Abstract
Objectives
Few studies have examined the long-term effect of age at implantation on outcomes using multiple data points in children with cochlear implants. The goal of this study was to determine if age at implantation has a significant, lasting impact on speech perception, language, and reading performance for children with prelingual hearing loss.
Design
A linear mixed model framework was utilized to determine the effect of age at implantation on speech perception, language, and reading abilities in 83 children with prelingual hearing loss who received cochlear implants by age 4. The children were divided into two groups based on their age at implantation: 1) under 2 years of age and 2) between 2 and 3.9 years of age. Differences in model specified mean scores between groups were compared at annual intervals from 5 to 13 years of age for speech perception, and 7 to 11 years of age for language and reading.
Results
After controlling for communication mode, device configuration, and pre-operative pure-tone average, there was no significant effect of age at implantation for receptive language by 8 years of age, expressive language by 10 years of age, reading by 7 years of age. In terms of speech perception outcomes, significance varied between 7 and 13 years of age, with no significant difference in speech perception scores between groups at ages 7, 11 and 13 years. Children who utilized oral communication (OC) demonstrated significantly higher speech perception scores than children who used total communication (TC). OC users tended to have higher expressive language scores than TC users, although this did not reach significance. There was no significant difference between OC and TC users for receptive language or reading scores.
Conclusions
Speech perception, language, and reading performance continue to improve over time for children implanted before 4 years of age. The current results indicate that the effect of age at implantation diminishes with time, particularly for higher-order skills such as language and reading. Some children who receive CIs after the age of 2 years have the capacity to approximate the language and reading skills of their earlier-implanted peers, suggesting that additional factors may moderate the influence of age at implantation on outcomes over time.
Introduction
It is well established that hearing loss in early childhood affects the development of speech perception, language, and reading skills. For children with profound hearing loss, the auditory access provided by hearing aids is not sufficient, resulting in delayed spoken language development. An alternative for children who receive limited benefit from hearing aids is a cochlear implant (CI). Research on the efficacy of CIs in children shows improvements over time in speech perception (Davidson et al., 2011; Uhler et al., 2011), speech production (Tomblin et al., 2008), and language and reading skills (Archbold et al., 2008; Ching et al., 2009). Moreover, children with CIs reach levels of performance that surpass those of their non-implanted peers who use hearing aids (Spencer et al., 1998; Yoshinaga-Itano et al., 2010). This technology enables many children with congenital deafness to be educated in regular-education school settings alongside their hearing peers.
Within the group of children with CIs, however, language and listening outcomes are quite variable. Some factors that influence outcomes include age at onset of deafness, duration of profound deafness, communication mode, pre-operative residual hearing, and nonverbal cognition (Cowan et al., 1997; Dowell et al., 2002; Fryauf-Bertschy et al., 1997; Moog & Geers, 2003; Sarant et al., 2001). Most of these factors are not malleable (i.e., under clinician control). However, the age at which children receive their implants garners much interest, and earlier implantation has been generally shown to yield better outcomes (Connor et al., 2006; Duchesne et al., 2009; Manrique et al., 2004; Nicholas & Geers, 2007). Evidence for the influence of age at implantation has changed clinical practice, with a steady decrease in the age at which children received CIs (Tomblin et al., 2007; Valencia et al., 2008; Wie, 2010).
Children who are implanted earlier tend to show speech perception outcomes that are significantly better than children who are implanted later (Manrique, Cervera-Paz, Huarte, & Molina, 2004). Manrique and colleagues found that children implanted between 0 to 3 years of age outperformed children implanted between 4 to 6 years of age on open-set speech perception measures. In addition, among children with at least five years of CI experience, those who received CIs prior to age 2 years achieved higher speech perception scores than children implanted after age 2 years. Svirsky et al. (2004) explored the effects of age at implantation on speech perception outcomes for children who received CIs at 1, 2, or 3 years of age. They found a significant improvement in speech perception for children who received CIs prior to age 2 years.
Age at implantation also may influence language and reading outcomes. Kirk et al. (2000) reported that children who received CIs before 2 years of age showed faster growth in receptive vocabulary and language compared to children who received CIs at later ages. Tomblin et al. (2005) examined expressive language outcomes in 29 children. Children who received CIs prior to 20 months of age demonstrated more rapid growth in expressive language than children who received CIs after 20 months. Furthermore, Niparko et al. (2010) showed that age at implantation influenced language comprehension and expression, with significantly steeper increases over time for children implanted younger than 18 months compared to children implanted after 18 months. Holt and Svirsky (2008) investigated children implanted before the age of 12 months and at ages 1, 2, or 3 years. For expressive language and speech perception outcomes, there were no differences between children implanted prior to 12 months and children implanted between 1 and 2 years of age, although the earliest implanted group was significantly different from the children implanted after 2 years of age. In contrast, for receptive language skills there was a significant advantage for implantation before 12 months of age, compared to implantation between 1 and 2 years. Thus, while there are clear benefits to earlier implantation, the evidence favoring implantation before 12 months of age compared to 1 to 2 years of age remains mixed.
With respect to reading outcomes, there are fewer published reports, but most results show an advantage for earlier implantation (Archbold, Harris, O'Donoghue, Nikolopoulos, White, & Richmond, 2008; Connor & Zwolan, 2004; Johnson & Goswami, 2010; Marschark et al., 2007). Johnson and Goswami (2010) divided children with CIs into two groups based on age at implantation (prior to 39 months or after 43 months) and found a significant difference in reading quotient scores in favor of the younger-implanted group. Archbold, Harris, O'Donoghue, Nikolopoulos, White, and Richmond (2008) assessed reading and writing skills for 105 children at 5 and 7 years post-implantation. Children were separated into an early-implantation group (before 42 months of age) and a late-implantation group (between 43 and 84 months of age). There was a strong association between reading outcomes and age at implantation. In both Johnson and Goswami (2010) and Archbold, Harris, O'Donoghue, Nikolopoulos, White, and Richmond (2008), the division between early and late implantation (around 3.5 years of age in both studies) is quite late, by current clinical standards. There is a need for more fine-grained examinations of the effect of age at implantation on reading, in order to determine if the age at CI receipt has a long-term impact on reading outcomes.
As we have described, a large number of studies have shown that age at implantation influences outcomes for children who are congenitally deaf. The vast majority of these studies have only examined children in the first few years of CI use, however. There are a few studies that address long-term outcomes of cochlear implantation, but most of those were either cross-sectional studies or studies that only examined one specific area of development (e.g., speech perception or receptive language). For example, Peng et al. (2004) reported on children with seven years of CI experience (age at implantation between 31 and 133 months of age), but focused solely on speech intelligibility outcomes at one test interval. Using linear regression modeling, they noted that there was a significant relationship between age at implantation and speech intelligibility. For every one year decrease in the age at implantation, there was a 5.5% increase in speech intelligibility scores (as measured by naïve listeners transcribing recorded speech samples). Geers and Nicholas (2012) examined vocabulary and receptive and expressive language skills with 60 children who were approximately 10.5 years of age and had received CIs between 12 and 38 months of age. They found that three factors – age at implantation, nonverbal cognitive skills, and pre-implant residual hearing – were significantly associated with better language skills. Finally, Geers et al. (2008) presented data on a large number of children (n=85) between 15 and 18 years of age, with an average of 13 years of CI experience (mean age at implantation = 3.5 years, SD = 0.8). Multiple regression analyses indicated that earlier implantation was moderately correlated with better language and reading outcomes (though this was only marginally significant for language), but not speech perception. While all these studies contribute to our knowledge regarding outcomes for older children with CIs, each paper uses a cross-sectional approach in analyzing the data. This approach only allows for testing and comparisons at a single point in time. The present study utilizes longitudinal data, in contrast, which allows us to follow the same children over their developmental growth period to compare groups at multiple ages and determine if the effect is shrinking as the children mature.
Based on this review of the literature, the long-term influence of age at implantation on speech perception, language, and reading is not yet clear. As Wie (2007) points out, the influence of early implantation is likely strongest in the years immediately following CI receipt. Geers et al. (2003) and Geers and Nicholas (2012) both note that most of the studies examining age at implantation effects only tested children up to 6 years old, or in the first 1 to 3 years of CI use, and it is possible that the influence of age at implantation may not be as strong in later childhood. In other words, studies may be observing an effect on the immediate performance, but the ultimate developmental potential of children could be less robustly related to age of implantation. As children grow older, other factors such as general cognitive development and educational environment have greater effects on outcomes, while the effects of age at implantation may diminish. Thus, if the goal is to expand evidence-based knowledge regarding language development in CI users over the long-term, it is important to investigate the long-term outcomes of children with CIs. Such investigations will allow researchers to assess whether differences in age at implantation functionally matter for outcomes. Assessing long-term outcomes can help determine whether performance of children implanted at very young ages continues to exceed that of children implanted at older ages, or if the older group catches up to their younger-implanted peers.
The goal of this study was to determine the longitudinal effect(s) of age at implantation on speech perception, language, and reading performance in a cohort of 83 children with prelingual deafness, in order to determine if the age at which children receive a CI has a long-term impact on outcomes in these areas. In the present study, children were separated into two groups: those who received their CI before 2 years of age and those who received their CI between 2 and 3.9 years. The rationale for the boundaries of these age groups was two-fold: 1) the two groups had a relatively equal number of subjects, and 2) general clinical consensus advocates for implantation prior to 2 years of age in children with prelingual deafness.
Method
We conducted a retrospective analysis of the Iowa Cochlear Implant Clinical Research Center’s longitudinal database of speech perception, language, and reading outcome measures.
Subjects
As of March, 2010, there were 105 children participating in a long-term research project who were implanted at the University of Iowa under the age of four years. After excluding children with severe cognitive, visual, or motor disabilities and children who did not use English as their primary spoken language, we examined the longitudinal data for 83 children with prelingual deafness. A team of audiologists and speech and language scientists collected data annually with this population since 1990. Seventy-five children received a Nucleus device and eight children received an Advanced Bionics (Clarion) device. The speech processing strategies are not specified, as it changed for most of the children over time due to advances in technology.
Participants were divided into two groups based on age at implantation. The first group (henceforth designated “earlier-implanted group”) included 38 children implanted under 2 years of age (mean age = 1.38 years; SD = 0.27; birth year 1995 to 2005) with an average of 7.8 years of CI experience at the time the data were extracted (SD = 2.71; range = 3.0 years to 12.8 years), although children were examined longitudinally. The second group (designated “later-implanted group”) included 45 children implanted between the ages of 2 to 3.9 years of age (mean age = 2.99 years; SD = 0.55; birth year 1983 to 2003) with an average of 12.2 years of CI experience at the time the data were extracted (SD = 5.04; range = 3.2 years to 22.4 years).
We compared the two groups on a number of measures that could affect language and reading outcomes. We first examined hearing and demographic variables. There was no significant difference in pre-operative residual hearing between the two groups (t[78] = −.795, p = .43; earlier-implanted group average = 110 dB HL[SD=11]; later-implanted group average = 108 dB HL [SD=11.54]). There was a significant difference in initial age of hearing aid use between the groups (t[77] = −5.4, p <.05; earlier-implanted group average = 6.8 months [SD=4.86]; later-implanted group average = 14.7 months [SD=7.39]) with the earlier-implanted group utilizing hearing aids at a younger age. A Chi-square test indicated that there was no significant difference in maternal education level (coded as six categorical levels) between the two groups (χ2 [5] = 3.53, p = 0.61; earlier-implanted group average = 14.8 years [SD=2.33]; later-implanted group average = 14.5 years [SD=2.03]). We also examined nonverbal cognitive skills using the parent-report Minnesota Child Development Inventory – Situation-Comprehension subscale (MCDI) (Ireton & Thwing, 1974), which was available for a subset of participants (earlier-implanted group: n = 25; later-implanted group: n = 20) between 3 and 4 years of age. Seventeen out of the 20 children in the later-implanted group had received their CIs at the time they were administered the MCDI. There was no significant difference in raw scores for nonverbal cognitive ability (t[43] = .153, p = .88; earlier-implanted group average = 33.2 [SD=5.85]; later-implanted group average = 33.5 [SD=4.94]). Nonverbal cognition was not consistently assessed during the school-age years, which prevented the comparison of nonverbal cognition with standardized assessment measures between the groups at later ages.
