Abstract
The CC (cumulus cell) proliferation index in relation to the expression and distribution of Cdk4 and Cx43 proteins, which are crucial factors for oocyte maturation, was investigated. Cumulus-oocyte complexes (COCs) were recovered from pubertal crossbred Landrace gilts and treated with collagenase, and separated CCs were cultured in standard TCM199 medium for 44 h. At each step of in vitro cultivation (IVC) of CCs (0, 12, 24 and 44 h), a normalized proliferation index was assessed. Cdk4 and Cx43 protein expression and the CC-specific cellular distribution were analyzed by confocal microscopic observation. The normalized proliferation index (number of cells attached, measured by impedance) was increased in the first 12 h of IVC (P<0.01) and differed between 12 h and 24 h of cultivation (P<0.001). Later, between 24 h–44 h of IVC, the CC proliferation rate was stable, and no significant differences were observed. Based on the confocal microscopic observation, increased expression of both Cdk4 and Cx43 was found after 44 h of IVC compared with the expression of these proteins before IVC. Moreover, after IVC, a substantial translocation of Cdk4 and Cx43 was noted from the nucleus to the cytoplasm of CCs. In conclusion, it was demonstrated for the first time that CCs can be cultured in vitro separately without oocytes and that the proliferation index was significantly increased in the first 12 h of IVC, which may reflect the process of ordinary cumulus cell expansion. Furthermore, the expression of both Cdk4 and Cx43 in CCs suggested that these proteins may be regarded as markers not only of proper oocyte maturation but also of CC differentiation. Translocation of these proteins into the cytoplasm of CCs after 44 h of IVC may be related to the expansion process.
Keywords: Connexin, Cumulus cells, Cyclin-dependent kinase, Oocyte, Pig
The potential of oocytes to attain full maturation competence is characterized by an appropriate course of nuclear and cytoplasmic maturation, which finishes with attainment of the MII stage both in vivo and in vitro. Only mature oocytes can be successfully fertilized, undergo zygote formation and attain normal embryo development [1,2,3]. The maturation process is determined by several intrinsic and extrinsic factors, involving the storage of large amounts of mRNA and proteins as well as the transfer of low molecular weight compounds and substrates between oocytes and the surrounding somatic cumulus cells (CCs) [4,5,6]. The communication between these cells is associated with the formation of protein channels, also called gap junction connections (GJCs), permitting the transfer of small molecules of molecular weights less than 1 kD. It was clearly demonstrated that this “cross-talk” is critical for proper oocyte maturation and resumption of meiosis [7,8,9]. Experiments based on knock-out models demonstrated that mutation in genes encoding proteins that form GJCs led to failed oocyte maturation and finally produced sterile females [10]. GJC channels are formed by connexins (Cx), including mainly Cx31, Cx37, Cx43 and Cx47. Although the presence of Cx molecules in cumulus cells has been reported [1, 11], no data are available on the profiling of differential expression of Cx genes and the distribution of the proteins in the course of real-time CCs proliferation. Furthermore, cyclin-dependent kinases (Cdks) have been shown to be also involved in proper oocyte maturation and attainment of the MII stage [12]. Using mouse knock-out models it was demonstrated that Cdks are the main regulators of cell divisions and represent a checkpoint of cell cycle progression [13]. Cdk4 belongs to the protein-serine kinase superfamily, which catalyzes phosphorylation of target proteins and promotes cell cycle progression. The formation of the complex between Cdk4 and type-D cyclin regulates cell proliferation during the G1 phase [14]. Since Cdk4 is the main target during the cell division, the expression of this protein in various phases of CC proliferation is examined in this study.
During the complex process of oocyte maturation in vivo and/or in vitro, cumulus cells undergo substantial expansion that is highly linked with the transfer of regulatory molecules and small substrates. However, it has not been fully recognized if this process is related to CC proliferation and at which steps of oocyte maturation it occurs. Since it was shown that CCs manifested adherence in monolayer culture systems, it is known that the proliferation of CCs should be associated with the expansion during maturation [15,16,17]. However, no evidence was provided for proliferation of COCs in vivo. The supposition of such proliferation comes from in vitro studies in which porcine COCs were cultivated for 44 h. Despite documented changes in CC abundance during maturation, there is no clear evidence that CCs proliferate during IVC and that the proliferation rate is determined by in vitro conditions. Therefore, the goal of this study was to show quantitative changes in CC proliferation in real time in relation to the Cdk4 and Cx43 mRNA expression profile and the distribution of encoded proteins during periods of in vitro maturation in separated porcine CCs.
