Abstract
Objective
Clinical guidelines for the acute management of emergency department (ED) patients with severe sepsis encourage the placement of central venous catheters (CVC). Data examining the timing of CVC insertion among critically ill patients admitted from the ED are limited. We examined the hypothesis that prompt CVC insertion during hospitalization among patients admitted from the ED acts as a surrogate marker for early aggressive care in the management of critically ill patients.
Design
Retrospective cross sectional analysis of ED visits using 2003-2006 discharge data from California, State Inpatient Databases (SID), Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality.
Setting
General medical or general surgical hospitals (n=310).
Patients
Patient hospitalizations beginning in the ED with the two most common diagnoses associated with CVC (sepsis and respiratory arrest.)
Interventions
None.
Measurements and Main Results
We identified the occurrence and timing of CVC using International Classification of Diseases, Clinical Modifications, 9th Revision procedure codes. The primary outcomes measured were annual CVCs per 1000 hospitalizations that began in the ED occurring emergently (procedure day 0), urgently (procedure day 1-2), or late (procedure day 3 or later). A total of 129,288 hospital discharges had evidence of CVC. In 2003 5,759 CVCs were placed emergently compared to 10,469 in 2006. The rate of emergent CVC/1000 increased annually from 228 in 2003, 239 in 2004, 257 in 2005, up to 269 in 2006. Urgent and late CVC rates trended down (p<.001). In a multilevel model the odds of undergoing emergent CVC relative to 2003 increased annually: 1.08 (95%CI, 1.03 to 1.12) in 2004, 1.19 (95%CI, 1.14 to 1.23) in 2005, and 1.28 (95%CI, 1.23 to 1.33) in 2006.
Conclusions
CVCs are inserted earlier and more frequently among critically ill patients admitted from the ED. Earlier CVC insertion may require systematic changes to meet increasing utilization and enhanced mechanisms to measure CVC outcomes.
Keywords: Catheterization, Central Venous, Emergency Medicine, Sepsis
Introduction
In the past 18 years the number of hospital visits with a code for central venous catheter insertion (CVC) more than quadrupled from 439,339 in 1993 to 1,968,244 in 2010.1 In the Emergency Department (ED), ultrasound technology and Surviving Sepsis guidelines have facilitated and encouraged early CVC among critically ill patients to guide resuscitation and decrease mortality.2-4 Additionally, the number of severely septic patients EDs will care for before admission to the intensive care unit (ICU) is on the rise. 5-7 According to the Healthcare Cost and Utilization Project (HCUP), the number of patients who entered the hospital from the ED with a principal diagnosis of sepsis increased from 198,909 in 1993 to 457,944 in 2006.1
The objective of this study was to examine utilization and timing of CVC among patients admitted from the ED in an environment of changing clinical approaches in the management of acute sepsis. CVC may be interpreted as a surrogate marker for aggressive management since invasive hemodynamic parameters (central venous pressure and central venous oxygen saturation) and the delivery of vasoactive medications both require large central vein access. There are currently no national databases that readily identify patterns of CVC utilization among patients admitted from the ED. Identifying the timing of aggressive resuscitation during hospitalization can provide information for system-wide severe sepsis strategies including efforts to meet demand and systems to help mitigate potential adverse evnts from CVCs inserted outside the ICU.
Methods
Databases and Study Population
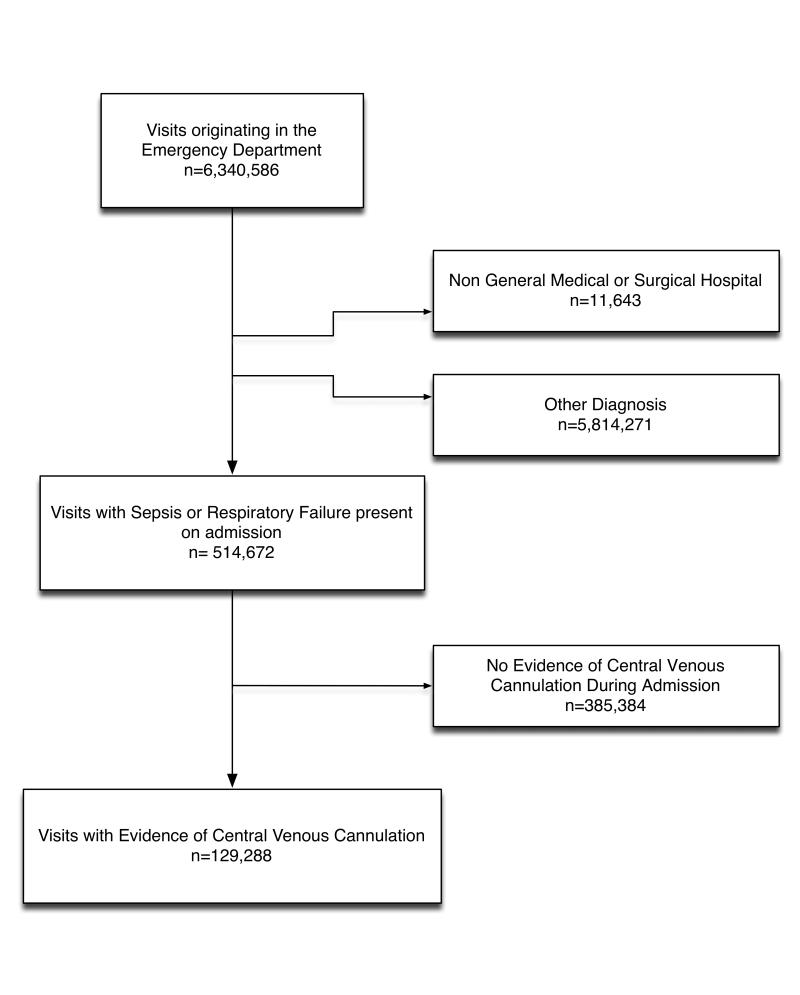
We performed a retrospective cross-sectional analysis of hospitalizations that began in the ED using data from the Agency for Healthcare Research and Quality’s (AHRQ) HCUP California State Inpatient Databases (SID) from 2003 to 2006.8 The California SID was chosen because of its large sample size, diverse patient population, and relevant clinical information. Records were limited to adult (18 years of age or older) stays in community acute-care (non-federal, non-rehabilitation) hospitals admitted from the ED. (Figure 1) Records were merged with The American Hospital Association (AHA) Annual Survey Database (Health Forum) and limited to general medical and general surgery service hospitals. Transfers between hospitals were excluded to eliminate the possibility of misclassifying the location and timing of procedures.
Figure 1.
