Abstract
The 13th International Congress on Combustion By-Products and their Health Effects was held in New Orleans, Louisiana from May 15–18, 2013. The congress, sponsored by the Superfund Research Program, National Institute of Environmental Health Sciences, and National Science Foundation, brought together international academic and government researchers, engineers, scientists and policymakers. With industrial growth, increased power needs and generation and coal consumption and their concomitant emissions, pernicious health effects associated with exposures to these emissions are on the rise. This congress provides a unique platform for interdisciplinary exchange and discussion of these topics. The formation, conversion, control and health effects of combustion by-products, including particulate matter and associated heavy metals, persistent organic pollutants and environmentally persistent free radicals, were discussed during the congress. This review will summarize and discuss the implications of the data presented.
Introduction
Combustion by-products are produced when carbon-based fuels such as gas, oil, kerosene, wood, charcoal, or tobacco are burned resulting in both outdoor and indoor pollution. These compounds represent a large spectrum of chemicals being formed as a result of incomplete degradation of the fuel components, secondary reactions of degradation products and de novo formation of molecules from small fragments. Conventional combustion pollutants comprise four main classes: particulate matter (PM), heavy metals, organic pollutants (e.g., dioxins and polycyclic aromatic hydrocarbons or PAHs) and, the more recently realized pollutant-particle systems known as environmentally persistent free radicals (EPFRs). Combustion by-products released from automotive sources account for nearly 80% of particulate air pollution near roadways. Depending on distance from a major combustion source (e.g., coal firing plant, roadway, etc.), indoor levels of combustion by-products from outdoor sources can vary considerably. More concerning, however, is that a considerable amount of combustion by-products can be produced indoors from unvented kerosene and gas space heaters, gas fireplaces, gas stoves, indoor use of charcoal or gas grills, poorly ventilated appliances, and cars idling in attached garages.
The variability of combustion sources greatly affects the composition of the pollutants formed during the combustion process. These pollutants are a complex mixture of chemicals, metals, gases, and particles derived from both natural and anthropogenic sources. These pollutant mixtures have significant effects on multiple systems including pulmonary, cardiovascular, immune and neurological systems causing numerous adverse health outcomes. Furthermore, the health effects associated with combustion by-products appears to vary with pollutant source and composition, developmental age at exposure and duration and route of exposure(s).
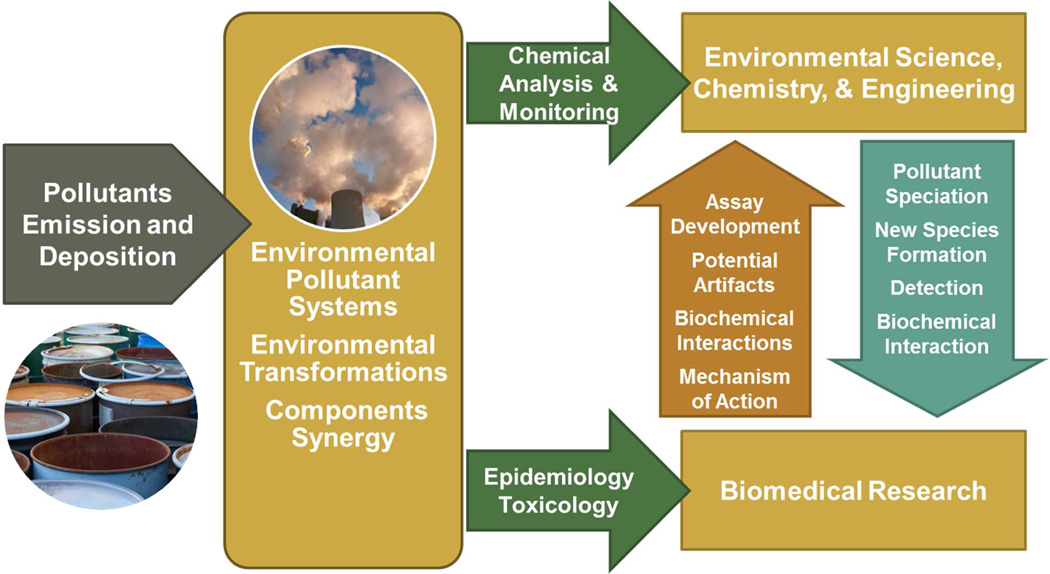
Drs. Stephania Cormier and Slawo Lomnicki chaired this congress. Dr. Bill Suk, Director of the National Institute of Environmental Health Sciences (NIEHS) Superfund Research Program opened the congress and introduced the Keynote Speaker, Dr. Linda Birnbaum, Director of NIEHS. Dr. Birnbaum discussed NIEHS’ long-term commitment and involvement in the congress which serves a special role in increasing synergy between investigators from all over the world in environmental sciences, chemistry and engineering and biomedical researchers to advance understanding of combustion related health effects, toxin formation, and remediation and prevention (Figure 1). She stressed the importance of community based participatory research in these processes and the inclusion of our emerging scientist, graduate students and post-doctoral fellows as keys to the success and sustainability of these efforts.
Figure 1.
The International Congress on Combustion By-Products and Their Health Effects consistently brings together scientists from all over the world and with different disciplines such as engineering, chemistry, remediation, biomedical research, and community based participatory research to advance our understanding of combustion pollution formation and exposure related health effects. Interactions such as these are rare but are catalysts for collaboration and rapid scientific advance necessary to alter policy.
Gulf Oil Spill: Emissions, Remediation, Combustion & Toxicity (Brian Gullett & Slawo Lomnicki
Maria Martinez (Chief, Air Quality Analysis Section, US EPA Region 6, Dallas, Texas) opened this session by summarizing the actions, approach, methodology, and geographical areas monitored by the Environmental Protection Agency (EPA) in response to the 2010 Deepwater Horizon Oil Spill. Key air pollutants monitored included ozone, PM10, PM2.5, volatile organic compounds (specifically, benzene, toluene, ethyl benzene, and xylenes), polyaromatic hydrocarbons (PAHs), and H2S. Air monitoring took place along the Gulf Coast including the states of Alabama, Florida, Louisiana, Mississippi and Texas with mobile and stationary devices. In short, EPA air monitoring data summaries failed to demonstrate any major impact on onshore air quality from oil spill-related activities. Based on observed air pollutant concentration levels, the oil spill did not contribute to increased long-term risks from breathing the onshore air in the Gulf region. In general, Air Quality Index (AQI) levels and air pollutant concentrations were not statistically different with historical values for the region. Data generally showed levels in the “good” to “moderate” range for both O3 and PM2.5.
Anthony Szema (Stony Brook University School of Medicine, New York) presented his studies on the emerging respiratory health effects being observed in US soldiers and veterans returning from in Iraq and Afghanistan. These soldiers and veterans were exposed to a number of inhalational irritants including smoke from various chemical fires (e.g. burning crude oil and sulfur-mines, by-products from improvised explosive device detonation, blast overpressure, and mortar fired rounds, etc.), smoke from burning of solid and human wastes in burn pits in the base camps, and blowing dust from storms. He discussed the potential role for such exposures as an important component of Iraq-Afghanistan War lung injury (IAW-LI) and for the development of new onset asthma1.
