwent 5-exo-dig cyclization with the ynamide leading to vinyl radical 9 of E-geometry.
Scheme 2.
3. Ring-Closing Metathesis
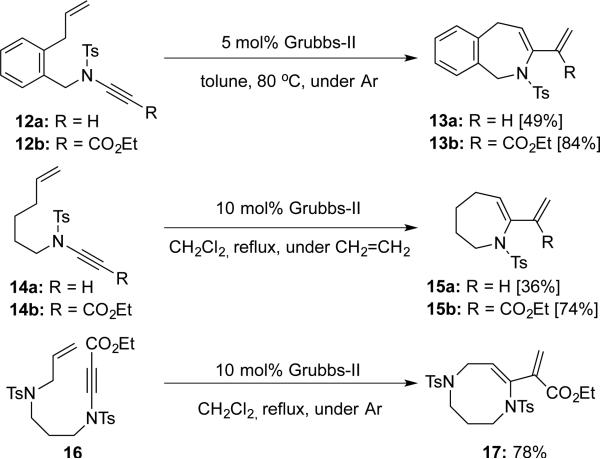
Wakamatsu and Mori9 reported further development of their ring-closing metathesis of ene-ynamides, featuring syntheses of 7-membered heterocycles 13, 15 and 8-membered heterocycles 17 using 2nd-generation Grubbs catalyst (Scheme 3).
Scheme 3.
4. Non-Transition Metal Mediated Cyclizations
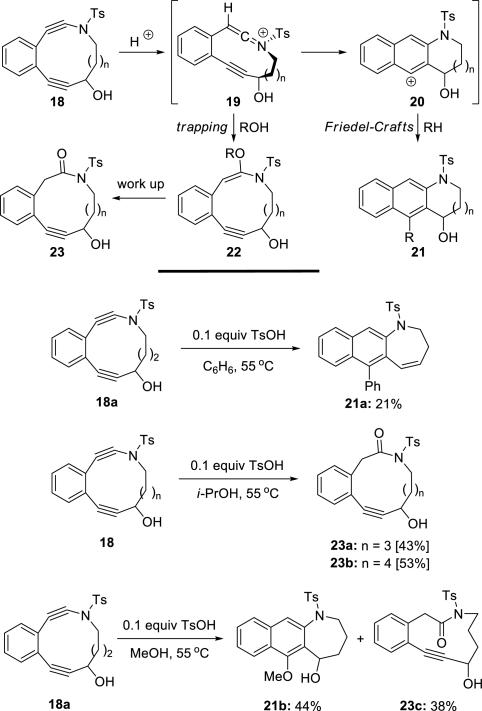
Popik10 reported acid catalyzed cycloaromatizations of cyclic ynamides 18 (Scheme 4). Protonation of the ynamide followed by addition of the alkyne onto the resulting keteniminium ion 19 provided cation 20, which underwent Friedel-Crafts additions. Alcoholic solvent trapping of 19 competed with cycloaromatization, especially with increasing in ring size.
Scheme 4.
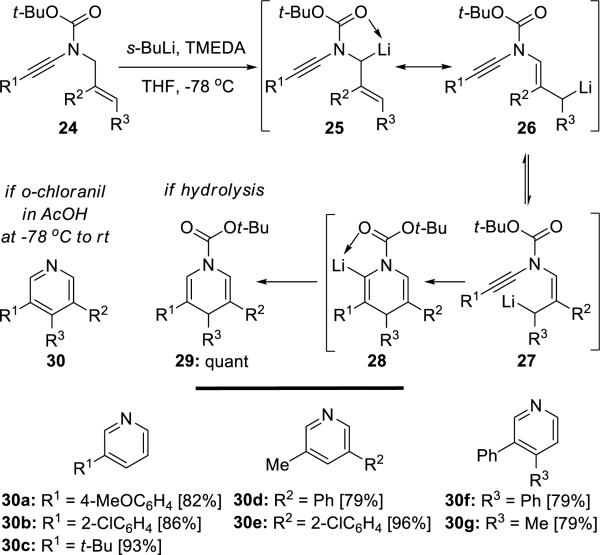
Evano11 developed a general and efficient approach toward 1,4-dihydropyridines 29 and pyridines 30 from readily available N-allyl-ynamides 24 via a tandem lithiation–isomerization–6-endo-dig intramolecular carbolithiation sequence (Scheme 5).
Scheme 5.
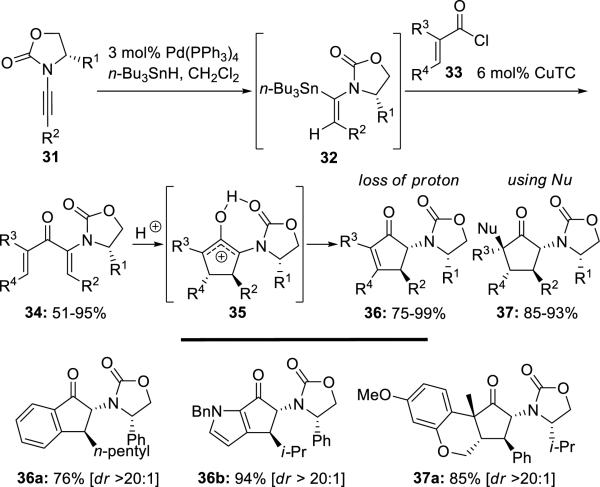
Flynn12 reported a highly torquoselective Nazarov cyclization of 2-amido divinyl ketones 34 derived from chiral oxazolidinone-substituted ynamides 31 (Scheme 6). The diastereoselectivity can be very high, and Nazarov arrested products such as 37a could also be obtained.
Scheme 6.
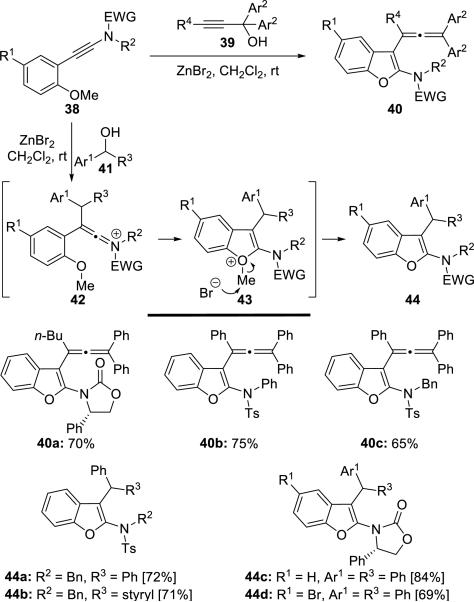
Cao13 described an efficient approach toward 3-allenyl-2-amidobenzofurans 40 and 3-alkyl-2-amidobenzofurans 44 via a novel carbocation-induced electrophilic cyclization of o-anisole-substituted ynamides with 1,1-diaryl-prop-2-yn-1-ol 39 and diarylmethanol 41, respectively (Scheme 7).
Scheme 7.
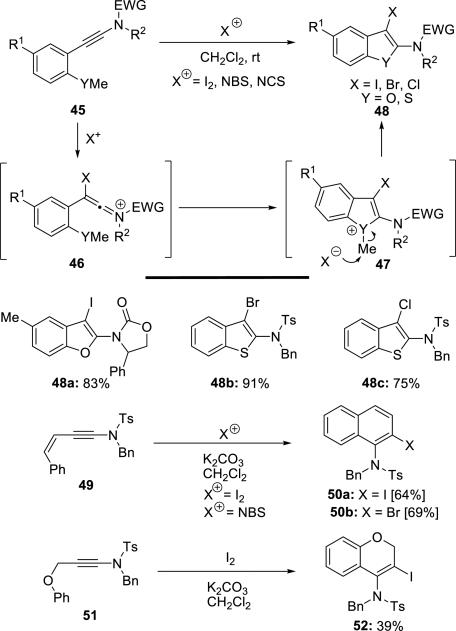
Later, Cao14 developed a novel synthesis of 2-amidobenzofurans and 2-amidobenzothiophenes via electrophilic cyclization of o-anisole- and o-thioanisole-substituted ynamides 45 with I2, NBS, and NCS. This strategy was also used to construct 1-amidonaphthalenes 50 and 1-amidobenzopyrans 52 from ynamides (Scheme 8).
Scheme 8.
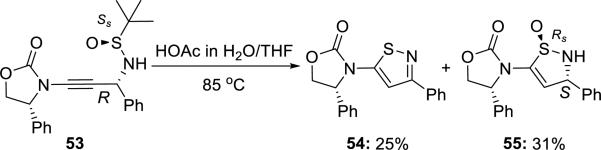
Hsung15 unveiled a novel acid promoted 5-endo-dig cyclization of chiral γ-amino-ynamide 53 concomitant with the loss of the t-Bu group that led to the formation of isothaizole 54 and dihydroisothaizole S-oxide 55. An inversion at the “S” center occurred in 55 (Scheme 9).
Scheme 9.
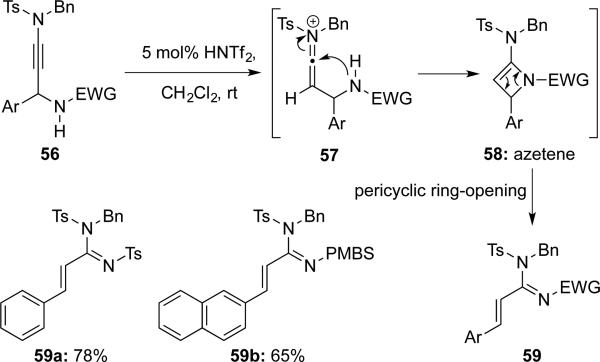
Hsung16 featured an aza-variant of a Meyer-Schuster rearrangement of γ-amino-ynamides 56, which involved the formation of azetene intermediate 58 and pericyclic ring-opening (Scheme 10).
Scheme 10.
5. Transition Metal Mediated Cyclizations
5.1. Rhodium
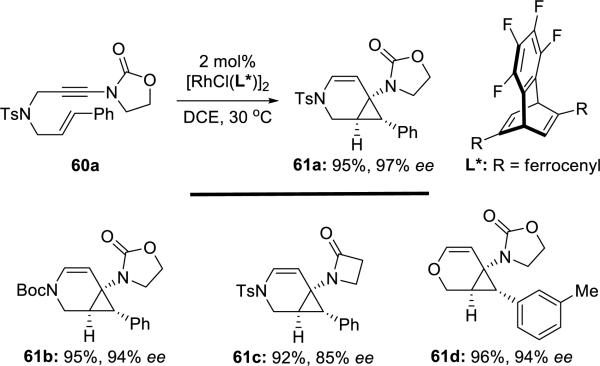
Nishimura and Hayashi17 disclosed a rhodium-catalyzed asymmetric cycloisomerization of heteroatom-bridged 1,6-enynamides such as 60 to afford 3-aza and oxabicyclo[4.1.0]heptene derivatives such as 61a (Scheme 11). 2-oxazolidinone and 2-azetidinone substituents of the ynamides were critical for high enantioselectivities, as the carbonyl oxygen might coordinate to the metal during the transformation.
Scheme 11.
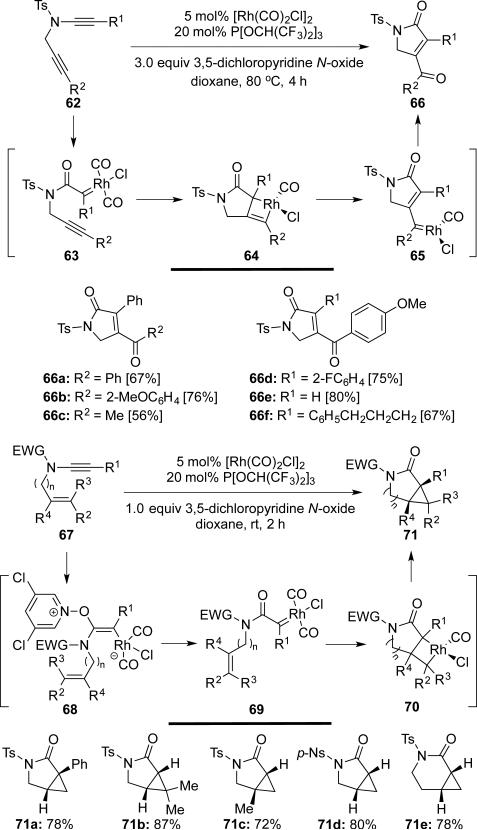
Tang18 developed an efficient method for the generation of α-oxo Rh(I) carbenes 63 and 69 from ynamides with 3,5-dichloropyridine N-oxide. The resulting Rh(I) carbenes then react intramolecularly with various alkynes or alkenes affording 2-oxopyrrolidines 66 and 3-azabicyclo[3.1.0]hexanes 71, respectively (Scheme 12).
Scheme 12.
5.2. Palladium
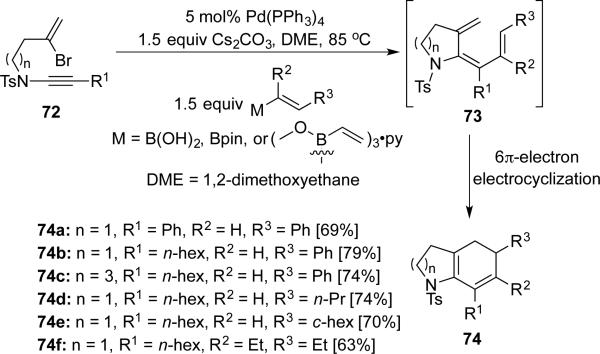
Anderson19 reported a palladium-catalyzed tandem cascade of cyclization–cross-coupling–6π-electron electrocyclization using bromoenynamides 72 (Scheme 13).