Material and Methods
Animals
A total number of 41 pubertal crossbred Landrace gilts (age 170 days, body weight 98 kg) were used. The animals were kept under the same conditions. The experiments were approved by the local Ethics Committee.
Collection of porcine ovaries and cumulus-oocyte complexes (COCs)
The ovaries and reproductive tracts were recovered at slaughter and transported to the laboratory within 10 min at 38 C in 0.9% NaCl. In order to provide optimal conditions for subsequent oocyte maturation and fertilization in vitro, the ovaries of each animal were placed in 5% fetal bovine serum solution (FBS; Sigma-Aldrich, St. Louis, MO, USA) in PBS [18, 19]. Thereafter, individual large follicles (>5 mm) were opened by puncturing with a 20-G needle connected to a 5-ml syringe in a sterile Petri dish, and COCs were recovered. COCs were washed three times in a modified PBS supplemented with 36 µg/ml pyruvate, 50 µg/ml gentamicin, and 0.5 mg/ml BSA (Sigma-Aldrich). COCs were selected under an inverted microscope (Axiovert 35, Zeiss, Lübeck, Germany), counted and morphologically evaluated using the scale suggested by Jackowska et al. [20]. Only COCs of grade I with homogeneous ooplasm and uniform and compact cumulus cells were considered for use in the following steps of the experiment.
Assessment of oocyte developmental competence by BCB test
To perform the BCB staining test (brilliant cresyl blue), COCs were washed twice in a modified Dulbecco's PBS (DPBS) (Sigma-Aldrich), supplemented with 50 IU/ml penicillin, 50 µg/ml streptomycin (Sigma-Aldrich), 0.4% [w/v] BSA, 0.34 mM pyruvate, and 5.5 mM glucose (DPBSm). Thereafter, they were treated with 26 µM BCB (Sigma-Aldrich) diluted in DPBSm at 38.5 C in 5% CO2 in air for 90 min. Next, the oocytes were transferred to DPBSm and washed two times. During the washing procedure, the oocytes were examined under an inverted microscope and classified as either stained blue (BCB+) or remaining colorless (BCB–). After harvesting cumulus-granulosa somatic cells (CCs), only BCB+-COCs were incubated with bovine testicular collagenase (50 to 200 units/ml in HBSS) (Sigma-Aldrich) for 10 min at 38 C. Thereafter, the cells were removed by vortexing and pipetting the BCB+ oocytes in 1% sodium citrate buffer and by mechanical displacement, using a small-diameter glass micropipette. The oocytes were discarded, and the CCs were used for real-time cultivation procedures to determine the proliferation index and perform the confocal microscope analysis.
In vitro CC cultivation using a real-time cell analyzer (RTCA)
Harvested GCCs were transferred into a real-time cell analyzer (RTCA, E-Plates 48, Roche Applied Science, Penzberg, Germany) consisting of an RTCA Analyzer, RTCA SP Station and RTCA Software. The CCs were then cultured in 200 μl standard tissue culture medium (TCM-199) with Earle's salts and L-glutamine, (Gibco BRL Life Technologies, Grand Island, NY, USA) supplemented with 2.2 mg/ml sodium bicarbonate (Nacalai Tesque, Kyoto, Japan), 0.1 mg/ml sodium pyruvate (Sigma-Aldrich), 10 mg/ml BSA (bovine serum albumin), (Sigma-Aldrich), 0.1 mg/ml cysteine (Sigma-Aldrich), 10% (v/v) filtered porcine follicular fluid and gonadotropin supplements at final concentrations of 2.5 IU/ml hCG (Ayerst Laboratories, Philadelphia, PA, USA) and 2.5 IU/ml eCG (Intervet, Whitby, ON, Canada). The CCs were cultured for 44 h at 38 C under 5% CO2 in air. After 44 h of cultivation, a set of CCs was treated with trypsin (0.25% trypsin in a balanced salt solution; Sigma-Aldrich), and the collected pool of proliferating cells was used for further confocal microscope analysis. After 12, 24 and 44 h of cultivation, the standardized cell index (CI) was used to evaluate quantitative changes in electrical impedance of cells. The cell status was determined using the RTCA software.