Asthma is an exclusion criterion for enlistment and active duty. Dr. Szema compared asthma rates in U.S. Iraq War Veterans to rates in veterans who never served in Iraq retrospectively from >6,000 veterans. Dr. Szema found increased rates of asthma among Iraq-deployed soldiers and the highest risk to develop asthma appeared to be in the 26–30 year-old and 36–40 year-old age groups for both men and women. PM10 levels averaged more than 100 μg/m3 and on several occasions exceeded 1000 μg/m3. Animals studies have verified that dusts collected from Iraq have a unique ability to induce enhance airway pathology and inflammation. It is clear that the air quality conditions in Iraq/Afghanistan were unfavorable. This research strongly suggests that exposure to high levels of PM can induce new onset respiratory diseases making it incumbent to review potential sources of PM air pollution in order to prevent their etiology.
Brian Gullett (US EPA Office of Research and Development) discussed the EPA’s efforts to sample the particulate emissions from the in situ oil burns performed during the cleanup of the BP Deepwater Horizon Spill. In situ oil burns involved the “collection” of oil into a fireproof collection boom from the Gulf surface by two boats. The collected oil was then ignited with gelled diesel and a road flare. Burn times varied from minutes to hours. Dr. Gullett and his team developed an aerostat-lofted sampler, which was maneuvered from a ship-mounted winch into the plumes for sampling emissions 2. The samples were analyzed for polychlorinated dibenzodioxin and polychlorinated dibenzofuran (PCDD/PCDF), total particulate matter (PM), and CO2. The total PCDD/PCDF was 4600 fg/m3 2, which was significantly above background levels, but on the order of those from wood burning. PM values were extremely high and averaged 4.4 g/m3 and by mass represented almost 9% of oil carbon burned. Conclusions were that the quality of the combustion was poor and ongoing pollutant characterization studies on plumes will enable comprehensive environmental cost/benefit analyses of in situ oil burning.
Slawo Lomnicki (Louisiana State University, Baton Rouge, LA) collected tar balls from the Louisiana and Florida coastline following the Deepwater Horizon Oil Spill and performed analyses including ICP MS, extraction/GCMS and EPR to identify chemical species within these tar balls. He detected the presence of oxygenated aromatic radical species. These radical species were found to be products of partial oxidation of crude oil components and result from the interaction of the oxidized aromatics with metal ion centers. These radicals were found to be similar to semiquinone-type, environmentally persistent free radicals (EPFRs) that Drs. Lomnicki and Barry Dellinger have previously observed in combustion-generated particulate and contaminated soil samples with pentachlorophenol 3. Tar ball radicals were found to be persistent and capable of generating hydroxyl radicals. Similar radicals on airborne particulate matter have been shown to cause oxidative stress, pulmonary dysfunction and lead to cardiovascular diseases4–12. It is currently unclear what the exact impacts are, but these tar balls are a potential direct health risk for marine organisms and when degraded to smaller particles, they become inhalable and therefore it is easily conceivable that they pose a human health risk.
Arthur Penn (Louisiana State University - School of Veterinary Medicine, Baton Rouge, LA) presented data from his laboratory on the direct exposure effects of zebrafish embryos to Deepwater Horizon (DH) crude oil emulsions. PAH-rich emulsions were collected from four sites (Fort Pickens, FL; Gulfport, MS; Fort Morgan, AL; Perdido Key, FL) along the Gulf of Mexico shoreline from July 1–4, 2010. Analysis of these samples revealed that they contained varying amounts of alkanes and PAHs, as well as sand and seawater. Since zebrafish embryos (ZFE) are a well-established model for vertebrate development and are exquisitely sensitive to a variety of environmental pollutants, Dr. Penn’ group exposed ZFEs to the collected emulsion samples to determine their cytotoxic and embryotoxic effects. In particular, they were interested in the effects of direct contact to these different emulsions on morphology and gene expression patterns of ZFE. ZFE at various times post-fertilization (i.e. 0, 24, 48 hours post-fertilization) were exposed to the different emulsions for 48 hours. Morphological changes (e.g. axial malformations, pericardial and yolk sac edema), altered swimming patterns, and altered gene expression patterns were apparent in response to these short-term exposures to DH-derived emulsions. In particular, phase I biotransformation-related genes including cytochrome P450 family members cyp1a, 1b1, 1c1, and 3a65; aryl hydrocarbon receptor; and sulfotransferase family member sult6b1 expression were increased, as were genes involved in regulating oxidative stress (e.g. nqo1, prdx1, hmox1). After ZFE were removed from the emulsions, a number of phase I gene expression levels returned to baseline levels; however, up-regulation of oxidative stress genes persisted. In general, there were strong similarities in the morphological, behavioral, and gene expression responses of ZFEs to the emulsions collected from different sampling sites, despite large differences in PAH levels (total and relative)13. This indicates that ZFEs are most likely responding to a limited number of PAHs, which are present at each of the 4 sites. Future studies will determine the long-term consequences of direct exposures to these emulsions on development, behavior, and reproduction. These data demonstrate the ZFE could serve as a rapid, high-throughput model for predicting developmental toxicity of combustion-derived soots from a variety of sources.
Dosimetry and Environmental Fate of Pollutants from Combustion Sources (Tobias Stöger)
Philip Demokritou (Harvard School of Public Health, Boston, MA) brought focus to the discussions by reminding everyone that in addition to the specific toxicity of a material, the respective exposure needs to be considered for hazard assessment. In this context, when discussing the response to nanoparticles (NP), the type of exposure and its respective dosimetry is a crucial and also complex issue in predicting potential NP exposure related health effects. Dr. Demokritou highlighted the importance of nanotoxicology for the safety of nanotechnology and the increasing advent of new engineered nanomaterials with a potential of subsequent exposure. To perform a well characterized but also environmentally relevant exposure in either cells or animals, NPs have to be either produced freshly or sampled in an appropriate way to avoid their modification. To this aim, devices have been developed allowing, (i) the efficient concentration of ambient ultrafine particles, regardless of their hygroscopicity by the Harvard Ultrafine Particle Concentrator (HUCAPS), and (ii) the Versatile Engineered Nanomaterial Generation System (VENGES) for toxicological characterization of freshly engineered nanomaterials directly released into the exposure chambers at a controlled agglomeration state. The use of such well-defined particle sources for toxicological investigations shall help to overcome the current lack of harmonization between different in vitro methodologies. Proper particle characterization and dosimetric considerations could be responsible for the current discrepancies found in the nanotoxicology literature 14,15. Dr. Demokritou proposed that an in vitro screening methodology for nanotoxicology requires three steps: (i) preparation and characterization of NP liquid suspension, by sonication, ROS generation, monodispersity, and stability measurements, (ii) assess NP transformations in physiological media for their agglomeration state, zeta potential, and density, followed by (iii) dosimetry considering particokinetics, administered vs. delivered dose, and exposure time. Once relevant toxicity endpoints have been identified, such as cell death or inflammatory response, a comparison of the in vitro dose-response relationships with those reported in vivo will have to be done for a final validation of the system as a screening platform. But the dose by which people might be exposed to NPs, incorporated via the lungs and subsequently either remaining or being cleared from the lungs, or translocated to secondary organs remains largely unknown.