Flow Chart Demonstrating Visits Included in the Study
Clinical Classification Software (CCS), developed to categorize International Classification of Diseases, 9th Revision (ICD-9-CM) diagnoses from large administrative databases into clinically meaningful groups for the purposes of research, was used to assign diagnosis categories.9,10 Records were included if the principal CCS diagnosis of sepsis (CCS= 2) or respiratory arrest (CCS=131) was present on admission meaning these conditions were present when the patient was admitted from the ED. Among all hospital records (not just those originating in the ED) sepsis and respiratory arrest were the two most common principal CCS diagnoses associated with CVC. Respiratory arrest was included because of the undifferentiated nature of critically ill ED patients and the common prevalence of respiratory arrest and infection in cases of presumed sepsis in the ED. The ICD-9-CM procedure code for CVC (38.93) was used to identify inpatient discharges with CVC. Discharges without evidence of CVC insertion were excluded from the analysis.
Power calculations were done assuming a 2% increase per year in the rate of early CVC. To account for comparison of multiple years, a Bonferroni correction was done with alpha = .0125. We estimated approximately 36,000 records would be necessary to detect a significant yearly difference with 80% power. Calculations were performed using G*Power (v 3.1.3, Dusseldorf, Germany).
Measures
Primary Dependent Variable
HCUP assigns the day that a procedure took place during the hospitalization relative to the hospital admission date. If a procedure takes place on the same day the patient is admitted, it is assigned to procedure day 0. Any procedure occurring on day 0 or day -1 was categorized as an “emergent CVC.” Procedures taking place on days 1-2 or on day 3 or more of hospital admission were categorized as cases that required “urgent” or “late” CVCs, respectively. The primary dependent variable of interest was expressed as emergent CVCs/1000 hospitalizations that began in the ED among patients with a principal diagnosis of sepsis or respiratory arrest present on admission.
Independent variables or covariates
Demographic, payer, and mortality data were collected from the SID. Medicare for individuals 18-65 years was categorized separately to represent the disabled or chronically ill. Elixhauser Comorbidity Software was used to assign comorbidities to each record.11
Hospital teaching status and urban or rural location originated from the AHA annual survey. The 2005 and 2006 California State Emergency Department Databases (SEDD), which contained treat and release visits, were concatenated with the 2005 and 2006 California SID discharge records to calculate annual ED volume. ED visits for years 2003 and 2004 reflect 2005 ED volume since no SEDD existed for those years.12
Data Analysis
Descriptive statistics were used to characterize the population.13 Continuous data were examined for normality expressed with the appropriate statistic. The Kruskal-Wallis test was used to examine differences between non-normally distributed variables. The Cochran-Armitage test was used to examine trends. The Chi-square test was used to detect categorical differences.
Variables with a P<.05 on Chi-square testing were considered for the multivariate model. The multivariable model was multi-level to account for the effect of clustering of physician practices within hospitals.14 Noting the co-linearity between age and payer status, models were run including the Medicare payer category in the payer reference group as well as a separate category. Since no appreciable differences were noted, we present our results keeping the Medicare payer in the reference category.
Two multivariable multi-level models were tested to examine the differences between emergent CVCs and CVCs inserted later during a hospitalization. The first compared emergent CVCs to all CVCs inserted during admission. The second compared emergent CVCs to urgent CVCs. We examined the distribution of residuals in order to assess model fit. Plots of residuals were examined to determine that they were normally distributed with a mean of zero. Interactions between ED annual volume and hospital teaching status were tested to explore associations among busier academic centers. All statistical analyses were performed in SAS (Cary, S.C) version 9.2.
Human Subject Protection
The University’s Human Research Protection Office deemed this study exempt from consent.
Results
Between 2003 and 2006, 6,340,586 hospital stays began in California EDs (Figure 1). A total of 514,672 ED visits were admitted to 310 general medical or general surgical hospitals with a principal CCS diagnosis of sepsis or respiratory arrest present on admission. A CVC was placed in 129,288 (25%) of these admissions.
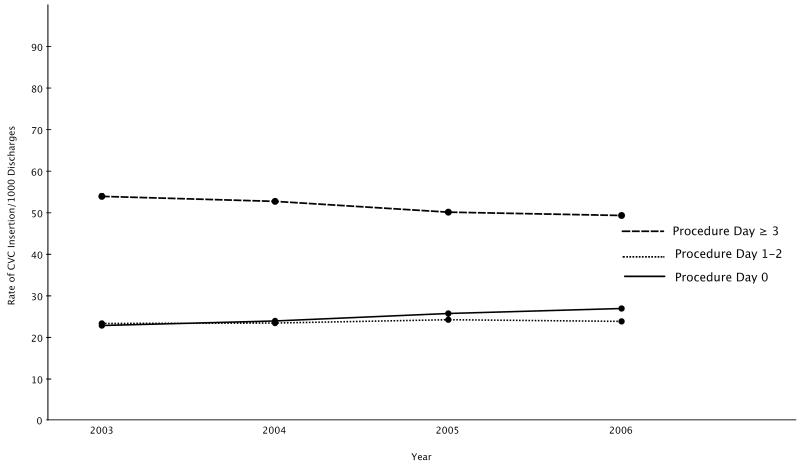
The number of emergent CVCs almost doubled from 5,759 in 2003 to 10,469 in 2006 (Table 1). The unadjusted rate of emergent CVC (inserted on day 0) over the 4 year period was 251 CVC /1000 hospitalizations compared to 237/1000 urgent CVCs (inserted on days 1-2) and 512/1000 late CVCs (inserted on day 3 or later). In comparison to urgent rates of CVC, the rate of emergent CVC increased from 2003 to 2006 (Cochran-Armitage, p<.001). Likewise, the rate of emergent CVC increased from 2003 to 2006 compared to late CVCs although the majority of CVCs were late insertions (Cochran-Armitage, p<.001) (Figure 2).
Table 1.
