Abstract
IgG4 related disease (IgG4-RD) is characterized by a lymphoplasmacytic infiltrate composed of IgG4+ plasma cells, tumefactive lesions, obliterative phlebitis, and mild to moderate eosinophilia. It has been suggested that IgG4-RD is characterized by allergic manifestations and is potentially driven by enhanced T helper type 2 (Th2) responses. We aimed to investigate the potential contribution of atopy to enhanced Th2 responses in IgG4-RD. Peripheral blood mononuclear cells from 39 patients were isolated and subjected to in vitro mitogenic stimulation with PMA and ionomycin. Following stimulation, gated CD3+CD4+ T cells were analyzed for production of the Th2 cytokines IL-4, IL-5 and IL-13. Among the 39 patients analyzed, only the 18 patients who had a history of atopy showed increases in circulating Th2 memory cells. Our results indicate that Th2 responses that have been reported in IgG4-RD may result from the concomitant atopic manifestation in diseased patients.
Keywords: IgG4-RD, Th2 response, Atopy, Allergy
IgG4-related disease (IgG4-RD) is a debilitating fibrotic and inflammatory disease that affects many organ systems and its pathogenesis is poorly understood. The conditions that make up the spectrum of IgG4-RD disorders can affect virtually every organ system of the body, and the defining features are tumefactive lesions, storiform fibrosis, obliterative phlebitis and the presence of IgG4 secreting plasma cells in affected tissues1. Elevated concentrations of serum IgG4 are also observed in the majority of subjects. Studies in type I autoimmune pancreatitis, a condition that falls under the IgG4-RD spectrum, have shown that a proportion of these patients have a long standing history of allergies, peripheral blood eosinophilia (PBE) and serum IgE elevation, or manifest atopic symptoms (rhinitis, atopic dermatitis, bronchial asthma) during the time that the full IgG4-RD phenotype develops2.
Allergic immune responses can be induced by Th2 cytokines: IL-4, IL-5 and IL-13, which may contribute to the IgG4/IgE class switch and also promote peripheral blood eosinophilia. The analysis of circulating T cells for Th1/Th2 polarization has led to conflicting results in IgG4-RD subjects. One study reported a Th1 skew in peripheral blood T cells in autoimmune pancreatitis3 but more recent studies involving patients with IgG4-RD lacrimal gland enlargement showed an increase in Th2 phenotype cells in peripheral blood4,5. All these studies involved small cohorts of 5–10 patients. More elaborate reports, based primarily on the detection of mRNA-levels of the cytokines IL-4, IL-5 and IL-13 in disease lesions, have suggested the role of Th2 responses in IgG4-RD pathogenesis6,7. These studies also showed the abundance of IL-4 in disease lesions but it is unclear if tissue Th2 cells are the source of this cytokine. Therefore, a direct evidence for a role of Th2 cells in disease pathogenesis is still lacking. This would require demonstration of an expansion in subjects of CD4+ T cells that can secrete Th2 cytokines when re-stimulated or the documentation of Th2 cytokines within T cells that infiltrate affected tissues. It remains unclear whether Th1 or Th2 cells, or possibly some other polarized T cell subset, contribute to disease pathogenesis.
In this study, we sought to investigate whether Th2-type memory responses can be observed in IgG4-RD patients and whether such a response reflects an underlying atopic diathesis or if it is an integral feature of the pathogenesis of IgG4-RD.
Methods
Thirty-nine subjects with IgG4-RD presenting to the Massachusetts General Hospital Rheumatology Clinic were included in this study. All subjects signed written, informed consent for the investigations described and all had a biopsy-proven diagnosis of IgG4-RD. Using the definitions of the European Academy of Allergy and Clinical Immunology, we classified the subjects as either atopic or non-atopic8 [Table 1]. Eight healthy volunteers from the clinic and the laboratory without a history of atopy were used as controls.
Table 1.