Confocal microscope analysis of Cdk4 and Cx43 expression and distribution in CCs
The CCs were analyzed before and after 44 h of IVC. After cultivation, CCs were collected and fixed using an acetone/methanol mixture (1:1) for 10 min at –20 C and washed three times in PBS/PVP (0.2%). In order to block nonspecific binding, samples were incubated in 3% BSA in PBS with 0.1% Tween 20 for 30 min at RT. CCs were incubated for 1 h at room temperature (RT) with rabbit polyclonal anti-Cdk4 antibody (Ab), H-22 and/or rabbit polyclonal anti-Cx43 Ab and (Ser 279/282)-R Ab, both from Santa Cruz Biotechnology (Santa Cruz, CA, USA), diluted 1:500 in PBS/1.5% BSA/0.1% Tween 20. After several washes with PBS/0.1% Tween 20, samples were incubated with rabbit polyclonal anti-Cdk4 Ab and H-22 for 1 h at RT with fluorescent isothiocyanate (FITC)-conjugated anti-rabbit IgGAb (Santa Cruz Biotechnology) and diluted 1:500 in PBS/0.1% Tween 20. Samples with rabbit polyclonal anti-Cx43 Ab and (Ser 279/282)-R Ab were incubated for 1 h at RT with fluorescent isothiocyanate (FITC)-conjugated anti-rabbit IgG Ab and diluted 1:500 in PBS/0.1% Tween 20. Following washing in PBS/0.1% Tween 20, the CCs were stained with 0.1 µg/ml 4,6-diamino-2-phenylindole (DAPI; Santa Cruz Biotechnology) in mineral oil, mounted on glass slides in an antifade drop and observed under an LSM 510 confocal system microscope (Olympus FluoView 10i). FITC was excited at 488 nm from an argon laser, and emissions were imaged through a 505–530 nm filter. All confocal microscopic images were analyzed using the Imaris 7.2 (Bitplane, Zurich, Switzerland) software.
Statistical analysis
One-way ANOVA followed by a Tukey post hoc test was used to compare the results of real-time quantification of the proliferation index. The experiments were carried out with at least two replications. The cell proliferation index was quantified using an RTCA system. The differences were considered to be significant at P<0.05, P<0.01 and P<0.001 and evaluated by comparing the results of analyzes between four replicates of the same recovered cumulus-granulosa cells. The statistical calculations were applied to compare all investigated groups to the highest normalized proliferation index in each time ratio. The GraphPad Prism software, version 4.0 (GraphPad Software, San Diego, CA, USA) was used for the statistical calculations.
Results
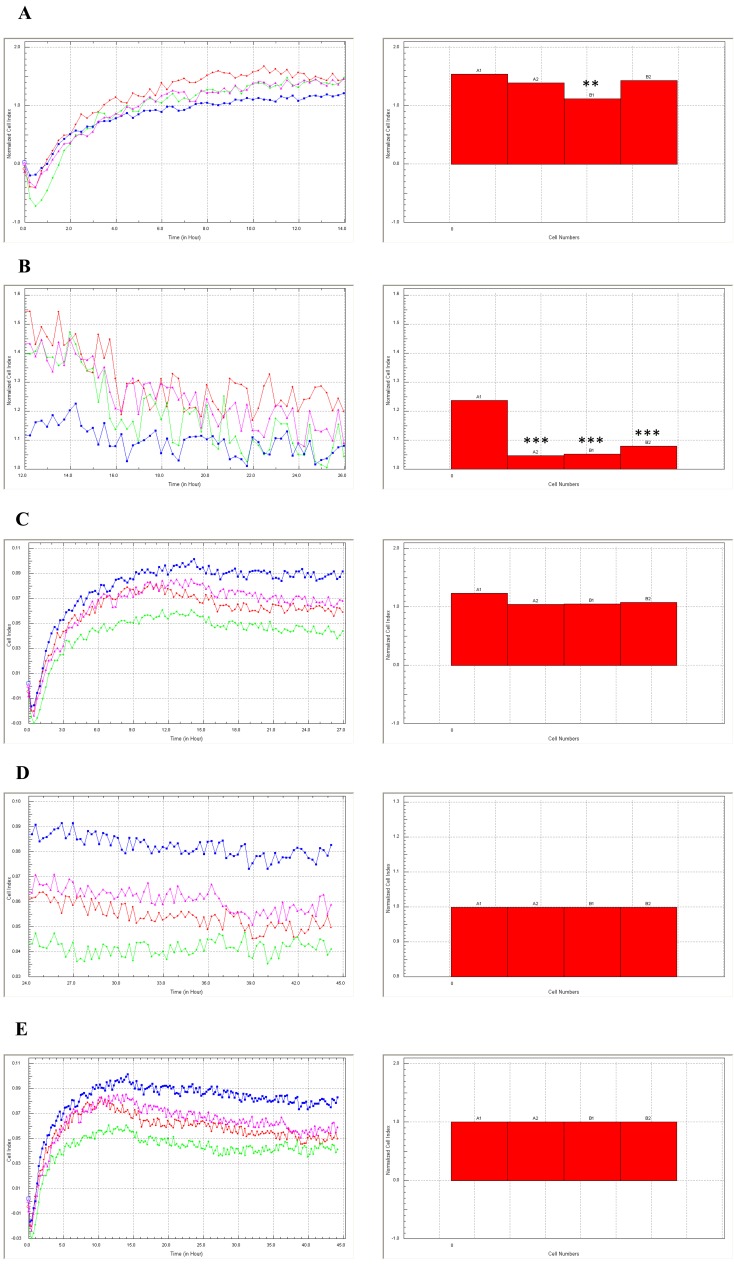
In this study, we analyzed the cumulus cell proliferation index between 0–44 h of IVC and assessed the expression and cellular distribution of Cdk4 and Cx43 using confocal microscope observation. The normalized cell index was assessed between 0–12 h, 12–24 h, 0–24 h, 24–44 h and 0–44 h of IVC (Fig. 1A–E), respectively. Differences in the proliferation index were found at 0–12 h and 12–24 h of IVC (P<0.01, P<0.001, respectively) (Fig. 1A and B). The proliferation index assessed at 0–24 h, 24–44 h and 0–44 h did not vary between groups (Fig. 1C–E).