The quantity and quality of early service provision and aural habilitation can have a strong impact on later outcomes. Therefore, we also sought to determine if there were differences in the amount of service provision per week at two points: preoperatively, and at a post-operative visit between 7 years, 9 months and 8 years, 9 months. The latter visit was selected because data were available for the largest number of participants in both groups during this time period. Amount of service provision was assessed by parent report of the number of minutes per week of services from a speech-language pathologist, teacher of the deaf/hard of hearing, and/or early childhood development specialist. These data were available for 67% of the earlier-implanted group and 82% of the later-implanted group preoperatively; 86% and 53% respectively, postoperatively. There were no significant differences between the two groups in pre-operative (t[47] = −1.014, p = .32) or post-operative (t[35] = −.991, p = .33) minutes of service provision. We acknowledge that there may have been qualitative differences in the type of intervention, but did not have access to that retrospective information.
The number of children in this study who reported using oral communication (OC) versus total communication (TC) as their primary communication modality did not vary based on age at implantation (χ2 [2] = 2.92, p =.23). At the most recent report, for the earlier-implanted group, 69% reported using OC, 29% reported using TC, and 3% reported using Signing Exact English (SEE). For the later-implanted group, 50% reported using OC, 48% used TC, and 2% used SEE.
Additional demographic information for each child is included in Table 1.
Table 1.
Device demographic information. Pure-tone average (PTA) calculated at 500, 1000, 2000 Hz. Implant Ear (first) is the ear of the first implant surgery. Implant Ear (second) is the ear of the second implant surgery. N=Nucleus device; AB= Advanced Bionics device. Activation is the month and year of the cochlear implant activation.
| Age at First Implant (years) |
Pre- Op PTA |
Gender | Implant Type |
Implant Ear (first) |
First Activation (month - year) |
Implant Ear (second) |
Second Activation (month - year) |
|---|---|---|---|---|---|---|---|
| 0.9 | UNK | M | AB | L | Apr-05 | ||
| 0.9 | 112 | M | N | R | Nov-02 | L | Jul-09 |
| 0.9 | 113 | M | N | L | Jun-03 | ||
| 1.1 | 80 | F | N | R | Sep-04 | ||
| 1.1 | 108 | F | N | R | Jan-03 | L | Jun-06 |
| 1.1 | 118 | F | N | L | Jan-02 | ||
| 1.1 | 118 | M | N | R | Nov-01 | ||
| 1.2 | UNK | F | N | L | Sep-01 | ||
| 1.2 | 107 | M | N | L | Mar-01 | ||
| 1.2 | 115 | M | N | B | May-99 | ||
| 1.2 | 118 | M | N | R | Jun-01 | L | Sep-05 |
| 1.2 | 118 | M | N | R | Sep-01 | L | Feb-10 |
| 1.2 | 118 | F | N | R | Feb-07 | ||
| 1.2 | 118 | F | N | B | Aug-05 | ||
| 1.2 | 118 | M | N | R | Feb-07 | ||
| 1.2 | 118 | M | N | R | Dec-02 | ||
| 1.3 | 78 | F | N | R | Oct-06 | ||
| 1.3 | 110 | M | N | R | Jun-03 | ||
| 1.3 | 118 | F | N | R | Sep-00 | L | Jun-07 |
| 1.4 | 118 | M | N | L | Apr-04 | ||
| 1.4 | 118 | M | N | R | May-98 | ||
| 1.5 | 92 | M | N | B | Apr-06 | ||
| 1.5 | 98 | F | N | L | Jun-01 | ||
| 1.5 | 107 | M | N | R | Apr-02 | L | Sep-02 |
| 1.6 | 112 | F | N | R | Dec-98 | L | Oct-06 |
| 1.6 | 115 | M | N | R | Aug-98 | ||
| 1.6 | 118 | F | N | R | Dec-97 | ||
| 1.6 | 118 | F | N | R | Mar-99 | ||
| 1.6 | 118 | F | N | R | Jun-99 | ||
| 1.7 | UNK | F | N | R | Jul-06 | ||
| 1.7 | 107 | M | N | R | Nov-00 | ||
| 1.7 | 113 | F | N | L | Apr-01 | ||
| 1.7 | 115 | M | N | L | Sep-94 | ||
| 1.7 | 118 | F | N | L | Apr-97 | ||
| 1.7 | 118 | M | N | R | Oct-00 | ||
| 1.7 | 118 | M | N | L | Dec-99 | ||
| 1.8 | 88 | F | N | R | Apr-04 | ||
| 1.8 | 90 | M | AB | R | Aug-04 | R | Apr-07 |
| 2 | 107 | F | N | R | Aug-01 | ||
| 2.1 | 113 | F | AB | R | Jun-96 | ||
| 2.1 | 118 | M | N | R | Oct-01 | L | Aug-09 |
| 2.2 | 103 | M | N | L | Sep-00 | ||
| 2.2 | 117 | F | N | L | Apr-93 | ||
| 2.2 | 117 | F | N | R | Jun-99 | L | Apr-07 |
| 2.2 | 118 | F | N | R | Feb-00 | ||
| 2.3 | 78 | M | AB | L | Jul-04 | R | Apr-06 |
| 2.3 | 118 | F | N | R | May-97 | ||
| 2.4 | 97 | M | N | R | Mar-04 | ||
| 2.5 | 98 | M | N | R | Aug-90 | ||
| 2.6 | 82 | F | N | L | Oct-05 | ||
| 2.6 | 92 | M | AB | R | Jun-04 | R | Sep-08 |
| 2.6 | 102 | M | N | R | Jun-98 | ||
| 2.7 | 115 | M | N | R | Jan-07 | ||
| 2.8 | 88 | F | N | L | Oct-03 | ||
| 2.8 | 112 | M | N | L | Nov-92 | ||
| 2.8 | 112 | M | N | R | Dec-92 | ||
| 2.8 | 118 | F | N | R | Apr-93 | ||
| 2.9 | 107 | M | N | L | May-97 | ||
| 3 | 92 | M | N | R | Apr-04 | ||
| 3 | 118 | F | AB | R | May-04 | L | Dec-09 |
| 3.1 | 107 | F | N | R | Apr-01 | ||
| 3.1 | 110 | M | N | L | Feb-94 | ||
| 3.1 | 118 | F | N | L | Jan-92 | ||
| 3.1 | 118 | M | N | R | Apr-02 | ||
| 3.2 | 105 | M | N | L | Jan-00 | ||
| 3.2 | 118 | M | N | R | Dec-06 | ||
| 3.3 | 97 | F | N | L | Mar-99 | ||
| 3.3 | 113 | M | N | R | Sep-99 | ||
| 3.3 | 117 | M | N | R | Sep-92 | ||
| 3.4 | 107 | F | N | L | May-99 | R | Dec-04 |
| 3.4 | 112 | M | N | R | Nov-01 | L | Mar-10 |
| 3.4 | 118 | M | N | L | Jul-94 | ||
| 3.5 | 110 | M | N | R | Sep-93 | ||
| 3.5 | 118 | F | N | L | Mar-90 | ||
| 3.5 | 118 | M | N | L | Oct-93 | ||
| 3.6 | 117 | F | AB | L | Mar-96 | L | Oct-97 |
| 3.6 | 117 | M | N | L | Nov-01 | L | Mar-04 |
| 3.7 | 95 | M | AB | R | Apr-96 | ||
| 3.7 | 118 | F | N | L | May-91 | ||
| 3.8 | 110 | M | N | R | Sep-87 | ||
| 3.8 | 113 | M | N | R | Apr-93 | ||
| 3.8 | 118 | F | N | L | Oct-90 | ||
| 3.9 | 83 | M | N | R | Nov-04 | L | Jan-09 |
Procedures and Test Measures
Speech Perception
Speech perception tests were recorded and presented in sound field in a double-walled sound booth. Speech materials were always presented from 0°-azimuth at 70 dB SPL. Speech perception was measured in quiet using pre-recorded Consonant-Nucleus-Consonant (CNC) monosyllabic words (Tillman & Carhart, 1966) and Phonetically Balanced-Kindergarten (PB-K) (Haskins, 1949) words. Due to the influence of vocabulary on the speech perception word lists, PB-K lists were administered to children as young as 4 or 5 years of age while CNC words were administered to children between the ages of 6–8 years or older. Both the CNC and PB-K scoring was based on percent-correct performance at the word level. One or two 50-word lists of CNC words were presented to each child per visit, depending upon the child’s attention span. All lists were randomized between children and no child received two of the same lists during the same visit. Two of the PB-K 50-word lists were used (list 1 and 2). Each child received one PB-K list per visit.
The CNC and PB-K scores were combined to create longitudinal speech perception scores for each child over time. In cases where examiners administered both the CNC and the PB-K to a particular child during one visit, the scores were averaged to create one score at that visit. If there was only one score available from either the CNC or PB-K tests, that score was included in the analysis. To verify that the scores were measuring the same underlying construct, we computed the concordance correlation coefficient (CCC) of Lin (Lin, 1989). The CCC measures the agreement between two variables and evaluates the degree to which the pairs of observations fall on the 45° line through the origin. That is, we measured not only the correlation between CNC and PB-K, but to what extent they gave the same percent correct value when both were administered at the same visit. For those children that completed both the CNC test and the PB-K test during the same visit, the CCC between the two measures was 83%, suggesting that the two scores were highly reliable measures of the same underlying construct.
Language
The Clinical Evaluation of Language Fundamentals-3rd edition (CELF-3) (Semel et al., 1995) was used to measure global receptive and expressive language skills. There are a number of subtests within the test battery; for the purposes of the current study we examined longitudinal data for one receptive language subtest, Concepts and Directions, and one expressive language subtest, Formulated Sentences, because both could be administered starting at age 6 and continuing through adulthood. In the Concepts and Directions subtest, an examiner presented pictures of black and white geometric shapes and asked the child to follow directions (e.g., “Point to the black circle, then the white square”). Items increased in length and linguistic complexity. In the Formulated Sentences subtest, the examiner presented a target word and a stimulus picture to the child. The child generated a novel sentence that referred to the picture and included the target word. Target words varied in syntactic category, and included nouns, verbs, adjectives, and conjunctions. Both subtests were administered using spoken language for children who relied on oral language for communication and in simultaneous sign and speech for children who utilized total communication or Signing Exact English (SEE). Standard scores were available for data analysis but because such scores control for chronological age, they did not allow for the examination of growth over time. As a result, we used raw scores for statistical analyses involving the CELF-3 subtests.
Reading
One subtest from the Woodcock Reading Mastery Test-Revised (WRMT-R) (Woodcock, 1987) was used to measure reading ability, starting at age 6. The Passage Comprehension subtest evaluates the ability of an individual to understand a short written passage. Each item consists of one or more sentences with one missing keyword. The child read the sentences and identified the missing word. Passages are designed so that it is difficult to identify the target word without reading the entire selection. Raw scores were transformed into W scores, which are transforms of the raw score into an equal-interval scale based on the difficulty of the item. W scores reflect the child’s ability in a domain and range from 338 to 608, with a score of 500 indicating fifth-grade achievement. They were utilized instead of raw or standard scores because they allowed growth at any point in the scale to be measured on the same scale; thus, they were well suited for growth analysis.