Clinical characteristics of patients affected by IgG4-RD.
| Atopic patients (n=18) |
Non-atopic patients (n=21) |
All patients (n=39) |
|
|---|---|---|---|
| Mean age in years (range) | 54.1 (24–75) | 60 (46–80) | 57.3 (24–80) |
| Male / Female ratio | 2.4 | 2.8 | 2.62 |
| Atopic symptoms, n (%) | |||
| Rhinitis | 12 (66.7%) | 0 | |
| Conjunctivitis | 3 (16.67%) | 0 | |
| Asthma | 5 (27.67%) | 0 | |
| Hives | 3 (16.67%) | 0 | |
| Gastrointestinal symptoms | 1 (5.55%) | 0 | |
| Anaphylaxis | 0 | 0 | |
| Organ involvement, n (%) | |||
| Salivary glands | 11 (61.1%) | 8 (38%) | 19 (48.7%) |
| Lacrimal glands | 4 (22.2%) | 2 (10%) | 6 (15.3%) |
| Orbit | 4 (22.2%) | 2 (10%) | 6 (15.3%) |
| Nasopharynx | 2 (11.1%) | 2 (10%) | 4 (10.2%) |
| Lymph nodes | 2 (11.1%) | 3 (14%) | 5 (12.8%) |
| Thyroid | 1 (5.5%) | 1 (2.5%) | |
| Lung | 3 (16.6%) | 4 (19%) | 7 (18%) |
| Pericardium | 1 (5.5%) | 1 (3%) | |
| Pancreas | 6 (33.3%) | 7 (33%) | 13 (34%) |
| Liver | 1 (5%) | 1 (3%) | |
| Biliary tree | 3 (16.6%) | 2 (10%) | 5 (12.8%) |
| Kidney | 2 (11.1%) | 4 (19%) | 6 (15.3%) |
| Aorta | 2 (11.1%) | 1 (5%) | 3 (8%) |
| Prostate | 1 (5.5%) | 1 (2.5%) | |
| Eye | 1 (5.5%) | 1 (2.5%) | |
| Peripheral nerves | 1 (5.5%) | 1 (2.5%) | |
| Neck | 1 (5.5%) | 1 (2.5%) | |
| Skin | 2 (11.1%) | 2 (5%) | |
Peripheral blood mononuclear cells were isolated using Ficoll-Paque gradient separation (GE Healthcare) prior to immunosuppressive therapy. These cells were re-stimulated for 4 hours with phorbol myristoyl acetate (PMA) [50ng/ml] and ionomycin (Sigma) [100 ng/ml] along with brefeldin A (BD biosciences) [2ug/ml]. Following stimulation, cells were blocked using Fc receptor blocking solution (Biolegend) [2.5 ug/million cells] followed by surface staining (30 minutes on ice) with Alexa fluor-700 conjugated anti-human CD3 (Clone HIT3a) and Brilliant violet-510 conjugated antihuman CD4 (Clone OKT4) (Biolegend). Cells were then fixed and permeabilized with a Foxp3 staining kit (eBioscience) according to the manufacturer’s protocol and incubated with PE Cy7 conjugated anti-GATA-3 (Clone L50-823) (BD Biosciences), Alexa fluor-488 conjugated anti-human IL-4 (Clone 8D4-8), PE conjugated anti-human IL-5 (Clone JES1-39D10) and PerCP Cy 5.5 conjugated anti-human IL-13 (Clone JES10-5A2) antibodies (Biolegend). Cells were then washed and acquired on a BD-LSR II analyser (BD bioscience) and samples analyzed using FACSDiva or Flowjo software.
Results
The mean age of the study subjects was 57.3 years (range 24–80), and the male:female ratio was 2.8 [Table 1]. The IgG4 levels at diagnosis were higher in atopic patients (907.1mg/dL ± S.E.M. 333) compared to the non-atopic subjects (388.7mg/dL±S.E.M. 120.6), but this difference was not significant (p = 0.09). Also, the spectrum of organ involvement was very similar across the two groups with the exception of salivary glands, lacrimal glands and orbits, which were slightly more involved in atopic patients [Table 1].