Yearly Central Venous Cannulations by Time of Insertion and Diagnosis
| Emergent (PRDAYa 0) |
Urgent (PRDAYa 1-2) |
Late (PRDAYa ≥ 3) |
Totals | ||||
|---|---|---|---|---|---|---|---|
| Year | n | % | n | % | n | % | |
| 2003 | 5,759 | (22.8) | 5,893 | (23.3) | 13,601 | (53.9) | 25,253 |
| Sepsis | 2,957 | (20.0) | 3,322 | (22.5) | 8,484 | (57.5) | 14,763 |
| Respiratory Arrest | 2,802 | (26.7) | 2,571 | (24.5) | 5,117 | (48.8) | 10,490 |
| 2004 | 7,152 | (23.9) | 6,998 | (23.4) | 15,784 | (52.7) | 29,934 |
| Sepsis | 4,036 | (21.6) | 4,238 | (22.7) | 10,402 | (55.7) | 18,676 |
| Respiratory Arrest | 3,116 | (27.7) | 2,760 | (24.5) | 5,382 | (47.8) | 11,258 |
| 2005 | 9,039 | (25.7) | 8,506 | (24.2) | 17,628 | (50.1) | 35,173 |
| Sepsis | 5,227 | (23.5) | 5,295 | (23.8) | 11,755 | (52.8) | 22,277 |
| Respiratory Arrest | 3,812 | (29.6) | 3,211 | (24.9) | 5,873 | (45.5) | 12,896 |
| 2006 | 10,469 | (26.9) | 9,263 | (23.8) | 19,196 | (49.3) | 38,928 |
| Sepsis | 6,290 | (25.1) | 5,725 | (22.8) | 13,053 | (52.1) | 25,068 |
| Respiratory Arrest | 4,179 | (30.1) | 3,538 | (25.5) | 6,143 | (44.3) | 13,860 |
Procedure Day.
Figure 2.
Rates of Central Venous Catheter Insertion by Procedure Day During the Years 2003-2006
The total number of emergent CVCs among sepsis discharges more than doubled from 2,957 in 2003 to 6,290 in 2006 (Table1). Among visits for sepsis, emergent CVC rates increased more than urgent or late insertions. Rates for emergent cannulation increased by 5.1% (95% CI, 4.2-5.9) while rates for urgent CVC remained unchanged (0.3% difference, 95% CI -.1-1.1) and rates among late sepsis cases decreased overall by 5.4% (95% CI, 4.4-6.4). Increased emergent CVC rates were also noted for cases of respiratory arrest though by only 3.4% from 2003 to 2006 (95% CI, 2.3-4.6). Among respiratory arrest cases, rates for urgent CVC remained unchanged (1% difference, 95%CI -.08%-2.1%) while rates for late CVCs declined by 4.5% (95%CI, 3.2-5.7). Mortality among all three groups decreased during the 4 year time period (Table 2). Mortality decreased in the emergent CVC group by 3.7% (95% CI 2.2-5.3), in the urgent group by 5.1 % (95%CI, 3.6-6.7), and in the late CVC group by 2% (95% CI 1.0-3.0). Length of stay among survivors decreased by one day.
Table 2.
Mortality and Length of Stay Among Survivors Undergoing Central Venous Cannulations Emergently (Procedure Day 0), Urgently (Procedure Day 1-2) or Late (Procedure Day 3 or Later) in the State of California from 2004-2006
| 2003 | 2004 | 2005 | 2006 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Diedb | % | % | % | % | p<.001a | ||||
| Emergent | 2,276/5,759 | (39.5) | 2,606/7,152 | (36.4) | 3,198/9,039 | (35.4) | 3,749/10,469 | (35.8) | |
| Urgent | 2,050/5,893 | (34.8) | 2,171/6,998 | (31.0) | 2,679/8,506 | (31.5) | 2,747/9,263 | (29.7) | |
| Late | 3,770/13,601 | (27.7) | 4,228/15,784 | (26.8) | 4,541/17,628 | (25.8) | 4,936/19,196 | (25.7) | |
| LOS Survivorsc (Days) | Median | IQR | Median | IQR | Median | IQR | Median | IQR | |
| Emergent | 10 | 6-16 | 9 | 6-16 | 9 | 5-15 | 9 | 5-15 | 0.005d |
| Urgent | 11 | 7-18 | 10 | 6-17 | 10 | 6-16 | 10 | 6-16 | 0.001d |
| Late | 17 | 10-27 | 16 | 10-26 | 16 | 10-25 | 16 | 10-25 | <.001d |
Cochrane-Armitage.
25 Missing.
Length of Stay.
Kruskal-Wallis.
Table 4 and Table 5 show that in both multilevel models the odds of emergent CVC increased over time. Emergent CVC was more likely in rural hospitals, among the uninsured, and among visits indicating uncomplicated diabetes, drug abuse and coagulopathy although coagulopathy was not significant in the model comparing emergent to urgent CVCs. Visits indicating liver disease, congestive heart failure, metastatic cancer, paralysis and obesity were less likely to undergo emergent CVC although obesity was not significant in the model comparing emergent to late CVC and paralysis was not significant in the model comparing emergent to urgent CVCs. Among females emergent CVC was more likely than late CVC however less likely than urgent CVC. Emergent CVC was less likely among visits by patients in the oldest quartile but this finding was non-significant in the model comparing emergent to urgent CVC placement. There were no significant effects of ED visit volume or teaching hospital status in both adjusted models.
Table 4.
Model 1 Comparing Emergent CVCs to CVCs Inserted After Procedure Day 0
| Variable | Odds Ratio | 95% Confidence Limits | p value |
|---|---|---|---|
| Year | |||
| 2003 | ref. | ||
| 2004 | 1.08 | 1.03 - 1.12 | 0.0005 |
| 2005 | 1.19 | 1.14 - 1.23 | <.0001 |
| 2006 | 1.28 | 1.23 - 1.33 | <.0001 |
| Hospital Level Variables | |||
| Urban Hospital | ref. | ||
| Rural Hospital | 1.84 | 1.37 - 2.47 | <.0001 |
| Non-Teaching Hospital | ref. | ||
| Teaching | 1.03 | 0.95 - 1.13 | NS |
| Annual ED Visits | |||
| <30,603 | ref. | ||
| 30,603-40,599 | 0.99 | 0.89 - 1.09 | NS |
| 40,600-51,270 | 1.10 | 0.98 - 1.25 | NS |
| >51,270 | 1.05 | 0.91 - 1.21 | NS |
| Encounter Level Variables | |||
| Female | 1.06 | 1.03 - 1.09 | <.0001 |
| Age | |||
| 65-74 | ref. | ||
| 18-45 | 1.00 | 0.94 - 1.05 | NS |
| 46-64 | 1.01 | 0.97 - 1.06 | NS |
| 75+ | 0.96 | 0.92 - 0.99 | 0.0066 |
| Primary Payer | |||
| Private Insurance/HMO | ref. | ||
| Medicaid | 0.98 | 0.93 -1.02 | S |
| No Insurance | 1.23 | 1.16 -1.30 | <.0001 |
| Medicare Age < 65 | 0.99 | 0.94 -1.05 | NS |
| Elixhauser Co-Morbidities | |||
| Fluid and Electrolyte Disorder | 1.05 | 1.02 -1.07 | 0.0010 |
| Diabetes | 1.12 | 1.09 - 1.16 | <.0001 |
| Coagulopathy | 1.07 | 1.03 - 1.11 | 0.0002 |
| Drug Abuse | 1.37 | 1.30 - 1.45 | <.0001 |
| Paralysis | 0.94 | 0.89 - 0.99 | 0.0263 |
| Liver Disease | 0.93 | 0.88 - 0.97 | 0.0023 |
| Congestive Heart Failure | 0.92 | 0.89 - 0.95 | <.0001 |
| Metastatic Cancer | 0.91 | 0.85 - 0.97 | 0.0052 |
| Diabetes w/Chronic Complications | 0.91 | 0.86 - 0.96 | 0.0002 |
| Solid Tumor | 0.97 | 0.90 - 1.06 | NS |
| Obesity | 1.01 | 0.96 - 1.07 | NS |
| Alcohol Abuse | 1.02 | 0.97 - 1.07 | NS |
| Renal Failure | 1.00 | 0.96 - 1.04 | NS |
| Chronic Lung Disease | 1.00 | 0.97 - 1.03 | NS |
Table 5.
