Abstract
LR11, also known as SorLA or SORL1, is a type-I membrane protein from which a large extracellular part, soluble LR11 (sLR11), is released by proteolytic shedding on cleavage with a disintegrin and metalloproteinase 17 (ADAM17). A shedding mechanism is presumed to have a key role in the functions of LR11, but the evidence for this has not yet been demonstrated. Tetraspanin CD9 has been recently shown to regulate the ADAM17-mediated shedding of tumor necrosis factor-α and intercellular adhesion molecule-1 on the cell surface. Here, we investigated the role of CD9 on the shedding of LR11 in leukocytes. LR11 was not expressed in THP-1 monocytes, but it was expressed and released in phorbol 12-myristate 13-acetate (PMA)-induced THP-1 macrophages (PMA/THP-1). Confocal microscopy showed colocalization of LR11 and CD9 proteins on the cell surface of PMA/THP-1. Ectopic neo-expression of CD9 in CCRF-SB cells, which are LR11-positive and CD9-negative, reduced the amount of sLR11 released from the cells. In contrast, incubation of LR11-transfected THP-1 cells with neutralizing anti-CD9 monoclonal antibodies increased the amount of sLR11 released from the cells. Likewise, the PMA-stimulated release of sLR11 increased in THP-1 cells transfected with CD9-targeted shRNAs, which was negated by treatment with the metalloproteinase inhibitor GM6001. These results suggest that the tetraspanin CD9 modulates the ADAM17-mediated shedding of LR11 in various leukemia cell lines and that the association between LR11 and CD9 on the cell surface has an important role in the ADAM17-mediated shedding mechanism.
Keywords: ADAM17, CD9, LR11, TNF-α converting enzyme, Tetraspanin
Introduction
LR11, also known as SorLA or SORL1, is a member of the low density lipoprotein receptor family, which binds apolipoprotein E.1, 2 LR11 is a type-I membrane protein from which a large extracellular part, referred to as soluble LR11 (sLR11), is released by proteolytic shedding. It has been shown that LR11 has a key role in migration of undifferentiated vascular smooth muscle cells and that the circulating sLR11 is a biomarker for atherosclerosis, coronary stenosis and diabetic retinopathy.3, 4, 5 Moreover, the mutations in LR11/SORL1 gene are predictive of Alzheimer's disease, and increased levels of sLR11 in the cerebrospinal fluid predict neurodegeneration in patients with Alzheimer's disease.6, 7, 8 We have previously demonstrated that the levels of serum sLR11 are significantly elevated in patients with acute leukemia and that the levels of sLR11 are associated with the percentage of peripheral blood blasts.9 In addition, we found that high levels of sLR11 have a significant negative prognostic impact on progression-free survival in patients with follicular lymphoma (FL).10 In the FL analysis, the immunohistological staining intensity of LR11 in lymph nodes of FL patients did not show a significant association with the levels of serum sLR11. Therefore, an ectodomain shedding mechanism is presumed to have a key role in the functions of LR11, including migration, adhesion and drug resistance, but evidence for this has not yet been demonstrated.
A disintegrin and metalloproteinase 17 (ADAM17, also known as tumor necrosis factor (TNF)-α converting enzyme) has been identified as the enzyme that cleaves the transmembrane precursor form of TNF-α, as well as the ectodomains of other cell surface proteins critically involved in development, cell growth, adhesion, differentiation and migration of leukocytes and tumor cells.11, 12, 13 LR11 is also cleaved by ADAM17.14, 15 Tetraspanin CD9 has been recently shown to regulate the shedding activity of ADAM17 on the cell surface.16 In this study, the authors reported that CD9 negatively regulated the ADAM17-mediated shedding of TNF-α and intercellular adhesion molecule-1 in leukocytes and endothelial cells. Thus, we hypothesized that the shedding of LR11 may also be regulated by CD9 in a mechanism similar to these other ADAM17 substrates. Here, we investigated the role of CD9 on the shedding of LR11 in leukocytes.