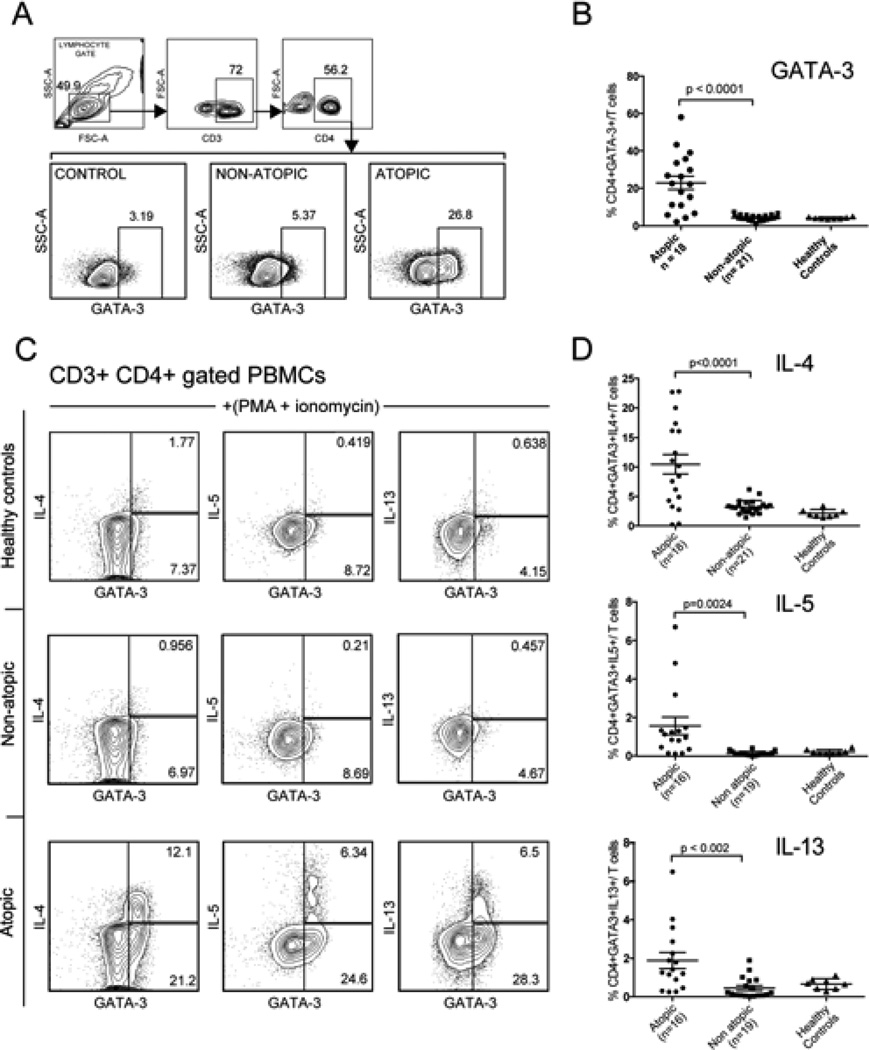
Given the limited published data about the prevalence of polarized Th2 responses in IgG4-RD, we sought to investigate our patient cohort for the presence of Th2-skewed CD4+ memory cells. Peripheral blood T cells were analyzed for the presence of CD4+GATA3+ memory T cells and also for the production of the Th2 cytokines, IL-4, IL-5 and IL-13 upon in vitro restimulation of CD4+ T cells with PMA and ionomycin. Atopic patients (18/39) displayed a marked increase in the proportion of CD4+GATA-3+ Th2 cells in peripheral blood (22.5% ± S.E.M. 3.5%) compared to the 21/39 non-atopic patients studied (4.5% ± S.E.M. 0.41%)) and healthy non-allergic controls (4.2% ± S.E.M. 0.18%) (Fig 1 A, B). Upon in vitro re-stimulation, a large fraction of the CD4+GATA-3+ cells produced IL-4, IL-5 and IL-13 and the proportion of cells making these cytokines was significantly higher in atopic subjects compared to the non-atopic IgG4RD patients and healthy controls (Figure 1 C–D). Thus, we found that the presence of circulating Th2 memory cells was largely restricted to IgG4-RD subjects with concurrent atopic manifestations, suggesting that previous studies analyzing the role of Th2 response in the pathogenesis of IgG4-RD were probably confounded by concomitant allergic disease.
Figure 1. Atopic IgG4-RD patients show enhanced circulating Th2-memory cells.