Model 2 Comparing Emergent CVCs (Inserted on Procedure Day 0) to Urgent CVCs (Inserted on Procedure Days 1-2)
| Variable | Odds Ratio | 95 % Confidence Interval | p value |
|---|---|---|---|
| Year | |||
| 2003 | ref. | ||
| 2004 | 1.05 | 1.00 - 1.11 | 0.0434 |
| 2005 | 1.10 | 1.05 - 1.15 | 0.0002 |
| 2006 | 1.17 | 1.11 - 1.23 | <.0001 |
| Hospital Level Variables | |||
| Urban Hospital | ref. | ||
| Rural Hospital | 1.39 | 1.05 - 1.84 | 0.0410 |
| Non-Teaching Hospital | ref. | ||
| Teaching | 1.07 | 0.97 - 1.18 | NS |
| Annual ED Visits | |||
| <30,603 | ref. | ||
| 30,603-40,599 | 0.99 | 0.89 - 1.10 | NS |
| 40,600-51,270 | 1.08 | 0.95 - 1.22 | NS |
| >51,270 | 1.06 | 0.91 - 1.22 | NS |
| Encounter Level Variables | |||
| Female | 0.95 | 0.92 - 0.98 | 0.0010 |
| Age | |||
| 65-74 | ref. | ||
| 18-45 | 0.94 | 0.88 - 1.00 | NS |
| 46-64 | 0.96 | 0.91 - 1.02 | NS |
| 75+ | 0.95 | 0.91 - 1.00 | NS |
| Primary Payer | |||
| Private Insurance/HMO | ref. | ||
| Medicaid | 1.05 | 0.99 - 1.10 | NS |
| No Insurance | 1.19 | 1.11- 1.28 | <.0001 |
| Medicare Age < 65 | 1.01 | 0.95 - 1.08 | NS |
| Elixhauser Co-Morbidities | |||
| Fluid and Electrolyte Disorder | 1.01 | 0.98 - 1.05 | NS |
| Diabetes | 1.06 | 1.02 - 1.10 | 0.0052 |
| Coagulopathy | 1.04 | 1.00 - 1.09 | NS |
| Drug Abuse | 1.33 | 1.24 - 1.42 | <.0001 |
| Paralysis | 0.95 | 0.89 - 1.02 | NS |
| Liver Disease | 0.86 | 0.81 - 0.92 | <.0001 |
| Congestive Heart Failure | 0.95 | 0.92 - 0.99 | 0.0087 |
| Metastatic Cancer | 0.91 | 0.84 - 0.99 | 0.0222 |
| Diabetes w/Chronic Complications | 0.98 | 0.92 - 1.05 | NS |
| Solid Tumor | 0.98 | 0.88 - 1.08 | NS |
| Obesity | 0.88 | 0.83 - 0.94 | <.0001 |
| Alcohol Abuse | 0.97 | 0.91 - 1.04 | NS |
| Renal Failure | 1.01 | 0.96 - 1.05 | NS |
| Chronic Lung Disease | 0.99 | 0.95 - 1.02 | NS |
Discussion
In this study, the rate of emergent CVC among patients with a diagnosis of sepsis or respiratory arrest admitted from the ED increased from 2003-2006 indicating that aggressive management of critically ill patients may be occurring sooner during the course of hospitalization, possibly in the ED. The rise in emergent CVC rates observed by our data suggests that, among critically ill ED admissions, aggressive sepsis therapeutic pathways may be initiated sooner than before conforming to published guidelines and the acceptance of early goal directed therapy (EGDT) protocols.3,15 If one is to accept that CVC represents more intense care, these data compliment prior research suggesting that aggressive therapy is occurring earlier among critically ill patients admitted from the ED.16
Though our objective was to characterize the timing of CVC utilization, we found patient outcomes similar to other sepsis studies using large databases. Unadjusted mortality and length of hospital stay decreased similar to Kumar’s study which used a national sample of inpatient discharges.17 There are data examining the association between CVC timing and outcomes. One small study found organ function worsened with each hour of CVC delay.18 In a large population-based study, Walkey et. al. found decreased mortality among septic shock patients in whom CVCs were inserted upon hospital admission compared to those in whom a CVC was never placed.19 In the initial EGDT study, patients in both the intervention and control arm underwent CVC in the first 6 hours. Decreases in mortality were attributed to those resuscitated to achieve specific hemodynamic goals, some of which were available only by CVC (e.g. central venous pressure, central venous oxygen saturation).3 Conversely, CVC placement is likely to improve outcomes only if it leads to therapies (e.g. fluid administration, transfusions, inotropes, intubation and sedation or prevention of sudden cardiopulmonary complications) which are timed correctly for reversal of imbalances between supply and demand.20 Levy et. al. found that increased sepsis bundle compliance decreased mortality although CVC related hemodynamic parameters were not shown to independently predict survival. 21,22 It’s possible that CVC hemodynamic parameters may play a critical management role in early quantitative resuscitation, but their effect may be tempered over the course of a full sepsis admission.23,24
In both multivariable models, emergent CVC placement was more likely in rural hospitals compared to urban hospitals although there were few rural cases. In contrast, Wang et al. used a national sample and found that 15% of all severe sepsis cases presented to non-metropolitan statistical areas (MSA). These differences may be partially explained by how the AHA data designates rural hospital status and that this study used only data from the state of California.25 Reasons for expeditious placement are likely related to delayed healthcare access in rural settings resulting in greater illness severity upon presentation.26-33 Barriers to healthcare access and greater co-morbidity burden may also explain why the uninsured were more likely to require emergent CVC during their hospital visit.31-33 Irrespective of the exact causes, populations traditionally challenged by access to the healthcare system may be more likely to require early CVC placement.