Data Analysis
The primary focus of this analysis was to compare children implanted under the age of two years to children implanted between two and four years of age. We made that comparison within a Linear Mixed Model (LMM) framework. The LMM assumes a normal distribution for the dependent variables (speech perception, CELF-3 Formulated Sentences and Concepts and Directions raw scores and WRMT-R Passage Comprehension W scores) and can be used to investigate the relationship of the dependent variable with each of the independent predictor variables. The LMM accounted for the correlation between data points collected over time, including a random intercept and random slope for time. A random intercept represented the individual influence each child had on repeated observations not captured by the other covariates. Akaike Information Criterion (AIC) was used to select the variables used in the model and the final covariance structure for the LMMs. Through the LMM we achieved a model-specified mean score for each group allowing that mean to vary by age at testing. A two-sample t-test was then used to compare the two group means at differing values of age at testing. One benefit of the model-specified mean score is that it permits use of all the available information for data that were collected at irregular intervals. For example, with the speech perception measures, four children were measured only one time and five more only had two measurements. On the other hand, five children had ten measurements and eight had eleven measurements. Half of the children had between three and eight measurements. It was because of these sporadic visit times that the longitudinal model is particularly appropriate. It allows for analysis of means at any testing age, as well as use of the model standard error in the hypothesis tests. Therefore, the present analysis is less impacted by subject attrition rates over time than traditional t-tests between groups. We did not adjust the alpha level for multiple comparisons because that would inflate the Type II error and we did not wish to artificially conclude that the two groups were equal if they were, in fact, not equal. The modeling was performed using Proc Mixed in SAS v9.3.
Age at testing, our primary dependent measure, was modeled as a continuous variable due, in part, to the irregular follow-up time periods across children. Growth over time did not follow a strictly linear pattern. As a result we adopted two approaches. For the language and reading measures, a quadratic effect of time was also included in the model to create the curvature seen in Figures 2, 3, and 4B. However, the speech perception performance showed a much more non-linear function. A sixth or seventh order polynomial may have been able to be fit to the speech perception curve but we felt other statistical approaches may be better suited to answer our research questions. Thus, we used spline regression of time, which is a semi-parametric regression model. Knot points were chosen to be every other year at ages 7, 9, and 11. Furthermore, because dependent measures are correlated over time, a compound symmetry correlation function was used to account for the correlated responses for all three measures. The compound symmetry formulation assumed that the correlations between all time points were equal and was selected over other correlation structures because it yielded the lowest AIC.
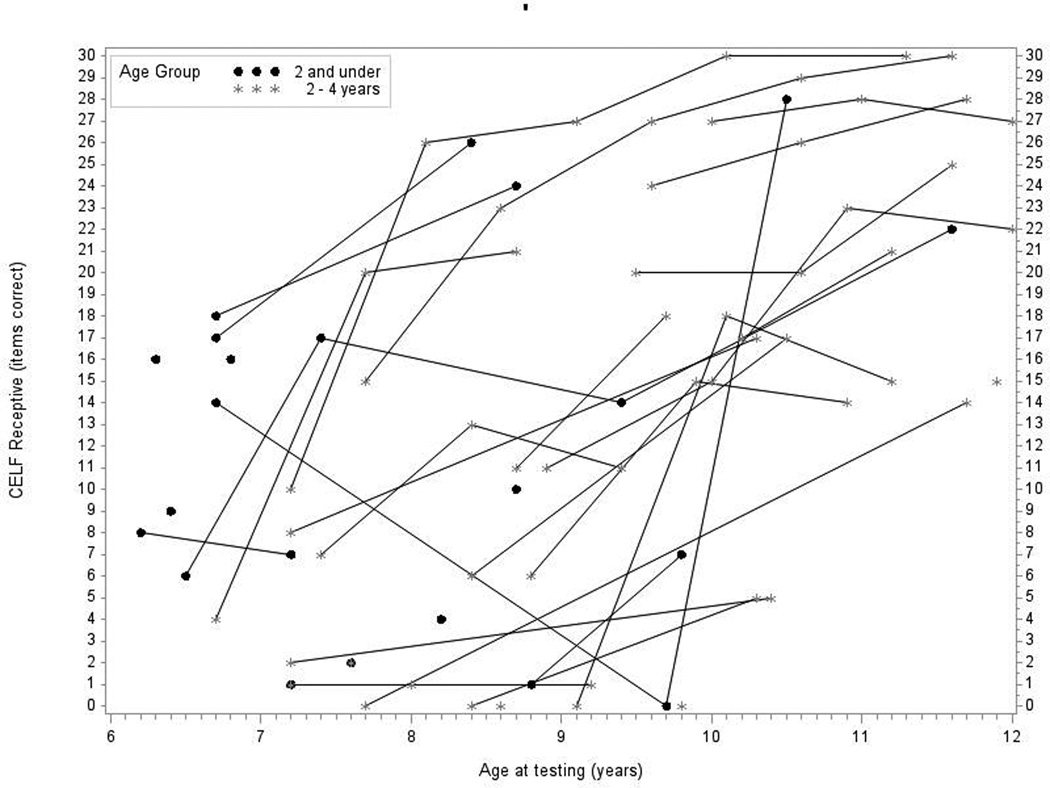
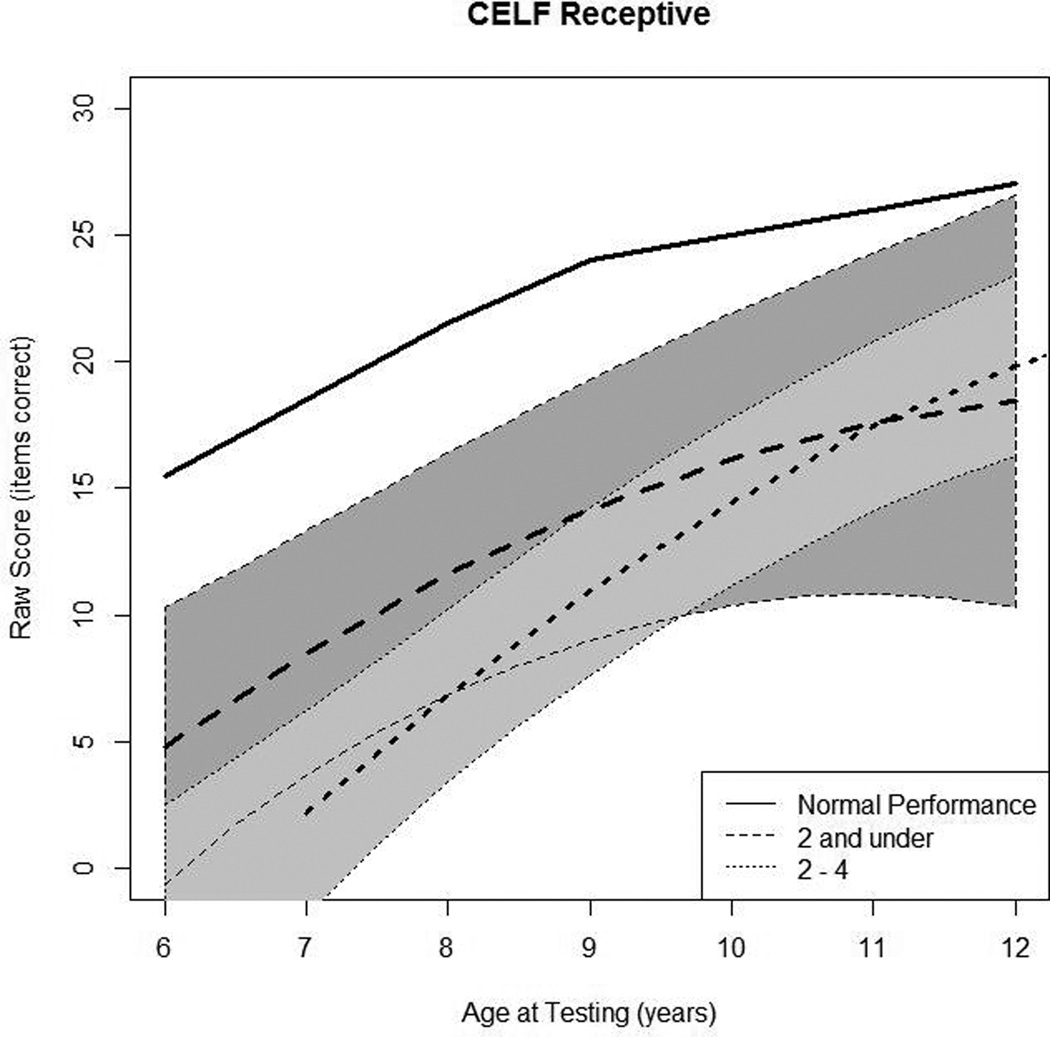
Figure 2.
In Panel 2A, raw values for CELF-3 Concepts and Directions (CELF-3 Receptive) are plotted over time by age at testing for the younger- and older-implanted groups. In Panel 2B, modeled values for receptive language are plotted over time by age at testing for the younger-implanted groups (solid bold line), for the older-implanted group (dashed bold line), and for normal hearing (dashed bold gray line). The other lines (solid and dashed) represent the 95% confidence intervals for the two groups.