Scheme 13.
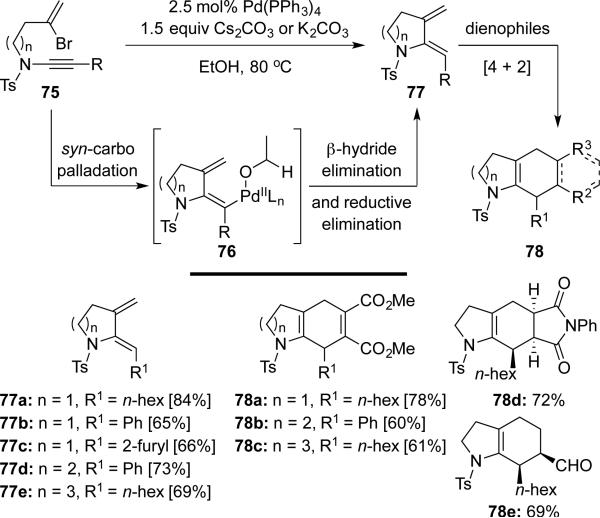
Anderson20 subsequently reported a related palladium-catalyzed cascade using bromoenynamides 75 affording cyclic 2-amidodienes 77 that could be used in ensuing Diels-Alder cycloadditions (Scheme 14). An alcohol served as a hydride source to terminate the carbopalladation process.
Scheme 14.
5.3. Platinum
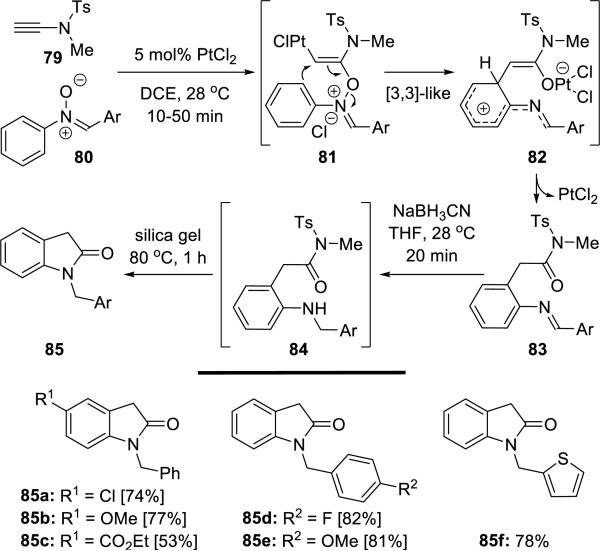
Liu21 reported an equivalent of Pt(II)-catalyzed oxo-arylations of ynamide 79 (Scheme 15). This process employs nitrones 80 and provides imines 83 via intermediates 81 and 82, and under reductive conditions using NaBH3CN, 2-indolones 85 were obtained.
Scheme 15.
5.4. Copper
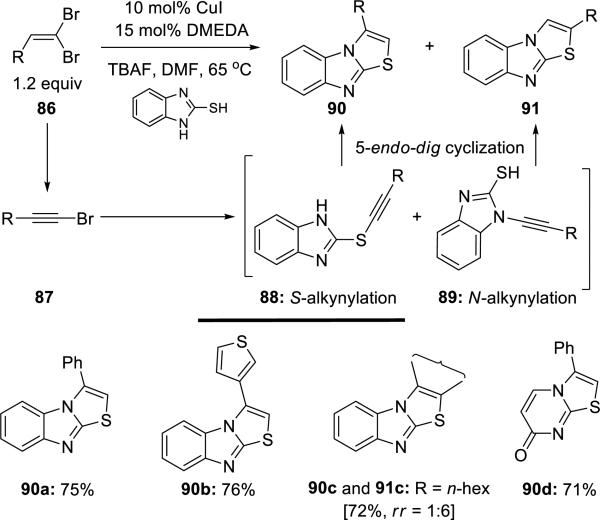
Chen22 reported a Cu(I)-catalyzed 1,2-aminothiolation of 1,1-dibromoalkenes 86 with 2-thiobenzoimidazole, leading to thiazolines 90 and 91 (Scheme 16). The regiochemistry of the 1,2-aminothiolation depends on whether it is 5-endo dig cyclization of S- or N-alkynylation intermediates (88 or 89).
Scheme 16.
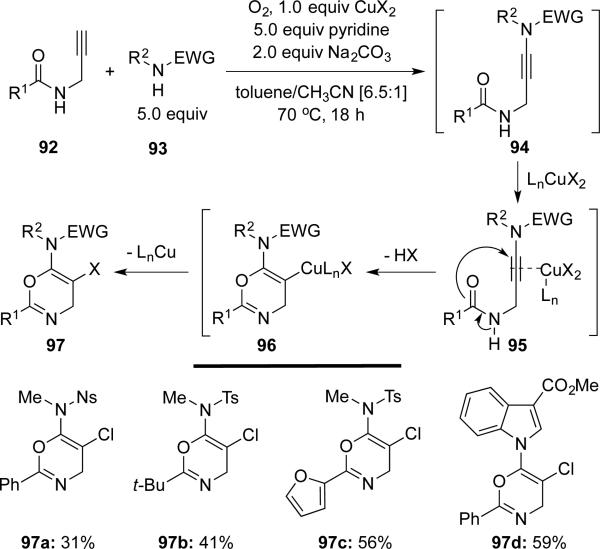
Hashmi23 reported a copper-mediated domino reaction of three simple components that included propargylcarboxamide 92, protected amine 93, and a chloride source. This cascade provides an efficient construction of highly functionalized oxazines 97 (Scheme 17).
Scheme 17.
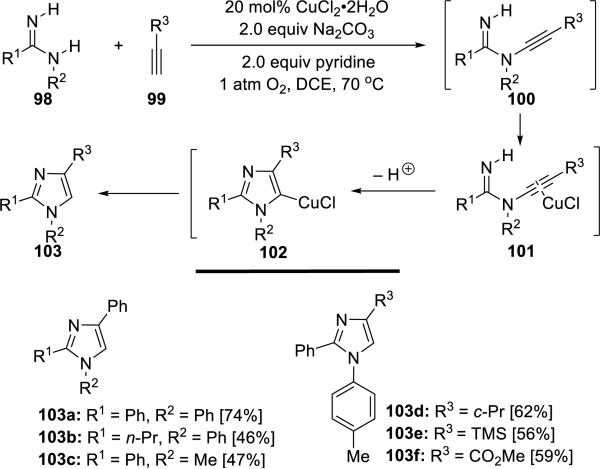
Neuville24 developed an efficient and regioselective approach to 1,2,4-trisubstituted imidazoles 103 via a copper-catalyzed oxidative diamination of terminal alkynes 99 by amidines 98 (Scheme 18). This transformation employs oxygen as the co-oxidant and proceeds through a direct N-alkynylation of the terminal acetylenes, thereby rendering the process atom economical.
Scheme 18.
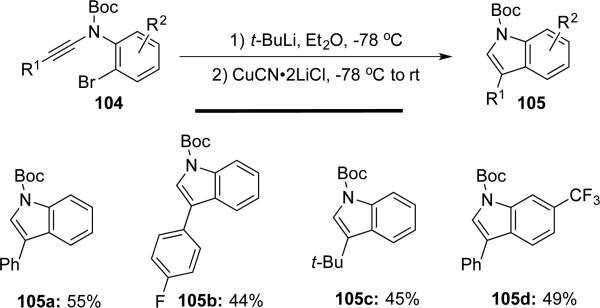
Evano25 reported a modular synthesis of polysubstituted indoles 105 from N-aryl-ynamides 104. A bromine/lithium exchange of N-(2-bromoaryl)ynamides 104, followed by a 5-endo-dig carbocupration afforded the substituted indoles efficiently (Scheme 19).
Scheme 19.
5.5. Silver
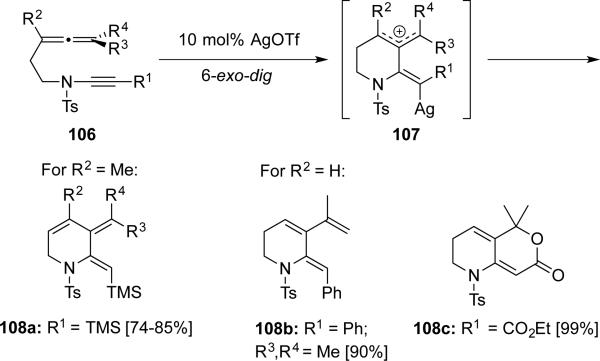
Malacria, Fentsterbank, and Aubert26 reported a silver-catalyzed cycloisomerization of de novo allene-ynamides 106, leading to amide-substituted cross-conjugated trienes 108 that are useful for tandem Diels-Alder cycloadditions (Scheme 20).
Scheme 20.
5.6. Gold
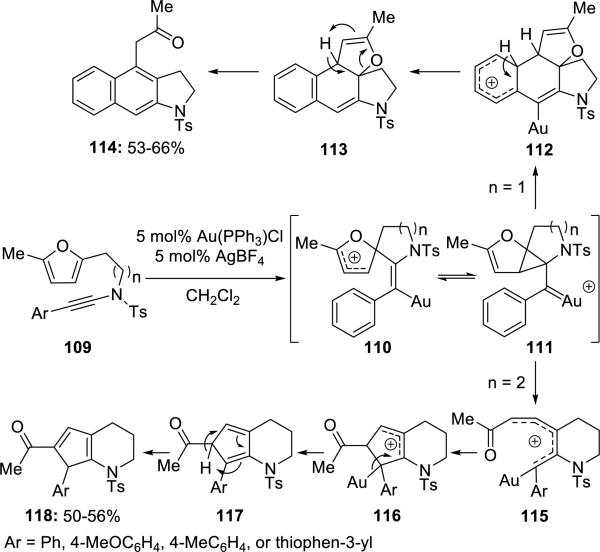
Hashimi27 reported a gold-catalyzed cyclization of furanyl-ynamides 109 (Scheme 21). The course of the cyclization depended upon the length of the tether. Benzoanellated heterocycles 114 were produced when n = 1, while cyclopentadiene fused piperidines 118 were obtained when n = 2.
Scheme 21.
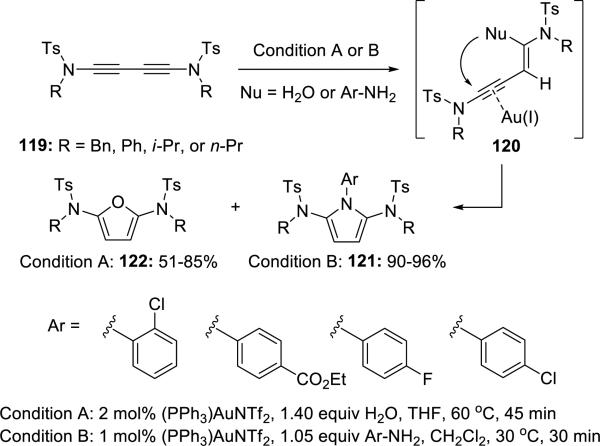
Skrydstrup28 reported Au(I)-catalyzed hydroaminations or hydrations of diynamides 119 to access 2,5-diamidopyrroles 121 and 2,5-diamidofurans 122,respectively (Scheme 22). This development represents a clever application of diynamides.
Scheme 22.
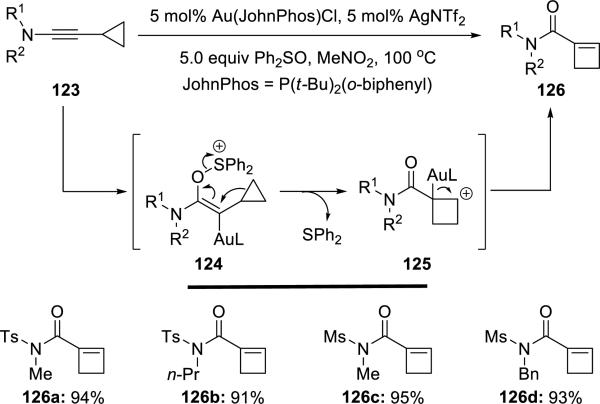
Liu29 reported highly regioselective Au(I)-catalyzed oxidative ring-expansions of cyclopropyl-substituted ynamides 123 using Ph2SO (Scheme 23). The ring expansion is not believed to proceed through an α-keto gold carbenoid intermediate but through 124. Subsequently, Li30 independently reported a similar gold-catalyzed oxidative ring-expansion (details not shown here).
Scheme 23.