Fig. 1.
Normalized proliferation index of cumulus cells cultivated for 44 h. The cumulus cells were recovered from porcine COCs after collagenase treatment for 10 min at 38.5 C. Then, the CCs were immediately transferred into a real-time cell analyzer (RTCA, E-Plates 48, Roche Applied Science, Penzberg, Germany). The experiment consisted of four replications involving cultivation of the same population of collected CCs. At every step of the experiment, the normalized proliferation index was assessed in real-time in vitro cultivation for time periods of 0–12 h (A), 12–24 h (B), 0–24 h (C), 24–44 h (D) and 0–44 h (E).
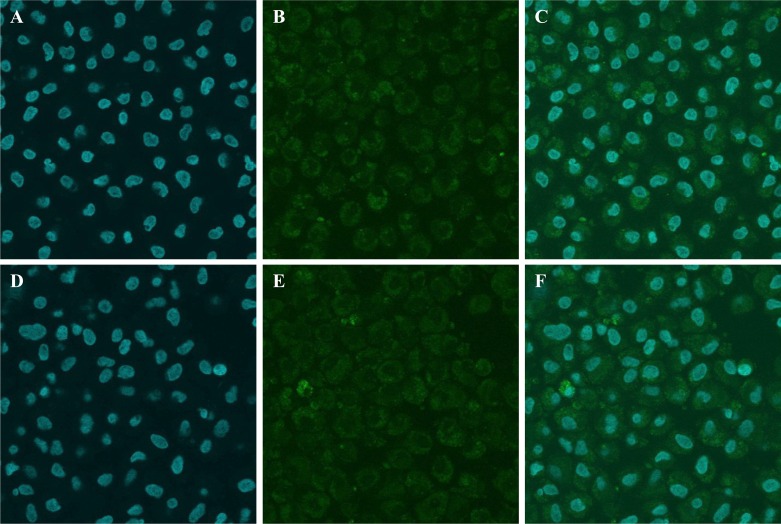
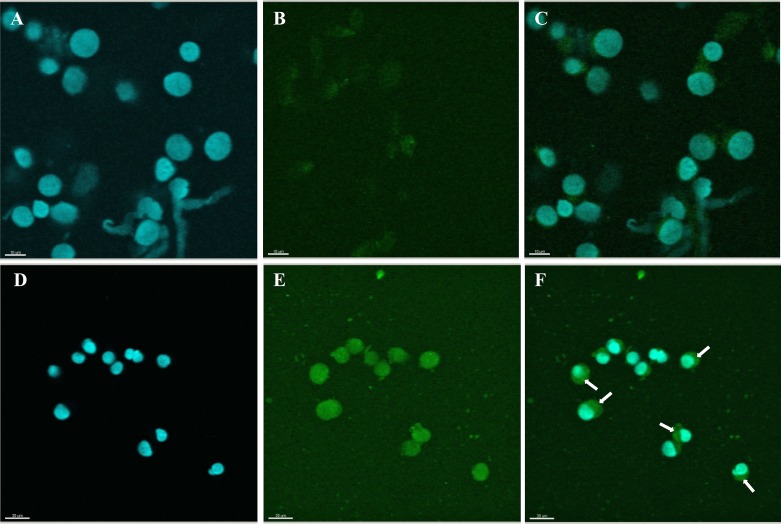
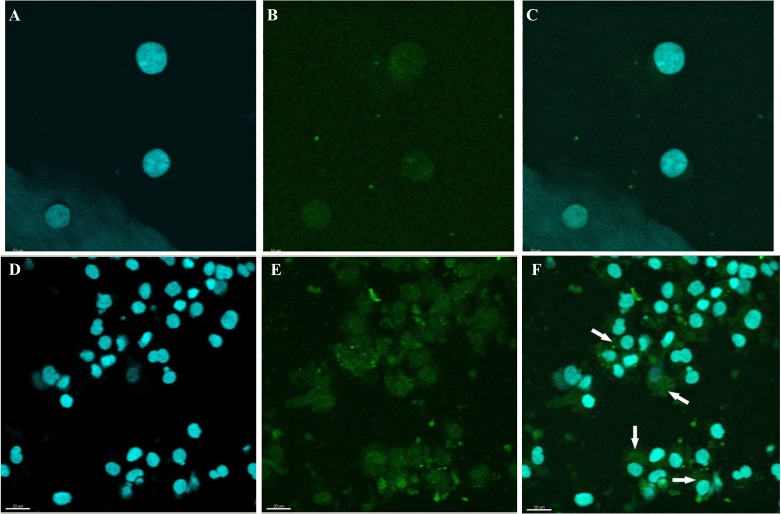
Using confocal microscopic observation, we analyzed Cx43 (Fig. 2A–C) and Cdk4 (Fig. 2D–F) expression and cellular distribution after 12 h of IVC. The Cx43 and Cdk4 cellular expression was determined in the first 12 h of CC proliferation during cultivation. Expression of Cdk4 protein was significantly higher in CCs after 44 h of cultivation compared with its expression before IVC (Fig. 3A–F). Furthermore, Cdk4 was localized in the nucleus of CCs before IVC, whereas after IVC this protein was distributed in the cytoplasm of CCs (Fig. 3C and F). Similarly, a substantially increased expression of Cx43 was observed after IVC as compared with CCs analyzed before IVC (Fig. 4A–F). Moreover, a translocation of Cx43 from the cell nucleus before IVC to the cytoplasm after IVC was observed (Fig. 4C and F).
Fig. 2.
Confocal microscopic observation of Cdk4 and Cx43 expression and cellular distribution in porcine CCsat 0–12 h of IVM. Expression and localization of Cx43 (Fig. 2A–C) and Cdk4 (Fig. 2D–F) at the 0–12 h of IVM. CCs were stained with 0.1 µg/ml 4,6-diamino-2-phenylindole (DAPI; Santa Cruz Biotechnology, Santa Cruz, CA, USA) in mineral oil (Fig. 2A