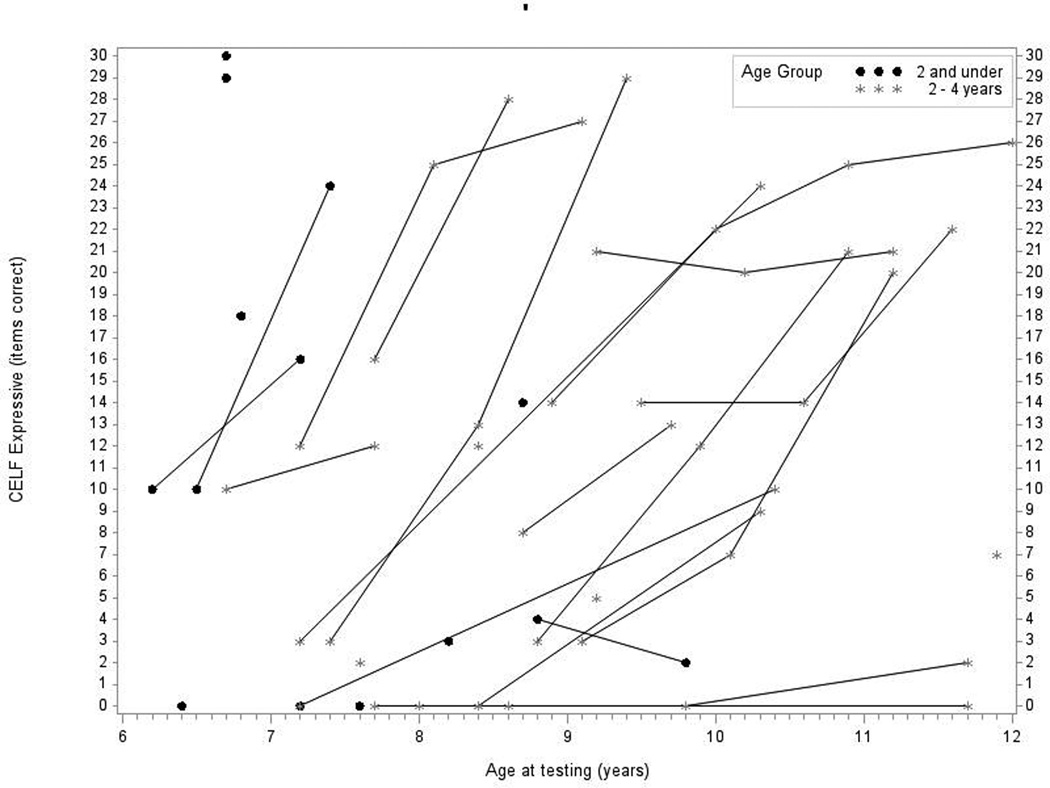
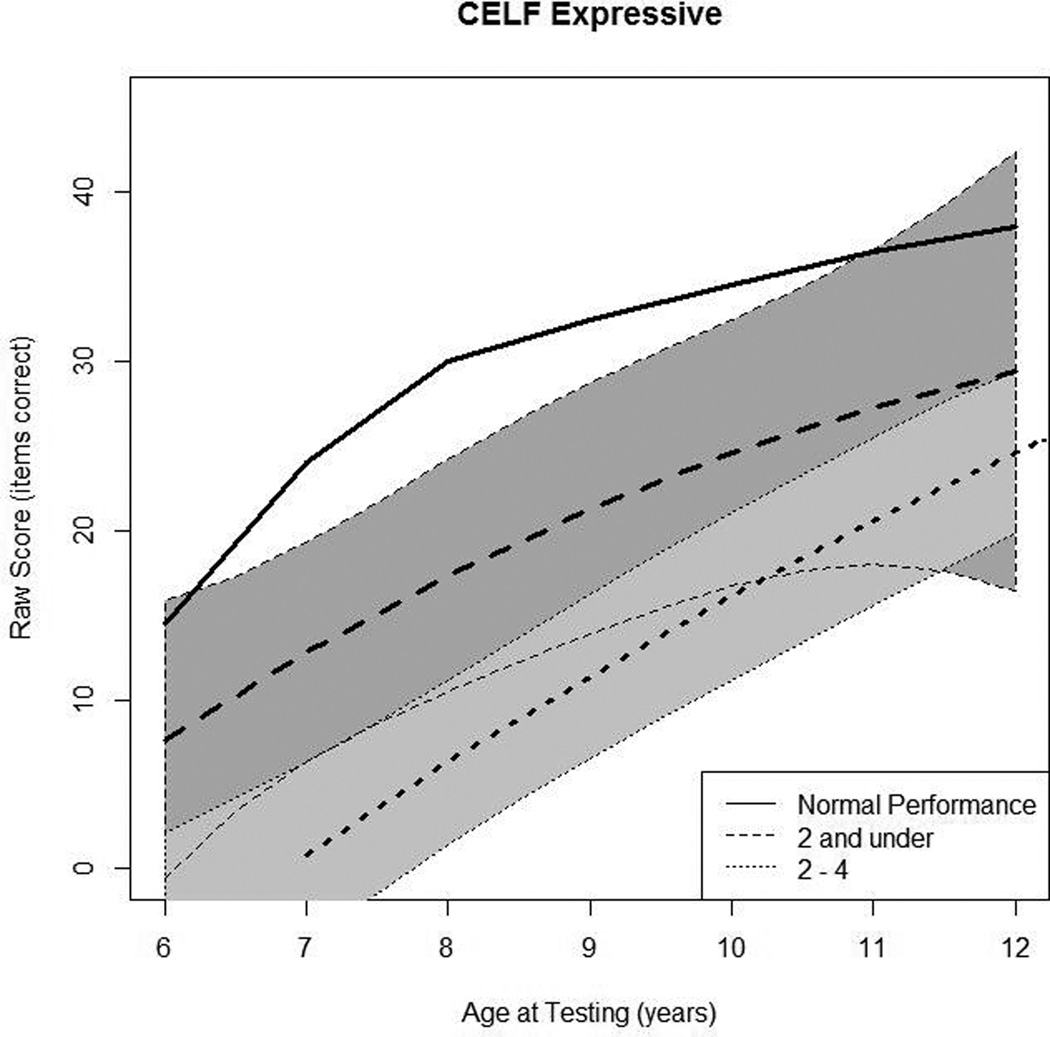
Figure 3.
In Panel 3A, raw values for CELF-3 Formulated Sentences (CELF-3 Expressive) are plotted over time by age at testing for the younger- and older-implanted groups. In Panel 3B, modeled values for expressive language are plotted over time by age at testing for the younger-implanted group (solid bold line), for the older-implanted group (dashed bold line), and for normal hearing (dashed bold gray line). The other lines (solid and dashed) represent the 95% confidence intervals for the two groups.
Figure 4.
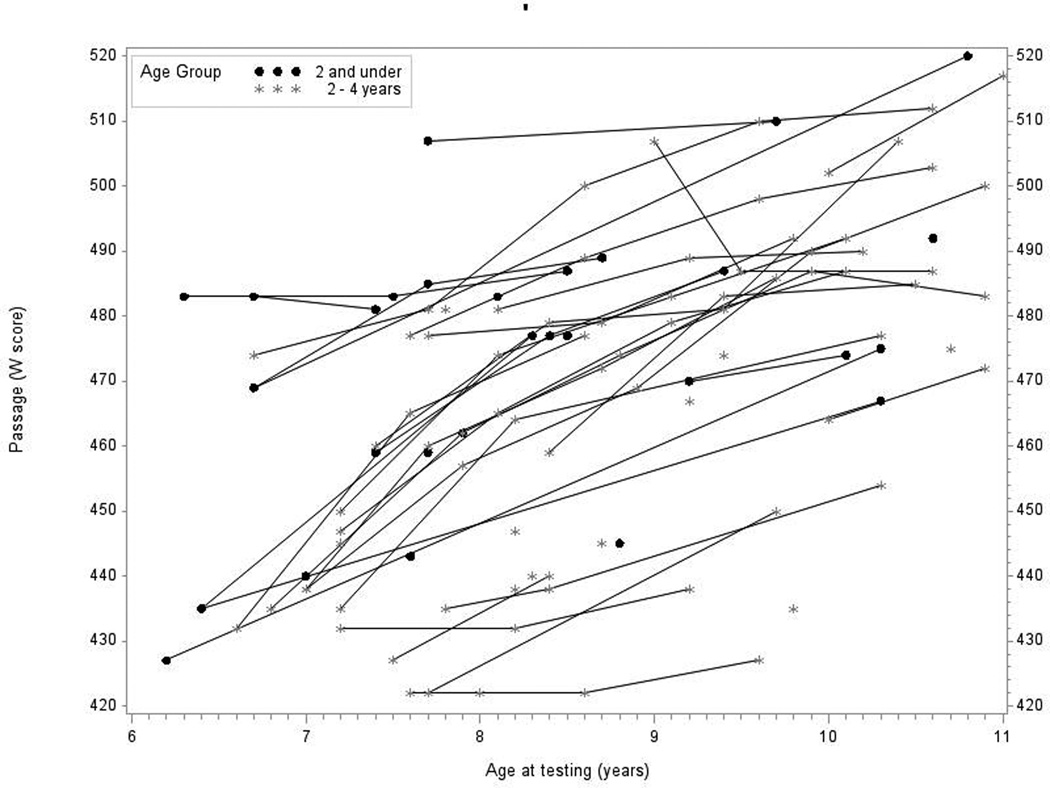
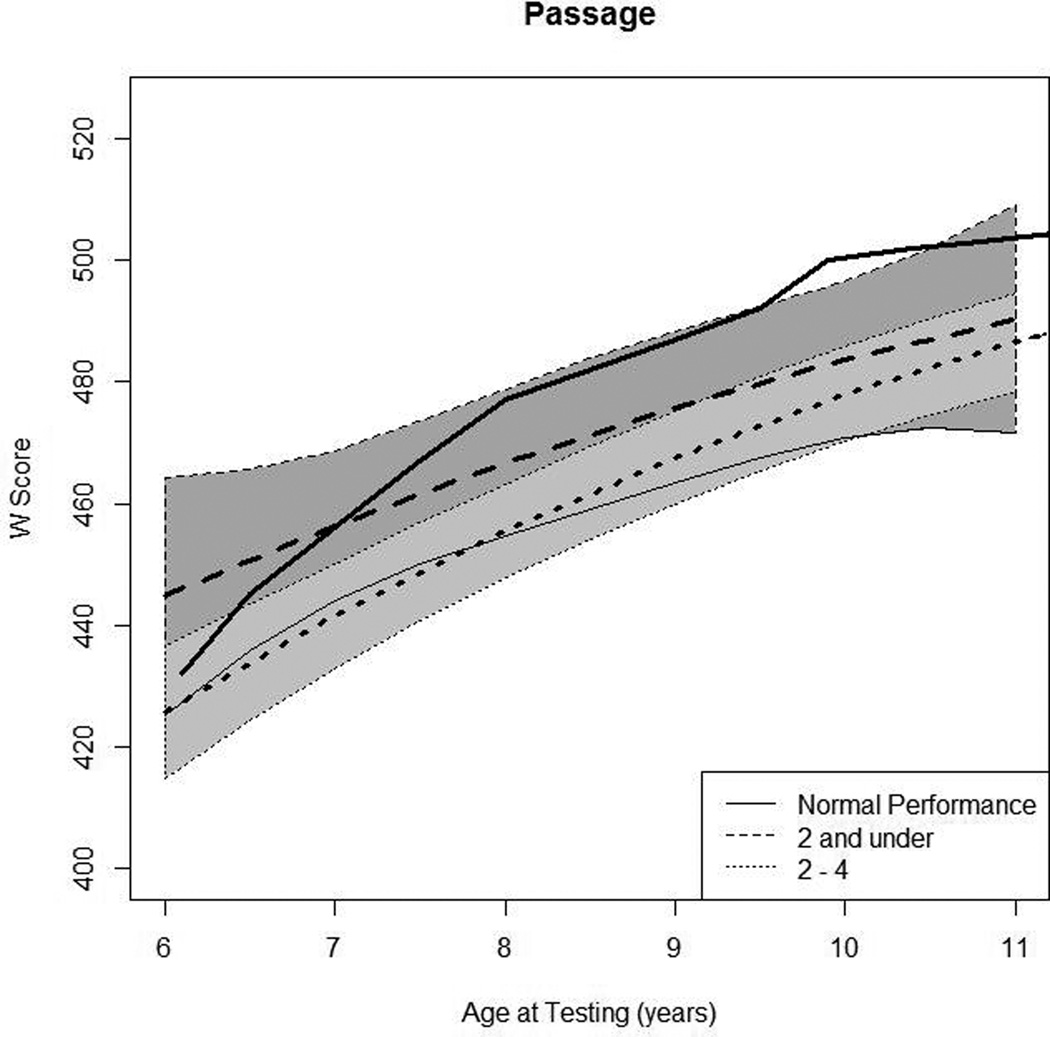
In Panel 4A, raw values for WRMT Passage Comprehension W-scores are plotted over time by age at testing for the younger- and older-implanted groups. In Panel 4B, modeled values for Passage Comprehension are plotted over time by age at testing for the younger-implanted group (solid bold line), for the older-implanted group (dashed bold line), and for normal hearing (dashed bold gray line). The other lines (solid and dashed) represent the 95% confidence intervals for the two groups.
Independent variables included communication mode, unilateral versus bilateral CI use, pre-operative PTA, time (age at testing), age at implantation (age group), and time (either quadratic or spline). All pairwise interaction terms were tested to be included in each model and the interaction between time and age-group was the only one that reduced AIC in all three models. The inclusion of the interaction between time and age-group was important because it allowed comparison of the effect of early versus late implantation on the growth of the dependent variable over time. Thus, as previously alluded to, we could determine if the difference between the groups was changing over time and how quickly it changed after adjusting for important covariates.