Similar to Walkey et. al.’s study, women were more likely to undergo emergent CVC on admission day 0 compared to later CVC.19 However, females were less likely to undergo emergent CVC when compared to urgent CVC (days 1-2). Among females, reports of delay in accepted ED care processes have been observed in cases of acute myocardial infarction, stroke, and appendicitis. 34-37 Variable disease presentation and diagnostic challenges among females may partially explain these delays. Additionally, critically ill females may seek ED care sooner than males and require less intense ED services.38 Lastly, differing prevalence of mediating co-morbidities between men and women in our first and second model may have also contributed to the inconsistent findings.
The likelihood of emergent CVC compared to later CVC decreased with age in our study though prior research indicates the incidence of sepsis is highest among those older than 70 years.6,7 Physicians may be reluctant to offer aggressive therapy to some elderly patients because they may be thought unlikely to benefit. These patterns are well documented in cases of myocardial infarction and unstable angina and may explain delays in aggressive resuscitation among the critically ill.39,40
The co-morbidities associated with emergent CVC placement in both multivariate models were drug abuse and diabetes. ED patients with drug abuse often lack readily available peripheral access.41 Acute diabetes management may require multi-lumen access for frequent blood draws and medication delivery. Delay in presentation among patients with drug abuse may also be a contributor. Although coagulopathy is cited as a relative contraindication, it did not seem to delay the timing of CVC in the acute setting.42
Emergent CVC was less likely in patients with metastatic cancer, congestive heart failure, liver failure and paralysis. Critical illness may be difficult to diagnose and act upon among chronically ill patients in the acute setting. Conversely, patients with chronic conditions may be closely monitored lessening the likelihood of presenting sicker to the health system.43 Cancer patients may also have long-term tunneled catheters mitigating the need for emergent venous access. Obesity decreased the likelihood of emergent CVC compared to urgent CVC. Lack of technology or expertise may explain why emergent CVC was delayed in anatomically challenging patients.44,45
These data could not reliably identify where emergent CVCs were placed, however, EDs were likely involved. From 2001-2004, EDs cared for over 500,000 severe sepsis patients annually, a number likely to rise due to increasing ED visits.6,46,47 Average length of ED stay for severely septic patients approaches 5 hours. Despite this window of opportunity, studies examining Surviving Sepsis guidelines find that only 25% of ED cases complete full bundle compliance. 6,21,22 These figures raise the possibility that EDs may not be sufficiently prepared to meet demands unless rates of emergent CVC rise. EGDT protocols may not be feasible without additional resources or without the development of non-CVC dependent EGDT protocols using non-invasive devices.48-51 To resuscitate patients within 6 hours of arrival, ICU/ED collaborative teams will likely play a role. Technologies that improve the safety and efficiency of CVC insertion, such as ultrasound guidance, must be made widely available.2,52,53 Central line associated blood stream infection (CLABSI) surveillance and prevention systems, widely implemented in ICUs but not EDs, will need to broaden their scope.54,55 Despite great attention given to CLABSI prevention in ICUs, only one single center study has addressed the ED CLABSI rate, concluding that it is similar to the ICU rate.56 There are no studies examining the feasibility and efficacy of CLABSI prevention strategies in the ED.
This study was constrained by limitations that pertain to administrative data.57 We relied on timing of billing codes to identify CVCs but have no clinical details to establish whether patients denied consent or whether the patient’s condition indeed merited a CVC. However, our focus was to track utilization to potentially inform system-wide strategies. While the specificity of ICD-9 procedures for major procedures is high, CVC may be considered a minor procedure and hence may be under-coded by some institutions.58 The variability in hospital coding patterns may bias the data either away or towards the null and this may vary over time. Furthermore, some Elixhauser co-morbidities relevant to our analysis may have been under-coded limiting the ability to adjust for severity of illness. Elixhauser co-morbidities may weakly predict death in high-mortality subsets of ED patients admitted to the ICU compared to physiologic scores.59,60 However, Elixhauser comorbidities have performed well in predicting mortality in other studies compared to the Charlson co-morbidity index.61 Lastly, we did not examine CVC complications and the association of earlier CVC with adverse events requires further study.
Conclusions
Early CVC insertion rates are increasing among critically ill patients admitted from the ED. Units that initially care for critically ill patients, such as the ED, may require additional resources to meet increased utilization and to follow CVC outcomes.
Table 3.
Hospital and Encounter Level Characteristics of 129,288 Hospital Discharges by Timing of Central Venous Cannulation Representing Emergent (n=32,419), Urgent (n=30,660), and Late (n=66,209) Procedures.