Materials and methods
Antibodies
Monoclonal antibodies (mAbs; A2-2-3, M3 and R14) against LR11 have been previously described.17 M3 was used for flow cytometry and ELISA, A2-2-3 for western blotting and R14 for immunofluorescence and ELISA. mAbs against CD9 (MM2/57, ALB-6, HI9a and M-L 13) were purchased from Merck Millipore (Billerica, MA, USA), Beckman Coulter (Brea, CA, USA), BioLegend (San Diego, CA, USA) and BD Biosciences (San Jose, CA, USA), respectively. MM2/57 was used for western blotting, ALB-6 as a neutralizing antibody, HI9a for flow cytometry and M-L 13 for immunofluorescence.
Cells
The human monocytic THP-1, the promonocytic U937 and the B lymphoblastoid CCRF-SB cell lines were all purchased from ATCC (Manassas, VA, USA). The cell lines were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS; Thermo Scientific, Waltham, MA, USA). Normal human peripheral blood was obtained from healthy volunteers. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using the Ficoll-Paque Plus (GE Healthcare, Pittsburgh, PA, USA). CD3+ T cells, CD14+ monocytes and CD19+ B cells were magnetically labeled with specific microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) and then enriched using MACS columns. Primary human macrophages were generated by culturing human monocytes for 7 days in RPMI 1640 medium supplemented with 10% FBS and 50 ng ml−1 M-CSF (Sigma-Aldrich, St Louis, MO, USA). For analysis of sLR11 in the culture supernatant, the cells were cultured with fresh serum-free media, which was collected and used for western blot analysis or ELISA of sLR11 after concentration of the media using Amicon Ultra centrifugal filter units (100 kDa NMWL membranes, Merck Millipore).
Generation of LR11-overexpressing cells, CD9-overexpressing cells and CD9-silencing cells
For the generation of LR11-overexpressing cells, THP-1 cells were transiently transfected with pBK-CMVhLR112 using the Neon electroporation device (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. For the generation of CD9-overexpressing cells, CCRF-SB cells were transiently transfected by electroporation with the pCMV6-AN-mGFP vector containing CD9 cDNA (OriGene Technologies, Rockville, MD, USA) or with an empty vector (mock control). Stable CD9-silencing THP-1 cells were generated by transfection with the TRCN0000291711 plasmid (Sigma-Aldrich) expressing CD9-targeted shRNA, using Lipofectamine 2000 transfection reagent (Invitrogen), and compared with the cells transfected with the empty vector. Transfected cells were selected for use in 0.5 μg ml−1 puromycin-supplemented medium for 2–3 weeks. After applying this limiting dilution method, the reduced expression of CD9 in the resulting cell population was verified by flow cytometry.
Western blot analysis
Cultured cells were lysed in ice-cold Radioimmunoprecipitation Assay Buffer (Wako, Osaka, Japan) supplemented with protease inhibitors (Complete Mini; Roche Applied Science, Upper Bavaria, Germany). The resulting cell lysates were recovered after centrifugation at 20 000 × g for 20 min. Protein concentrations were determined using the BCA Protein Assay Kit (Thermo Scientific). Samples were mixed with an equal volume of Laemmli Sample Buffer (Bio-Rad, Hercules, CA, USA), containing 5% β-mercaptoethanol, and heated for 5 min at 95 °C. Protein samples were subjected to SDS-PAGE, and the immunoreactive signals were detected by western blot analysis using mouse mAbs against LR11 (A2-2-3) and CD9 (MM2/57), followed by horseradish peroxidase-conjugated anti-mouse IgG. ECL detection reagents (GE Healthcare) were used to detect the horseradish peroxidase enzyme activity signals, which were quantified using the ChemiDoc XRS system with the Image Lab software (Bio-Rad).
RNA extraction and quantitative real-time PCR analysis
Total RNA was isolated using the RNeasy Mini Kit (Qiagen, Venlo, Netherlands), eluted and then quantified using the Nanodrop spectrometer (Thermo Scientific). The reverse transcription step was performed with the PrimeScript RT Reagent Kit (Takara Bio, Shiga, Japan) according to the manufacturer's instructions. The resulting cDNA samples were subjected to quantitative real-time PCR to measure the levels of LR11 mRNA, using the TaqMan Assay-on-Demand kit with the StepOne Real-Time PCR System (Applied Biosystems, Norwalk, CT, USA). The PCR primer sequences were previously mentioned.4 We quantified and normalized the levels of LR11 mRNA to those of the house-keeping gene glyceraldehyde-3-phosphate dehydrogenase.