Ian M Kennedy (University of California, Davis, CA) introduced lanthanide-labeled metal oxide NPs to improve the accuracy of measurement of in vivo clearance and translocation of nanoparticles. Previous studies have used either radioactive 16,17 or radioactive labeled particles for deposition, clearance and translocation studies, but unfortunately in the latter case the label may come off leading to dramatic over-interpretation 18–20. NP from the Lanthanide series generated by spray flame synthesis offer unique advantageous properties such as magnetic phosphorescence, and due to their extreme low natural background signal, can be detected by ICP-MS with high sensitivity. In a recent proof of concept study 21 with Eu and Gd NPs, it was confirmed that 24h after intratracheal delivery, the majority of the dose remained in the lungs (59%), with a cleared fraction of 20% found in the feces and only 0.2% detected in the liver or even less for other secondary organs.
Phillip M. Potter (Trainee, Louisiana State University, Baton Rouge, LA) discussed the impact of aluminas on the formation of EPFRs in fly ash PM. This study indicates that besides the known potency of metal oxides and chlorinated aromatics, also α-alumina, and aluminosilicates effectively promote the formation of polychlorinated dibenzo-p-dioxins and polychlorinated furans (PCDD/Fs). Since alumina and aluminosilicates are frequently found in fly ash particles, this pathway of chlorinated dioxin formation might be of high relevance for the toxicity of byproducts from combustion and thermal processes.
Lavrent Khachatryan (Louisiana State University, Baton Rouge, LA) demonstrated potential artifacts when investigating EPFRs activity in OH radical formation by DMPO spin trapping in conjunction with EPR spectroscopy. He highlighted different behaviors of surface available and internal radicals. In general, for most of the tested PM2.5 samples, as well as for laboratory generated EPFRs, high EPFR concentrations could be associated with a high oxidative capacity (OH production). Some PM2.5 samples however, showed a high radical content but did not display any increased redox activity (OH generation). He concluded that external radicals should be distributed on the outer layer of the particle surface and accessible to chemicals during the redox cycling; candidates are CuO/SiO2/EPFRs and “active” PM2.5 samples. Internal radicals in contrast are distributed in inner layers of the particle (pockets, bays, between branches etc.) and thus not accessible for reaction, as in the case of “passive” PM2.5 samples.
Mariana Ghosh (Southampton University and University of Reading, United Kingdom) discussed her work to assess free radical formation from the oxidation of acoustically levitated α-pinene droplets using electron spin resonance (ESR). Her work has been primarily focused on the terrestrial vegetation release of terpenes into the atmosphere, which are a component of the “blue haze” that can be seen in areas with large amounts of pine trees (e.g., Smoky Mountain region of the Southeastern area of US). Photo-oxidation of these terpenes and isoprenes yield products that partially remain in the gas phase, but some, less volatile, photo-oxidation products accumulate in the condensed phase and contribute to ambient particulate mass. She is specifically interested in assessing the variability of free radical formation from reactions of these monoterpenes (e.g. α -pinene) with ozone, which is important for understanding the health effects associated with the ambient particulate mass. Previous studies have shown that a significant amount of reactive oxygen species, during the reaction of α-pinene with ozone, are associated with secondary organic aerosols (SOA) 21. Mechanisms of free radical formation are important to understand since health effects have been associated with radical-containing PM; and SOAs can have direct and indirect effects on climate change.
To detect and identify radical species formed from the α-pinene/ozone reaction, she combined acoustic levitation with one of two different methods to detect free radicals. Acoustic (ultrasonic) levitation, which consists of an acoustic levitator coupled to a Raman microscope via a fiber-optic probe, was used to study the properties of droplets in an aerosol. This instrument provided the capability to trap and monitor aerosol particles (i.e., droplets) and control environmental parameters in the chamber (e.g., gas phase, temperature, humidity). Free radical formation was monitored both directly using flow-electron paramagnetic resonance (EPR) and indirectly using spin-trapping techniques. The flow-EPR method allowed for identification of free radicals that have a very short life-time (<1 min); whereas the addition of a spin-trap increased the lifetime of the free radicals allowing for more detailed chemical composition of free radical species 22. The spin-trapping technique forms a spectrum that serves as “fingerprint” or a spectral “snapshot” of the radical under investigation. With these techniques, she and her colleagues have demonstrated that, at room temperature and in presence of ozone, internal double bonds in acoustically levitated terpene droplets are unstable. They were also able to trap the first-generation radical intermediates of α-pinene oxidation and determined the structure of the products. Their study demonstrates that it is possible to analyze free radicals using these techniques to study mechanisms of other reactions in atmospheric science.
Shengyong Lu (Zhejiang University, Hangzhou, China) explored the chemistry involved in the formation of chlorobenzene(s) (CBz) and both polychlorinated dibenzodioxins (PCDD) and dibenzofurans (PCDF) during incineration processes. Prior reports demonstrated that increased oxygen concentrations generally resulted in increased PCDD/F formation. The objective of Dr. Lu’s studies was to understand the conditions involved in the formation of CBz and PCDD/F so as to optimize operating conditions of incinerators. Model fly ash was produced in the presence of CuO, along with increasing concentrations of O2. A total of 136 isomers of CBz and PCDD/Fs were measured using state-of-the-art techniques including gas chromatography (GC) with electron capture detection and high-resolution GC with high resolution mass spectrometry, respectively. As observed previously, production of chlorinated products generally increased with O2 concentration. O2 concentration had a stronger effect on less chlorinated CBzs compared to high chlorinated products. It also had a stronger effect on PCDD compared to PCDF formation, and on less chlorinated PCDD/Fs. The group furthermore found a good correlation between the formation of toxic dioxins and O2 concentration. Thus, an understanding of the mechanisms of PCDD/F formation may indeed lead to optimization of operating conditions for incinerators, so as to reduce the risks associated with pollutant formation.
Environmental Sampling: Advances, Challenges, and Implications (Ian Kennedy)
Richard Tropp (Desert Research Institute, Reno, NV) discussed air sampling networks in the United States with particular reference to the monitoring of combustion-generated pollutants. Understanding the risk that is posed by combustion-generated gases and particles requires an appropriate knowledge of exposures. This can only be accomplished with a suitable network of air quality monitoring stations. The USA has a number of air quality monitoring networks. These include the IMPROVE network for monitoring the visual quality of ambient air as well as the RADNET network that monitors radiation in the environment. The latter network of 124 sites monitors radiation levels in aerosol particles in the atmosphere, in atmospheric precipitation, in drinking water and in milk. The SLAMS network (state and local air monitoring stations) was designed to ensure compliance with the National Ambient Air Quality Standards (NAAQS). This network measures the so-called criteria pollutants, comprised of ozone, carbon monoxide, SO2, NO2, PM2.5, PM10, and lead. This network has seen, in general, a decrease in the number of monitoring stations over the last 20 years, with the exception of those for ozone. The sampling locations are concentrated on the East and West coasts of the United States. The SLAMS network data show significant improvements in ambient air quality between 1980 and 2010. For example, carbon monoxide levels have dropped by 82% over that period. Lead concentrations have shown the most remarkable decrease, as might be expected, decreasing by 90% between 1980 and 2010. The National Core Network (NCore) constitutes a subset of the SLAMS network. This sampling network provides more sensitive measurements of pollutants, and provides speciation of PM2.5 and PM10. The photochemical assessment monitoring stations (PAMS) were set up to monitor ozone pollution and its precursors. This includes measurements of ozone, nitrogen oxides, and volatile organic compounds, all components of photochemical pollution. There are currently approximately 75 sites in the PAMS network. This network is currently being reconfigured to provide more relevant and useful data. The National Air Toxics Trends Stations (NATTS) started as a pilot program in 2001–2002 and was fully implemented in 2003–2004. Its goal is to provide high quality estimates of air toxic concentrations over time. The toxic network measures concentrations of metals, volatile organic compounds and carbonyls. The network reports a general improvement in air quality with regard to toxics between 2005 to 2010. Unfortunately, increasing levels of three volatile organic compounds were measured: carbon tetrachloride, vinyl chloride, and chloroform. Emerging issues in the area of air quality monitoring including giving greater attention to populations at risk such as children living near roadways and expanding air sampling networks with greater sensitivity and specificity were discussed. In addition, Dr. Tropp discussed cost-effective emerging technologies that may be used in the future including handheld monitoring devices, low-power severely miniaturized sensors, and improved real-time connectivity.