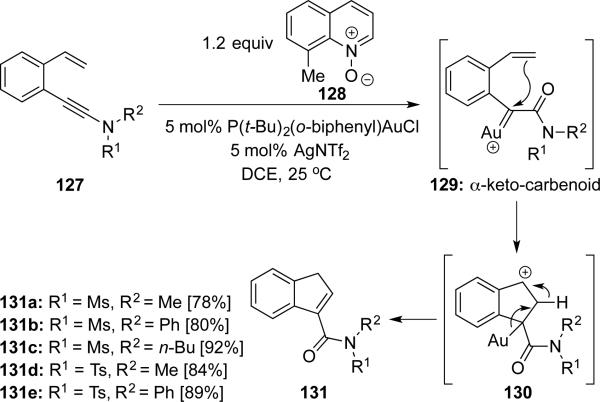
Liu31 then explored 1,5-enynamides 127, developing a Au(I)-catalyzed oxidative cyclization using 8-methylquinoline N-oxide 128 as the external oxidant to construct 3-carboxyamidoindenes 131 (Scheme 24). The transformation is believed to proceed through α-ketocarbenoid 129.
Scheme 24.
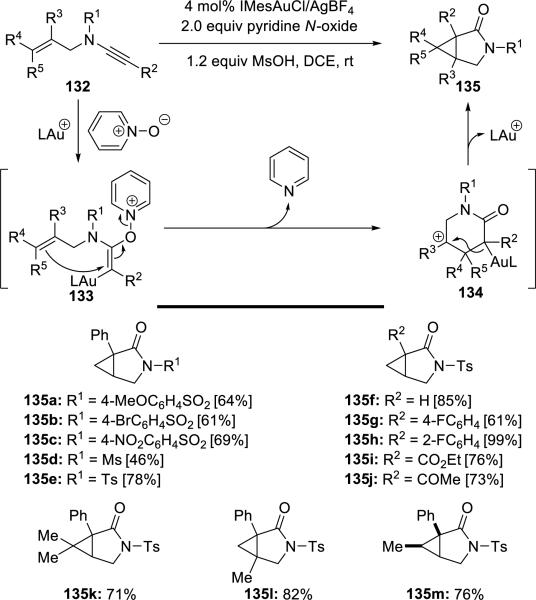
Li32 reported a clever synthesis of 3-aza-bicyclo[3.1.0]hexan-2-one derivatives 135 via Au(I)-catalyzed oxidative cyclopropanations of N-allylynamides 132 using pyridine N-oxide as the external oxidant (Scheme 25).
Scheme 25.
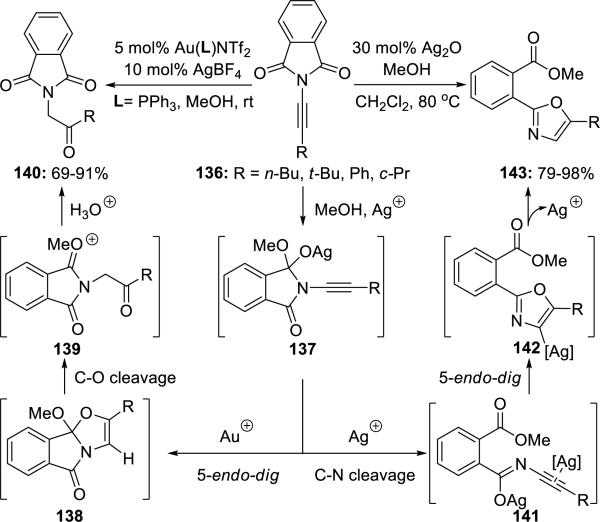
Sueda33 reported both Ag(I)- and/or Au(I)-catalyzed cyclizations of de novo ynimides 136, which could be accessed for the first time. These cyclizations gave β-ketoimides 140 when using both Au(I) and Ag(I), while providing oxazoles 143 when using only Ag(I) (Scheme 26).
Scheme 26.
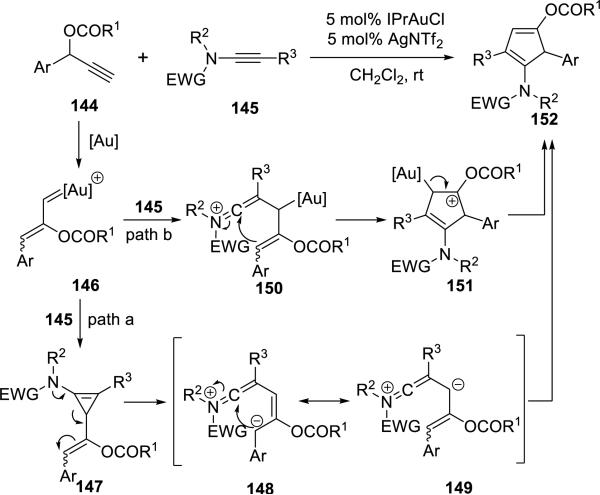
Hashmi34 reported synthesis of highly functionalized cyclopentadienes 152 in moderate to good yields via Au(I)-catalyzed intermolecular cyclization of propargylic carboxylates 144 and ynamides 145 (Scheme 27).
Scheme 27.
Sahoo35 developed Au(I)-catalyzed hydrative cyclization of easily accessible 5-yne-ynamides 153, giving substituted 1,6-dihydropyridin-2(3H)ones 157 in good to excellent yields (Scheme 28). A mechanism involving a 6-endo-dig cyclization of intermediate 155 was proposed.
Scheme 28.
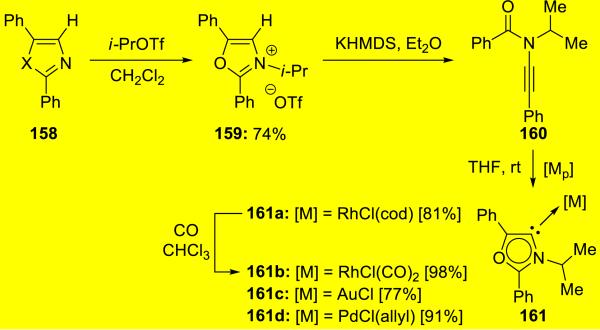
Bertrand36 reported that deprotonation of oxazolium salt 159 initiated an interesting ring opening process giving ynamide 160 (Scheme 29). The acyclic ynamide readily reacts with various transition metals affording robust mesoionic carbene complexes 161.
Scheme 29.
6. Cycloadditions and Formal Cycloadditions
6.1 [2 + 1]
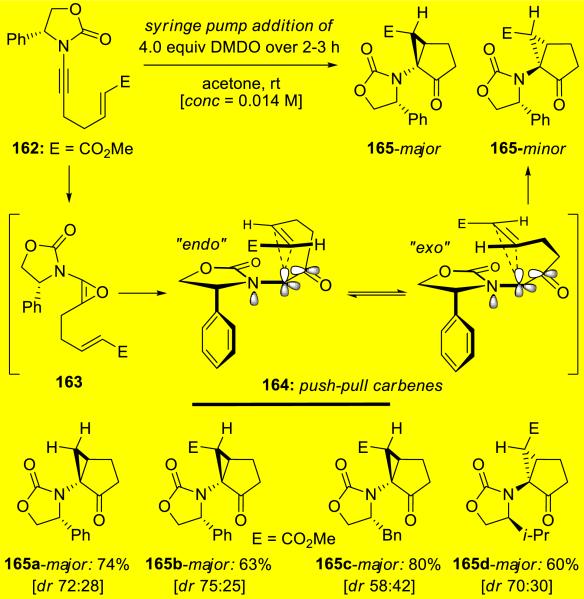
Hsung37 reported the first examples of stereoselective intramolecular cyclopropanations via a de novo class of push-pull carbenes derived from DMDO-epoxidations of chiral ynamides 162 (Scheme 30). This tandem epoxidation-cyclopropanation afforded a series of structurally unique amido-cyclopropanes 165.
Scheme 30.
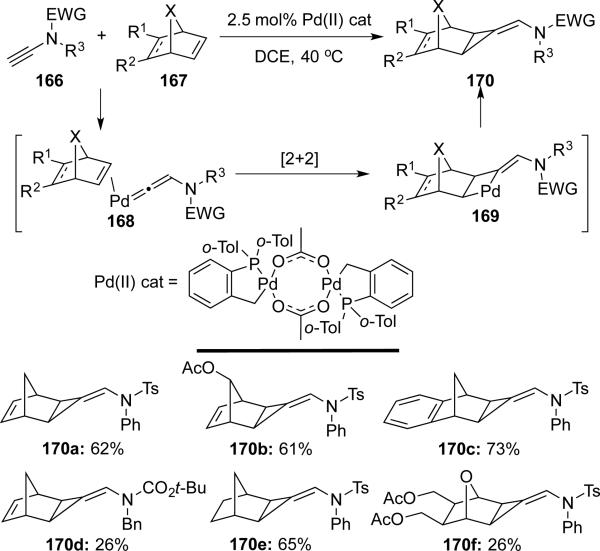
Buono38 reported an unusual palladium catalyzed [2 + 1] cycloaddition of ynamides 166 with norbornene derivatives 167 giving various substituted aminomethylenecyclopropanes 170 (Scheme 31). Based on their previous study in alkyne system,39 this process may involve a [2 + 2] cycloaddition of palladium vinylidene species with the double bonds of norbornene derivatives, followed by reductive elimination to furnish cyclopropanes.
Scheme 31.
6.2 [2 + 2]
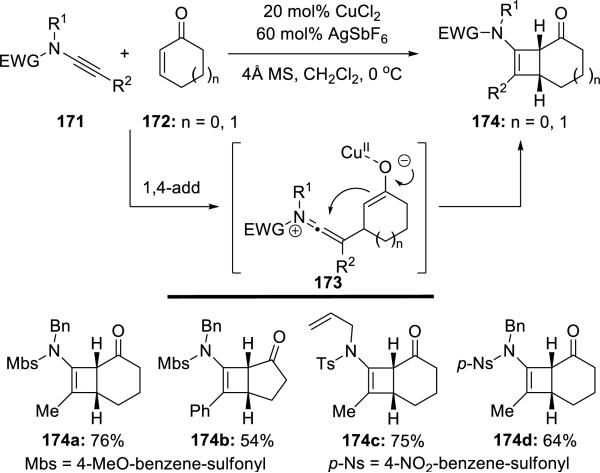
Hsung40 reported the first successful example of Ficini's [2 + 2] cycloaddition of ynamides 171 with enones 172 using CuCl2 and AgSbF6 as catalysts (Scheme 32).
Scheme 32.
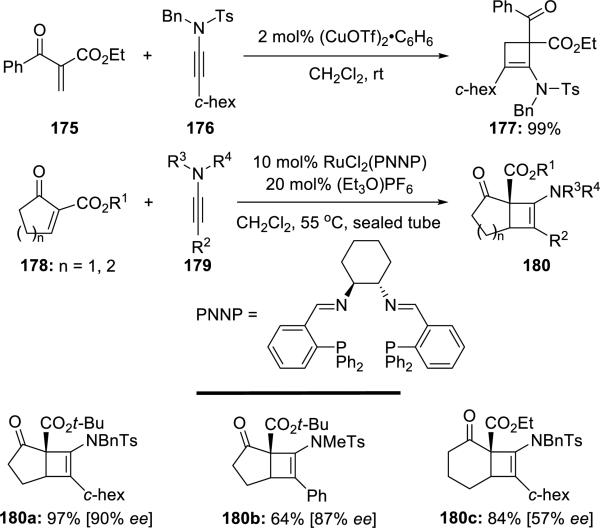
Mezzetti41 subsequently reported Cu(OTf)2 promoted Ficini [2 + 2] cycloadditions of ynamides with unsaturated β-keto esters in addition to a beautiful asymmetric variant using dicationic ruthenium(II)/PNNP complex (Scheme 33).
Scheme 33.
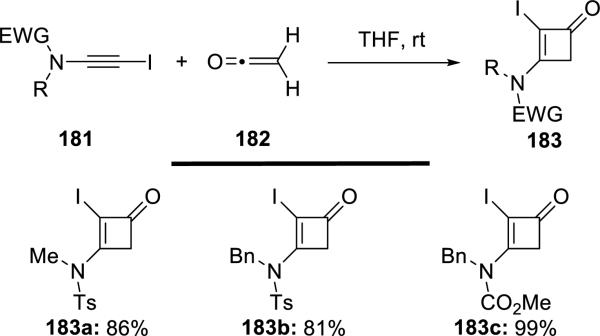
Danheiser42 reported the first examples of thermal [2 + 2] cycloadditions of 2-iodoynamides 181 with ketenes 182, leading to 3-amido-2-iodocyclobutenones 183 (Scheme 34).
Scheme 34.
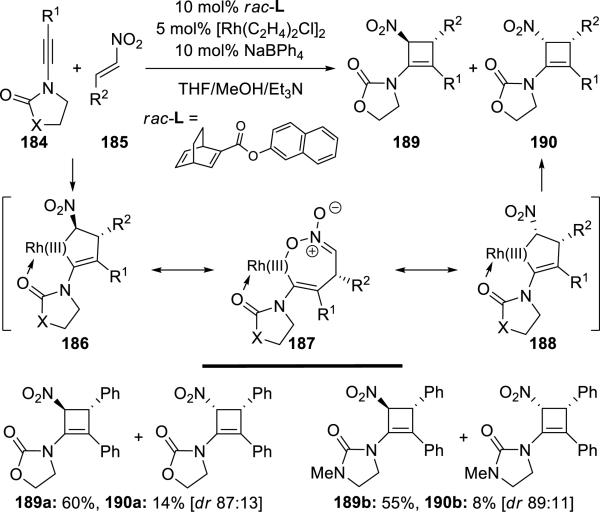
Lam43 reported a [2 + 2] cycloaddition of ynamides 184 with nitroalkenes 185 catalyzed by a dirhodium complex and sodium tetraphenylborate, leading to nitro-substituted cyclobutenamides 189 and 190 (Scheme 35).