| Emergent CVC (PRDAYb 0) |
Urgent CVC (PRDAY 1-2) |
Late CVC (PRDAY ≥3) |
||||||
|---|---|---|---|---|---|---|---|---|
| n | (%)a | n | (%)a | n | (%)a | Totals | p valuec | |
|
Hospital Level Non Teaching Hospital |
21,940 | (24) | 22,475 | (25) | 45,992 | (51) | 90,407 | <.0001 |
| Teaching Hospital | 10,479 | (27) | 8,185 | (21) | 20,217 | (52) | 38,881 | |
| Urban Hospital | 31,704 | (25) | 30,171 | (24) | 65,545 | (51) | 127,420 | <.0001 |
| Rural Hospital | 715 | (38) | 489 | (26) | 664 | (36) | 1,868 | |
| ED Annual Visits | <.0001 | |||||||
| <30,603 | 7,965 | (24) | 7,934 | (24) | 16,853 | (51) | 32,752 | |
| 30,603 to 40,599 | 7,271 | (23) | 7,461 | (23) | 17,179 | (54) | 31,911 | |
| 40,600 to 51,270 | 8,515 | (26) | 8,034 | (25) | 16,027 | (49) | 32,576 | |
| >51,270 | 8,668 | (27) | 7,231 | (23) | 16,150 | (50) | 32,049 | |
| Encounter Level | ||||||||
| Sex d | ||||||||
| Female | 16,061 | (25) | 16,080 | (25) | 32,006 | (50) | 64,147 | <.0001 |
| Age | ||||||||
| 18-45 | 5,281 | (28) | 4,493 | (24) | 9,275 | (49) | 19,049 | |
| 46-64 | 10,768 | (27) | 9,783 | (24) | 20,057 | (49) | 40,608 | |
| 65-74 | 6,096 | (24) | 5,885 | (23) | 13,159 | (52) | 25,139 | |
| 75+ | 10,274 | (23) | 10,499 | (24) | 23,718 | (53) | 44,491 | |
| Insurance Status e | ||||||||
| Privt Ins/HMO | 5,498 | (18) | 5,505 | (18) | 19,969 | (64) | 30,972 | <.0001 |
| Medicaid | 6,306 | (27) | 5,368 | (23) | 11,999 | (51) | 23,673 | |
| No Insurance | 2,797 | (33) | 1,998 | (23) | 3,792 | (44) | 8,587 | |
| Medicare Old | 14,164 | (23) | 14,319 | (24) | 32,344 | (53) | 60,827 | |
| Medicare Young | 3,647 | (26) | 3,462 | (24) | 7,094 | (50) | 14,203 | |
| Elixhauser Co-Morbidities | ||||||||
| Fluid and Electrolyte Disorders | 18,185 | (25) | 17,133 | (24) | 36,367 | (51) | 71,685 | 0.0005 |
| Congestive Heart Failure | 9,632 | (23) | 9,764 | (24) | 21,764 | (53) | 41,160 | <.0001 |
| Chronic Lung Disease | 9,383 | (25) | 9,245 | (24) | 19,330 | (51) | 37,958 | 0.0016 |
| Uncomplicated Diabetes | 7,173 | (27) | 6,667 | (25) | 13,130 | (49) | 26,970 | <.0001 |
| Coagulopathy | 5,434 | (26) | 4,905 | (24) | 10,502 | (50) | 20,841 | 0.0012 |
| Renal Failure | 5,447 | (24) | 5,358 | (23) | 12,308 | (53) | 23,113 | <.0001 |
| Liver Disease | 3,003 | (26) | 2,872 | (25) | 5,477 | (48) | 11,352 | <.0001 |
| Alcohol Abuse | 2,966 | (29) | 2,533 | (24) | 4,871 | (47) | 10,370 | <.0001 |
| Drug Abuse | 2,846 | (34) | 1,873 | (22) | 3,606 | (43) | 8,325 | <.0001 |
| Obesity | 2,330 | (26) | 2,556 | (28) | 4,206 | (46) | 9,092 | <.0001 |
| Complicated Diabetes | 2,274 | (22) | 2,367 | (23) | 5,845 | (56) | 10,486 | <.0001 |
| Paralysis | 2,182 | (24) | 2,150 | (23) | 4,844 | (53) | 9,176 | 0.0028 |
| Elixhauser Co-Morbidities | ||||||||
| Metastatic Cancer | 1,257 | (22) | 1,335 | (24) | 3,071 | (54) | 5,663 | <.0001 |
| Arthritis | 896 | (24) | 902 | (24) | 1,983 | (52) | 3,781 | 0.126 |
| Solid Tumor (no metastasis) | 851 | (23) | 853 | (23) | 1,936 | (53) | 3,640 | 0.0263 |
Row percentages indicated.
Procedure Day.
Chi-square.
112 Missing.
26 Missing.
Acknowledgments
Work Performed at Washington University School of Medicine in St. Louis
Financial Support: This work was sponsored by a grant from the Agency for Research Healthcare and Quality (AHRQ) K08 HS18092 (DLT), by 5K12RR023249(VF), and by the Center for Administrative Data Research at Washington University School of Medicine supported in part by AHRQ 1R24 HS19455 (MAO, VF) and the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources (NCRR) at the National Institutes of Health (NIH) UL1 RR024992.
Dr. Theodoro received grant support from the Agency of Healthcare Research and Quality. Dr. Olsen’s institution received grant support from AHRQ (1R24 HS19455), the National Institutes of Health (UL1 TR000448), Sanofi Pasteur, and Optimer. Dr. Fraser’s institution received grant support from the AHRQ, NIH, and the Centers for Disease Control and Prevention.
Dr. Fraser consulted for Battelle and The Foundation for Barnes-Jewish Hospital. Dr. Olsen consulted for Sanofi Pasteur. Dr. Fraser is employed by Washington University in St. Louis.
Drs. Theodoro, Olsen, Owens, and Fraser received support for article research from NIH and AHRQ.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Daniel Theodoro, Division of Emergency Medicine Washington University School of Medicine St. Louis, MO, USA.
Pamela L. Owens, Division of Infectious Diseases, Department of Medicine Washington University School of Medicine St. Louis, MO, USA.
Margaret A. Olsen, Division of Infectious Diseases, Department of Medicine Division of Public Health Sciences, Department of Surgery Washington University School of Medicine St. Louis, MO, USA.
Victoria Fraser, Division of Infectious Diseases, Department of Medicine Washington University School of Medicine St. Louis, MO, USA.
References Cited
- 1.HCUPnet. Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality. Rockville, MD: 1993-2010. http://hcupnet.agrq.gov/ [PubMed] [Google Scholar]
- 2.Leung J, Duffy M, Finckh A. Real-Time Ultrasonographically-Guided Internal Jugular Vein Catheterization in the Emergency Department Increases Success Rates and Reduces Complications: A Randomized, Prospective Study. Ann Emerg Med. 2006;48(5):540–547. doi: 10.1016/j.annemergmed.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001 Nov 8;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 4.Jones AE, Focht A, Horton JM, Kline JA. Prospective external validation of the clinical effectiveness of an emergency department-based early goal-directed therapy protocol for severe sepsis and septic shock. Chest. 2007 Aug;132(2):425–432. doi: 10.1378/chest.07-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banta JE, Joshi KP, Beeson L, Nguyen HB. Patient and hospital characteristics associated with inpatient severe sepsis mortality in California, 2005-2010. Crit Care Med. 2012 Nov;40(11):2960–2966. doi: 10.1097/CCM.0b013e31825bc92f. [DOI] [PubMed] [Google Scholar]
- 6.Wang HE, Shapiro NI, Angus DC, Yealy DM. National estimates of severe sepsis in United States emergency departments. Crit Care Med. 2007 Aug;35(8):1928–1936. doi: 10.1097/01.CCM.0000277043.85378.C1. [DOI] [PubMed] [Google Scholar]
- 7.Strehlow MC, Emond SD, Shapiro NI, Pelletier AJ, Camargo CA., Jr National study of emergency department visits for sepsis, 1992 to 2001. Ann Emerg Med. 2006 Sep;48(3):326–331. 331 e321–323. doi: 10.1016/j.annemergmed.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 8.HCUP State Inpatient Databases (SID) Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality. Rockville, MD: 2003-2006. http://www.hcup-us.ahrq.gov/sidoverview.jsp. [PubMed] [Google Scholar]
