Abstract
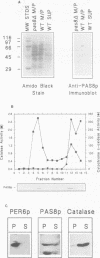
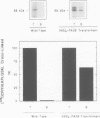
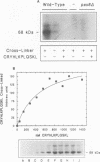
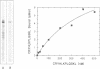
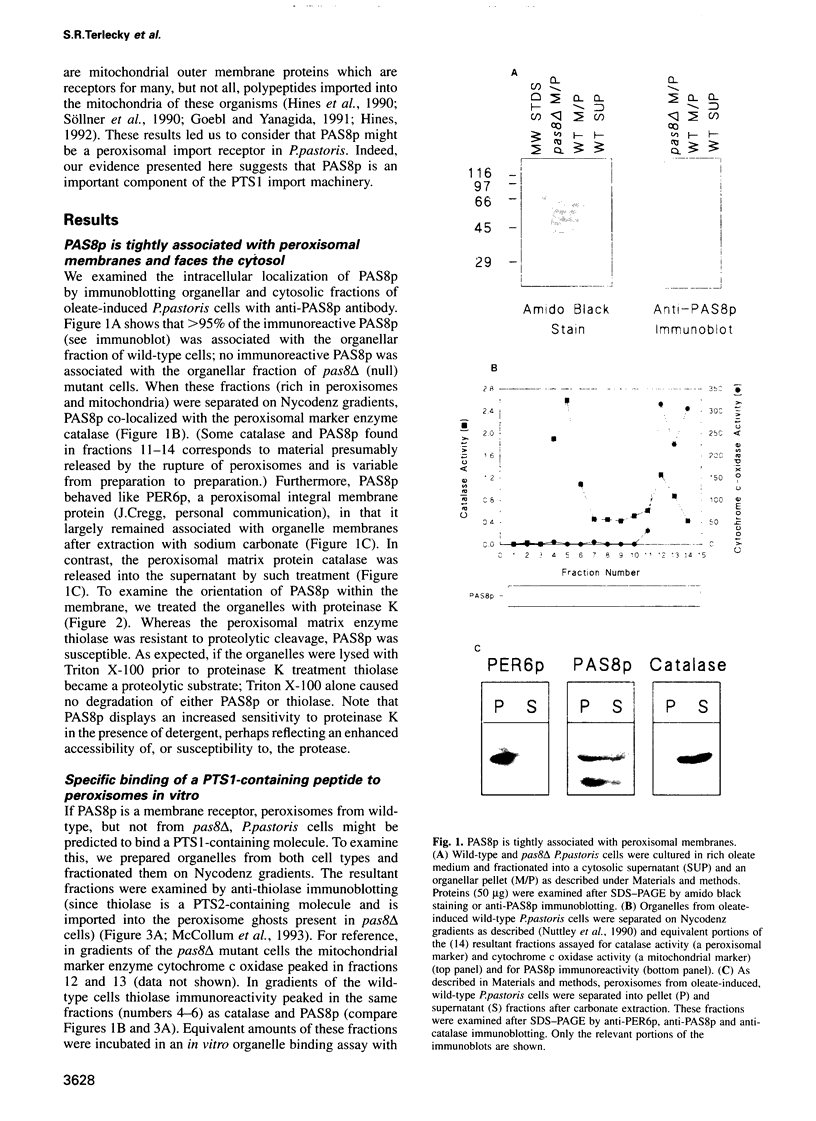
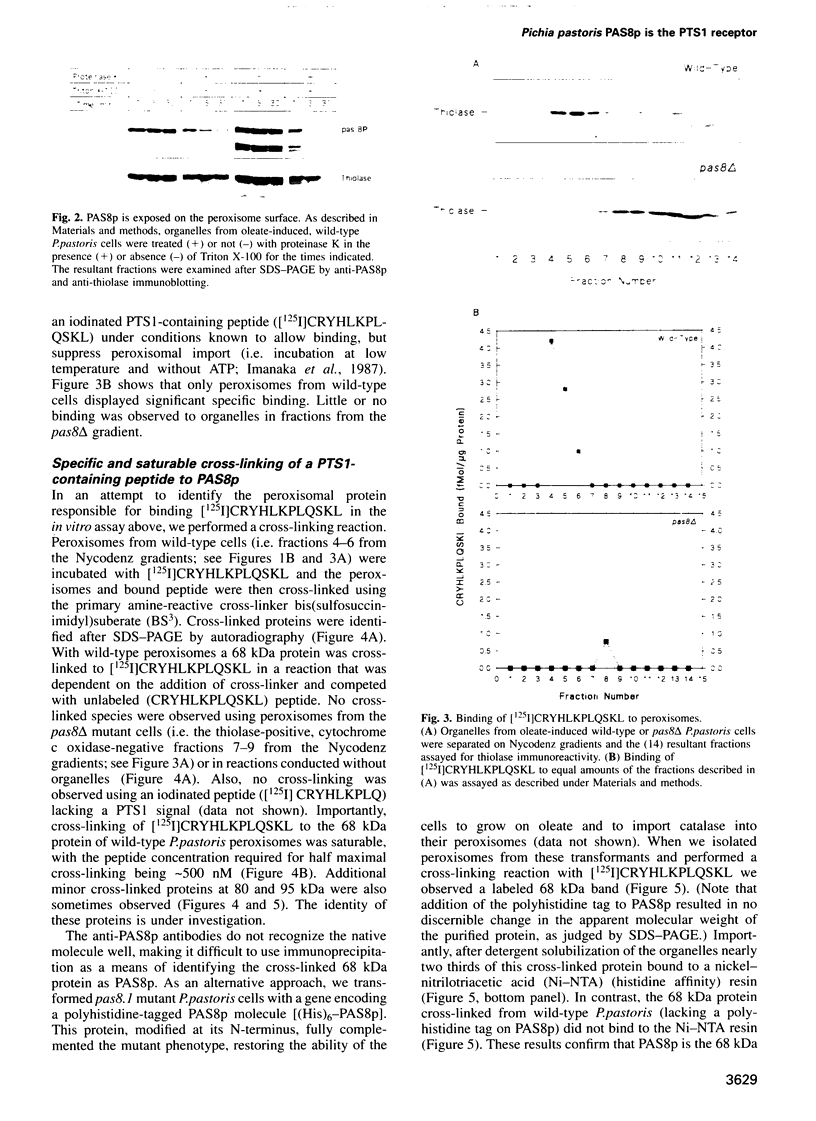
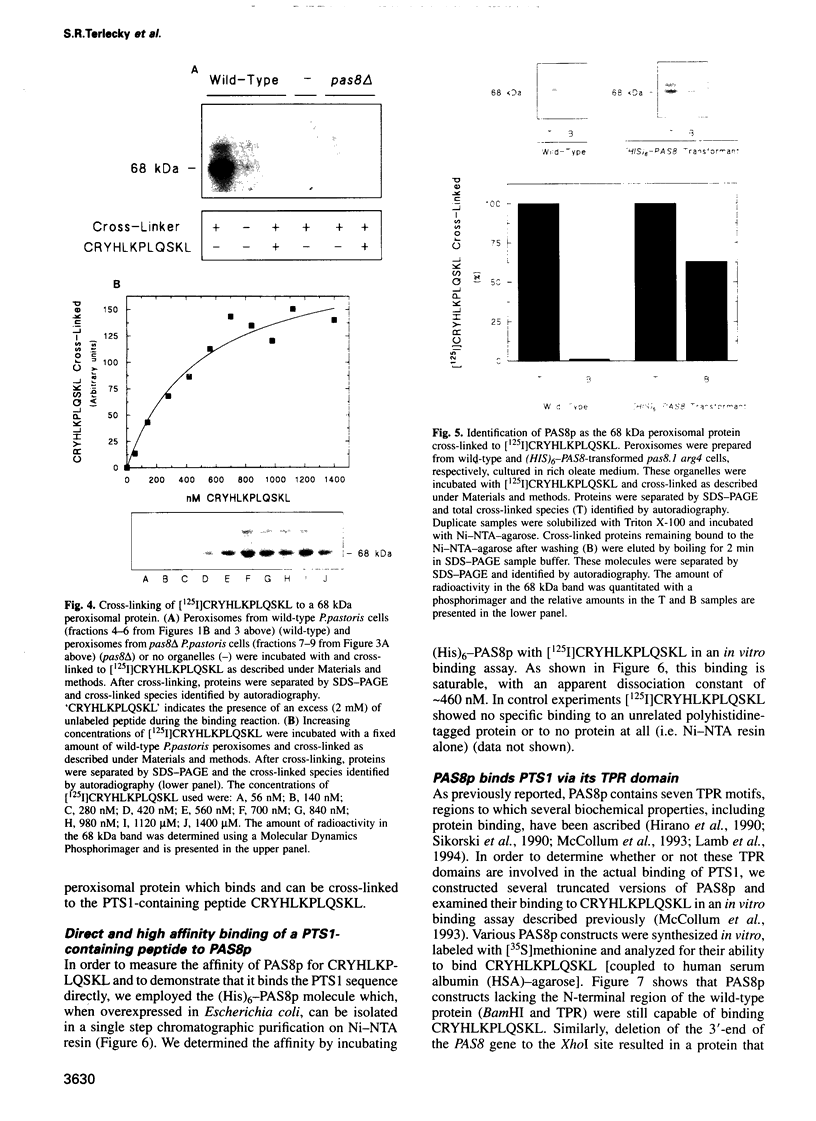
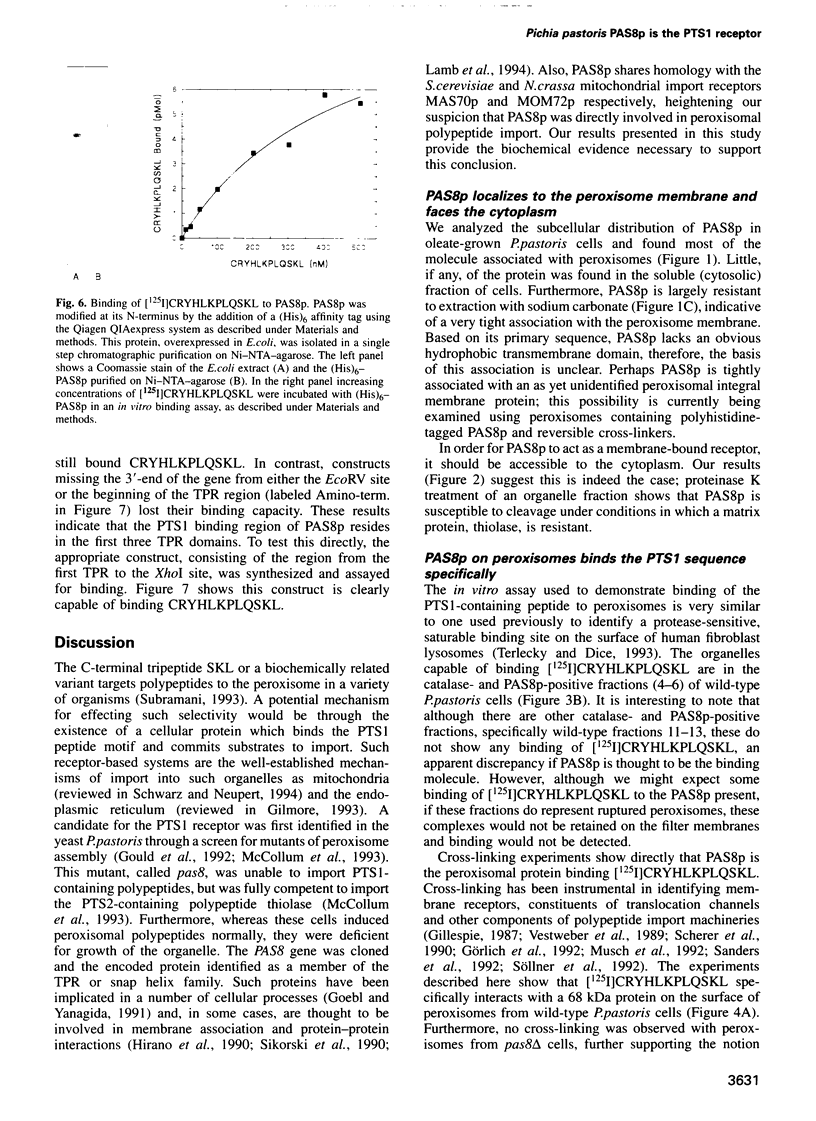
The peroxisomal targeting signal 1 (PTS1), consisting of a C-terminal tripeptide (SKL and variants), directs polypeptides to the peroxisome matrix in evolutionarily diverse organisms. Previous studies in the methylotrophic yeast Pichia pastoris identified a 68 kDa protein, PAS8p, as a potential component of the PTS1 import machinery. We now report several new properties of this molecule which, taken together, show that it is the peroxisomal PTS1 receptor. (i) PAS8p is localized to and tightly associated with the cytoplasmic side of the peroxisomal membrane, (ii) peroxisomes of wild-type, but not of pas8 delta (null) mutant, P.pastoris cells bind a PTS1-containing peptide (CRYHLKPLQSKL), (iii) CRYHLKPLQSKL can be cross-linked to PAS8p after binding at the peroxisome membrane and (iv) purified PAS8p binds CRYHLKPLQSKL with high affinity (nanomolar dissociation constant). In addition, the tetratricopeptide repeat (TPR) domain of PAS8p is identified as the PTS1 binding region.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dodt G., Braverman N., Wong C., Moser A., Moser H. W., Watkins P., Valle D., Gould S. J. Mutations in the PTS1 receptor gene, PXR1, define complementation group 2 of the peroxisome biogenesis disorders. Nat Genet. 1995 Feb;9(2):115–125. doi: 10.1038/ng0295-115. [DOI] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie L. L. Identification of an outer mitochondrial membrane protein that interacts with a synthetic signal peptide. J Biol Chem. 1987 Jun 15;262(17):7939–7942. [PubMed] [Google Scholar]