Scheme 35.
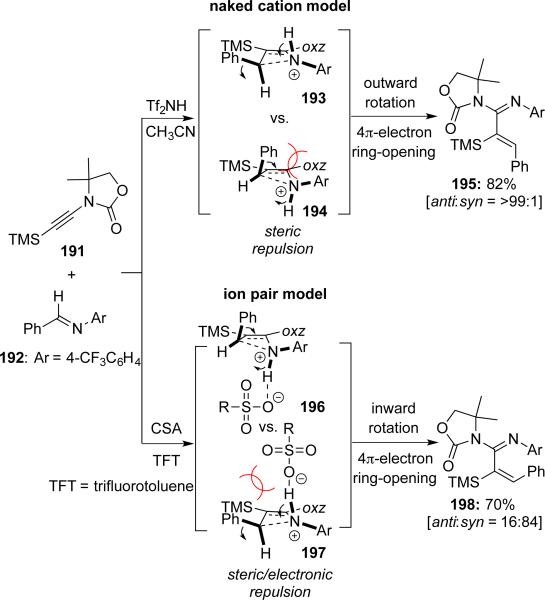
Takasu and Takemoto44 reported selective syntheses of either syn or anti isomers of α,β-unsaturated amidines 195 and 198 through a tandem cascade of aza-[2 + 2] cycloaddition–4π-electron pericyclic ring-opening by using Tf2NH or CSA as catalyst, respectively (Scheme 36). The torquoselectivity of ring-opening was controlled by the Brønsted acidity of the catalyst and the polarity of the solvent.
Scheme 36.
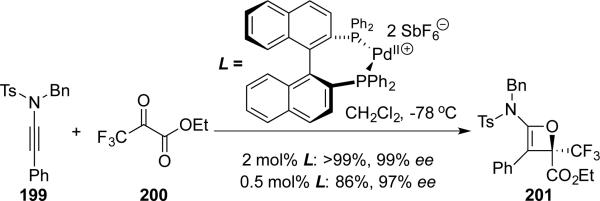
Mikami45 reported an asymmetric [2 + 2] cycloaddition of ynamide 199 with ethyl trifluoropyruvate 200 using a dicationic (S)-BINAP-Pd catalyst with excellent yield and enantioselectivity. This is also the first enantioselective synthesis of a stable oxetene derivative (Scheme 37).
Scheme 37.
6.3. [3 + 2]
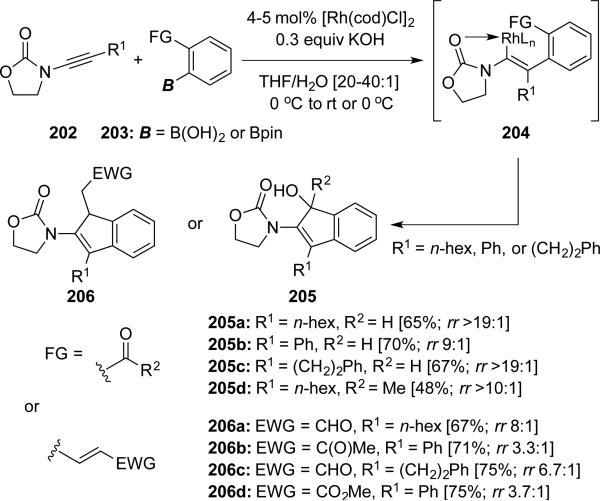
Lam46 reported a Rh-catalyzed formal [3 + 2] cycloaddition of ynamides 202 with arylboronic acids or esters 203 containing an electrophilic functional group at the ortho-position. This transformation effectively provides 2-amido-indenols 205 or 2-amido-indenes 206 in good regioselectivities (Scheme 38).
Scheme 38.
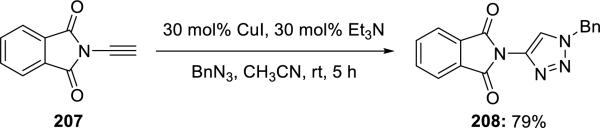
Sueda47 applied their ynimides 207 to the copper-mediated Huisgen's azide-[3 + 2] cycloaddition giving 4-phthalimido-1-benzyl-1,2,3-triazole 208 (Scheme 39).
Scheme 39.
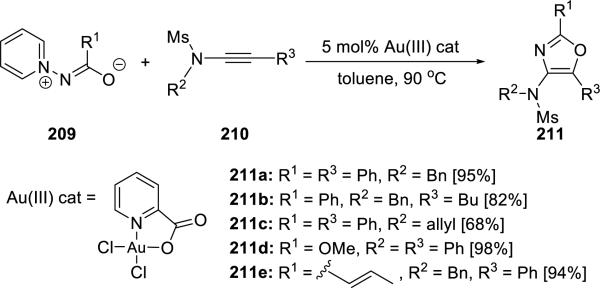
Davies48 reported that 2,4,5-trisubstituted oxazoles 211 could be synthesized from ynamides 210 and 1,3-N,O-dipole equivalents 209 in a gold (III) catalyzed process (Scheme 40).
Scheme 40.
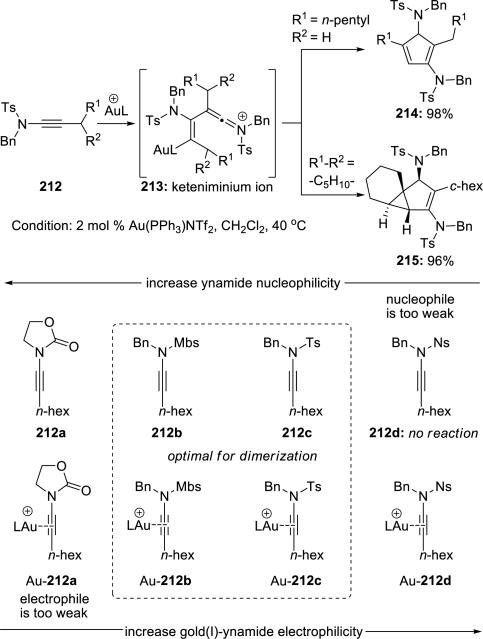
Gagosz and Skrydstrup49 reported syntheses of cyclopentadienes 214 or tricycles 215 from dimerizations of ynamides 212 in the presence of a Au(I) complex (Scheme 41). While the divergence in this dimerization depends upon the substitution pattern, its efficiency is directly dependent on the electronic properties of the ynamide, which acts both as the electrophile and the nucleophile in the process.
Scheme 41.
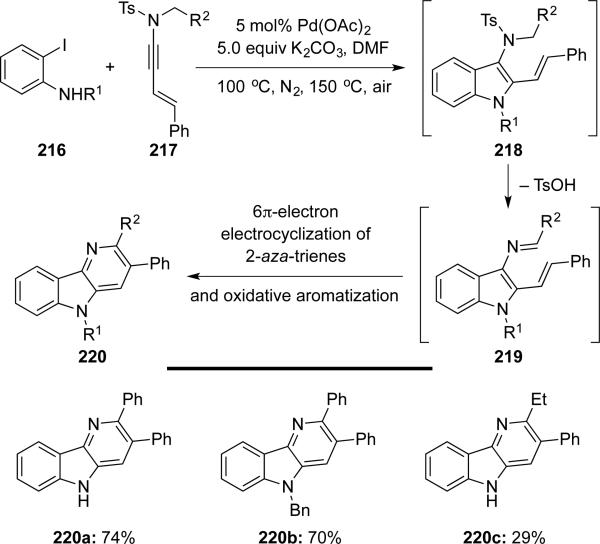
Lai50 reported the synthesis of δ-carbolines 220 from 2-iodoanilines 216 and N-tosyl-enynamides 217 via a Pd(0)-catalyzed cascade (Scheme 42). This cascade involves Larock's heteroannulation giving indoles 218 and an electrocyclization of 2-aza-trienes 219 after loss of TsOH.
Scheme 42.
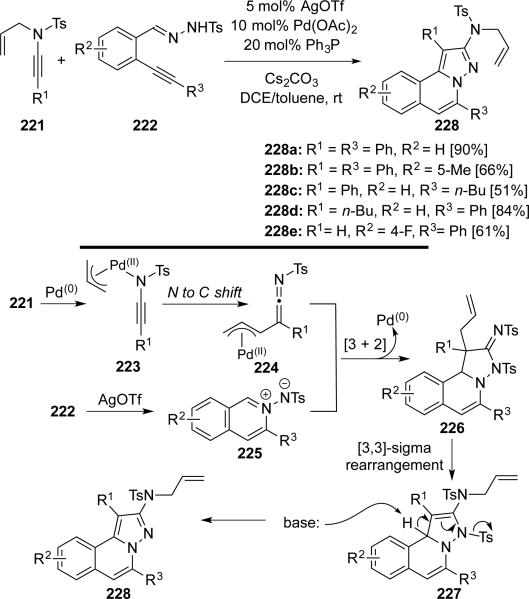
Peng51 reported a AgOTf/Pd(OAc)2 co-catalyzed [3 + 2]-cycloaddition of N-allyl-N-sulfonyl ynamides 221 with N’-(2-alkynyl-benzylidene)hydrazides 222 giving 2-amino-H-pyrazolo[5,1-a]isoquinolines 228 (Scheme 43). The transformation proceeds through 6-endo-dig cyclization of 222, [3 + 2] cycloaddition between 224 and 225, 3,3-sigmatropic rearrangement, and aromatization.
Scheme 43.
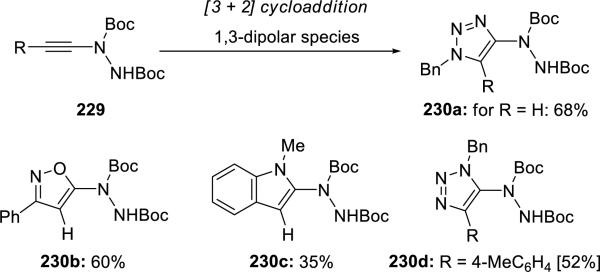
Batey52 reported a series of 1,3-dipole cycloadditions using ynehydrazides 229 that were first synthesized in these authors’ lab. This exercise demonstrates the synthetic potential of these novel ynamides (Scheme 44).
Scheme 44.
6.4. [4 + 1]
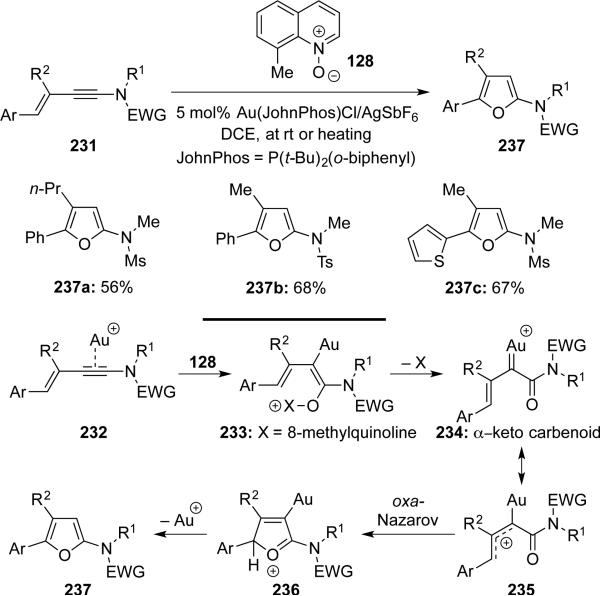
Liu53 reported a gold-catalyzed formal [4 + 1]-cycloaddition of ynamides 231 with 8-methylquinoline oxide 128, leading to a series of substituted 2-amido-furans 237 (Scheme 45). Mechanistically, this formal cycloaddition likely proceeded through α–keto carbenoid 234 via an initial gold-catalyzed addition of 8-methylquinoline oxide 128 to ynamides 231 followed by an oxa-Nazarov cyclization.
Scheme 45.
6.5. [4 + 2]
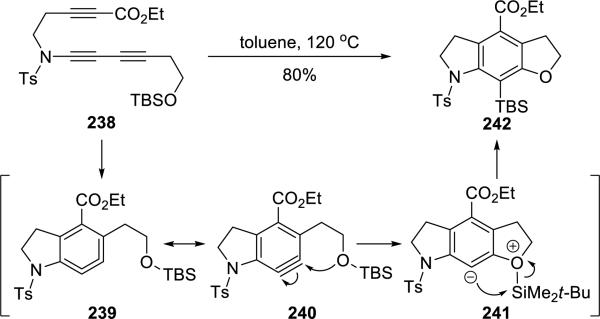
Hoye54 disclosed an intramolecular hexadehydro-Diels-Alder cycloaddition reaction of ynamide 238 (Scheme 46). The hexadehydro-Diels-Alder reaction of 238 led to the key benzyne intermediate 240, which was trapped by the pendant silyloxy group, giving the tricycle 242 in 80% yield after an O-to-C silyl migration of the zwitter ionic intermediate 241.
Scheme 46.