Shengyong Lu (Zhejiang University, Hangzhou, China) reported on recent advances in catalytic decomposition technology for the control of dioxin and dichlorobenzene. They used nanoscale titanium dioxide as a catalyst, and found that the presence of ozone and UV light enhanced the destruction of dichlorobenzene. The optimum ozone concentration was found to be about 165 ppm. They achieved up to 90% destruction of incoming dichlorobenzene. However, the need for “a specific wavelength of UV light may limit the practical application of this technology”. The authors also explored the catalytic degradation of dioxin. They showed that it was important to include a copper oxide catalyst along with titanium dioxide to achieve good degradation of dioxin. They noted that carbon nanotubes are extremely effective adsorbents of dioxin and, when added to the metal oxide catalysts, were able to improve the degradation of dioxin significantly. Oxygen was postulated to play an important role, with oxygen in the catalyst being a source of oxidation of the organic compound, with oxygen in the atmosphere serving to replenish lost oxygen from the catalysts.
New, fast and cheap methods for the measurement of environmental toxins are always needed. Jeffrey Crosby (UC Berkeley and Lawrence Berkeley National Laboratory) described his work with Don Lucas and Cathy Koshland to apply gold nanoparticles as sensors of environmental mercury. In particular, they explored the application of gold nanorods, which were particularly effective in this application. Gold nanoparticles display a phenomenon known as plasmon resonance. At specific wavelengths, the free electrons in the nanoparticles oscillate at a resonant wavelength that depends on the size of the particle and its morphology. The wavelength of the resonance shifts as mercury is adsorbed onto the surface of the particles. The gold particles are coated onto a substrate to form a thin film. Previous work with airborne mercury showed that this provides an effective sensor. The current report describes application of the technology to the detection of mercury in water following a chemical pre-processing step to convert all mercury to ionic mercury in solution. The gold nanorod sensor was able to detect mercury at a level below the necessary EPA limit. The device promises to be cheap and effective following some further optimization.
Andrew Larkin (Trainee, Oregon State University, Corvallis, OR) has investigated the use of mobile telephone devices for the assessment and prediction of air borne products of combustion, and the dissemination of the results to concerned members of the public. These smart phones have been successfully linked with environmental models to personalize pollutant exposure information. The application running on a smart phone will report predicted pollutant levels in the neighborhood of the user, with warnings about unwanted predicted exposures. The software has been developed as open source, which will minimize the operational costs and make the application more generally available.
M. Paul Herring (Trainee, Louisiana State University, Baton Rouge, LA) along with Lavrent Khachatryan, Slawo Lomnicki, and Barry Dellinger identified the paramagnetic centers in soot formed from the oxidative pyrolysis of 1-methylnaphthalene from a reactor maintained at 1100°C. Electron Paramagnetic Resonance was used to evaluate the mechanism of radical formation associated with soot following up on earlier work by the group that has shown persistent free radicals are present in particulate and are associated with adverse health effects. An EPR spectrum consisting of multiple radicals was observed and was determined to include the indenyl, cyclopentadienyl, and naphthalene 1-methylene radical. Future work will evaluate the impact of metal oxide on the production of free radical during the oxidative pyrolysis of 1-methylnapthalene.
Combustion Toxicology, Epidemiology, and Environmental Studies (Jim Diaz)
Joe Mauderly (National Environmental Respiratory Center, Lovelace Respiratory Research Institute, Albuquerque, NM) addressed the identification of causal components of the respiratory and cardiovascular health effects of repeated inhalation exposures to near-source exhaust from diesel and gasoline engines and wood stoves, and simulated “downwind” coal combustion emissions. The following conclusions resulted from Multiple Additive Regression Tree analysis of the combined database for the detailed composition of the controlled laboratory exposures and numerous adverse health responses. Few, if any, health effects of combustion emissions appear likely to be caused by single components of the complex mixtures. High-ranking predictors of response (putative causal components) varied among the different health outcomes, and were approximately equally distributed among components of particulate matter and non-particulate organic and inorganic gases and vapors. The shapes of partial dependence concentration-response plots for individual components in the presence of the rest of the mixture reflected a range of threshold and non-threshold response functions. The results indicate that it cannot be assumed that exposure-response relationships for pollutants within a mixture are non-threshold, progressive with “dose”, and the same for all outcomes – even related outcomes. The appearance of particulate total organic carbon as a key predictor of several health responses suggests that it would be useful to partition the effects of PM organic carbon into its thermal fractions. Lastly, the unexpected appearance of NH3 as a key predictor of several responses suggests that further exploration of the effects of repeated low-concentration exposures to NH3 bears further investigation.
Rebecca Klemm (Klemm Analysis Group, Washington, DC) compared the estimated effects of air pollution on human mortality in the southern US. The cities studied included Atlanta, Birmingham, and Dallas. Five death categories were created and included all, cardiovascular, respiratory, cancer, and all other non-accidental causes. Eight pollutants were measured daily or every three days and included PM2.5, EC, OC, NO2, CO, SO4, NO3, and O3. Only single pollutant models were analyzed. Statistically significant results for each city were observed for models involving cardiovascular-related mortality. Interestingly, very few statistically significant results were observed for other specified death categories. Dr. Klemm and her colleagues concluded that the relationship between air pollution and mortality differs by city despite some similarities in air pollution concentrations and other atmospheric conditions. She felt that this may be due to differences in measurement frequency and procedures across sites.
Shengyong Lu (Clean Energy Laboratory of the Institute for Thermal Power Engineering, Hangzhou, China) presented a life cycle assessment of a typical medical waste incineration plant in eastern China with the following results: global warming, acidification, nutrient enrichment, and human toxicity are the most serious environmental problems caused by medical waste incineration from a life cycle assessment viewpoint. CO2 contributes 99% to the global warming effect. Among acidic gases, NOx contributes 52.3% and 100%, respectively to acidification and nutrient enrichment. This date emphasize the need of improvement of deNOx system in China incinerators. Among emitted metals, Hg, As, and Sb contribute significantly to human toxicity constituting 23.5%, 33.7%, and 40.1% to the total human toxicity, respectively. In contrast, PCDD/Fs only contribute to 1.04% of the total impact; thus, demonstrating that the medical waste incineration plants in China are effectively controlling PCDD/F emissions. On the other hand, fly ash and bottom ash are a very serious burden on the environment and human health because of the high concentrations of heavy metals and sending them directly to hazardous waste landfills is inappropriate in China. In addition, the medical waste incineration plants in China are not equipped with energy recovery systems.