- Gilmore R. Protein translocation across the endoplasmic reticulum: a tunnel with toll booths at entry and exit. Cell. 1993 Nov 19;75(4):589–592. doi: 10.1016/0092-8674(93)90476-7. [DOI] [PubMed] [Google Scholar]
- Glover J. R., Andrews D. W., Rachubinski R. A. Saccharomyces cerevisiae peroxisomal thiolase is imported as a dimer. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10541–10545. doi: 10.1073/pnas.91.22.10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebl M., Yanagida M. The TPR snap helix: a novel protein repeat motif from mitosis to transcription. Trends Biochem Sci. 1991 May;16(5):173–177. doi: 10.1016/0968-0004(91)90070-c. [DOI] [PubMed] [Google Scholar]
- Gould S. J., Keller G. A., Hosken N., Wilkinson J., Subramani S. A conserved tripeptide sorts proteins to peroxisomes. J Cell Biol. 1989 May;108(5):1657–1664. doi: 10.1083/jcb.108.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. J., McCollum D., Spong A. P., Heyman J. A., Subramani S. Development of the yeast Pichia pastoris as a model organism for a genetic and molecular analysis of peroxisome assembly. Yeast. 1992 Aug;8(8):613–628. doi: 10.1002/yea.320080805. [DOI] [PubMed] [Google Scholar]
- Görlich D., Prehn S., Hartmann E., Kalies K. U., Rapoport T. A. A mammalian homolog of SEC61p and SECYp is associated with ribosomes and nascent polypeptides during translocation. Cell. 1992 Oct 30;71(3):489–503. doi: 10.1016/0092-8674(92)90517-g. [DOI] [PubMed] [Google Scholar]
- Hines V., Brandt A., Griffiths G., Horstmann H., Brütsch H., Schatz G. Protein import into yeast mitochondria is accelerated by the outer membrane protein MAS70. EMBO J. 1990 Oct;9(10):3191–3200. doi: 10.1002/j.1460-2075.1990.tb07517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Kinoshita N., Morikawa K., Yanagida M. Snap helix with knob and hole: essential repeats in S. pombe nuclear protein nuc2+. Cell. 1990 Jan 26;60(2):319–328. doi: 10.1016/0092-8674(90)90746-2. [DOI] [PubMed] [Google Scholar]