Flow cytometry
Cells were washed with PBS containing 2% FBS and then incubated with human serum type AB at 4 °C for 30 min to block the Fc receptors. Next, the cells were stained at 4 °C for 30 min in the dark with fluorescein isothiocyanate-conjugated anti-LR11 antibody M3, phycoerythrin-conjugated anti-CD9 antibody HI9a and antibodies against CD3, CD14 or CD19 (BioLegend). The isotype control antibody (BD Biosciences) was used as a negative control. Flow cytometry was performed with the FACSCanto II or the FACSCalibur flow cytometers (BD Biosciences).
Immunofluorescence
Cells were fixed in 100% methanol at −20 °C for 15 min. Fixed cells were incubated with a biotinylated mAb against LR11 (R14) followed by an Alexa Fluor 594 streptavidin conjugate (Invitrogen). Next, the cells were incubated with a fluorescein isothiocyanate-conjugated anti-CD9 antibody M-L 13. Cells were mounted with VECTASHIELD Mounting Medium (Vector Laboratories, Burlingame, CA, USA). Slides were examined with the Zeiss LSM5 PASCAL confocal laser scanning microscope (Carl Zeiss, Oberkochen, Germany).
ELISA
The amount of sLR11 released into the culture medium was determined by sandwich ELISA, as previously described.17 In brief, samples were reacted with the capture mAb M3, and then incubated with the biotinylated reporter rat mAb R14. The LR11-mAb complex was reacted with horseradish peroxidase-conjugated streptavidin. A standard curve was constructed using the purified LR11 protein.
Statistics
All data are presented as the mean±s.d. or s.e. for each index. The two-tailed Student's t-test was used to compare between two groups and Dunnett's test or Tukey's HSD test was used for multiple group comparisons. A value of P<0.05 was considered to be statistically significant.
Results
Expression of LR11 and CD9 in normal human PBMCs
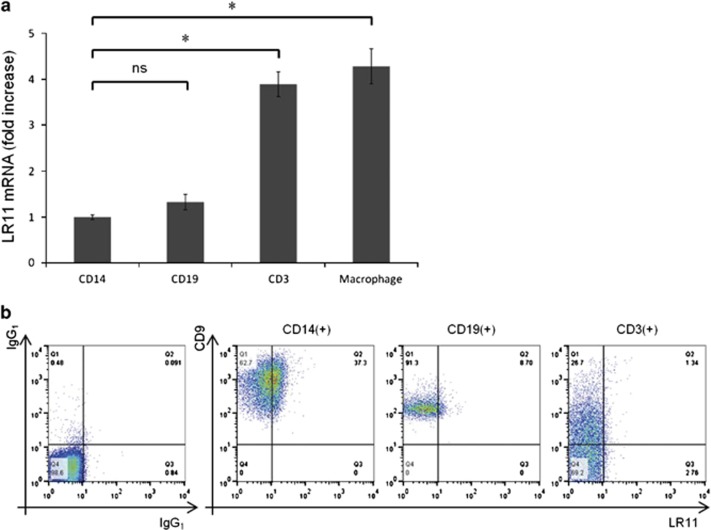
First, we examined the expression levels of LR11 in normal human PBMCs by quantitative real-time PCR. The expression levels of LR11 were significantly higher in CD3+ T cells than in CD14+ monocytes, whereas there was no difference between monocytes and CD19+ B cells. In addition, the gene expression levels of LR11 were significantly higher in human monocyte-derived macrophages than in CD14+ monocytes (Figure 1a). However, as we previously demonstrated,9 LR11 was only expressed on the cell surface of monocytes in normal human PBMCs, as detected by flow cytometry, whereas CD9 was broadly expressed on the cell surface of all PBMCs (Figure 1b).
Figure 1.