and D) following staining with porcine Cx43 (rabbit polyclonal anti-Cx43 Ab) (Fig. 2B) and Cdk4 (rabbit polyclonal anti-Cdk4 Ab) (Fig. 2E). Secondary antibodies were labeled with FITC (fluorescence isothiocyanate), which emits a green fluorescent signal after excitation at 488 nm. Double staining of CCs is presented for Cx43 (Fig. 2C) and Cdk4 (Fig. 2F).
Fig. 3.
Confocal microscopic observation of Cdk4 expression and cellular distribution in porcine CCs before and after IVC. The collected CCs before (Fig. 3A–C) and after IVC (Fig. 3D–F) were stained with 0.1 µg/ml 4,6-diamino-2-phenylindole (DAPI; Santa Cruz Biotechnology, Santa Cruz, CA, USA) in mineral oil (Fig. 3A and D), following staining with porcine Cdk4 (rabbit polyclonal anti-Cdk4 Ab), (Fig. 3B and E). Secondary antibodies were labeled with FITC (fluorescence isothiocyanate), which emits a green fluorescent signal after excitation at 488 nm. Double staining of CCs is presented as before (Fig. 3C) and after (Fig. 3F) IVC. The arrows point to the cytoplasmic localization of Cdk4 in CCs after IVC. Scale bars represent 10 µm (Fig. 3A–C) and 20 µm (Fig. 3D–F).
Fig. 4.
Confocal microscopic observation of Cx43 expression and cellular distribution in porcine CCs before and after IVC. The collected CCs before (Fig. 4A–C) and after IVC (Fig. 4D–F) were stained with 0.1 µg/ml 4,6-diamino-2-phenylindole (DAPI; Santa Cruz Biotechnology, Santa Cruz, CA, USA) in mineral oil (Fig. 4A and D), following staining with porcine Cx43 (rabbit polyclonal anti-Cx43 Ab), (Fig. 4B and E). Secondary antibodies were labeled with FITC (fluorescence isothiocyanate), which emits a green fluorescent signal after excitation at 488 nm. Double staining of CCs is presented as before (Fig. 4C) and after IVC (Fig. 4F). The arrows point to the cytoplasmic localization of Cx43 in CCs after IVC. Scale bars represent 10 µm (Fig. 4A–C) and 20 µm (Fig. 4D–F).