Lucy Kiruri (LSU, Baton Rouge, LA) presented a discussion on the effect of CuO concentration in particles on the formation of environmentally persistent free radicals (EPFRs) and reactive oxygen species (ROS). She concluded that EPFRs yields are dependent on the concentration of Cu on the surface of the particle; however, this relationship was not linear and in fact the dose response curve was near Gaussian in shape. The EPFRs produced are stable and do not decay rapidly in air; EPFRs generate •OH in solution and undergo redox cycling as confirmed by spin trapping experiments. Dr. Kiruri observed an inverse relationship between the half-life of the Cu(II)O/silica particles and the relative amount of •OH generation. Lastly, centrifuged particles yielded more •OH compared to non-centrifuged particles, which she believed was due to Cu mass loading. This last bit of data strongly suggests that methods to collect and/or concentrate combustion derived products for their study will have immense impact on their biological effects.
In the last talk of the session, Eric Vejerano (Virginia Tech, Blacksburg, VA) discussed the growth in nanotechnology and how some fraction of nanomaterial-laden waste will be incinerated as end-of-life treatment. And yet, the influence of nanomaterials on the formation of hazardous pollutant under high-temperature, oxidative conditions is not well understood. Dr. Vejerano reported that the incineration of paper and plastic wastes containing various nanomaterials in a laboratory-scale furnace affected the emission of polycyclic aromatic hydrocarbons (PAHs) and polychlorinated dibenzofurans (PCDFs). The type and quantity of the nanomaterial determined the amount and speciation of PAH emitted, with the major PAH formed being phenanthrene and anthracene. The PAH emission factors totaled over 16 PAH species and were on average six times higher for the waste spiked with the nanomaterials compared to those with the bulk counterparts 23. Wastes containing nanosilver and nanotitania emitted the highest amount of PCDF. This is of concern as these nanomaterials are produced and used in greater volumes. In summary, Dr. Vejerano’s work suggests an increased pollutant burden and environmental and health impacts of nanomaterials as their use in industry, science, and medicine continue to increase.
Pollutants from Combustion Sources: Mechanisms of Toxicity and Dysfunction (Danielle Carlin, Stephania A. Cormier, Tammy Dugas and Kurt Varner)
Timothy Nurkiewicz (West Virginia University, Morgantown, WV) opened the discussion of the adverse cardiopulmonary effects of airborne particulates with his plenary presentation highlighting the microvascular consequences of inhalation exposures of combustion-generated particles. Dr. Nurkiewicz summarized a large body of work from his laboratory delineating the effects of inhaled fine, ultrafine and nanoparticles from a variety of sources including residual oil fly ash (ROFA), titanium dioxide, phenanthrenequinone, diesel exhaust, cerium dioxide and particles generated by mountaintop coal mining operations on arteriolar and venular function. Although it has been established that exposure to airborne particulates leads to vascular injury and dysfunction in humans and experimental animals, the mechanisms underlying these processes have remained largely unknown. Using two techniques: 1) intravital microscopy with the rat spinotrapezius muscle preparation and 2) isolated/cannulated/perfused microvessels with coronary arterioles and bone principal nutrient arterioles, Dr. Nurkiewicz showed that inhalation exposure to ROFA/TiO2 dose-dependently reduced the arteriolar vasodilatory responses elicited by the calcium ionophore, A2318724. The degree of vascular dysfunction was dependent on particle size, with the nanoparticles producing a greater level of disruption in the lung and greater physiological effects than the larger fine particles. The reduction in vascular responsiveness was attributed to a particle-mediated impairment of nitric oxide-dependent and independent mechanisms. In contrast, the vascular responses to vasoconstrictor substances were not affected by exposure to particulates. The particle-mediated vascular dysfunction was accompanied by increased polymorphonuclear leukocyte rolling and adhesion. Many of the adherent leukocytes contained high levels of myeloperoxidase deposited within the vessel walls. Additionally, the vessel walls showed signs of localized oxidative stress. The link between particle-mediated pulmonary inflammation and vascular dysfunction was further demonstrated by increases in the plasma levels of inflammatory cytokines such as IL-1α, ICAM-1 and IL-13 and increased dihydroethidine staining (marker of oxidative stress) in the vessels walls after pulmonary exposure to nanoparticles. Together, these studies provide new insight into the effects pulmonary exposure to particulates can have on vasodilatory responses and highlights causative roles played by inflammation and oxidative stress in producing particle-mediated vascular dysfunction.
Sarah Robertson (Postdoctoral Trainee; University of New Mexico, Albuquerque, NM) presented her work from Matthew J. Campen’s laboratory exploring the induction of vasoactive circulating factors by inhalation of diverse pollutants. Mice were exposed to either combined diesel and gasoline exhaust or ozone. Serum from these mice was then incubated with aortic rings from naive mice. Both types of exposures impaired vascular responses to acetylcholine (compared to filtered air control exposures) suggesting endothelial dysfunction. Interesting, removal of the particulate component from the combined exhaust product prevented the observed PM-induced reductions in vascular responses, while elimination of the gaseous component only partially attenuated PM-mediated vascular dysfunction. Plasma collected from humans 24 h after a 2 h exposure to either diesel exhaust or NO2 increased the expression of adhesion molecules in cultured human vascular endothelial cells25. These data cumulatively suggested that the vascular responses induced by PM exposure were not simply a consequence of blood borne PM but that a second circulating factor must be released from the lungs following inhalation exposures that had a causal role in promoting vascular dysfunction. To test this hypothesis, the group assessed the role of inhaled ozone, which cannot be transported into the circulation, in promoting vascular dysfunction. A similar experimental paradigm was followed, except this time, CD36 deficient mice and wild-type (WT) mice were exposed to ozone. Serum was isolated from these mice and incubated with vessels from naive, WT mice. (CD36 is a pattern recognition receptor on cells that is known to bind to damage-related circulating molecules). In reciprocal experiments, serum taken from mice exposed to ozone was incubated with vessels from CD36 deficient or WT mice. These elegant studies demonstrated that although CD36 was important for lung inflammatory and systemic vascular effects, the presence of vasoactive circulating factors was independent of CD36 and pulmonary inflammatory responses26.