- Imanaka T., Small G. M., Lazarow P. B. Translocation of acyl-CoA oxidase into peroxisomes requires ATP hydrolysis but not a membrane potential. J Cell Biol. 1987 Dec;105(6 Pt 2):2915–2922. doi: 10.1083/jcb.105.6.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Michaud W. A., Sikorski R. S., Hieter P. A. Cdc16p, Cdc23p and Cdc27p form a complex essential for mitosis. EMBO J. 1994 Sep 15;13(18):4321–4328. doi: 10.1002/j.1460-2075.1994.tb06752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarow P. B., Fujiki Y. Biogenesis of peroxisomes. Annu Rev Cell Biol. 1985;1:489–530. doi: 10.1146/annurev.cb.01.110185.002421. [DOI] [PubMed] [Google Scholar]
- McCammon M. T., McNew J. A., Willy P. J., Goodman J. M. An internal region of the peroxisomal membrane protein PMP47 is essential for sorting to peroxisomes. J Cell Biol. 1994 Mar;124(6):915–925. doi: 10.1083/jcb.124.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum D., Monosov E., Subramani S. The pas8 mutant of Pichia pastoris exhibits the peroxisomal protein import deficiencies of Zellweger syndrome cells--the PAS8 protein binds to the COOH-terminal tripeptide peroxisomal targeting signal, and is a member of the TPR protein family. J Cell Biol. 1993 May;121(4):761–774. doi: 10.1083/jcb.121.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNew J. A., Goodman J. M. An oligomeric protein is imported into peroxisomes in vivo. J Cell Biol. 1994 Dec;127(5):1245–1257. doi: 10.1083/jcb.127.5.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motley A., Hettema E., Distel B., Tabak H. Differential protein import deficiencies in human peroxisome assembly disorders. J Cell Biol. 1994 May;125(4):755–767. doi: 10.1083/jcb.125.