Device configuration (unilateral versus bilateral CI use) and pre-operative PTA did not contribute a significant amount of variance to any of the outcome variables and were removed from the final model. Communication mode influenced some, but not all of the outcome variables, as described in the results section. Residuals were analyzed in all models but yielded no evidence of violating model assumptions.
Results
Speech perception
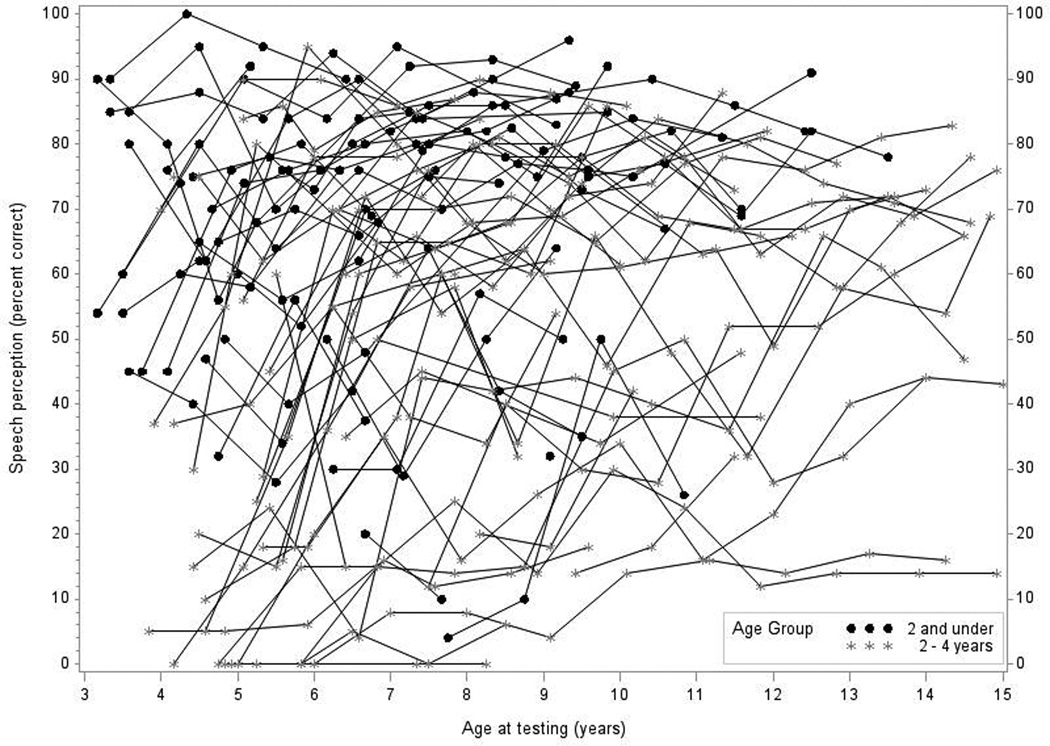
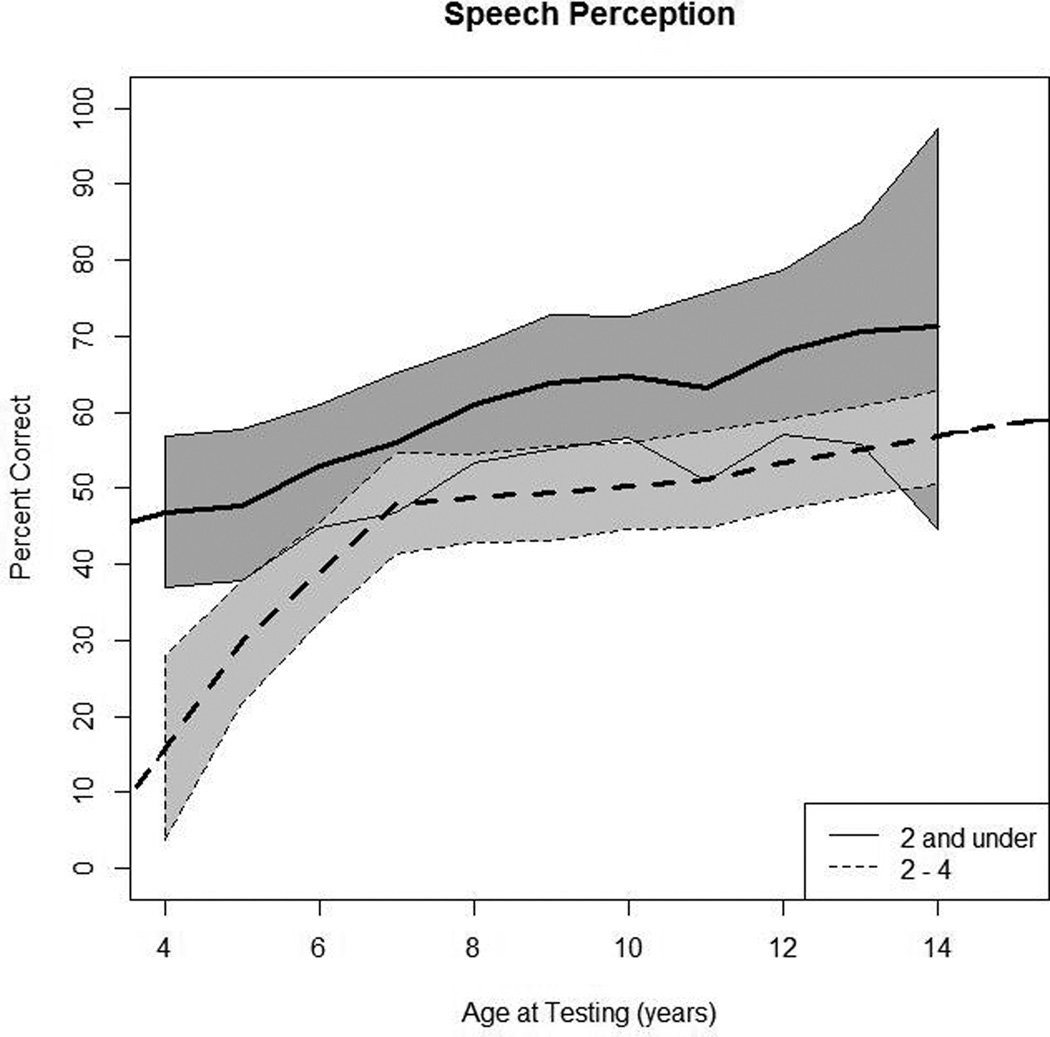
Figure 1A shows all of the individual trajectories with dots representing children implanted at under 2 years of age, and stars representing children implanted between 2 and 3.9 years of age (Figures 2A, 3A, and 4A show similar individual trajectories for language and reading data). Data from 67 children were used in this analysis (28 children in group 1 and 39 children in group 2). Figure 1B shows the mean predicted response curve along with 95% confidence intervals for each group generated by the statistical model. The thick solid curve is the model-predicted mean of the younger-implanted group with the thin solid curves representing the 95% confidence interval. The dotted curves are for the older age group. The curves represent where the average child would start for each group and the trajectory if that child was measured each subsequent year (Figures 2B–4B depict the same visual representations for language and reading data). As expected, speech perception scores in both groups generally increased over time.
Figure 1.
In Panel 1A, raw values for speech perception are plotted over time by age at testing for the younger-implanted group and the older-implanted group. In Panel 1B, modeled values for speech perception are plotted over time by age at testing for the younger-implanted group (solid bold line) and for the older-implanted group (dashed bold line). The other lines (solid and dashed) represent the 95% confidence intervals for the two groups.
Our primary research question focused on the difference in performance between the younger-implanted and the older-implanted group at specific testing ages. The younger-implanted group had higher speech perception scores at 5 years of age, compared to the older-implanted group (p < 0.001). From Figure 1B we see the gap between the two groups narrows at age 7 (p = 0.16). After age 7, there was much slower growth for both groups and the two appeared to have fairly similar trends in performance. Significance varied between 8 and 13 years of age with scores significantly different between the two groups at 8, 9, 10, and 12 years (p < 0.05). There was no significant difference at ages 11 and 13 years. Finally, communication mode was the only other factor in the model that had a significant effect on speech perception scores (p < 0.0001), with children using OC obtaining scores that were 33.8% higher than children using TC, on average.
Language
Figure 2A shows the raw longitudinal data for receptive language skills, as measured by the CELF-3 Concepts and Directions subtest. Data from 38 children were used in this analysis (13 children in group 1 and 25 children in group 2). In general, language scores increased for nearly all of the individuals in the study. One individual stood out as unique in terms of trajectory (scoring a 14 at age 7 years, 0 at age 9 years, and 28 at 10 years of age). That person was removed from the group analysis. Figure 2B shows the mean predicted response curve for CELF-3 Concepts and Directions along with 95% confidence intervals for each group generated by the statistical model and a gray dashed line representing average raw scores for children with NH based on CELF-3 normative data at each age interval as a reference. Regarding the primary research question – the difference between older- and younger-implanted groups – at age 7, the younger-implanted group achieved receptive language scores that were 7 points higher than the older-implanted group, on average, which was a significant difference (p = 0.04). By age 8, there was no statistical difference between the two groups (p = 0.09), a trend that continued across all older ages (see Table 3). Finally, communication mode did not have a significant impact on receptive language (p = 0.26).
Figure 3A shows the raw scores for expressive language skills, as measured by the CELF-3 Formulated Sentences subtest. Data from 39 children were used in this analysis (13 children in group 1 and 26 children in group 2). Overall, there was a general increase in scores over time for most of the children. Several children did not show increases over time, but were left in the analysis. Figure 3B shows the mean predicted response curve for CELF-3 Formulated Sentences along with 95% confidence intervals for each group generated by the statistical model and a gray dashed line representing average raw scores for children with NH at each age interval as a reference. The comparison between groups indicated that at age 7, the younger-implanted group achieved scores that were 12 points higher than the older implanted group, on average. This difference was significant (p = 0.01). Both groups showed trends of scores increasing over time, with the means narrowing and confidence intervals widening. By age 10, there was no significant difference between the two groups (p = 0.07), a trend that continued at age 11 as well (p = 0.20). Communication mode approached significance for expressive language scores (p = 0.06), with higher scores for OC users than TC users.
Reading
Figure 4A shows the raw scores for WRMT-R Passage Comprehension test. Data from 49 children were used in this analysis (16 children in group 1 and 33 children in group 2). Overall, there was a general increasing trend by almost all of the children regardless of baseline value. In Figure 4B we see the mean predicted response curve for WRMT-R Passage Comprehension along with 95% confidence intervals for each group generated by the statistical model and a gray dashed line representing average W scores for children with NH at each age interval as a reference. It is important to note that in Figure 4B, it appears that the younger-implanted group demonstrated better scores on the reading measure than the average W scores for NH children. This is a consequence of the minimal scoring demands for younger ages on the WRMT-R Passage Comprehension measure. Children only need to get a few items correct to achieve a score in the average to above-average range at ages 6 or 7, resulting in a compression of scores in this age range. This is true only at that younger age of testing. The comparison between groups indicated that at age 7, the younger-implanted group achieved scores that were almost 15 points higher than the older-implanted group, on average. However, this difference at the baseline interval just missed significance (p = 0.054). Both groups demonstrated similar growth rates across the tested age ranges for WRMT-R Passage Comprehension scores. Thus, there was no statistically significant difference between the two groups at any of the test intervals. Communication mode did not have a significant impact on reading scores (p = 0.13).