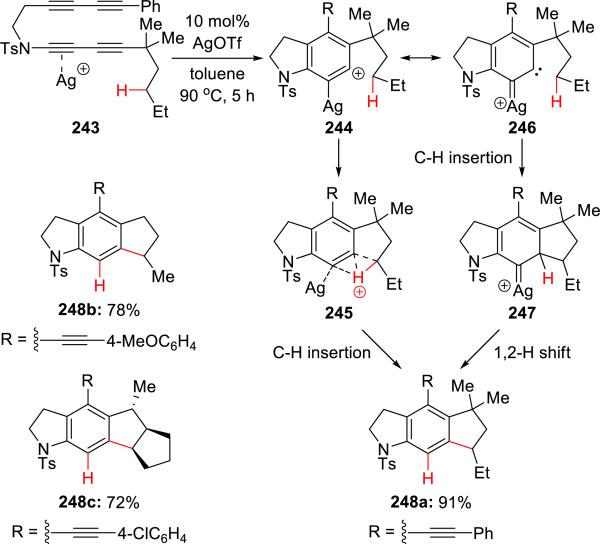
Lee55 independently reported a similar hexadehydro-Diels-Alder cycloaddition of ynamide 243 catalyzed with silver (Scheme 47). What distinguishes this beautiful work from Hoye's is the alkane C-H insertion of the silver complex aryne to form carbon-carbon bonds.
Scheme 47.
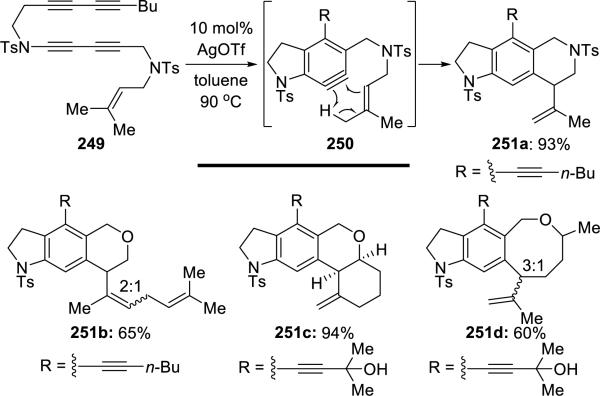
Lee56 subsequently reported a clever use of the Alder-ene process to trap the aryne intermediates derived from hexadehydro-Diels-Alder cycloaddition of ynamide 249 (Scheme 48). The metal catalyst was not essential, but increased the reaction's rate.
Scheme 48.
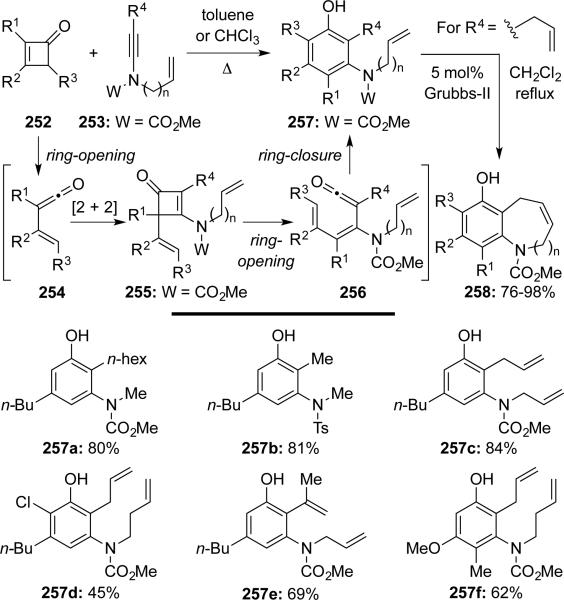
Danheiser57 developed an ynamide-benzannulation using cyclobutenones 252 to synthesize highly substituted anilides 257 (Scheme 49). This benzannulation proceeds beautifully via four consecutive pericyclic processes, thereby constituting a formal [4 + 2] annulation. With olefin substitutions in R4 and on the nitrogen atom, these anilides could undergo ring-closing metathesis to generate complex N-heterocycles such as 258.
Scheme 49.
6.6. [2 + 2 + 2]
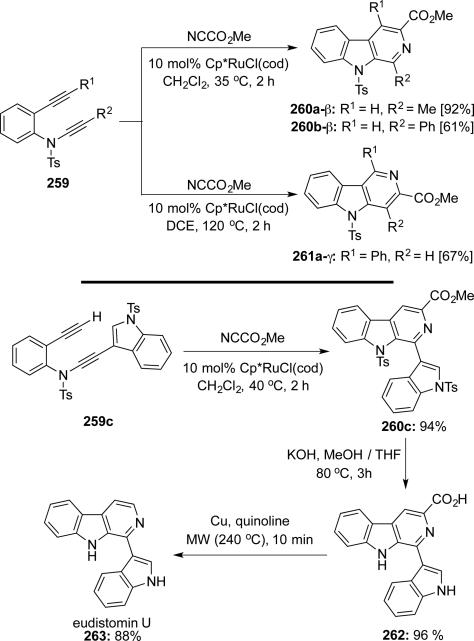
Witulski58 reported a ruthenium-catalyzed hetero-[2 + 2 + 2] cycloaddition of yne-ynamides 259 with Mander's reagent giving β- and γ-carbolines 260 and 261 (Scheme 50). The regioselectivity could be controlled by the steric hindrance of substitutions to the alkynes and ynamides. A total synthesis of eudistomin U 263 was achieved using regioselective β-carboline synthesis.
Scheme 50.
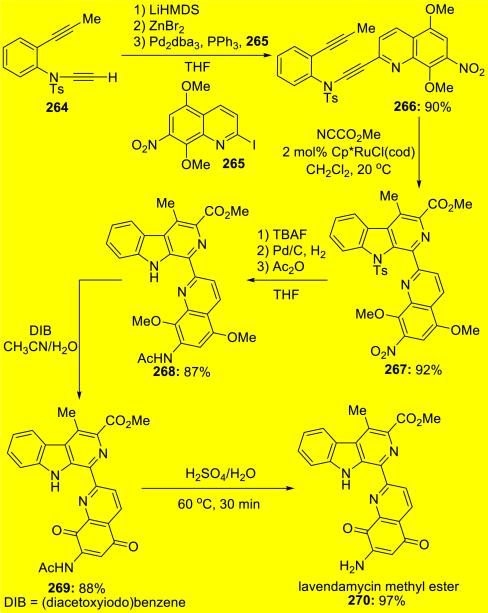
Nissen59 subsequently reported the total synthesis of lavendamycin by Ru(II)-catalyzed hetero-[2 + 2 + 2] cycloaddition of ynamide 266 and an electron deficient nitrile to prepare the carboline scaffold (Scheme 51).
Scheme 51.
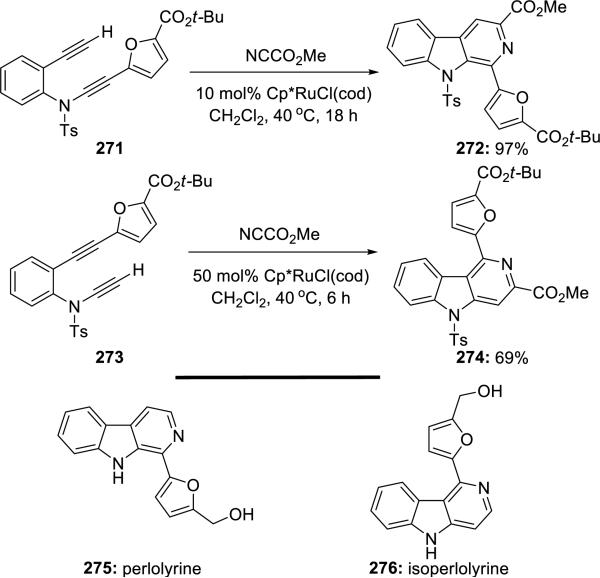
Witulski and Detert60 later reported the total syntheses of perlolyrine 275 and isoperlolyrine 276, featuring this Ru(II)-catalyzed hetero-[2 + 2 + 2] cycloaddition using yne-ynamides 271 and 273 (Scheme 52).
Scheme 52.
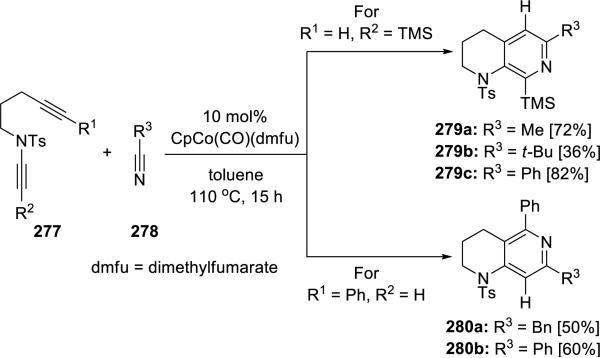
Malacria, Aubert and Gandon61 reported a Co(I)-catalyzed regioselective [2 + 2 + 2]-cycloaddition between ynamides 277 and nitriles 278 (Scheme 53). Through adjusting the substituent on the ynamides, regioselectivity of this cycloaddition could be tuned to favor either 3-aminopyridines 279 or 4-aminopyridines 280.
Scheme 53.
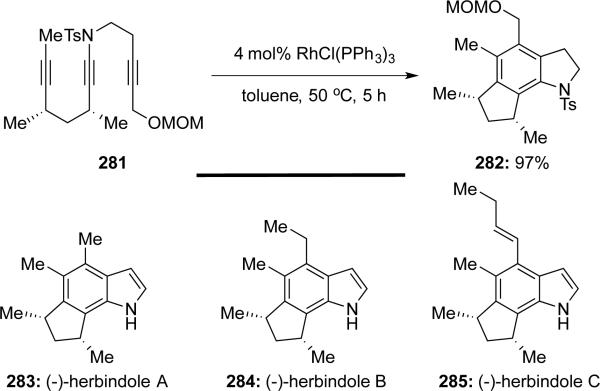
Saito and Sato62 reported divergent total syntheses of (-)-herbindoles A-C through intramolecular [2 + 2 + 2] cycloaddition of ynamide 281 catalyzed by Wilkinson's catalyst (Scheme 54). All three herbindoles could be constructed from the common indoline intermediate 282.
Scheme 54.
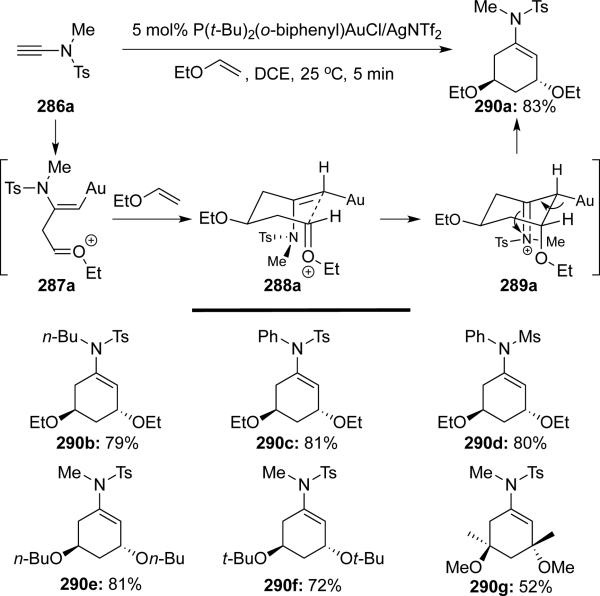
Liu63 reported a Au(I)-catalyzed formal [2 + 2 + 2] cycloaddition of ynamides 286 with two equivalents of enol ethers (Scheme 55). Under activation by gold, two consecutive nucleophilic attacks by the enol ether followed by Prins-type cyclization furnished cyclic enamides 290 stereoselectively.
Scheme 55.
6.7. [3 + 2 + 2]
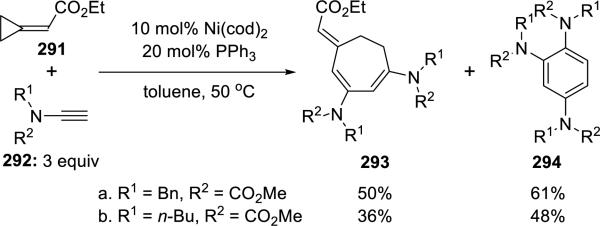
Saito64 unveiled a novel Ni(0)-catalyzed [3 + 2 + 2] cycloaddition of ethyl cyclopropylideneacetate 291 with ynamides 292 (Scheme 56). The desired cycloadducts 293 were obtained in moderate yields with significant amounts of trisubstituted benzenes 294 resulting from trimerizations of the corresponding ynamides.
Scheme 56.
7. Rearrangements
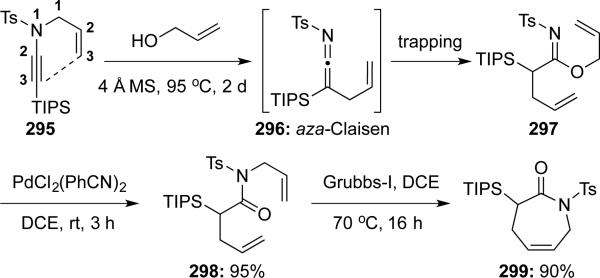
Hsung65 communicated the synthesis of azapin-2-one 299 via a sequence of aza-Claisen rearrangement, Pd(0)-catalyzed Overman rearrangement after trapping of ketenimine 296 with allyl alcohol, and ring-closing metathesis (Scheme 57).