Discussion
Cumulus cells undergo substantial morphological changes during maturation of COCs in vivo and in vitro, which are recognized as CC expansion. This process is accompanied by the transfer of large amounts of small molecules and substrates between the mature oocyte and surrounding somatic cells [15, 21,22,23]. However, the molecular changes, visible as differential expression profiles of genes and proteins responsible for COC maturation, are still not fully recognized. It was shown in several studies that the achievement of a fully mature stage of oocytes is regulated via an appropriate communication between the oocyte and the surrounding cumulus-granulosa cells, and it is assumed that attainment of the MII stage by the oocyte is accompanied by CC expansion [8, 18, 19, 24, 25]. The mechanisms responsible for this process are not entirely known. In our study, we postulated that the expression and cellular distribution of Cdk4 and Cx43 regulate not only oocyte maturation but also the mechanisms responsible for cumulus cell differentiation.
Since the bidirectional communication between the oocyte and CCs is acknowledged, analysis of the expression of specific proteins in oocytes and/or CCs may determine important molecular factors regulating oocyte-CC maturation and differentiation. Regassa et al. [26] investigated the global expression profile of genes in separated bovine oocytes and CCs. They found several hundred genes that were differentially expressed when CCs were cultured with or without oocytes. A bidirectional effect of cultivation on CCs and oocytes was observed because several genes were expressed in oocytes or CCs only or in both cell types, respectively. Likewise, we observed an expression of Cdk4 and Cx43 proteins in porcine CCs that has previously been described in oocytes [1, 27]. This indicates that both proteins might play a key role not only during oocyte maturation but that they also might regulate processes of CC differentiation. The assumption that CC expansion is associated with attainment of the MII stage in oocytes is supported only by biochemical data of the transfer of substances between oocytes and CCs [8, 23, 28]. Our observation of Cdk4 and Cx43 translocation from the nucleus to the cytoplasm of CCs during IVC provides clues regarding mechanisms of CC differentiation. Translocation of Cx43 and Cdk4 proteins into cytoplasm after IVC might be associated with an increased transport of molecules between the oocyte and CCs and with acquisition of the MII stage. However, in our experiment, we cultured CCs without oocytes, and the results indicate that even in a separated culture system both Cdk4 and Cx43 exert an effect on CC maturation. Almost all previously published data described oocytes enclosing cumulus cells and the possible association between oocyte and CC communication during IVC procedures [29,30,31]. In addition to the analysis of protein expressions after short-term cultivation of separated CC, we recorded in our study the CC proliferation index. Differences in the proliferation index were observed only between 0 h and 24 h of IVC. This period may be interpreted as the adherent phase and the pre-proliferation stage. During the subsequent period, no differences in the proliferation index were observed, and it can be recognized as a stabile period. Besides the translocation of Cdk4 and Cx43 into the cytoplasm of CCs during 44 h of IVC, the time window of 0–20 h of IVC may be crucial for CC growth. Only a few studies have shown that CCs may proliferate in vitro, but in all cases, this was based on the culture of CC-enclosed oocytes. Gilchrist et al. [23] stated that the process of CC expansion may occur without oocytes, and this was demonstrated in three out of four examined mammalian species. They used porcine and mouse COCs and oocyte-free complexes (OOXs) for evaluation the SMAD signaling pathway, which contributes to the efficiency of CC expansion. They found that inhibition of the ERK1/2 and p38 MAPK pathways affects porcine COC expansion. Although they did not culture separated CCs, as in our study, it can be concluded that porcine oocyte-free CCs may expand during IVC. Our results demonstrated that oocyte free-CCs are able to proliferate in vitro, which is presumably related to in vitro CC expansion.
For the first time, we could demonstrate the proliferation ability of separated oocyte-free CCs in vitro. Moreover, substantial changes in the expression and translocation of Cdk4 and Cx43 in CCs during IVC were observed. The results of our study show that the concentration levels of both analyzed proteins were higher after IVM. Moreover, similarity was also observed in the distribution of proteins, with the localization of proteins before IVM being mainly nuclear and after IVM being mainly cytoplasmic. Both processes are thought to be crucial for full maturation of porcine oocytes.
Acknowledgment
This study was made possible by grant number 0233/IP1/2011/71 of the “Iuventus Plus” program of the Polish Ministry of Scientific Research and Higher Education. MW was supported by “Scholarship support for Ph.D. students specializing in majors strategic for Wielkopolska's development”, Sub-measure 8.2.2 Human Capital Operational Programme, co-financed by the European Union under the European Social Fund (No. POKL 8.2.2/30-165-11).