Discussion
The University of Iowa has a large population of children with CIs who have been engaged in clinical research and were followed incrementally over time for up to 23 years. Because the acceptable age at implantation has expanded to implanting younger children in recent years, we evaluated the long-term effects of age at implantation while controlling for confounds including communication mode, pre-implant residual hearing, and unilateral versus bilateral CI use.
Age at Implantation
Our results demonstrated that age at implantation initially influenced speech perception in children with CIs. When both groups were tested at 5 and 6 years of age, the younger-implanted group performed significantly better than the older-implanted group. Many previous studies showed similar results, with children implanted at younger ages displaying better speech perception skills than children implanted at older ages, particularly in the immediate years following implantation (Geers, Tobey, Moog, & Brenner, 2008; Kaplan & Puterman, 2010; Manrique, Cervera-Paz, Huarte, & Molina, 2004; McConkey Robbins et al., 2004; Zwolan et al., 2004). The significant difference early in development is at least partially explained by duration of CI experience; the children implanted under 2 had more experience (around 3–4 years) with their CI before initial testing at 5 years of age, while children in the other group had less experience with their CI at the initial testing intervals.
Our results also showed that the impact of age at implantation on speech perception gradually weakened over time with the older group narrowing the gap quickly once they gained experience with their device. At age 7, statistical analysis indicated no significant difference between the two groups on speech perception scores. However, between ages 8 and 13 years, the growth in performance for both groups slowed down and statistical analyses indicated the two groups were not significantly different at 11 and 13 years of age. Thus, at 8, 9, 10, and 12 years of age, there was a significant difference in scores between the two groups. It is difficult to determine if these results are due to the fact that the younger-implanted group was starting to reach a ceiling level in performance. Perhaps if a more demanding speech perception measure (e.g., listening in noise or with multiple talkers) had been available for analysis, we would have seen a stronger effect of age at implantation on long-term outcomes. Additional research is needed to determine the effects of age at implantation on speech perception in complex listening situations.
Age at implantation also initially influenced language scores in children with CIs. These results are consistent with the speech perception findings and are due in part to an advantage of longer duration of CI experience for children implanted under 2 years. Both expressive and receptive language scores showed a consistent pattern in which there was no significant difference between the earlier- and later-implanted groups at later ages. Specifically, statistical analyses indicated that the older- and younger-implanted groups no longer had significantly different scores by age 8 for receptive language and age 10 for expressive language. These results may suggest that some children, who are implanted after age 2 years, but before age 4, are able to show similar growth trends as their younger-implanted peers, once they have attained several years of CI experience. We express caution, however, in interpreting these findings as a clear indication that the older-implanted group “caught up” to the younger-implanted group, given the widening variance at older ages for both groups.
In many regards, the lack of strong effects of age-at-implantation for language skills is quite surprising. Previous studies are fairly consistent with regards to the relationship between age at implantation and expressive language (Holt & Svirsky, 2008) (Holt & Svirsky, 2008; Nicholas & Geers, 2007; Niparko et al., 2010; Tomblin, Barker, Spencer, Zhang, & Gantz, 2005) with clear benefits of early implantation being seen across studies. The majority of previous studies also suggest that younger age at implantation is related to positive outcomes in receptive language performance (Holt & Svirsky, 2008; Niparko, Tobey, Thal, Eisenberg, Wang, Quittner, & Fink, 2010). It is important to note, however, that in these previous studies, children were not tested past age 8 and for the most part, the children only had 1 to 3 years of CI experience. In the present study, children in the younger-implanted group performed significantly better on both receptive and expressive language tests at age 7, which is consistent with previous findings. As chronological age increased, however, the differences in language scores between groups decreased, resulting in no significant difference by age 8 for receptive language and age 10 for expressive language. Geers, Nicholas, and Sedey (2003) also found no effect of age at implantation in a group of children who were implanted between 2 and 5 years of age, and tested at 8 and 9 years of age. On the other hand, Geers and Sedey (2011) found that duration of deafness prior to implantation (a proxy measure for age at implantation) accounted for a significant proportion of variance in receptive and expressive language when the children were re-tested at 15 to 18 years of age. Geers and Nicholas (2012) reported similar findings in 10-year-olds with CIs. It should be acknowledged that both studies utilized different statistical techniques in their research (i.e., hierarchical multiple linear regression) compared to the present study (i.e., linear mixed modeling). We expect that if we had employed regression analysis for the whole group, we would have reached similar findings as Geers and Sedey (2011) and Geers and Nicholas (2012) in that age at implantation would have accounted for a significant proportion of the variance in language skills. Nevertheless, the current results offer an expanded view of long-term language outcomes for children implanted after age 2. Some of these later-implanted children are capable of performing within the same range of earlier-implanted children. Although the two groups demonstrated no significant differences in maternal education level, preschool-age nonverbal cognition, pre-operative residual hearing, and quantity of pre- and post-operative service provision, it is likely that other variables are supporting language development in these children. Possibilities include quality of parental linguistic input and service provision, school-age nonverbal cognitive skills, and frequency of daily CI use. A goal of future prospective research should be to further investigate alternative factors that ameliorate the negative effects of later implantation, such as early intervention, home environment, or consistency of device use (Szagun & Stumper, 2012).
The present data also revealed that age at implantation did not have a strong impact on reading skills in the early elementary years. Children implanted before age 2 years showed an initial, but non-significant, advantage over children implanted between 2 and 3.9 years of age. These initial reading results at age 6 should be interpreted with caution, because of the likelihood of compressed variance and floor effects. Reading performance indicated no significant difference between groups over time, and effect sizes decreased with increases in chronological age. These findings are inconsistent with previous research on reading outcomes and age at implantation. James et al. (2008) and Johnson and Goswami (2010) reported that age at implantation had a significant effect on reading scores, but it should be noted that the cut-off for younger- vs. older-implanted groups was around 4 to 5 years of age. If the present study had included a group of children implanted after age 4, we predict that there may have been stronger effects for age at implantation in reading. Another study by Geers and Hayes (2011) investigated children with 10 or more years of CI experience. They found that duration of deafness accounted for a significant proportion of the variance in high-school reading performance. Again, we acknowledge that different statistical methods were utilized in the current study, and it is possible that we would have found that age at implantation would account for variance in the whole group. At the same time, the present dataset demonstrated small effect sizes for reading over time (Table 3), indicating that the contribution of age at implantation was minimal, especially as children grew older. These conflicting findings lead us to concur with the position elucidated by Marschark, Rhoten, and Fabich (2007): more longitudinal research on reading and academic skills for children with CIs is needed, in order to effectively demonstrate that age at implantation has a lasting impact on reading outcomes.
When viewed altogether, what might explain the unexpected findings of the current study? An important motivation for earlier implantation derives from literature on the auditory development of hearing infants, showing that speech perception develops quite early and is largely tuned to the native language by 12 months of age (Eimas et al., 1971; Kuhl, 1979; Werker & Tees, 1984). This implies that infants need auditory input early, during this critical plastic period. However, more recent work suggests considerable plasticity after infancy (Davis et al., 2005; Kraljic & Samuel, 2005; Lively et al., 1993; Norris et al., 2003). For example, Sharma et al. (2002) used P1 cortical auditory evoked potentials in children with CIs to demonstrate that maximum plasticity for central auditory development exists up to 3.5 years of age, and some plasticity still exists up to age 7. Most of the children in the present study were implanted before 3.5 years and were within this period of maximum plasticity when they received CIs. In addition, a number of studies are now identifying critical changes in infant speech perception that occur during the second year of life as children learn how to map acoustic/phonetic cues to words (Dietrich et al., 2007; Rost & McMurray, 2009, 2010). There is also evidence for continued perceptual organization of speech sounds well into the early school years and beyond (Slawinski, 1998). When considered in combination with an emerging view that perceptual organization for speech is highly plastic and slow to develop, the present language and reading results may offer some support to the notion that it is acceptable to give parents more time to accept the diagnosis of hearing loss and feel comfortable with their decisions regarding implantation (Geers & Nicholas, 2012).
Communication Mode
Another contributing factor in performance on various outcome measures is communication mode. Our demographic information showed that 69% and 50% of the children implanted under the age of 2 years and between 2 and 3.9 years, respectively, reported using oral/aural communication. Consistent with Geers, Brenner, and Davidson (2003), we found that communication mode was highly predictive of speech perception outcomes. Specifically, children whose educational programs emphasized auditory-oral communication (OC) were better at perceiving speech than children who used total communication (TC).
At the same time, the effects of communication mode on measures of higher-level language ability were mixed. Expressive and receptive language results diverged with respect to the influence of communication mode. The results for expressive language indicated that communication mode influenced performance (although statistical results did not quite reach significance), with OC users demonstrating better expressive language scores than TC users. Kirk et al. (2002) also demonstrated an advantage of OC over TC on expressive language outcomes. On the other hand, the receptive language and reading data indicated no significant difference in communication mode (OC vs TC). This finding is also consistent with previous literature for receptive language (Holt & Svirsky, 2008; Kirk, Hay-McCutcheon, Sehgal, & Miyamoto, 2000; Kirk, Miyamoto, Lento, Ying, O'Neill, & Fears, 2002) as well as reading (Connor & Zwolan, 2004). With regards to language comprehension performance, the limited influence of communication mode on receptive language is likely due to the receptive measure being administered in the child’s preferred communication mode. Children who use TC may benefit from the iconicity of signs, which could influence performance on the CELF Concepts and Directions subtest. The TC group would not experience that same visual advantage on speech perception or expressive language measures.
Limitations and Future Directions
There are a number of limitations in the present work, most of which are related to the difficulties inherent in conducting a retrospective analyses over a 20-year period, particularly in a period that has undergone so many dramatic changes in the field of pediatric audiology. Although there was no significant difference in pre-operative hearing thresholds between the two groups, the possibility exists that some children had progressive or acquired hearing loss in the first year of life, and thus had an early advantage over children with more severe hearing loss. Other studies demonstrated the importance of pre-operative residual hearing on developmental outcomes (Geers & Nicholas, 2012; Nicholas & Geers, 2006). Therefore, one limitation is that we were unable to obtain the first diagnostic ABR or audiogram for most of the children because these were obtained at centers other than the University of Iowa. Furthermore, many children were identified prior to the advent of newborn hearing screening. These two factors make it difficult to establish the severity of hearing loss during infancy. Children with acquired hearing loss later than 12 months of age were excluded from the present analysis, in an attempt to control for this confound, but the possibility exists that there could have been a significant difference in pre-operative thresholds at birth or shortly thereafter.
Another limitation of conducting a retrospective analysis is dropout rate of participants and incomplete data sets over time. Specifically, as with most longitudinal studies, the number of subjects available for analysis decreases as the number of years increases. The figures in this manuscript showing the growth curves demonstrate this as the confidence intervals become wider at the latter ages in the study. This represents a lack of confidence in the true growth at those ages. This has been accounted for in the statistical model which assumes data are missing at random which is a valid assumption for this dataset. The missingness does not depend on how they would have scored. This means that those scoring poorly have the same likelihood of having missing data as those who score well. Thus, the results of the statistical analysis hold if the missing at random assumption is met. We can hypothesize that the current trend will continue but we will continue to follow the individuals for future followups in our continued studies.