James R. Reed (Louisiana State University Health Sciences Center, New Orleans, LA) examined the inhibitory effects of combustion-derived ultrafine nanoparticles on cytochrome P450-specific enzymatic activities. During combustion processes, the chemisorption of aromatic hydrocarbons onto metal oxide-containing fine and ultrafine particles leads to the generation of environmentally persistent free radicals (EPFRs). Exposure to ultrafine EPFRs has been associated with an array of pulmonary and cardiovascular morbidities. Dr. Reed investigated the potential for the model EPFR, 2-monochlorophenol chemisorbed to copper oxide-silica particles at 230C (MCP230), to alter metabolism by P450s in rat liver microsomes. Using probe substrates that are selective for specific P450 isoforms, the model EPFR dramatically inhibited all P450 activities examined (i.e., CYP1A1, CYP1A2, CYP2B, CYP2E1, CYP2D2, and CYP3A). In comparison, the same concentration of untreated silica particles had no effect. Moreover, EPFR-mediated inhibition was dose-dependent; and for the CYP2D2-catalyzed reaction, the EPFR did not appear to compete with substrate for binding to the P450. P450-mediated metabolism is very inefficient, as typically more than 50% of the electrons received from cytochrome P450 reductase (CPR) are spent in making the “uncoupled” reaction products, superoxide, hydrogen peroxide, and excess water. The effect of MCP230 on the efficiency of P450-mediated metabolism was also assessed. Interestingly, the EPFR influenced metabolism by two P450s differently. Although MCP230 inhibited the P450-dependent rate of NADPH consumption by CYP2D2 in rat liver microsomes, it did not change the efficiency by which NADPH was used for substrate metabolism. When metabolism by a purified P450 (rabbit CYP2B4) was compared, MCP230 reduced both the rate of P450-dependent NADPH oxidation and the efficiency of NAPDP utilization. Thus, as compared to silica particles, EPFRs show a profound ability to inhibit P450-mediated metabolism. This could have important ramifications on xenobiotic metabolism and elimination in individuals exposed to EPFRs. The specific mechanisms underlying EPFR-mediated inhibition of P450s appear to be dependent on the P450 isoform. For CYP2D2-mediated metabolism in rat liver, MCP230 does not appear to interact with the P450 binding site and/or change the way in which the substrate is metabolized; however, MCP230 inhibits the ability of the P450 to receive electrons from the CPR. The effect of MCP230 on CYP2B-mediated metabolism is more complicated and may involve multiple mechanisms of inhibition. These results demonstrate that EPFRs can lead to toxicity by direct inhibition of P450-dependent oxidation drugs and other toxicants. Because the P450 system is essential for the elimination of the majority of drugs and foreign compounds, inhibition of these enzymes could prolong their lifetime within the organism, and as a consequence, either produce an enhanced pharmacologic effect of administered drugs, or increased toxicity from co-exposure to other environmental pollutants.
Dr. Sharon A. Meyer (University of Louisiana at Monroe, Monroe, LA) summarized the various scenarios responsible for exposure to crude oil and the major health effects associated with such exposures. The discussion then focused upon use of chemometrics to correlate constituents of analytical “fingerprints” of crude oil mixtures with a variety of toxic endpoints. Chemometrics has been employed in the petroleum industry to optimize conditions for process chemistry and the question was posed that a similar approach could reveal principal constituents mediating effect in mixtures in situ. As such, chemometrics would offer a novel approach to the difficult analysis of toxicant effects and interactions of mixtures. Chemometrics employs application of multivariate statistical methods to high density output that can be obtained with analytical techniques such as 1H- and 13C-NMR and EPR27. One method especially useful for discovering correlation of signal from a “causal” constituent is partial least square (PLS) regression because of its relative insensitivity to overfitting datasets with large numbers of spectral signals that may not be independent. Dr. Meyer presented pilot studies applying this chemometric approach to effects of crude oil obtained from different locations (LA Sweet, Nigeria (Qua Iboe), and Iraq (Basrah Light)) in orally exposed rats. Increase in liver weight and liver CYP1A1 induction varied substantially among oil source and correlated with asphaltene free radical and 1H NMR signals for toluene, 2-ring polycyclic aromatic hydrocarbons (PAHs) and 3-ring PAHs. Similarly, correlations were suggested for loss of bone marrow granulocyte macrophage progenitor cells with benzene and nitric oxide (NO) loss with aliphatic hydrocarbon content and asphaltene free radical. The work presented by Dr. Meyer was unique from several perspectives including the comprehensive toxicological approach, the absence of health effects data due to exposure to different types of crude oils, and her use of mathematical modeling techniques to identify specific chemical constituents responsible for the adverse health effects observed. Together, her studies suggested that high density multi-signal chemical analysis of complex mixtures has the potential to identify causal principles of observed health effects in situ. The modeling of other types of exposures and the application of methodologies as applied by Dr. Meyer should allow for better prediction of health effects following complex exposures.
Dr. Lyndsey Darrow (Emory University, Atlanta, GA) presented her work on short-term changes in outdoor air pollution concentrations and their association with emergency department visits for respiratory event in children ages 0–4 years. Her work is unique in that few studies have focused on respiratory disease during these first few years of life, even though susceptibility may be greater during this stage than in later stages of life. Infants and children are more susceptible to air pollution because their lungs and immune systems are still developing; respiratory morbidity is common in early life; children have higher ventilatory rates than adults; they have different behaviors that lead to more outdoor air exposures, and anatomically, they have narrower peripheral airways.
To determine if there was an association between changes in outdoor air pollution and respiratory events in this age group, she conducted epidemiological studies using two existing high-quality databases. The first database consisted of ~530,000 emergency department visits and data on pediatric respiratory conditions (asthma, wheeze, bronchiolitis, bronchitis, pneumonia, and upper respiratory infection) collected from 41 Atlanta metropolitan area hospitals between January 1, 1993 and December 31, 2004. The second database included daily measurements of PM2.5 and PM10 analyzed for elemental carbon, organic carbon, water-soluble metals, and sulfate, and gaseous pollutants (ozone (O3), carbon monoxide (CO), nitrogen dioxide (NO2)) which was available from U.S. Environmental Protection Agency (EPA) monitors. The combination of air quality data along with pediatric respiratory events are being used to help identify sources of air pollution that are particularly harmful to this age group and to provide insight into the potential mechanisms responsible for adverse pulmonary events.
Dr. Darrow and her colleagues found associations between emergency department visits for respiratory events in children 0–4 years with ozone as well as primary traffic-related pollutants (e.g., elemental carbon, NO2, CO) and organic carbon. Interestingly, upper respiratory infections were associated with elevations in PM, ozone and primary traffic pollutants.