Scheme 57.
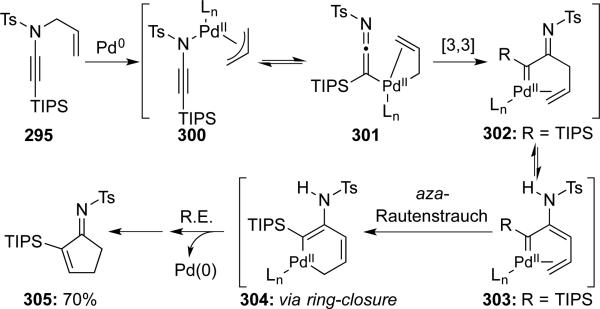
Hsung66 reported a novel synthesis of α,β-unsaturated cyclopentenimine 305 via a Pd-catalyzed aza-Rautenstrauch-type cyclization67 (Scheme 58). It was proposed that the ynamido-π-allyl complex 301 derived from the oxidative addition of TIPS-terminated ynamide 295 underwent a Pd-[3,3] sigmatropic rearrangement giving the α-imino palladium carbenoid 302, which is related to the key intermediates proposed in the Rautenstrauch cyclization.
Scheme 58.
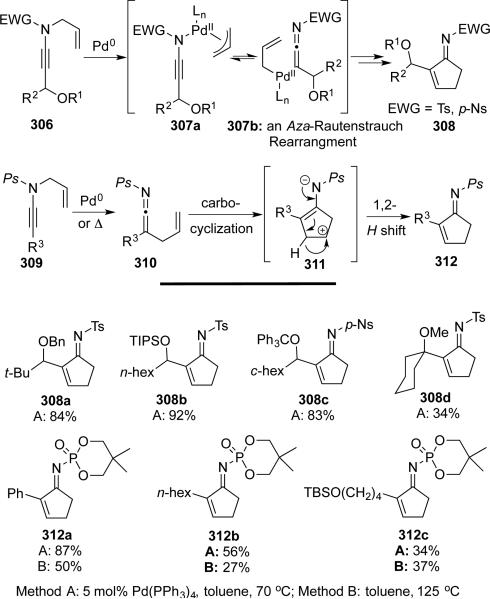
Later, Hsung and DeKorver68 discovered that the substrate scope for the Pd-catalyzed carbocyclization was quite broad. A variety of functionalized N-allyl-γ-branched ynamides were employed in cyclopentenimine synthesis. With N-sulfonyl ynamides 306, palladium catalysis is required, as facile 1,3-sulfonyl shifts dominate under thermal conditions. However, since no analogous 1,3-phosphoryl shift is operational, N-phosphoryl ynamides 309 were used to prepare similar cyclopentenimines under thermal conditions through zwitter ionic intermediates 311 that underwent N-promoted H-shifts (Scheme 59).
Scheme 59.
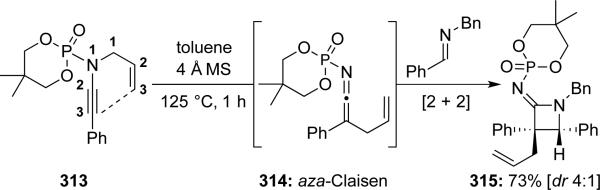
DeKorver and Hsung69 described a moderately stereoselective Staudinger-type ketenimine-imine [2 + 2] cycloaddition using N-phosphoryl ynamide 313 giving azetidin-2-imine 315 bearing a quaternary carbon center (Scheme 60). The ketenimine intermediate 314 was generated in situ via an aza-Claisen reaction.
Scheme 60.
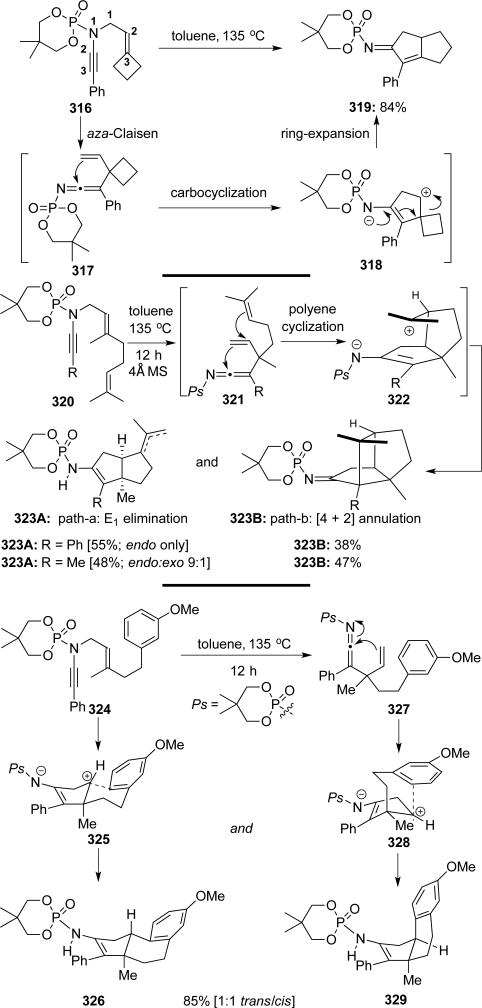
DeKorver and Hsung70 showcased a tandem aza-Claisencarbocyclization of N-phosphoryl-N-allyl-ynamides that included possibilities such as ring-expansion via Meerwein-Wagner rearrangement and polyene-type cyclizations, thereby rapidly building structural complexity leading to fused bi- and tricyclic scaffolds (Scheme 61).
Scheme 61.
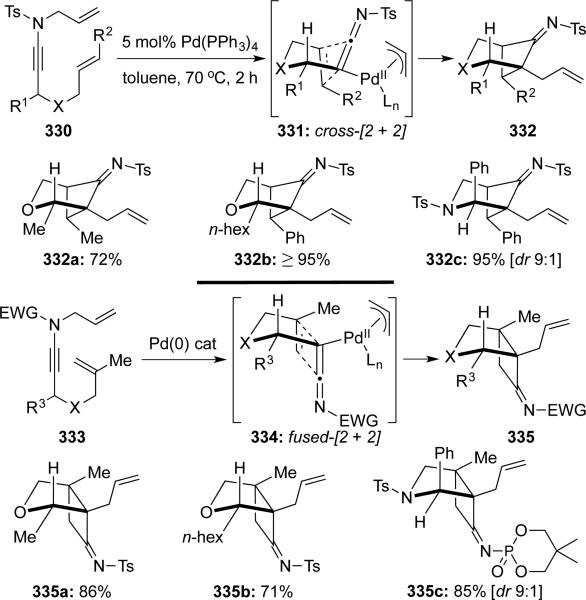
Hsung71 communicated a stereoselective synthesis of bridged or fused bicycloimines through a crossed or fused intramolecular [2 + 2] cycloaddition of ketenimines via palladium-catalyzed aza-Claisen rearrangements of N-allylynamides 330 and 333 (Scheme 62). Preference of cycloaddition pathways depended upon alkene substitutions.
Scheme 62.
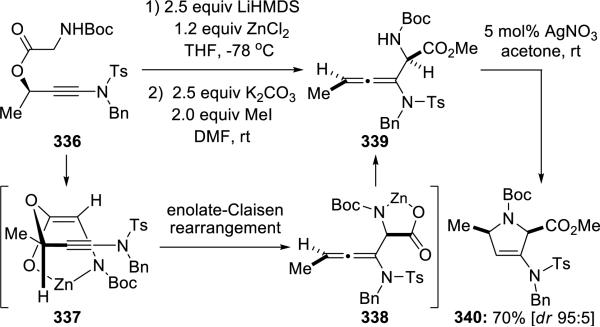
Meyer and Cossy72 described an interesting Saucey-Marbet rearrangement of ynamide 336 containing an N-Bocglycinate motif, providing stereoselective access to functionalized allenamide 33973,74 (Scheme 63). These de novo allenamides underwent silver catalyzed cyclization affording 3-pyrrolidine derivatives 340 having 2,5-syn relative stereochemistry.
Scheme 63.
8. Conclusion
This manuscript has highlighted recent advances in ynamide cyclization reactions that are useful in the rapid synthesis of cyclic and polycyclic structural motifs. The transformations presented are significant due to their rapid assembly of structural complexity, and inclusion of nitrogen within, or in close proximity to, the newly formed ring or rings. Thus, ynamides have emerged as powerful synthons for nitrogen-containing heterocycles and nitrogen-substituted rings. We hope this Account will help promote continued interest in the chemistry of ynamides.
Acknowledgments
We wish to thank NIH [GM066055] for funding. BLK also acknowledges the UW-Oshkosh Faculty Development Program for financial support.
Biographies
Xiao-Na Wang received a B.S. in chemistry from Nanyang Normal University in 2006, and her Ph.D. degree in 2011 at Institute of Chemistry, Chinese Academy of Sciences with Professor Song Ye. Currently, she is conducting postdoctoral research with Professor Richard Hsung at the University of Wisconsin.
Hyu-Suk Yeom received a B.S in chemistry from Hanyang University in 2007 and his Ph.D. degree in 2012 with Professor Shin at Hanyang University. Currently, he is conducting postdoctoral research with Professor Richard Hsung at the University of Wisconsin.
Lichao Fang received a B.S. in chemistry from Nankai University in 2007. He carried out doctoral research with Professor Zhen Yang at Peking University and obtained his Ph.D. degree in 2012. Currently, he is conducting postdoctoral research with Professor Richard Hsung at the University of Wisconsin.
Shuzhong He received his B.S. degree from Peking University in 2004. He then joined Professor Chi-Sing Lee's group in Peking University Shenzhen Graduate School and obtained his Ph.D. degree in 2011. Currently, he is a postdoctoral research scholar with Professor Richard Hsung at the University of Wisconsin.
Zhi-Xiong Ma received a B.S. in chemistry from University of Science and Technology of China in 2005, and his Ph.D. degree in 2010 at Shanghai Institute of Organic Chemistry with Professor Gang Zhao. Currently, he is conducting postdoctoral research with Professor Richard Hsung at the University of Wisconsin.
Brant L. Kedrowski obtained a B.S. in chemistry in 1994, and Ph.D. in organic chemistry in 2000 with Professor Wayland Noland at the University of Minnesota. He did postdoctoral work with Professor Clayton Heathcock at the University of California Berkeley before his appointment as an assistant professor at the University of Wisconsin Oshkosh in 2002. He was promoted to associate professor in 2008 and to full professor in 2013.
Richard P. Hsung obtained his B.S. in chemistry and mathematics from Calvin College and attended The University of Chicago for his M.S. and Ph.D. degrees in organic chemistry, respectively, with Professors Jeff Winkler and Bill Wulff. After postdoctoral stays with Professor Larry Sita in Chicago and Professor Gilbert Stork at Columbia University, he moved to University of Minnesota as an assistant professor in 1997. He was promoted to associate professor in 2002 and to full professor after moving to University of Wisconsin in 2006. He has coauthored over 200 publications and supervised over 150 students and postdoctoral fellows with research interests in developing stereoselective methods using allenamides, ynamides, enamides, and cyclic acetals, and applications in natural product syntheses.
References
- 1.DeKorver KA, Li H, Lohse AG, Hayashi R, Lu Z, Zhang Y, Hsung RP. Ynamides: A Modern Functional Group for the New Millennium. Chem. Rev. 2010;110:5064–5106. doi: 10.1021/cr100003s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evano G, Coste A, Jouvin K. Ynamides: Versatile Tools in Organic Synthesis. Angew. Chem. Int. Ed. 2010;49:2840–2859. doi: 10.1002/anie.200905817. [DOI] [PubMed] [Google Scholar]
- 3.For Partial Reviews, see: Ackermann L, Potukuchi HK. Regioselective Syntheses of Fully-Substituted 1,2,3-Triazoles: The CuAAC/C - H Bond Functionalization Nexus. Org. Biomol. Chem. 2010;8:4503–4513. doi: 10.1039/c0ob00212g.Domínguez G, Perez-Castells J. Recent Advances in [2 + 2 + 2] Cycloaddition Reactions. Chem. Soc. Rev. 2011;40:3430–3444. doi: 10.1039/c1cs15029d.Weding N, Hapke M. Preparation and Synthetic Applications of Alkene Complexes of Group 9 Transition Metals in [2 + 2 + 2] Cycloaddition Reactions. Chem. Soc. Rev. 2011;40:4525–4538. doi: 10.1039/c0cs00189a.Madelaine C, Valerio V, Maulide N. Revisiting Keteniminium Salts: More than the Nitrogen Analogs of Ketenes. Chem. Asian J. 2011;6:2224–2239. doi: 10.1002/asia.201100108.