Furthermore, in retrospective studies with children, it is often challenging to find outcome measures that are consistent over a number of years. For example, global language measures, such as the CELF-3, begin at age 6. Although language assessment measures were available for some participants at younger ages, we could not conduct a growth curve analysis using these data because they were not from the CELF-3. Similar limitations existed for speech perception scores; reliable responses on open-set speech perception measures were not available for many of the children until they were 6 years old. We acknowledge this limitation of the study, and direct readers to previous literature regarding growth trajectories of children in the initial years of implant use (Tomblin, Barker, Spencer, Zhang, & Gantz, 2005).
There are additional factors besides age at implantation that likely predict long-term speech perception, language, and reading outcomes, which we were unable to account for here. One variable that has received little to no attention in the literature is the amount of time children wear the CI on a daily basis. Research on children who are hard of hearing and wear hearing aids indicates wide variation in daily use time, particularly for younger children (Walker et al., 2012). Furthermore, increased consistency of daily hearing aid use strengthens the impact of aided audibility on communication outcomes of preschoolers with moderate-to-severe hearing loss (Tomblin et al., in revision). There is little reason to expect different outcomes in children with CIs, although there has been virtually no research in this area. Future CI technology should include data logging capacities, similar to what is currently available in most hearing aids. This would allow audiologists to determine the consistency of CI use in pediatric patients, without having to rely on parent report as a proxy measure. It would also allow researchers to conduct prospective analyses that include daily use as an independent predictor variable for outcomes.
Another important predictor variable of language and reading outcomes, in particular, is the richness of the linguistic input to the child. Previous literature on children with normal hearing clearly demonstrates the importance of early child-directed speech on language proficiency at later ages (Fernald et al., in press; Hart & Risley, 1995) . A recent study by Szagun and Stumper (2012) indicated that the quality of maternal language input had a stronger influence on language outcomes than age at implantation. Unfortunately, the current retrospective analysis was unable capture the quality and quantity of early linguistic input in parent-child interactions following CI stimulation. Recent advances in technology, such as the Language ENvironment Analysis system (LENA) allows for automated, full-day recordings of children’s auditory environments (Gilkerson & Richards, 2008). This device could be a powerful tool for examining the natural language use of families of children with CIs. It could also be used prospectively to determine how much linguistic input in the home accounts for variance in later communication outcomes, relative to the variables of interest in the present report, namely age at implantation.
We did not assess differences between groups in pragmatics, psychosocial, academics, and executive function abilities. This is an important point to make in light of the present results and conclusions. Although there were no long-term significant differences for age at implantation in language and reading performance, there was a significant or near-significant difference in the intercept for all test measures, indicating an early advantage for children who received their CIs at younger ages. This early advantage could have a cascading influence on early and later psychosocial competence, as well as academics and executive functions, but would likely be mediated by the influence of language skills. There has been very little research examining psychosocial adjustment in relation to long-term CI use. Moog, Geers, Gustus, and Brenner (2011) used a social skills questionnaire to examine psychosocial development in high schoolers with CIs, but did not consider the relationship between age at implantation and psychosocial skills. Spencer, Tomblin, and Gantz (2012) reported on education and vocational outcomes of CI users who were 16 years or older, but also did not consider these outcomes in relation to age at implantation. Executive function abilities and CIs have drawn recent interest in the literature (Hauser et al., 2008; Pisoni et al., 2008), but have not been examined in children with long-term CI experience to test age at implantation effects. An important future direction in research would be to investigate age at implantation effects on pragmatic/psychosocial abilities, academic achievement, and executive functions in children with long-term CI use, including communication skills as a mediator in the analysis.
Conclusion
In translating these findings to clinical practice, we contend that earlier implantation is better, but there may be diminishing returns over time. Specifically, for speech perception, between ages 7 and 13 years, the effect of age at implantation varied with no significant difference in performance between groups at ages 7, 11 and 13 years. Furthermore, our findings indicated that there was no significant difference in long-term language and reading performance between the groups of children implanted under the age of 2 years and between 2 to 3.9 years of age. Thus, children who receive CIs between age 2 and 4 years have the capacity to approximate the speech perception, language and reading skills of their earlier-implanted peers. We note, however, that effect sizes varied across test measures and ages and variance increased between the two groups with increasing chronological age. Future studies should address what other malleable factors protect children from the negative effects of later implantation, which will allow researchers and service providers to identify which children may succeed or struggle in terms of functional outcomes in high school and beyond.
As clinical trends in pediatric cochlear implantation seem to be advocating for earlier implantation (prior to 12 months of age), future studies should evaluate the risk versus benefits of implantation prior to 12 months of age. Before proceeding with implantation, we advocate for proper behavioral testing to take place that will provide the child’s family and care providers with a reliable measurement of the child’s residual hearing. The benefits of preserving residual acoustic hearing with newer hearing preservation cochlear implant electrodes might outweigh the need to proceed with implantation prior to obtaining reliable audiometric measures, although future studies will need to confirm this hypothesis. Until the risk versus benefit of early implantation is properly assessed, perhaps knowing that the effects of age at implantation diminish over time on some outcome measures, parents and professionals might feel less urgency to implant children prior assessment of residual hearing.
Table 2.
Static demographic information. Data is shown in percent. Maternal education level is reported at time of implantation; Communication mode is reported at most recent appointment.
| Group 1 | Group 2 | ||
|---|---|---|---|
| Maternal education level | |||
| Kindergarten-8th Grade | 3 | 0 | |
| 9–12th Grade | 3 | 7 | |
| High School Diploma | 14 | 15 | |
| Some College Coursework | 25 | 37 | |
| College Degree | 47 | 34 | |
| Post-graduate Degree | 8 | 7 | |
| Communication mode | |||
| Total (TC) | 29 | 48 | |
| Auditory Oral (AO) | 69 | 50 | |
| Manual (MA) | 3 | 2 | |
| Ethnicity | |||
| Asian | 3 | 0 | |
| Black | 8 | 4 | |
| Hispanic/Latino | 3 | 2 | |
| White | 84 | 93 | |
| other | 3 | 0 | |
Acknowledgement
This research was supported in part by research grant 2P50DC000242-26A1 from the National Institutes on Deafness and Other Communication Disorders, National Institutes of Health; grant RR00059 from the General Clinical Research Centers Program, Division of Research Resources, National Institutes of Health; the Lions Clubs International Foundation; and the Iowa Lions Foundation. We would like to acknowledge Brittan Barker, Ph.D, for her edits and additions to this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Archbold S, Harris M, O'Donoghue G, et al. Reading abilities after cochlear implantation: The effect of age at implantation on outcomes at 5 and 7 years after implantation. International Journal of Pediatric Otorhinolaryngology. 2008;72(10):1471–1478. doi: 10.1016/j.ijporl.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Ching TY, Dillon H, Day J, et al. Early language outcomes of children with cochlear implants: Interim findings of the nal study on longitudinal outcomes of children with hearing impairment. Cochlear Implants International. 2009;10(Suppl 1):28–32. doi: 10.1002/cii.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor CM, Craig HK, Raudenbush SW, et al. The age at which young deaf children receive cochlear implants and their vocabulary and speech-production growth: Is there an added value for early implantation? Ear and Hearing. 2006;27(6):628–644. doi: 10.1097/01.aud.0000240640.59205.42. [DOI] [PubMed] [Google Scholar]
- Connor CM, Zwolan TA. Examining multiple sources of influence on the reading comprehension skills of children who use cochlear implants. J Speech Lang Hear Res. 2004;47(3):509–526. doi: 10.1044/1092-4388(2004/040). [DOI] [PubMed] [Google Scholar]
- Cowan RS, DelDot J, Barker EJ, et al. Speech perception results for children with implants with different levels of preoperative residual hearing. The American Journal of Otology. 1997;18(6 Suppl):S125–S126. [PubMed] [Google Scholar]
- Davidson LS, Geers AE, Blamey PJ, et al. Factors contributing to speech perception scores in long-term pediatric cochlear implant users. Ear and Hearing. 2011;32(1 Suppl):19S–26S. doi: 10.1097/AUD.0b013e3181ffdb8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis Matthew H, Johnsrude Ingrid S, Hervais-Adelman Alexis, et al. Lexical information drives perceptual learning of distorted speech: Evidence from the comprehension of noise-vocoded sentences. Journal of Experimental Psychology: General. 2005;134(2):222–241. doi: 10.1037/0096-3445.134.2.222. [DOI] [PubMed] [Google Scholar]
- Dietrich Christiane, Swingley Daniel, Werker Janet F. Native language governs interpretation of salient speech sound differences at 18 months. Proceedings of the National Academy of Sciences. 2007;104(41):16027–16031. doi: 10.1073/pnas.0705270104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell RC, Dettman SJ, Blamey PJ, et al. Speech perception in children using cochlear implants: Prediction of long-term outcomes. Cochlear Implants International. 2002;3(1):1–18. doi: 10.1179/cim.2002.3.1.1. [DOI] [PubMed] [Google Scholar]
- Duchesne L, Sutton A, Bergeron F. Language achievement in children who received cochlear implants between 1 and 2 years of age: Group trends and individual patterns. Journal of Deaf Studies and Deaf Education. 2009;14(4):465–485. doi: 10.1093/deafed/enp010. [DOI] [PubMed] [Google Scholar]
- Eimas Peter D, Siqueland Einar R, Jusczyk Peter, et al. Speech perception in infants. Science. 1971;(171):303–306. doi: 10.1126/science.171.3968.303. [DOI] [PubMed] [Google Scholar]
- Fernald A, Marchman VA, Weisleder A. Ses differences in language processing skill and vocabulary are evident at 18 months. Developmental Science. doi: 10.1111/desc.12019. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryauf-Bertschy H, Tyler RS, Kelsay DM, et al. Cochlear implant use by prelingually deafened children: The influences of age at implant and length of device use. Journal of Speech, Language, and Hearing Research. 1997;40(1):183–199. doi: 10.1044/jslhr.4001.183. [DOI] [PubMed] [Google Scholar]
- Geers A, Brenner C, Davidson L. Factors associated with development of speech perception skills in children implanted by age five. Ear and Hearing. 2003;24(1 Suppl):24S–35S. doi: 10.1097/01.AUD.0000051687.99218.0F. [DOI] [PubMed] [Google Scholar]
- Geers A, Hayes H. Reading, writing, and phonological processing skills of adolescents with 10 or more years of cochlear implant experience. Ear and Hearing. 2011;32(1):49S–59S. doi: 10.1097/AUD.0b013e3181fa41fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geers A, Nicholas J. Enduring advantages of early cochlear implantation for spoken language development. J Speech Lang Hear Res. 2012 doi: 10.1044/1092-4388(2012/11-0347). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geers A, Nicholas JG, Sedey AL. Language skills of children with early cochlear implantation. Ear and Hearing. 2003;24(1 Suppl):46S–58S. doi: 10.1097/01.AUD.0000051689.57380.1B. [DOI] [PubMed] [Google Scholar]
- Geers A, Sedey A. Language and verbal reasoning skills in adolescents with 10 or more years of cochlear implant experience. Ear and Hearing. 2011;32(1):39S–48S. doi: 10.1097/AUD.0b013e3181fa41dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geers A, Tobey E, Moog J, et al. Long-term outcomes of cochlear implantation in the preschool years: From elementary grades to high school. International Journal of Audiology. 2008;47(Suppl 2):S21–S30. doi: 10.1080/14992020802339167. [DOI] [PubMed] [Google Scholar]
- Gilkerson J, Richards A. The lena foundation natural language study (lena foundation technical report ltr-02-2) 2008 Retrieved from LENA Foundation: http://www.lenafoundation.org/TechReport.aspx/Natural_Language_Study/LTR-02-2.