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müsch A., Wiedmann M., Rapoport T. A. Yeast Sec proteins interact with polypeptides traversing the endoplasmic reticulum membrane. Cell. 1992 Apr 17;69(2):343–352. doi: 10.1016/0092-8674(92)90414-8. [DOI] [PubMed] [Google Scholar]
- Nuttley W. M., Bodnar A. G., Mangroo D., Rachubinski R. A. Isolation and characterization of membranes from oleic acid-induced peroxisomes of Candida tropicalis. J Cell Sci. 1990 Mar;95(Pt 3):463–470. doi: 10.1242/jcs.95.3.463. [DOI] [PubMed] [Google Scholar]
- Osumi T., Tsukamoto T., Hata S., Yokota S., Miura S., Fujiki Y., Hijikata M., Miyazawa S., Hashimoto T. Amino-terminal presequence of the precursor of peroxisomal 3-ketoacyl-CoA thiolase is a cleavable signal peptide for peroxisomal targeting. Biochem Biophys Res Commun. 1991 Dec 31;181(3):947–954. doi: 10.1016/0006-291x(91)92028-i. [DOI] [PubMed] [Google Scholar]
- Rapp S., Soto U., Just W. W. Import of firefly luciferase into peroxisomes of permeabilized Chinese hamster ovary cells: a model system to study peroxisomal protein import in vitro. Exp Cell Res. 1993 Mar;205(1):59–65. doi: 10.1006/excr.1993.1058. [DOI] [PubMed] [Google Scholar]
- Sanders S. L., Whitfield K. M., Vogel J. P., Rose M. D., Schekman R. W. Sec61p and BiP directly facilitate polypeptide translocation into the ER. Cell. 1992 Apr 17;69(2):353–365. doi: 10.1016/0092-8674(92)90415-9. [DOI] [PubMed] [Google Scholar]
- Scherer P. E., Krieg U. C., Hwang S. T., Vestweber D., Schatz G. A precursor protein partly translocated into yeast mitochondria is bound to a 70 kd mitochondrial stress protein. EMBO J. 1990 Dec;9(13):4315–4322. doi: 10.1002/j.1460-2075.1990.tb07880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E., Neupert W. Mitochondrial protein import: mechanisms, components and energetics. Biochim Biophys Acta. 1994 Aug 30;1187(2):270–274. doi: 10.1016/0005-2728(94)90125-2. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Boguski M. S., Goebl M., Hieter P. A repeating amino acid motif in CDC23 defines a family of proteins and a new relationship among genes required for mitosis and RNA synthesis. Cell. 1990 Jan 26;60(2):307–317. doi: 10.1016/0092-8674(90)90745-z. [DOI] [PubMed] [Google Scholar]
- Storrie B., Madden E. A. Isolation of subcellular organelles. Methods Enzymol. 1990;182:203–225. doi: 10.1016/0076-6879(90)82018-w. [DOI] [PubMed] [Google Scholar]
- Subramani S. Protein import into peroxisomes and biogenesis of the organelle. Annu Rev Cell Biol. 1993;9:445–478. doi: 10.1146/annurev.cb.09.110193.002305. [DOI] [PubMed] [Google Scholar]
- Swinkels B. W., Gould S. J., Bodnar A. G., Rachubinski R. A., Subramani S. A novel, cleavable peroxisomal targeting signal at the amino-terminus of the rat 3-ketoacyl-CoA thiolase. EMBO J. 1991 Nov;10(11):3255–3262. doi: 10.1002/j.1460-2075.1991.tb04889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner T., Pfaller R., Griffiths G., Pfanner N., Neupert W. A mitochondrial import receptor for the ADP/ATP carrier. Cell. 1990 Jul 13;62(1):107–115. doi: 10.1016/0092-8674(90)90244-9. [DOI] [PubMed] [Google Scholar]
- Söllner T., Rassow J., Wiedmann M., Schlossmann J., Keil P., Neupert W., Pfanner N. Mapping of the protein import machinery in the mitochondrial outer membrane by crosslinking of translocation intermediates. Nature. 1992 Jan 2;355(6355):84–87. doi: 10.1038/355084a0. [DOI] [PubMed] [Google Scholar]
- Terlecky S. R., Dice J. F. Polypeptide import and degradation by isolated lysosomes. J Biol Chem. 1993 Nov 5;268(31):23490–23495. [PubMed] [Google Scholar]