- 4.Evano G, Jouvin K, Coste A. General Amination Reactions for the Synthesis of Ynamides. Synthesis. 2013;45:17–26. [Google Scholar]
- 5.Also see: Mulder JA, Kurtz KCM, Hsung RP. In Search of an Atom-Economical Synthesis of Chiral Ynamides. Synlett. 2003;10:1379–1390.Dehli JR, Legros J, Bolm C. Synthesis of Enamines, Enol Ethers and Related Compounds by Cross-coupling Reactions. Chem. Commun. 2005;43:973–986. doi: 10.1039/b415954c.Tracey MR, Hsung RP, Antoline JA, Kurtz KCM, Shen L, Slafer BW, Zhang Y. Three Carbon-Heteroatom Bonds: Amides and Derivatives; Peptides; Lactams. Product Class 4: NArylalkanamides, Ynamides, Enamides, Dienamides, and Allenamides. In: Weinreb SM, editor. Science of Synthesis, Houben-Weyl Methods of Molecular Transformations. Georg Thieme Verlag KG; Stuttgart, Germany: 2005. Chapter 21.4.Evano G, Blanchard N, Toumi M. Copper-Mediated Coupling Reactions and Their Applications in Natural Products and Designed Biomolecules Synthesis. Chem. Rev. 2008;108:3054–3131. doi: 10.1021/cr8002505.
- 6.a Chen Z, Zheng D, Wu J. A Facile Route to Polysubstituted Indoles via Three-Component Reaction of 2-Ethynylaniline, Sulfonyl Azide, and Nitroolefin. Org. Lett. 2011;13:848–851. doi: 10.1021/ol102775s. [DOI] [PubMed] [Google Scholar]; b Yavari I, Nematpour M. Copper-Catalyzed One-Pot Synthesis of Functionalized 1,4-Dihydroazete Derivatives from Sulfonyl Azides, Terminal Alkynes, and Tetramethylguanidine. Synlett. 2012;23:2215–2218. [Google Scholar]; c Jiang Z, Lu P, Wang Y. Three-Component Reaction of Propargyl Amines, Sulfonyl Azides, and Alkynes: One-Pot Synthesis of Tetrasubstituted Imidazoles. Org. Lett. 2012;14:6266–6269. doi: 10.1021/ol303023y. [DOI] [PubMed] [Google Scholar]; d Yavari I, Nematpour M. Copper-Catalyzed Tandem Synthesis of Tetrasubstituted Pyrimidines from Alkynes, Sulfonyl Azides, Trichloroacetonitrile, and Tetramethylguanidine. Synlett. 2013;24:165–168. [Google Scholar]; e Cheng D, Ling F, Li Z, Yao W, Ma C. Three-Component Assembly of Conjugated Enyne Scaffolds via E-Selective Olefination of Ynals. Org. Lett. 2012;14:3146–3149. doi: 10.1021/ol3012277. [DOI] [PubMed] [Google Scholar]; f Li B-S, Yang B-M, Wang S-H, Zhang Y-Q, Cao X-P, Tu Y-Q. Copper(I)-Catalyzed Intramolecular [2 + 2] Cycloaddition of 1,6-Enyne-Derived Ketenimine: An Efficient Construction of Strained and Bridged 7-Substituted-3-heterobicyclo[3.1.1]heptan-6-one. Chem. Sci. 2012;3:1975–1979. [Google Scholar]
- 7.Balieu S, Toutah K, Carro L, Chamoreau L-M, Rousselière H, Courillon C. Radical Cyclization of Ynamides into Six- or Eight-membered Rings. Application to the Synthesis of a Protoberberine Analog. Tetrahedron Lett. 2011;52:2876–2880. [Google Scholar]
- 8.Chemla F, Dulong F, Ferreira F, Nüllen MP, Pérez-Luna A. Radical Zinc-Atom Transfer Based Multicomponent Approaches to 3-Alkylidene-Substituted Tetrahydrofurans. Synthesis. 2011;9:1347–1360. [Google Scholar]
- 9.Wakamatsu H, Sakagami M, Hanata M, Takeshita M, Mori M. Ring-Closing Metathesis of Ene-Ynamide: Application to the Synthesis of Medium-Sized Cyclic Dienamide. Macromol. Symp. 2010;293:5–9. [Google Scholar]
- 10.Poloukhtine A, Rassadin V, Kuzmin A, Popik VV. Nucleophilic Cycloaromatization of Ynamide-Terminated Enediynes. J. Org. Chem. 2010;75:5953–5962. doi: 10.1021/jo101238x. [DOI] [PubMed] [Google Scholar]
- 11.a Gati W, Rammah MM, Rammah MB, Couty F, Evano G. De Novo Synthesis of 1,4-Dihydropyridines and Pyridines. J. Am. Chem. Soc. 2012;134:9078–9081. doi: 10.1021/ja303002a. [DOI] [PubMed] [Google Scholar]; b Gati W, Rammah MM, Rammah MB, Evano G. Intramolecular Carbolithiation of N-Allylynamides: An Efficient Entry to 1,4-Dihydropyridines and Pyridines – Application to a Formal Synthesis of Sarizotan. Beilstein J. Org. Chem. 2012;8:2214–2222. doi: 10.3762/bjoc.8.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerr DJ, Miletic M, Chaplin JH, White JM, Flynn BL. Oxazolidinone-Promoted, Torquoselective Nazarov Cyclizations. Org. Lett. 2012;14:1732–1735. doi: 10.1021/ol300316a. [DOI] [PubMed] [Google Scholar]
- 13.Kong Y, Jiang K, Cao J, Fu L, Yu L, Lai G, Cui Y, Hu Z, Wang G. Synthesis of 3-Alkyl- or 3-Allenyl-2-amidobenzofurans via Electrophilic Cyclization of o-Anisole-Substituted Ynamides with Carbocations. Org. Lett. 2013;15:422–425. doi: 10.1021/ol303474a. [DOI] [PubMed] [Google Scholar]
- 14.Kong Y, Yu L, Fu L, Cao J, Lai G, Cui Y, Hu Z, Wang G. Electrotrophilic Cyclization of o-Anisole- and o-Thioanisole-Substituted Ynamides: Synthesis of 2-Amidobenzofurans and 2-Amidobenzothiophenes. Synthesis. 2013;45:1975–1982. [Google Scholar]
- 15.Wang X-N, Hsung RP, Qi R, Fox SK, Lv M-C. A Highly Stereoselective Addition of Lithiated Ynamides to Ellman-Davis Chiral N-tert-Butanesulfinyl Imines. Org. Lett. 2013;15:2514–2517. doi: 10.1021/ol400989x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rui Q, Wang X-N, DeKorver KA, Tang Y, Wang C-C, Li Q, Li H, Lv M-C, Yu Q, Hsung RP. A Convenient Synthesis of γ-Amino-Ynamides via Additions of Lithiated Ynamides to Aryl Imines; Observation of an Aza-Meyer–Schuster Rearrangement. Synthesis. 2013;45:1749–1758. doi: 10.1055/s-0033-1338476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishimura T, Takiguchi Y, Maeda Y, Hayashi T. Rhodium-Catalyzed Asymmetric Cycloisomerization of 1,6-Eneynamides. Adv. Synth. Catal. 2013;355:1374–1382. [Google Scholar]
- 18.Liu R, Winston-McPherson GN, Yang Z-Y, Zhou X, Song W, Guzei IA, Xu X, Tang W. Generation of Rhodium(I) Carbenes from Ynamides and Their Reactions with Alkynes and Alkenes. J. Am. Chem. Soc. 2013;135:8201–8204. doi: 10.1021/ja4047069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenaway RL, Campbell CD, Holton OT, Russell CA, Anderson EA. Palladium-Catalyzed Cascade Cyclization of Ynamides to Azabicycles. Chem.-Eur. J. 2011;17:14366–14370. doi: 10.1002/chem.201102880. [DOI] [PubMed] [Google Scholar]
- 20.Greenaway RL, Campbell CD, Chapman HA, Anderson EA. Reductive Cyclization of Bromoenynamides with Alcohols as Hydride Source: Synthesis and Reactions of 2-Amidodienes. Adv. Synth. Catal. 2012;354:3187–3194. [Google Scholar]
- 21.Bhunia S, Chang C-J, Liu R-S. Platinum-Catalyzed Oxoarylations of Ynamides with Nitrones. Org. Lett. 2012;14:5522–5525. doi: 10.1021/ol302621z. [DOI] [PubMed] [Google Scholar]
- 22.Xu H, Zhang Y, Huang J, Chen W. Copper-Catalyzed Synthesis of N-Fused Heterocycles through Regioselective 1,2-Aminothiolation of 1,1-Dibromoalkenes. Org. Lett. 2010;12:3704–3707. doi: 10.1021/ol101563f. [DOI] [PubMed] [Google Scholar]
- 23.Hashmi ASK, Schuster AM, Zimmer M, Rominger F. Synthesis of 5-Halo-4H-1,3-oxazine-6-amines by a Copper-Mediated Domino Reaction. Chem.-Eur. J. 2011;17:5511–5515. doi: 10.1002/chem.201100423. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Neuville L. Copper-Catalyzed Oxidative Diamination of Terminal Alkynes by Amidines: Synthesis of 1,2,4-Trisubstituted Imidazoles. Org. Lett. 2013;15:1752–1755. doi: 10.1021/ol400560m. [DOI] [PubMed] [Google Scholar]
- 25.Gati W, Couty F, Boubaker T, Rammah MM, Rammah MB, Evano G. Intramolecular Carbocupration of N-Aryl-ynamides: A Modular Indole Synthesis. Org. Lett. 2013;15:3122–3125. doi: 10.1021/ol4013298. [DOI] [PubMed] [Google Scholar]
- 26.Garcia P, Harrak Y, Diab L, Cordier P, Ollivier C, Gandon V, Malacria M, Fensterbank L, Aubert C. Silver-Catalyzed Cycloisomerization of 1,n-Allenynamides. Org. Lett. 2011;13:2952–2955. doi: 10.1021/ol201041h. [DOI] [PubMed] [Google Scholar]
- 27.Hashmi ASK, Pankajakshan S, Rudolph M, Enns E, Bander T, Rominger F, Frey W. Gold Catalysis: Anellated Heterocycles and Dependency of the Reaction Pathway on the Tether Length. Adv. Synth. Catal. 2009;351:2855–2875. [Google Scholar]
- 28.Kramer S, Madsen JLH, Rottländer M, Skrydstrup T. Access to 2,5-Diamidopyrroles and 2,5-Diamidofurans by Au(I)-Catalyzed Double Hydroamination or Hydration of 1,3-Diynes. Org. Lett. 2010;12:2758–2761. doi: 10.1021/ol1008685. [DOI] [PubMed] [Google Scholar]
- 29.Li C-W, Pati K, Lin G-Y, Abu Sohel SM, Hung H-H, Liu R-S. Gold-Catalyzed Oxidative Ring Expansions and Ring Cleavages of Alkynylcyclopropanes by Intermolecular Reactions Oxidized by Diphenylsulfoxide. Angew. Chem., Int. Ed. 2010;49:9891–9894. doi: 10.1002/anie.201004647. [DOI] [PubMed] [Google Scholar]
- 30.Xu C-F, Xu M, Jia Y-X, Li C-Y. Gold-Catalyzed Synthesis of Benzil Derivatives and α-Keto Imides via Oxidation of Alkynes. Org. Lett. 2011;13:1556–1559. doi: 10.1021/ol200270t. [DOI] [PubMed] [Google Scholar]
- 31.Vasu D, Hung H-H, Bhunia S, Gawade SA, Das A, Liu R-S. Gold-Catalyzed Oxidative Cyclization of 1,5-Enynes Using External Oxidants. Angew. Chem., Int. Ed. 2011;50:6911–6914. doi: 10.1002/anie.201102581. [DOI] [PubMed] [Google Scholar]
- 32.Wang K-B, Ran R-Q, Xiu S-D, Li C-Y. Synthesis of 3-Azabicyclo[3.1.0]hexan-2-one Derivatives via Gold-Catalyzed Oxidative Cyclopropanation of N-Allylynamides. Org. Lett. 2013;15:2374–2377. doi: 10.1021/ol4007629. [DOI] [PubMed] [Google Scholar]
- 33.Sueda T, Kawada A, Urashi Y, Teno N. Ag- and Au-Catalyzed Addition of Alcohols to Ynimides: β-Regioselective Carbonylation and Production of Oxazoles. Org. Lett. 2013;15:1560–1563. doi: 10.1021/ol400338x. [DOI] [PubMed] [Google Scholar]
- 34.Rettenmeier E, Schuster AM, Rudolph M, Rominger F, Gade CA, Hashmi ASK. Gold Catalysis: Highly Functionalized Cyclopentadienes Prepared by Intermolecular Cyclization of Ynamides and Propargylic Carboxylates. Angew. Chem. Int. Ed. 2013;52:5880–5884. doi: 10.1002/anie.201301382. [DOI] [PubMed] [Google Scholar]
- 35.Ghosh N, Nayak S, Sahoo AK. Gold(I)-Catalyzed 6-Endo-Dig Hydrative Cyclization of an Alkyne-Tethered Ynamide: Access to 1,6-Dihydropyridin-2(3H)ones. Chem.-Eur. J. 2013;19:9428–9433. doi: 10.1002/chem.201301599. [DOI] [PubMed] [Google Scholar]
- 36.Ung G, Mendoza-Espinosa D, Bertrand G. Ynamides: Stable Ligand Equivalents of Unstable Oxazol-4-ylidenes (Novel Mesoionic Carbenes). Chem. Commun. 2012;48:7088–7090. doi: 10.1039/c2cc33319h. [DOI] [PubMed] [Google Scholar]