References
- 1.Antosik P, Kempisty B, Jackowska M, Bukowska D, Woźna M, Lianeri M, Brüssow KP, Jaśkowski JM. Assessment of transcript and protein levels contributing to cell cycle control and gap junction connections in morphologically variable groups of porcine cumulus-oocyte complexes. Vet Med-Czech 2010; 55: 512–521 [Google Scholar]
- 2.Duranthon V, Renard JP. The developmental competence of mammalian oocytes: a convenient but biologically fuzzy concept. Theriogenology 2001; 55: 1277–1289 [DOI] [PubMed] [Google Scholar]
- 3.Kempisty B, Piotrowska H, Walczak R, Śniadek P, Dziuban J, Bukowska D, Antosik P, Jackowska M, Woźna M, Jaśkowski JM. Morphological and molecular aspects of zygote formation and early stages of embryo development in pigs in light of genetic and microfluidic research. Medycyna Wet 2011; 67: 435–439(In Polish) [Google Scholar]
- 4.Ju S, Rui R. Effects of cumulus cells on in vitro maturation of oocytes and development of cloned embryos in the pig. Reprod Domest Anim 2012; 47: 521–529 [DOI] [PubMed] [Google Scholar]
- 5.Luciano AM, Lodde V, Beretta MS, Colleoni S, Lauria A, Modina S. Developmental capability of denuded bovine oocyte in a co-culture system with intact cumulus-oocyte complexes: role of cumulus cells,cyclicadenosine3',5'-monophosphate, and glutathione. Mol Reprod Dev 2005; 71: 389–397 [DOI] [PubMed] [Google Scholar]
- 6.Moor R, Dai Y. Maturation of pig oocytes in vivo and in vitro. Reprod Suppl 2001; 58: 91–104 [PubMed] [Google Scholar]
- 7.Anderson E, Albertini DF. Gap junctions between the oocyte and companion follicle cells in the mammalian ovary. J Cell Biol 1976; 71: 680–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santiquet NW, Develle Y, Laroche A, Robert C, Richard FJ. Regulation of gap-junctional communication between cumulus cells during in vitro maturation in swine, a gap-FRAP study. Biol Reprod 2012; 87: 46 [DOI] [PubMed] [Google Scholar]
- 9.Thomas RE, Armstrong DT, Gilchrist RB. Bovine cumulus cell-oocyte gap junctional communication during in vitro maturation in response to manipulation of cell-specific cyclic adenosine 3',5'-monophosophate levels. Biol Reprod 2004; 70: 548–556 [DOI] [PubMed] [Google Scholar]
- 10.Simon AM, Goodenough DA, Li E, Paul DL. Female infertility in mice lacking connexin 37. Nature 1997; 385: 525–529 [DOI] [PubMed] [Google Scholar]
- 11.Nitta M, Yogo K, Ohashi M, Akiyama M, Kunitomo Y, Ogawa T, Ishida-Kitagawa N, Miyoshi J, Sato E, Takeya T. Identification and expression analysis of connexin-45 and connexin-60 as major connexins in porcine oocytes. J Anim Sci 2010; 88: 3269–3279 [DOI] [PubMed] [Google Scholar]
- 12.Adhikari D, Zheng W, Shen Y, Gorre N, Ning Y, Halet G, Kaldis P, Liu K. Cdk1, but not Cdk2, is the sole Cdk that is essential and sufficient to drive resumption of meiosis in mouse oocytes. Hum Mol Genet 2012; 21: 2476–2484 [DOI] [PubMed] [Google Scholar]
- 13.Malumbres M. Physiological relevance of cell cycle kinases. Physiol Rev 2011; 91: 973–1007 [DOI] [PubMed] [Google Scholar]
- 14.De Falco M, Fedele V, Cobellis L, Mastrogiacomo A, Giraldi D, Leone S, De Luca L, Laforgia V, De Luca A. Pattern of expression of cyclin D1/CDK4 complex in human placenta during gestation. Cell Tissue Res 2004; 317: 187–194 [DOI] [PubMed] [Google Scholar]
- 15.Brůcková L, Soukup T, Moos J, Moosová M, Pavelková J, Rezábek K, Vísek B, Mokrý J. The cultivation of human granulosa cells. Acta Medica (Hradec Kralove) 2008; 51: 165–172 [DOI] [PubMed] [Google Scholar]
- 16.Voznesenskaia TIU, Blashkiv TV, Portnichenko AG. Effect of cumulus and granulosa cells on meiosis resumption in murine oocytes in vitro. Tsitologiia 2001; 43: 250–253(In Russian) [PubMed] [Google Scholar]