- Van der Leij I., Franse M. M., Elgersma Y., Distel B., Tabak H. F. PAS10 is a tetratricopeptide-repeat protein that is essential for the import of most matrix proteins into peroxisomes of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11782–11786. doi: 10.1073/pnas.90.24.11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestweber D., Brunner J., Baker A., Schatz G. A 42K outer-membrane protein is a component of the yeast mitochondrial protein import site. Nature. 1989 Sep 21;341(6239):205–209. doi: 10.1038/341205a0. [DOI] [PubMed] [Google Scholar]
- Walton P. A., Gould S. J., Feramisco J. R., Subramani S. Transport of microinjected proteins into peroxisomes of mammalian cells: inability of Zellweger cell lines to import proteins with the SKL tripeptide peroxisomal targeting signal. Mol Cell Biol. 1992 Feb;12(2):531–541. doi: 10.1128/mcb.12.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton P. A., Wendland M., Subramani S., Rachubinski R. A., Welch W. J. Involvement of 70-kD heat-shock proteins in peroxisomal import. J Cell Biol. 1994 Jun;125(5):1037–1046. doi: 10.1083/jcb.125.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland M., Subramani S. Cytosol-dependent peroxisomal protein import in a permeabilized cell system. J Cell Biol. 1993 Feb;120(3):675–685. doi: 10.1083/jcb.120.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland M., Subramani S. Presence of cytoplasmic factors functional in peroxisomal protein import implicates organelle-associated defects in several human peroxisomal disorders. J Clin Invest. 1993 Nov;92(5):2462–2468. doi: 10.1172/JCI116854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemer E. A., Subramani S. Protein import deficiencies in human peroxisomal disorders. Mol Genet Med. 1994;4:119–152. doi: 10.1016/b978-0-12-462004-9.50008-6. [DOI] [PubMed] [Google Scholar]