This research will advance our understanding of the relationships between ambient air pollutants and pediatric respiratory health. In addition, these studies should be followed by animal and cell models to help identify causal agents or mixtures to develop a better understanding of the age-specific health impacts of ambient air pollutants. and cardiac function
Dr. Gin C. Chuang (Postdoctoral Trainee; Louisiana State University Health Sciences Center, New Orleans, LA) explored mechanisms by which inhalation exposure to EPFR-containing PM may produce cardiac toxicity. Several epidemiological studies showed that PM increase cardiac morbidity and mortality10,11. In addition, earlier studies from his mentor’s, Dr. Kurt Varner, laboratory demonstrated that EPFR-containing PM significantly reduced left ventricular function and produced oxidative stress and inflammation after inhalational or intratracheal exposure4,9. However, it was unclear whether the adverse cardiac effects following exposure to EPFRs were due to systemic factors (i.e. inflammation or oxidative stress) or the direct effects of EPFRs, which translocated from the lungs to the heart. This study was designed to test the hypothesis that EPFR-containing PM could produce cardiac toxicity by direct actions on HL-1 cardiac myocytes in vitro. Lactate dehydrogenase (LDH) release, a marker of late cell death was used to show that EPFRs dose-dependently induced cardiac myocyte cell death within eight hours of a low-dose exposure (0 to 200 μg/ml). Cell death was accompanied by dose-dependent increases in the cleavage of pro-caspases 9 and 3 and poly (ADP-ribose) polymerase 1, indicating the activation of apoptotic signaling pathways. In contrast, caspase 9 cleavage was detected in absence of cell death after only 2 hours of exposure to EPFR-containing PM, indicating initiation of the early intrinsic apoptotic signaling pathway, which usually correlates with mitochondrial dysfunction. Confocal microscopy showed that mitochondrial membrane potential, a marker of mitochondrial dysfunction, was decreased after 2 hours of exposure to EPFR-containing PM. Taken together, these data indicate that EPFRs can depolarized mitochondria in cardiomyocytes, leading to the activation of canonical intrinsic apoptotic signaling pathways and resulting in cell death. Future studies will assess mitochondrial permeability transition and autophagy as contributing mechanisms. Identification of mechanisms responsible for adverse cardiac events following elevations in combustion by-products will provide insight into potential drug targets and therapeutics to reduce exposure-associated cardiac morbidity and mortality.
Dr. Jordy Saravia (Postdoctoral Trainee; University of Tennessee Health Science Center, Memphis, TN) presented his dissertation research which focused on understanding the mechanisms responsible for lung dysfunction following early, acute exposure to combustion-generated EPFRs. His work was unique in that few laboratories have attempted to study exposure during this critical stage of development (i.e. mouse neonate age 0–7d, which is comparable immunological and biologically to a human infant28); and serendipitously, it offered insight into potential mechanisms for the epidemiological data presented by Dr. Darrow - why infants are so vulnerable to lung disease (e.g., asthma, severe influenza infection) in areas of high ambient air pollution?
Using an acute exposure model in neonatal mice, he showed that exposure to EPFRs induced a tolerant/suppressive immune environment in the lungs. Such an environment affects how the body responds to other immunostimulatory agents such as allergens (e.g. pollen, ovalbumin, etc.) or even pathogens (e.g. influenza). For example, in allergic asthma, the immune system responds to a particular allergen by inducing an allergic immune response characterized by increased T helper type 2 (Th2) cell induction and recruitment to the lungs and associated elevations in IgE antibodies, pulmonary eosinophilia and Th2 cytokines. His data demonstrated that neonatal exposure to EPFRs for as little as seven days induced an immunosuppressive environment in the lung. This resulted in an inability for the exposed mice to develop an allergic asthma phenotype. While this may seem protective against allergies, he presented additional data that demonstrated that mice exposed to EPFRs as neonates are actually more prone to develop a hyper-allergic reaction (i.e., severe asthma) later in life when the tolerance diminishes. Even more concerning, he presented a glimpse of other data emerging from his mentor’s lab (Dr. Stephania Cormier) which demonstrated that the EPFR-induced immunosuppression is conducive to enhanced respiratory disease following influenza infection. This type of change in the programming of the immune system in the very young due to combustion by-product exposure has not been previously explored. Together, this research as presented by Drs. Darrow and Saravia will help to inform regulatory policies that provide protection for susceptible subpopulations, reduce economic costs associated with pediatric respiratory morbidities, and decrease the respiratory insults in early childhood that will elicit long-term consequences on respiratory health.
Discussion
Conventional combustion by-products consist mainly of four main classes of components: heavy metals, particulate matter (PM), organic pollutants (e.g., dioxins and polycyclic aromatic hydrocarbons or PAHs) and oxides of sulfur nitrogen. A new class is emerging and as a novel issue and possibly unifying component - EPFRs. Each of these groups is generally considered separately from a view-point of environmental protection and health impact, as well as, its related social and scientific issues. A major focus of this congress is to provide a holistic approach by bringing technical, health and policy issues to the forefront of discussion.
Thermal treatment of waste and clean coal technologies are two critical research topics, in many developing countries including China, India and Western Europe. Advanced diagnostic methods and measurement tools are essential in pollutant control and for environmental protection. Multi-pollutant monitoring sensors are being developed and there is growing emphasis on identifying and characterizing the numerous sources of pollution, related to combustion processes. A future is envisioned where a combination of mobile, handheld devices and social networking will provide new modalities for real-time community air-quality monitoring.
Much research remains to be accomplished in several broad areas related to the health impacts of combustion by-products. A comprehensive approach is necessary as evidenced by the lack of harmonization in recent nanotoxicology studies. Information exchange when studying health effects and on the sources and abatement of combustion by-products necessitates a dialogue among biomedical researchers, combustion engineers and policy makers. This interchange is quite necessary to properly perform in vitro and in vivo exposures that most closely resemble real-world exposure scenarios and to accelerate the understanding of health impacts of general or more specific by-products, improve risk analysis, develop new monitoring methods, as well as more advanced technology to abate pollutants, and, eventually, promulgate sensible yet sufficiently stringent regulations and implement these rules in the various realms of combustion.
Finally, it is becoming apparent that single exposure models do not fully explain epidemiology data and that the effects of exposures to complex mixtures do not mimic the effects observed with the individual component exposures. Multiple ways to address the toxicity of mixtures has emerged including mixture exposure models that are then intercalated with mathematical modeling techniques (e.g., multiple additive regression tree analysis and chemometrics) to identify the causal components. What has become evident is that it cannot be assumed that 1) even though a single pollutant in the mixture is below threshold levels that it will not exert an adverse effect in the mixture; 2) adverse effects will become progressively worse with the dose; or 3) the same single pollutant in a different mixture will exert the same adverse health effect.
Obviously, there are still major questions that need to be answered in order to reduce the environmental and health impacts of combustion by-products. And it is clear, that these questions can only be addressed by transdisciplinary research. It is expected that progress toward these answers will be made by the time of the 14th International Congress on Combustion By-Products and their Health Effects to be held in Umea, Sweden in 2015. For more information about this meeting, please visit http://www.lsu.edu/piccongress/.
Acknowledgements
The success of the 13th congress is in part due to the wonderful and often underappreciated administrative and technical support from Mrs. Tina Black and Mrs. Cori Fountain. The 13th congress was supported by funds from NIEHS (P42ES013648 to SL,TD, KV, BD and SAC and PA-12-212 to BD) and NSF (1060103 to BD).
Footnotes
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of NIH or the International Congress on Combustion By-Products and Their Health Effects.