- 9.Elixhauser A, Steiner CA, Whittington C. Clinical classifications for health policy research: Hospital inpatient statistics, 1995. Agency for Health Care Policy and Research; Rockville, MD: 1998. AHCPR Pub. No. 98-0049. [Google Scholar]
- 10.HCUP Clinical Classification Software (CCS) for ICD-9-CM . Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality; Rockville, MD: [Accessed May 14, 2013]. 2006-2009. http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. [Google Scholar]
- 11.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998 Jan;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 12.HCUP State Emergency Department Databases (SEDD) Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality. Rockville, MD: 2005-2006. http://www.hcup-us.ahrq.gov/seddoverview.jsp. [PubMed] [Google Scholar]
- 13.Houchens R. Inferences with HCUP State Databases Final Report. U.S. Agency for Healthcare Research and Quality; HCUP Methods Series Report # 2010-05. Online October 12, 2010. Available: http://www.hcupus.ahrq.gov/reports/methods.jsp. [Google Scholar]
- 14.Houchens R, Chu B, Steiner C. Heirarchical Modeling using HCUP Data. U.S. Agency for Healthcare Research and Quality; HCUP Methods Series Report #2007-01 Online. January 10, 2007. http://www.hcupus.ahrq.gov/reports/methods.jsp. [Google Scholar]
- 15.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008 Jan;36(1):296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 16.Herring AA, Ginde AA, Fahimi J, et al. Increasing critical care admissions from U.S. emergency departments, 2001-2009. Crit Care Med. 2013 May;41(5):1197–1204. doi: 10.1097/CCM.0b013e31827c086f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar G, Kumar N, Taneja A, et al. Nationwide Trends of Severe Sepsis in the 21st Century (2000-2007)National Trends of Severe Sepsis in 21st Century. CHEST Journal. 2011;140(5):1223–1231. doi: 10.1378/chest.11-0352. [DOI] [PubMed] [Google Scholar]
- 18.Kashiouris M, Kashyap Rahul, Jaffer-Sathick Insara, Gajic Ognjen, Cartin-Ceba Rodrigo. 514: Association between delays in central venous catherter utilization and organ dysfunction in patients with severe sepsis and septic shock. Crit Care. 2011;40(12):1–328. [Google Scholar]
- 19.Walkey AJ, Wiener RS, Lindenauer PK. Utilization Patterns and Outcomes Associated With Central Venous Catheter in Septic Shock: A Population-Based Study*. Crit Care Med. 2013 Jun;41(6):1450–1457. doi: 10.1097/CCM.0b013e31827caa89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011 Feb;39(2):259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 21.Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Medicine. 2010 Feb;36(2):222–231. doi: 10.1007/s00134-009-1738-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy MM, Artigas A, Phillips GS, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. The Lancet infectious diseases. 2012 Dec;12(12):919–924. doi: 10.1016/S1473-3099(12)70239-6. [DOI] [PubMed] [Google Scholar]
- 23.Varpula M, Tallgren M, Saukkonen K, Voipio-Pulkki LM, Pettila V. Hemodynamic variables related to outcome in septic shock. Intensive Care Medicine. 2005 Aug;31(8):1066–1071. doi: 10.1007/s00134-005-2688-z. [DOI] [PubMed] [Google Scholar]
- 24.Hayatdavoudi S, Crowell R, Rincon T, Winchell J, Yee A, Ikeda D. 505: Does Aggressive Fluid Resucitation Using Cvp Monitoring Decrease Mortality Below the Apache IV Predicted Outcomes? Crit Care Med. 2012;40(12):1–328. 310.1097/1001.ccm.0000424723.0000467161.de. [Google Scholar]
- 25.AHA Hospital Statistics. Health Forum LLC; Chicago, IL: 2008. [Google Scholar]
- 26.Shen YC, Hsia RY. Changes in emergency department access between 2001 and 2005 among general and vulnerable populations. Am J Public Health. 2010 Aug;100(8):1462–1469. doi: 10.2105/AJPH.2009.175828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sihler KC, Hemmila MR. Injuries in nonurban areas are associated with increased disability at hospital discharge. J Trauma. 2009 Nov;67(5):903–909. doi: 10.1097/TA.0b013e3181aebec2. [DOI] [PubMed] [Google Scholar]
- 28.Rust G, Baltrus P, Ye J, et al. Presence of a Community Health Center and Uninsured Emergency Department Visit Rates in Rural Counties. Journal of Rural Health. 2009;25(1):8–16. doi: 10.1111/j.1748-0361.2009.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Withy K, Davis J. Followup after an emergency department visit for asthma: urban/rural patterns. Ethn Dis. 2008 Spring;18(2 Suppl 2):S2–247. [PMC free article] [PubMed] [Google Scholar]
- 30.The Australasian Resuscitation in Sepsis Evaluation (ARISE) Investigators and the Australian and New Zealand Intensive Care Society (ANZICS) Adult Patient Database (APD) Management Committee The outcome of patients with sepsis and septic shock presenting to emergency departments in Australia and New Zealand. Crit Care Resusc. 2007 Mar;9(1):8–18. [PubMed] [Google Scholar]
- 31.Lowe RA, Fu R, Ong ET, et al. Community characteristics affecting emergency department use by Medicaid enrollees. Med Care. 2009 Jan;47(1):15–22. doi: 10.1097/MLR.0b013e3181844e1c. [DOI] [PubMed] [Google Scholar]
- 32.Smolderen KG, Spertus JA, Nallamothu BK, et al. Health care insurance, financial concerns in accessing care, and delays to hospital presentation in acute myocardial infarction. JAMA. 2010 Apr 14;303(14):1392–1400. doi: 10.1001/jama.2010.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spencer DL, Richardson SK, McCormick MK. Inpatient hospital utilization among the uninsured near elderly: data and policy implications for West Virginia. Health Serv Res. 2007 Dec;42(6 Pt 2):2442–2457. doi: 10.1111/j.1475-6773.2007.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bangalore S, Fonarow GC, Peterson ED, et al. Age and gender differences in quality of care and outcomes for patients with ST-segment elevation myocardial infarction. The American Journal of Medicine. 2012 Oct;125(10):1000–1009. doi: 10.1016/j.amjmed.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 35.Chandra NC, Ziegelstein RC, Rogers WJ, et al. Observations of the treatment of women in the United States with myocardial infarction: A report from the National Registry of Myocardial Infarction-I. Archives of Internal Medicine. 1998;158(9):981–988. doi: 10.1001/archinte.158.9.981. [DOI] [PubMed] [Google Scholar]