- Hart B, Risley T. Meaningful differences in everyday parenting and intellectual development in young american children. Baltimore: Brookes; 1995. [Google Scholar]
- Haskins HA. Master's thesis. 1949. A phonetically balanced test of speech discrimination for children. [Google Scholar]
- Hauser PC, Lukomski J, Hillman T. Development of deaf and hard-of-hearing students' executive function. Deaf cognition: Foundations and outcomes. 2008:286–308. [Google Scholar]
- Holt RF, Svirsky MA. An exploratory look at pediatric cochlear implantation: Is earliest always best? Ear and Hearing. 2008;29(4):492–511. doi: 10.1097/AUD.0b013e31816c409f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton H, Thwing E. Minnesota child development inventory. Minneapolis, MN: University of Minnesota; 1974. [Google Scholar]
- James D, Rajput K, Brinton J, et al. Phonological awareness, vocabulary, and word reading in children who use cochlear implants: Does age of implantation explain individual variability in performance outcomes and growth? Journal of Deaf Studies and Deaf Education. 2008;13(1):117–137. doi: 10.1093/deafed/enm042. [DOI] [PubMed] [Google Scholar]
- Johnson C, Goswami U. Phonological awareness, vocabulary, and reading in deaf children with cochlear implants. Journal of speech, language, and hearing research : JSLHR. 2010;53(2):237–261. doi: 10.1044/1092-4388(2009/08-0139). [DOI] [PubMed] [Google Scholar]
- Kaplan DM, Puterman M. Pediatric cochlear implants in prelingual deafness: Medium and long-term outcomes. Isr Med Assoc J. 2010;12(2):107–109. [PubMed] [Google Scholar]
- Kirk KI, Hay-McCutcheon M, Sehgal ST, et al. Speech perception in children with cochlear implants: Effects of lexical difficulty, talker variability, and word length. The Annals of Otology, Rhinology & Laryngology Supplement. 2000;185:79–81. doi: 10.1177/0003489400109s1234. [DOI] [PubMed] [Google Scholar]
- Kirk KI, Miyamoto RT, Lento CL, et al. Effects of age at implantation in young children. Annals of Otology Rhinology and Laryngology. 2002;111(5):69–73. doi: 10.1177/00034894021110s515. [DOI] [PubMed] [Google Scholar]
- Kraljic T, Samuel Arthur G. Perceptual learning for speech: Is there a return to normal? Cognitive Psychology. 2005;51(2):141–178. doi: 10.1016/j.cogpsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Kuhl Patricia K. Speech perception in early infancy: Perceptual constancy for spectrally dissimilar vowel categories. Journal of the Acoustical Society of America. 1979;66:1668–1679. doi: 10.1121/1.383639. [DOI] [PubMed] [Google Scholar]
- Lin Lawrence I-Kuei. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–268. [PubMed] [Google Scholar]
- Lively Scott E, Logan JS, Pisoni David B. Training japanese listeners to identify english/r/and/l/ii: The role of phonetic environment and talker variability in learning new perceptual categories. Journal of the Acoustical Society of America. 1993;94:1242–1255. doi: 10.1121/1.408177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manrique M, Cervera-Paz FJ, Huarte A, et al. Prospective long-term auditory results of cochlear implantation in prelinguistically deafened children: The importance of early implantation. Acta Otolaryngology Supplement. 2004;(552):55–63. doi: 10.1080/03655230410017148. [DOI] [PubMed] [Google Scholar]
- Marschark M, Rhoten C, Fabich M. Effects of cochlear implants on children's reading and academic achievement. J Deaf Stud Deaf Educ. 2007;12(3):269–282. doi: 10.1093/deafed/enm013. [DOI] [PubMed] [Google Scholar]
- McConkey Robbins A, Koch DB, Osberger MJ, et al. Effect of age at cochlear implantation on auditory skill development in infants and toddlers. Archives of otolaryngology--head & neck surgery. 2004;130(5):570–574. doi: 10.1001/archotol.130.5.570. [DOI] [PubMed] [Google Scholar]
- Moog JS, Geers AE. Epilogue: Major findings, conclusions and implications for deaf education. Ear Hear. 2003;24(1 Suppl):121S–125S. doi: 10.1097/01.AUD.0000052759.62354.9F. [DOI] [PubMed] [Google Scholar]
- Moog JS, Geers AE, Gustus CH, et al. Psychosocial adjustment in adolescents who have used cochlear implants since preschool. Ear Hear. 2011;32(1 Suppl):75S–83S. doi: 10.1097/AUD.0b013e3182014c76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas JG, Geers AE. Effects of early auditory experience on the spoken language of deaf children at 3 years of age. Ear Hear. 2006;27(3):286–298. doi: 10.1097/01.aud.0000215973.76912.c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas JG, Geers AE. Will they catch up? The role of age at cochlear implantation in the spoken language development of children with severe to profound hearing loss. Journal of Speech, Language, and Hearing Research. 2007;50(4):1048–1062. doi: 10.1044/1092-4388(2007/073). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niparko JK, Tobey EA, Thal DJ, et al. Spoken language development in children following cochlear implantation. JAMA. 2010;303(15):1498–1506. doi: 10.1001/jama.2010.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris Dennis, McQueen James, Cutler Anne. Perceptual learning in speech. Cognitive Psychology. 2003;47(2):204–238. doi: 10.1016/s0010-0285(03)00006-9. [DOI] [PubMed] [Google Scholar]
- Peng SC, Spencer LJ, Tomblin JB. Speech intelligibility of pediatric cochlear implant recipients with 7 years of device experience. Journal of speech, language, and hearing research : JSLHR. 2004;47(6):1227–1236. doi: 10.1044/1092-4388(2004/092). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni DB, Conway CM, Kronenberger W, et al. Efficacy and effectiveness of cochlear implants in deaf children. Deaf cognition: Foundations and outcomes. 2008:52–101. [Google Scholar]
- Rost Gwyneth C, McMurray Bob. Speaker variability augments phonological processing in early word learning. Developmental Science. 2009;12(2):339–349. doi: 10.1111/j.1467-7687.2008.00786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rost Gwyneth C, McMurray Bob. Finding the signal by adding noise: The role of non-contrastive phonetic variability in early word learning. Infancy. 2010;15(6):608. doi: 10.1111/j.1532-7078.2010.00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarant JZ, Blamey PJ, Dowell RC, et al. Variation in speech perception scores among children with cochlear implants. Ear and Hearing. 2001;22(1):18–28. doi: 10.1097/00003446-200102000-00003. [DOI] [PubMed] [Google Scholar]
- Semel E, Wiig EH, Secord WA. Clinical evaluations of language fundamentals: Third edition (celf-3. San Antonio: The Psychological Corporation; 1995. [Google Scholar]
- Sharma A, Dorman MF, Spahr AJ. A sensitive period for the development of the central auditory system in children with cochlear implants: Implications for age of implantation. Ear and Hearing. 2002;23(6):532–539. doi: 10.1097/00003446-200212000-00004. [DOI] [PubMed] [Google Scholar]
- Slawinski EB, Fitzgerald LK. Perceptual development of the categorization of the/r-w/contrast in normal children. Journal of Phonetics. 1998;(26):27–43. [Google Scholar]
- Spencer LJ, Tomblin JB, Gantz BJ. Growing up with a cochlear implant: Education, vocation, and affiliation. J Deaf Stud Deaf Educ. 2012;17(4):483–498. doi: 10.1093/deafed/ens024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer LJ, Tye-Murray N, Tomblin JB. The production of english inflectional morphology, speech production and listening performance in children with cochlear implants. Ear and Hearing. 1998;19(4):310–318. doi: 10.1097/00003446-199808000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svirsky MA, Teoh SW, Neuburger H. Development of language and speech perception in congenitally, profoundly deaf children as a function of age at cochlear implantation. Audiology & Neuro-otology. 2004;9(4):224–233. doi: 10.1159/000078392. [DOI] [PubMed] [Google Scholar]
- Szagun G, Stumper B. Age or experience? The influence of age at implantation and social and linguistic environment on language development in children with cochlear implants. Journal of speech, language, and hearing research : JSLHR. 2012;55(6):1640–1654. doi: 10.1044/1092-4388(2012/11-0119). [DOI] [PubMed] [Google Scholar]
- Tillman TW, Carhart R. An expanded test for speech discrimination utilizing cnc monosyllabic words. Northwestern University Auditory Test No. 6 Technical report No.SAM-TR-66- 55.USAF School of Aerospace Medicine, Brooks Air Force Base, Texas, cochlear implant. 1966 doi: 10.21236/ad0639638. [DOI] [PubMed] [Google Scholar]
- Tomblin JB, Barker BA, Hubbs S. Developmental constraints on language development in children with cochlear implants. International Journal of Audiology. 2007;46(9):512–523. doi: 10.1080/14992020701383043. [DOI] [PubMed] [Google Scholar]
- Tomblin JB, Barker BA, Spencer LJ, et al. The effect of age at cochlear implant initial stimulation on expressive language growth in infants and toddlers. Journal of Speech, Language, and Hearing Research. 2005;48(4):853–867. doi: 10.1044/1092-4388(2005/059). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomblin JB, Oleson J, Ambrose SE, et al. The influence of hearing aids on speech and language development in children with hearing loss. Pediatrics. doi: 10.1001/jamaoto.2014.267. (in revision). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomblin JB, Peng SC, Spencer LJ, et al. Long-term trajectories of the development of speech sound production in pediatric cochlear implant recipients. Journal of Speech, Language, and Hearing Research. 2008;51(5):1353–1368. doi: 10.1044/1092-4388(2008/07-0083). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhler K, Yoshinaga-Itano C, Gabbard SA, et al. Longitudinal infant speech perception in young cochlear implant users. Journal of the American Academy of Audiology. 2011;22(3):129–142. doi: 10.3766/jaaa.22.3.2. [DOI] [PubMed] [Google Scholar]
- Valencia DM, Rimell FL, Friedman BJ, et al. Cochlear implantation in infants less than 12 months of age. International Journal of Pediatric Otorhinolaryngology. 2008;72(6):767–773. doi: 10.1016/j.ijporl.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Walker EA, Spratford M, Moeller MP, et al. Predictors of hearing aid use time in children with mild-severe hearing loss. Lang Speech Hear Serv Sch. 2012 doi: 10.1044/0161-1461(2012/12-0005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werker Janet F, Tees Richard C. Cross-language speech perception: Evidence for perceptual reorganization during the first year of life. Infant Behavior and Development. 1984;7:49–63. [Google Scholar]
- Wie OB. Language development in children after receiving bilateral cochlear implants between 5 and 18 months. International Journal of Pediatric Otorhinolaryngology. 2010;74(11):1258–1266. doi: 10.1016/j.ijporl.2010.07.026. [DOI] [PubMed] [Google Scholar]
- Wie OB, Falkenberg ES, Tvete O, et al. Children with a cochlear implant: Characteristics and determinants of speech recognition, speech-recognition growth rate, and speech production. International Journal of Audiology. 2007;46(5):232–243. doi: 10.1080/14992020601182891. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, editor. Woodcock reading mastery test. (revised edn) Allen, TX: Teaching Resources; 1987. [Google Scholar]
- Yoshinaga-Itano C, Baca RL, Sedey AL. Describing the trajectory of language development in the presence of severe-to-profound hearing loss: A closer look at children with cochlear implants versus hearing aids. Otology & Neurotology. 2010;31(8):1268–1274. doi: 10.1097/MAO.0b013e3181f1ce07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwolan TA, Ashbaugh CM, Alarfaj A, et al. Pediatric cochlear implant patient performance as a function of age at implantation. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2004;25(2):112–120. doi: 10.1097/00129492-200403000-00006. [DOI] [PubMed] [Google Scholar]