- 37.Li H, Antoline JE, Yang J-H, Al-Rashid ZF, Hsung RP. A Stereoselective Intramolecular Cyclopropanation via a de novo Class of Push - Pull Carbenes Derived from DMDO-Epoxidations of Chiral Ynamides. New J. Chem. 2010;34:1309–1316. doi: 10.1039/c0nj00063a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clavier H, Lepronier A, Bengobesse-Mintsa N, Gatineau D, Pellissier H, Giordano L, Tenaglia A, Buono G. Palladium-Mediated [2 + 1] Cycloaddition of Norbornene Derivatives with Ynamides. Adv. Synth. Catal. 2013;355:403–408. [Google Scholar]
- 39.Bigeault J, Giordano L, Buono G. [2 + 1] Cycloadditions of Terminal Alkynes to Norbornene Derivatives Catalyzed by Palladium Complexes with Phosphinous Acid Ligands. Angew. Chem., Int. Ed. 2005;44:4753–4757. doi: 10.1002/anie.200500879. [DOI] [PubMed] [Google Scholar]
- 40.Li H, Hsung RP, DeKorver KA, Wei Y. Copper-Catalyzed Ficini [2 + 2] Cycloaddition of Ynamides. Org. Lett. 2010;12:3780–3783. doi: 10.1021/ol101418d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.a Schotes C, Bigler R, Mezzetti A. Bicyclo[3.2.0]heptane-Based Enamides by Ru/PNNP-Catalyzed Enantioselective Ficini Reactions: Scope and Application in Ligand Design. Synthesis. 2012;44:513–526. [Google Scholar]; b Schotes C, Althaus M, Aardoom R, Mezzetti A. Asymmetric Diels−Alder and Ficini Reactions with Alkylidene β-Ketoesters Catalyzed by Chiral Ruthenium PNNP Complexes: Mechanistic Insight. J. Am. Chem. Soc. 2012;134:1331–1343. doi: 10.1021/ja210372u. [DOI] [PubMed] [Google Scholar]; c Schotes C, Mezzetti A. Enantioselective Ficini Reaction: Ruthenium/PNNP-Catalyzed [2 + 2] Cycloaddition of Ynamides with Cyclic Enones. Angew. Chem., Int. Ed. 2011;50:3072–3074. doi: 10.1002/anie.201007753. [DOI] [PubMed] [Google Scholar]; d Schotes C, Mezzetti A. Cu(I)- and Cu(II)-Catalyzed Cyclo- and Michael Addition Reactions of Unsaturated β-Ketoesters. J. Org. Chem. 2011;76:5862–5866. doi: 10.1021/jo200776c. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y-P, Danheiser RL. Synthesis of 2-Iodoynamides and Regioselective [2 + 2] Cycloadditions with Ketene. Tetrahedron Lett. 2011;52:2111–2114. doi: 10.1016/j.tetlet.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith DL, Chidipudi SR, Goundry WR, Lam HW. Rhodium-Catalyzed [2 + 2] Cycloaddition of Ynamides with Nitroalkenes. Org. Lett. 2012;14:4934–4937. doi: 10.1021/ol302259v. [DOI] [PubMed] [Google Scholar]
- 44.Shindoh N, Kitaura K, Takemoto Y, Takasu K. Catalyst-Controlled Torquoselectivity Switch in the 4π Ring-Opening Reaction of 2-Amino-2-azetines Giving β-Substituted α,β-Unsaturated Amidines. J. Am. Chem. Soc. 2011;133:8470–8473. doi: 10.1021/ja202576e. [DOI] [PubMed] [Google Scholar]
- 45.Aikawa K, Hioki Y, Shimizu N, Mikami K. Catalytic Asymmetric Synthesis of Stable Oxetenes via Lewis Acid-Promoted [2 + 2] Cycloaddition. J. Am. Chem. Soc. 2011;133:20092–20095. doi: 10.1021/ja2085299. [DOI] [PubMed] [Google Scholar]
- 46.Gourdet B, Rudkin ME, Lam HW. Rhodium-Catalyzed Annulation of Ynamides with Bifunctional Arylboron Reagents. Org. Lett. 2010;12:2554–2557. doi: 10.1021/ol100769p. [DOI] [PubMed] [Google Scholar]
- 47.Sueda T, Oshima A, Teno N. N-Alkynyl Imides (Ynimides): Synthesis and Use as a Variant of Highly Labile Ethynamine. Org. Lett. 2011;13:3996–3999. doi: 10.1021/ol2014973. [DOI] [PubMed] [Google Scholar]
- 48.Davies PW, Cremonesi A, Dumitrescu L. Intermolecular and Selective Synthesis of 2,4,5-Trisubstituted Oxazoles by a Gold-Catalyzed Formal [3 + 2] Cycloaddition. Angew. Chem. Int. Ed. 2011;50:8931–8935. doi: 10.1002/anie.201103563. [DOI] [PubMed] [Google Scholar]
- 49.Kramer S, Odabachian Y, Overgaard J, Rottländer M, Gagosz F, Skrydstrup T. Taking Advantage of the Ambivalent Reactivity of Ynamides in Gold Catalysis: A Rare Case of Alkyne Dimerization. Angew. Chem. Int. Ed. 2011;50:5090–5094. doi: 10.1002/anie.201100327. [DOI] [PubMed] [Google Scholar]
- 50.Cao J, Xu Y, Kong Y, Cui Y, Hu Z, Wang G, Deng Y, Lai G. Synthesis of δ-Carbolines via a Pd-Catalyzed Sequential Reaction from 2-Iodoanilines and N-Tosyl-enynamines. Org. Lett. 2012;14:38–41. doi: 10.1021/ol2027762. [DOI] [PubMed] [Google Scholar]
- 51.Huang P, Chen Z, Yang Q, Peng Y. Silver Triflate and Palladium Acetate Co-catalyzed Reaction of N'-(2-Alkynylbenzylidene)hydrazide with N-Allyl Ynamide. Org. Lett. 2012;14:2790–2793. doi: 10.1021/ol301023m. [DOI] [PubMed] [Google Scholar]
- 52.Beveridge RE, Batey RA. Terminal Alkyne Addition to Diazodicarboxylates: Synthesis of Hydrazide Linked Alkynes (Ynehydrazides). Org. Lett. 2012;14:540–543. doi: 10.1021/ol2031608. [DOI] [PubMed] [Google Scholar]
- 53.Dateer RB, Pati K, Liu R-S. Gold-Catalyzed Synthesis of Substituted 2-Aminofurans via Formal [4 + 1]-Cycloadditions on 3-En-1-ynamides. Chem. Commun. 2012;48:7200–7202. doi: 10.1039/c2cc33030j. [DOI] [PubMed] [Google Scholar]
- 54.Hoye TR, Baire B, Niu D, Willoughby PH, Woods BP. The Hexadehydro-Diels-Alder Reaction. Nature. 2012;490:208–212. doi: 10.1038/nature11518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yun SY, Wang K-P, Lee N-K, Mamidipalli P, Lee D. Alkane C–H Insertion by Aryne Intermediates with a Silver Catalyst. J. Am. Chem. Soc. 2013;135:4668–4671. doi: 10.1021/ja400477r. [DOI] [PubMed] [Google Scholar]
- 56.Karmakar R, Mamidipalli P, Yun SY, Lee D. Alder-Ene Reactions of Arynes. Org. Lett. 2013;15:1938–1941. doi: 10.1021/ol4005905. [DOI] [PubMed] [Google Scholar]
- 57.Mak XY, Crombie AL, Danheiser RL. Synthesis of Polycyclic Benzofused Nitrogen Heterocycles via a Tandem Ynamide Benzannulation/Ring-Closing Metathesis Strategy. Application in a Formal Total Synthesis of (+)-FR900482. J. Org. Chem. 2011;76:1852–1873. doi: 10.1021/jo2000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nissen F, Richard V, Alayrac C, Witulski B. Synthesis of β- and γ-Carbolines via Ruthenium and Rhodium Catalysed [2 + 2 + 2] Cycloadditions of Yne-ynamides with Methylcyanoformate. Chem. Commun. 2011;47:6656–6658. doi: 10.1039/c1cc11298h. [DOI] [PubMed] [Google Scholar]
- 59.Nissen F, Detert H. Total Synthesis of Lavendamycin by a [2 + 2 + 2] Cycloaddition. Eur. J. Org. Chem. 2011:2845–2853. [Google Scholar]
- 60.Dassonneville B, Witulski B, Detert H. [2 + 2 + 2] Cycloadditions of Alkynylynamides-A Total Synthesis of Perlolyrine and the First Total Synthesis of “Isoperlolyrine”. Eur. J. Org. Chem. 2011:2836–2844. [Google Scholar]
- 61.a Garcia P, Evanno Y, George P, Sevrin M, Ricci G, Malacria M, Aubert C, Gandon V. Regioselective Cobalt-Catalyzed Formation of Bicyclic 3- and 4-Aminopyridines. Org. Lett. 2011;13:2030–2033. doi: 10.1021/ol200417p. [DOI] [PubMed] [Google Scholar]; b Garcia P, Evanno Y, George P, Sevrin M, Ricci G, Malacria M, Aubert C, Gandon V. Synthesis of Aminopyridines and Aminopyridones by Cobalt-Catalyzed [2 + 2 + 2] Cycloadditions Involving Yne-Ynamides: Scope, Limitations, and Mechanistic Insights. Chem. Eur. J. 2012;18:4337–4344. doi: 10.1002/chem.201103906. [DOI] [PubMed] [Google Scholar]
- 62.Saito N, Ichimaru T, Sato Y. Total Synthesis of (−)-Herbindoles A, B, and C via Transition-Metal-Catalyzed Intramolecular [2 + 2 + 2] Cyclization between Ynamide and Diynes. Org. Lett. 2012;14:1914–1917. doi: 10.1021/ol300571b. [DOI] [PubMed] [Google Scholar]
- 63.Dateer RB, Shaibu BS, Liu R-S. Gold-Catalyzed Intermolecular [4 + 2] and [2 + 2 + 2] Cycloadditions of Ynamides with Alkenes. Angew Chem., Int. Ed. 2012;51:113–117. doi: 10.1002/anie.201105921. [DOI] [PubMed] [Google Scholar]
- 64.Yamasaki R, Terashima N, Sotome I, Komagawa S, Saito S. Nickel-Catalyzed [3 + 2 + 2] Cycloaddition of Ethyl Cyclopropylideneacetate and Heteroatom-Substituted Alkynes: Application to Selective Three-Component Reaction with 1,3-Diynes. J. Org. Chem. 2010;75:480–483. doi: 10.1021/jo902251m. [DOI] [PubMed] [Google Scholar]
- 65.DeKorver KA, North TD, Hsung RP. An Efficient Synthesis of de novo Imidates via Aza-Claisen Rearrangements of N-Allyl Ynamides. Synlett. 2010:2397–2402. doi: 10.1055/s-0030-1258544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.DeKorver KA, Hsung RP, Lohse AG, Zhang Y. A Divergent Mechanistic Course of Pd(0)-Catalyzed Aza-Claisen Rearrangement and Aza-Rautenstrauch-Type Cyclization of N-Allyl Ynamides. Org. Lett. 2010;12:1840–1843. doi: 10.1021/ol100446p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rautenstrauch V. 2-Cyclopentenones from 1-Ethynyl-2-propenyl Acetates. J. Org. Chem. 1984;49:950–952. [Google Scholar]
- 68.Wang X-N, Winston-McPherson GN, Walton MC, Zhang Y, Hsung RP, DeKorver KA. Synthesis of Cyclopentenimines from N-Allyl Ynamides via a Tandem Aza-Claisen Rearrangement−Carbocyclization Sequence. J. Org. Chem. 2013;78:6233–6244. doi: 10.1021/jo400960e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.DeKorver KA, Walton MC, North TD, Hsung RP. Introducing a New Class of N-Phosphoryl Ynamides via Cu(I)-Catalyzed Amidations of Alkynyl Bromides. Org. Lett. 2011;13:4862–4865. doi: 10.1021/ol201947b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.DeKorver KA, Wang X-N, Walton MC, Hsung RP. Carbocyclization Cascades of Allyl Ketenimines via Aza-Claisen Rearrangements of N-Phosphoryl-N-allyl-ynamides. Org. Lett. 2012;14:1768–1771. doi: 10.1021/ol300366e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.DeKorver KA, Hsung RP, Song W-Z, Wang X-N, Walton MC. An Intramolecular [2 + 2] Cycloaddition of Ketenimines via Palladium-Catalyzed Rearrangements of N-Allyl-Ynamides. Org. Lett. 2012;14:3214–3217. doi: 10.1021/ol3013233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brioche J, Meyer C, Cossy J. Synthesis of Functionalized Allenamides from Ynamides by Enolate Claisen Rearrangement. Org. Lett. 2013;15:1626–1629. doi: 10.1021/ol400402n. [DOI] [PubMed] [Google Scholar]
- 73.Wei L-L, Xiong H, Hsung RP. The Emergence of Allenamides In Organic Synthesis. Acc. Chem. Res. 2003;36:773–782. doi: 10.1021/ar030029i. [DOI] [PubMed] [Google Scholar]
- 74.Lu T, Lu Z, Ma Z-X, Zhang Y, Hsung RP. Allenamides: A Versatile Synthetic Building Block in Organic Synthesis. Chem. Rev. 2013;113:4862–4904. doi: 10.1021/cr400015d. [DOI] [PMC free article] [PubMed] [Google Scholar]