A. Gating strategy for determining the frequency of CD3+ CD4+ GATA-3+ T lymphocytes
B. Frequency of CD3+CD4+GATA-3+ T cells in healthy controls and IgG4-RD subjects with and without atopy.
C. Representative plots from healthy controls and atopic and non-atopic IgG4-RD patients, examining CD3+CD4+ gated T lymphocytes that make IL-4, IL-5 and IL-13 following in vitro stimulation with PMA + ionomycin (with Brefeldin A).
D. The frequency of activated CD4+ T cells from healthy controls and IgG4-RD patients with and without atopy, that make IL-4, IL-5 and IL-13. The number of patients analyzed in each group is mentioned on the x-axis. Horizontal bars represent mean frequencies from studied patients ±standard error of the mean. P-values < 0.05 were considered significant.
Discussion
In this study, we found a strong correlation between the presence of circulating Th2 memory cells and an underlying atopic diathesis in IgG4-RD subjects. A large number of individuals with severe IgG4-RD were found to have no Th2 memory cells despite the lack of immunosuppressive therapy. Therefore, we propose that a Th2 immune response in IgG4-RD, when present, likely reflects intercurrent allergic conditions and may not represent a fundamental feature of IgG4-RD. Although it remains technically possible that IgG4-RD is initiated by an aberrant Th2 response, no such memory response seems necessary to maintain the chronic inflammation that is characteristic of IgG4-RD. This study is focused on recirculating T cells and clearly shows in a cohort of 39 patients that a large subset with severe IgG4-RD, but lacking atopic manifestations, do not have the accumulation of Th2 cells that have been reported previously4–7. The strong correlation between IgG4-RD patients with an allergic history with the presence of circulating Th2 cells suggests that studies implicating a pathogenic role for Th2 cells in IgG4-RD should be re-examined in this context.
Acknowledgments
This study was funded by grants AI 064930 and AI 076505 from the NIH to SP
Footnotes
Author Contributions
HM and VSM thought of and conducted laboratory studies with guidance from SP. EDT and HM analyzed the clinical material, supervised by JHS. HM and EDT analyzed the data and HM and SP drafted the manuscript. All authors read and approved the final version.
Conflict of Interest
The authors declare that they have no relevant conflict of interest
References
- 1.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539–551. doi: 10.1056/NEJMra1104650. [DOI] [PubMed] [Google Scholar]
- 2.Kamisawa T, Anjiki H, Egawa N, Kubota N. Allergic manifestations in autoimmune pancreatitis. Eur J Gastroenterol Hepatol. 2009;21:1136–1139. doi: 10.1097/meg.0b013e3283297417. [DOI] [PubMed] [Google Scholar]
- 3.Okazaki K, Uchida K, Ohana M, et al. Autoimmune-related pancreatitis is associated with autoantibodies and Th1/Th2-type cellular immune response. Gastroenterology. 2000;118:573–581. doi: 10.1016/s0016-5085(00)70264-2. [DOI] [PubMed] [Google Scholar]
- 4.Saito Y, Kagami S, Kawashima S, et al. Roles of CRTH2+CD4+ T cells in immunoglobulin G4-related lacrimal gland enlargement. International archives of allergy and immunology. 2012;158(Suppl):42–46. doi: 10.1159/000337761. [DOI] [PubMed] [Google Scholar]
- 5.Kanari H, Kagami S, Kashiwakuma D, et al. Role of Th2 cells in IgG4-relted lacrimal gland enlargement. International archives of allergy and immunology. 2010;152(Suppl):47–53. doi: 10.1159/000312125. [DOI] [PubMed] [Google Scholar]
- 6.Zen Y, Fujii T, Harada K, Kawano M, Yamada K, Takahira M, et al. Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology. 2007;45:1538–1546. doi: 10.1002/hep.21697. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka A, Moriyama M, Nakashima H, Miyake K, Hayashida JN, Maehara T, et al. Th2 and regulatory immune reactions contribute to IgG4 production and the initiation of Mikulicz disease. Arthritis Rheum. 2012;64:254e63. doi: 10.1002/art.33320. [DOI] [PubMed] [Google Scholar]
- 8.Johansson SG, Hourihane JO, Bousquet J, Bruijnzeel-Koomen C, Dreborg S, Haahtela T, et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy. 2001;56:813–824. doi: 10.1034/j.1398-9995.2001.t01-1-00001.x. [DOI] [PubMed] [Google Scholar]