- 17.Wongsrikeao P, Kaneshige Y, Ooki R, Taniguchi M, Agung B, Nii M, Otoi T. Effect of the removal of cumulus cells on the nuclear maturation, fertilization and development of porcine oocytes. Reprod Domest Anim 2005; 40: 166–170 [DOI] [PubMed] [Google Scholar]
- 18.Al-aghbari AM, Menino AR. Survival of oocytes recovered from vitrified sheep ovarian tissues. Anim Reprod Sci 2002; 71: 101–110 [DOI] [PubMed] [Google Scholar]
- 19.Nascimento AB, Albornoz MS, Che L, Visintin JA, Bordignon V. Synergistic effect of porcine follicular fluid and dibutyryl cyclic adenosine monophosphate on development of parthenogenetically activated oocytes from pre-pubertal gilts. Reprod Domest Anim 2010; 45: 851–859 [DOI] [PubMed] [Google Scholar]
- 20.Jackowska M, Kempisty B, Antosik P, Bukowska D, Budna J, Lianeri M, Rosińka E, Woźna M, Jagodziński PP, Jaśkowski JM. The morphology of porcine oocytes is associated with zona pellucida glycoprotein transcript contents. Reprod Biol 2009; 9: 79–85 [DOI] [PubMed] [Google Scholar]
- 21.Alvarez GM, Dalvit G, Cetica P. Influence of the cumulus and gonadotropins on the metabolic profile of porcine cumulus-oocyte complexes during in vitro maturation. Reprod Domest Anim 2012; 47: 856–864 [DOI] [PubMed] [Google Scholar]
- 22.Areekijseree M, Chuen-Im T. Effects of porcine follicle stimulating hormone, luteinizing hormone and estradiol supplementation in culture medium on ultrastructures of porcine cumulus oocyte complexes (pCOCs). Micron 2012; 43: 251–257 [DOI] [PubMed] [Google Scholar]
- 23.Gilchrist RB, Ritter LJ. Differences in the participation of TGFB superfamily signalling pathways mediating porcine and murine cumulus cell expansion. Reproduction 2011; 142: 647–657 [DOI] [PubMed] [Google Scholar]
- 24.Das SK, Chauhan MS, Palta P, Tomer OS. Influence of cumulus cells on in vitro maturation of denuded buffalo oocytes. Vet Rec 1997; 141: 522–523 [DOI] [PubMed] [Google Scholar]
- 25.Maedomari N, Kikuchi K, Ozawa M, Noguchi J, Kaneko H, Ohnuma K, Nakai M, Shino M, Nagai T, Kashiwazaki N. Cytoplasmic glutathione regulated by cumulus cells during porcine oocyte maturation affects fertilization and embryonic development in vitro. Theriogenology 2007; 67: 983–993 [DOI] [PubMed] [Google Scholar]
- 26.Regassa A, Rings F, Hoelker M, Cinar U, Tholen E, Looft C, Schellander K, Tesfaye D. Transcriptome dynamics and molecular cross-talk between bovine oocyte and its companion cumulus cells. BMC Genomics 2011; 12: 57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antosik P, Kempisty B, Jackowska M, Woźna M, Bukowska D, Brüssow KP, Bryja A, Jaśkowski JM. Are the levels of Cdk4 and Cx43 proteins of porcine oocytes associated with follicular size? Anim Biol 2011; 61: 211–224 [Google Scholar]
- 28.Gilula NB, Epstein ML, Beers WH. Cell-to-cell communication and ovulation. A study of the cumulus-oocyte complex. J Cell Biol 1978; 78: 58–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Mao G, Xia G. FSH modulates PKAI and GPR3 activities in mouse oocyte of COC in a gap junctional communication (GJC)-dependent manner to initiate meiotic resumption. PLoS One 2012; 7: e37835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Somfai T, Kikuchi K, Onishi A, Iwamoto M, Fuchimoto D, Papp AB, Sato E, Nagai T. Relationship between the morphological changes of somatic compartment and the kinetics of nuclear and cytoplasmic maturation of oocytes during in vitro maturation of porcine follicular oocytes. Mol Reprod Dev 2004; 68: 484–491 [DOI] [PubMed] [Google Scholar]
- 31.Tatemoto H, Sakurai N, Muto N. Protection of porcine oocytes against apoptotic cell death caused by oxidative stress during in vitro maturation: role of cumulus cells. Biol Reprod 2000; 63: 805–810 [DOI] [PubMed] [Google Scholar]