- 36.Kaul P, Armstrong PW, Sookram S, Leung BK, Brass N, Welsh RC. Temporal trends in patient and treatment delay among men and women presenting with ST-elevation myocardial infarction. American Heart Journal. 2011;161(1):91–97. doi: 10.1016/j.ahj.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 37.Greenberg MR, Bond WF, MacKenzie RS, et al. Gender disparity in emergency department non-ST elevation myocardial infarction management. J Emerg Med. 2012 May;42(5):588–597. doi: 10.1016/j.jemermed.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 38.McGann Donlan S, Mycyk MB. Is female sex associated with ED delays to diagnosis of appendicitis in the computed tomography era? Am J Emerg Med. 2009 Sep;27(7):856–858. doi: 10.1016/j.ajem.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Giugliano RP, Camargo CA, Jr., Lloyd-Jones DM, et al. Elderly patients receive less aggressive medical and invasive management of unstable angina. Arch Intern Med. 1998;158:1113–1120. doi: 10.1001/archinte.158.10.1113. [DOI] [PubMed] [Google Scholar]
- 40.Lee DC, Pancu DM, Rudolph GS, Sama AE. Age-associated time delays in the treatment of acute myocardial infarction with primary percutaneous transluminal coronary angioplasty. Am J Emerg Med. 2005;23:20–23. doi: 10.1016/j.ajem.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 41.Melniker L, Milling TJ, Briggs WM. Development and Validation of a Scale for the Clarity of Anatomical Landmarks for Use in Outcomes Research of Ultrasound Guidance of Central Venous Cannulation. Academic Emergency Medicine. 2006;13(3):368. doi: 10.1197/j.aem.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Adams BD, Lyon ML, DeFlorio PT. Central Venous Cannulation and Central Venous Pressure Monitoring. 5th ed Saunders Elsevier; Philadelphia: 2010. [Google Scholar]
- 43.Kurichi JE, Stineman MG, Kwong PL, Bates BE, Reker DM. Assessing and using comorbidity measures in elderly veterans with lower extremity amputations. Gerontology. 2007;53(5):255–259. doi: 10.1159/000101703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore CL, Molina AA, Lin H. Ultrasonography in community emergency departments in the United States: access to ultrasonography performed by consultants and status of emergency physician-performed ultrasonography. Ann Emerg Med. 2006 Feb;47(2):147–153. doi: 10.1016/j.annemergmed.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 45.Mansfield P, Hohn D, Fornage B, Gregurich M, Ota D. Complication and failures of subclavian-vein catheterization. N Engl J Med. 1994;331:1735–1738. doi: 10.1056/NEJM199412293312602. [DOI] [PubMed] [Google Scholar]
- 46.McCaig LF, Nawar EN. Advace data from vital and health statistics. National Center for Health Statistics; Hyattsville, MD: 2006. National Hospital Ambulatroy Medical Care Survey: 2004 emergency department summary. [PubMed] [Google Scholar]
- 47.Niska R, Bhuiya F, Xu J. National Ambulatory Medical Care Survey: 2007 Emergency Department Summary. National Center for Health Statistics; Hyattsville, MD: 2010. [PubMed] [Google Scholar]
- 48.Monnet X, Teboul JL. Passive leg raising. Intensive Care Med. 2008 Apr;34(4):659–663. doi: 10.1007/s00134-008-0994-y. [DOI] [PubMed] [Google Scholar]
- 49.Hadian M, Kim HK, Severyn DA, Pinsky MR. Cross-comparison of cardiac output trending accuracy of LiDCO, PiCCO, FloTrac and pulmonary artery catheters. Critical Care. 2010;14(6):R212. doi: 10.1186/cc9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Monnet X, Anguel N, Naudin B, Jabot J, Richard C, Teboul JL. Arterial pressure-based cardiac output in septic patients: different accuracy of pulse contour and uncalibrated pressure waveform devices. Critical Care. 2010;14(3):R109. doi: 10.1186/cc9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Backer D, Marx G, Tan A, et al. Arterial pressure-based cardiac output monitoring: a multicenter validation of the third-generation software in septic patients. Intensive Care Medicine. 2011 Feb;37(2):233–240. doi: 10.1007/s00134-010-2098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Milling TJ, Jr., Rose J, Briggs WM, et al. Randomized, controlled clinical trial of point-of-care limited ultrasonography assistance of central venous cannulation: the Third Sonography Outcomes Assessment Program (SOAP-3) Trial. Crit Care Med. 2005 Aug;33(8):1764–1769. doi: 10.1097/01.ccm.0000171533.92856.e5. [DOI] [PubMed] [Google Scholar]
- 53.Karakitsos D, Labropoulos N, De Groot E, et al. Real-time ultrasound-guided catheterisation of the internal jugular vein: a prospective comparison with the landmark technique in critical care patients. Critical Care. 2006;10(6):R162. doi: 10.1186/cc5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pronovost P, Needham D, Berenholtz S, et al. An Intervention to Decrease Catheter-Related Bloodstream Infections in the ICU. N Engl J Med. 2006 Dec 28;355(26):2725–2732. doi: 10.1056/NEJMoa061115. 2006. [DOI] [PubMed] [Google Scholar]
- 55.Marschall J. Catheter-associated bloodstream infections: Looking outside of the ICU. American Journal of Infection Control. 2008;36(10):S172.e175–S172.e178. doi: 10.1016/j.ajic.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 56.LeMaster CH, Schuur JD, Pandya D, et al. Infection and natural history of emergency department-placed central venous catheters. Ann Emerg Med. 2010 Nov;56(5):492–497. doi: 10.1016/j.annemergmed.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 57.Jollis JG, Ancukiewicz M, DeLong ER, Pryor DB, Muhlbaier LH, Mark DB. Discordance of databases designed for claims payment versus clinical information systems. Implications for outcomes research. Annals of Internal Medicine. 1993 Oct 15;119(8):844–850. doi: 10.7326/0003-4819-119-8-199310150-00011. [DOI] [PubMed] [Google Scholar]
- 58.Quan H, Parsons G, Ghali WA. Validity of Procedure Codes in International Classification of Diseases, 9th revision, Clinical Modification Administrative Data. Medical Care. 2004;42(8):801–809. doi: 10.1097/01.mlr.0000132391.59713.0d. [DOI] [PubMed] [Google Scholar]
- 59.Enfield KB, Schafer K, Zlupko M, et al. A comparison of administrative and physiologic predictive models in determining risk adjusted mortality rates in critically ill patients. PLoS ONE. 2012;7(2):e32286. doi: 10.1371/journal.pone.0032286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quan H, Parsons GA, Ghali WA. Validity of information on comorbidity derived rom ICD-9-CCM administrative data. Medical Care. 2002 Aug;40(8):675–685. doi: 10.1097/00005650-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 61.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. American journal of epidemiology. 2011 Mar 15;173(6):676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]