References
- 1.Szema AM, Peters MC, Weissinger KM, Gagliano CA, Chen JJ. New-onset asthma among soldiers serving in Iraq and Afghanistan. Allergy Asthma Proc. 2010;31(5):e67–e71. doi: 10.2500/aap.2010.31.3383. [DOI] [PubMed] [Google Scholar]
- 2.Aurell J, Gullett BK. Aerostat Sampling of PCDD/PCDF Emissions from the Gulf Oil Spill In Situ Burns. Environ Sci Technol. 2010;44(24):9431–9437. doi: 10.1021/es103554y. [DOI] [PubMed] [Google Scholar]
- 3.Dela Cruz ALN, Gehling W, Lomnicki S, Cook R, Dellinger B. Detection of Environmentally Persistent Free Radicals at a Superfund Wood Treating Site. Environ Sci Technol. 2011;45(15):6356–6365. doi: 10.1021/es2012947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lord K, Moll D, Lindsey JK, et al. Environmentally persistent free radicals decrease cardiac function before and after ischemia/reperfusion injury in vivo. J Recept Signal Transduct Res. 2011;31(2):157–167. doi: 10.3109/10799893.2011.555767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balakrishna S, Lomnicki S, McAvey KM, Cole RB, Dellinger B, Cormier SA. Environmentally persistent free radicals amplify ultrafine particle mediated cellular oxidative stress and cytotoxicity. Part Fibre Toxicol. 2009;6:11. doi: 10.1186/1743-8977-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balakrishna S, Saravia J, Thevenot P, et al. Environmentally persistent free radicals induce airway hyperresponsiveness in neonatal rat lungs. Part Fibre Toxicol. 2011;8:11. doi: 10.1186/1743-8977-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fahmy B, Ding L, You D, Lomnicki S, Dellinger B, Cormier SA. In vitro and in vivo assessment of pulmonary risk associated with exposure to combustion generated fine particles. Environ Toxicol Pharmacol. 2010;29(2):173–182. doi: 10.1016/j.etap.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halonen JI, Lanki T, Yli-Tuomi T, Tiittanen P, Kulmala M, Pekkanen J. Particulate air pollution and acute cardiorespiratory hospital admissions and mortality among the elderly. Epidemiol Camb Mass. 2009;20(1):143–153. doi: 10.1097/EDE.0b013e31818c7237. [DOI] [PubMed] [Google Scholar]
- 9.Mahne S, Chuang GC, Pankey E, et al. Environmentally persistent free radicals decrease cardiac function and increase pulmonary artery pressure. Am J Physiol Heart Circ Physiol. 2012;303(9):H1135–H1142. doi: 10.1152/ajpheart.00545.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pope CA, Burnett RT, Thurston GD, et al. Cardiovascular Mortality and Long-Term Exposure to Particulate Air Pollution Epidemiological Evidence of General Pathophysiological Pathways of Disease. Circulation. 2004;109(1):71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 11.Sun Q, Hong X, Wold LE. Cardiovascular Effects of Ambient Particulate Air Pollution Exposure. Circulation. 2010;121(25):2755–2765. doi: 10.1161/CIRCULATIONAHA.109.893461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang P, Thevenot P, Saravia J, Ahlert T, Cormier SA. Radical-containing particles activate dendritic cells and enhance Th17 inflammation in a mouse model of asthma. Am J Respir Cell Mol Biol. 2011;45(5):977–983. doi: 10.1165/rcmb.2011-0001OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bui A, Xiao R, Perveen Z, Kleinow K, Penn A. Zebrafish embryos sequester and retain petrochemical combustion products: Developmental and transcriptome consequences. Aquat Toxicol. 2012;108:23–32. doi: 10.1016/j.aquatox.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 14.Teeguarden JG, Hinderliter PM, Orr G, Thrall BD, Pounds JG. Particokinetics In Vitro: Dosimetry Considerations for In Vitro Nanoparticle Toxicity Assessments. Toxicol Sci. 2007;95(2):300–312. doi: 10.1093/toxsci/kfl165. [DOI] [PubMed] [Google Scholar]
- 15.Taurozzi JS, Hackley VA, Wiesner MR. Ultrasonic dispersion of nanoparticles for environmental, health and safety assessment – issues and recommendations. Nanotoxicology. 2011;5(4):711–729. doi: 10.3109/17435390.2010.528846. [DOI] [PubMed] [Google Scholar]
- 16.Kreyling WG, Semmler-Behnke M, Seitz J, et al. [Accessed October 24, 2013];Size dependence of the translocation of inhaled iridium and carbon nanoparticle aggregates from the lung of rats to the blood and secondary target organs. 2009 doi: 10.1080/08958370902942517. Available at: http://informahealthcare.com/doi/abs/10.1080/08958370902942517. [DOI] [PubMed] [Google Scholar]
- 17.Semmler-Behnke M, Kreyling WG, Lipka J, et al. Biodistribution of 1.4- and 18-nm Gold Particles in Rats. Small. 2008;4(12):2108–2111. doi: 10.1002/smll.200800922. [DOI] [PubMed] [Google Scholar]
- 18.Nemmar A, Hoet PHM, Vanquickenborne B, et al. Passage of Inhaled Particles Into the Blood Circulation in Humans. Circulation. 2002;105(4):411–414. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- 19.Nemmar A, Hoet PHM, Nemery B. Translocation of Ultrafine Particles. Environ Health Perspect. 2006;114(4):A211–A212. doi: 10.1289/ehp.114-a211b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geiser M, Kreyling WG. Deposition and biokinetics of inhaled nanoparticles. Part Fibre Toxicol. 2010;7:2. doi: 10.1186/1743-8977-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pavlovic J, Hopke PK. Technical Note: Detection and identification of radical species formed from α-pinene/ozone reaction using DMPO spin trap. Atmospheric Chem Phys Discuss. 2009;9(6):23695–23717. [Google Scholar]
- 22.Ghosh M, Ionita P, Almabrok S, Marston G, Pfrang C. Investigation of Free Radicals Formed in the Oxidation of Acoustically Levitated Alpha-Pinene Droplets by Electron Spin Resonance. Spain: Granada; 2012. [Google Scholar]
- 23.Vejerano EP, Holder AL, Marr LC. Emissions of Polycyclic Aromatic Hydrocarbons, Polychlorinated Dibenzo-p-Dioxins, and Dibenzofurans from Incineration of Nanomaterials. Environ Sci Technol. 2013;47(9):4866–4874. doi: 10.1021/es304895z. [DOI] [PubMed] [Google Scholar]
- 24.Nurkiewicz TR, Porter DW, Barger M, et al. Systemic Microvascular Dysfunction and Inflammation after Pulmonary Particulate Matter Exposure. Environ Health Perspect. 2006;114(3):412–419. doi: 10.1289/ehp.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Channell MM, Paffett ML, Devlin RB, Madden MC, Campen MJ. Circulating Factors Induce Coronary Endothelial Cell Activation Following Exposure to Inhaled Diesel Exhaust and Nitrogen Dioxide in Humans: Evidence From a Novel Translational In Vitro Model. Toxicol Sci. 2012;127(1):179–186. doi: 10.1093/toxsci/kfs084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robertson S, Colombo ES, Lucas SN, et al. CD36 Mediates Endothelial Dysfunction Downstream of Circulating Factors Induced by O3 Exposure. Toxicol Sci. 2013;134(2):304–311. doi: 10.1093/toxsci/kft107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varmuza K, Filzmoser P. Introduction to Multivariate Statistical Analysis in Chemometrics. CRC Press; 2009. [Google Scholar]
- 28.Cormier SA, You D, Honnegowda S. The use of a neonatal mouse model to study respiratory syncytial virus infections. Expert Rev Anti Infect Ther. 2010;8(12):1371–1380. doi: 10.1586/eri.10.125. [DOI] [PMC free article] [PubMed] [Google Scholar]