Expression of LR11 and CD9 in normal human PBMCs. (a) The gene expression levels of LR11 in CD14+ monocytes, CD19+ B cells, CD3+ T cells and monocyte-derived macrophages from healthy volunteers. Monocyte-derived macrophages were generated by culturing human CD14+ monocytes for 7 days in RPMI 1640 medium supplemented with 10% FBS and 50 ng ml−1 M-CSF. The gene expression levels of LR11 were determined by quantitative real-time PCR as described in Materials and methods. Data are shown as the fold increase relative to the control (CD14+ monocytes) and presented as the mean±s.d. (error bars, n=3). *P<0.05; ns, not significant. (b) The cell surface expression levels of LR11 and CD9 in CD14+ monocytes, CD19+ B cells and CD3+ T cells, as evaluated by flow cytometry.
Expression of LR11 and CD9 in undifferentiated/differentiated THP-1 cells
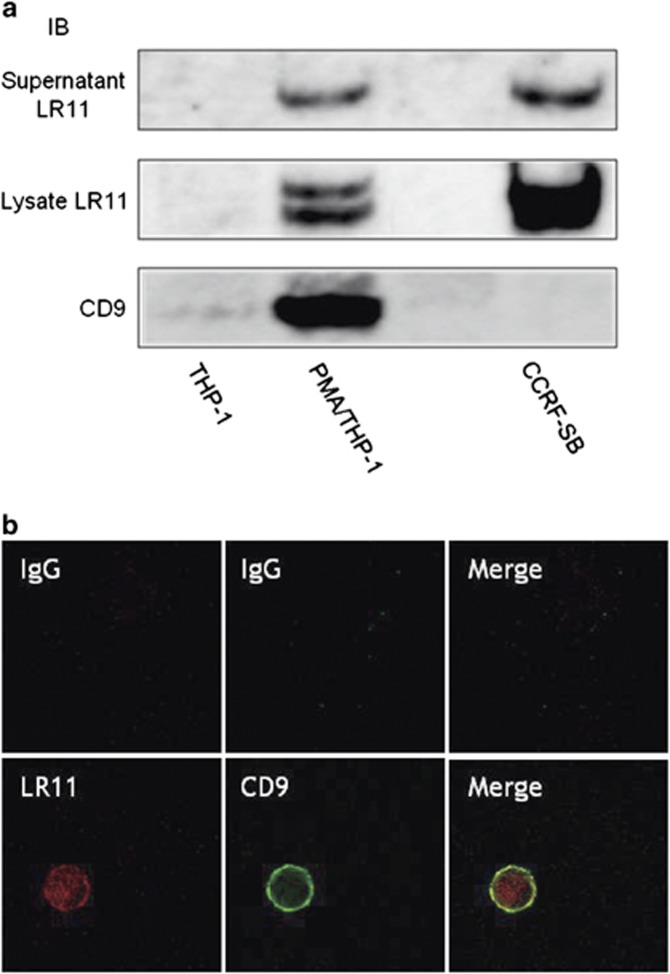
Because it has been reported that CD9 interacts with ADAM17 on the cell surface to exert a negative regulatory role on the sheddase activity of ADAM17,16 we hypothesized that CD9 is associated with the shedding of LR11 on the cell surface. To verify this hypothesis, we chose the monocytic cell line THP-1 because monocytes express both LR11 and CD9 on their surface (Figure 1b). First, we investigated the cellular expression and the released protein levels of LR11, as well as the cellular expression of CD9 in THP-1 cells by western blot analysis. Second, because the gene expression levels of LR11 were significantly higher in human monocyte-derived macrophages than in CD14+ monocytes (Figure 1a), we investigated the expression of LR11 and CD9 in phorbol 12-myristate 13-acetate (PMA)-induced THP-1 macrophages (PMA/THP-1). Although LR11 was not expressed in undifferentiated THP-1 cells, but it was expressed and released in differentiated PMA/THP-1. CD9 was poorly expressed in THP-1 cells, but the expression levels of CD9 increased in PMA/THP-1. Because we previously demonstrated that the molecular size of secreted sLR11 is smaller than that of the membrane-bound LR11 in cultured smooth muscle cells,18 whole cell lysates containing both membrane-bound and cytoplasmic LR11 displayed different molecular sizes of LR11. We previously investigated the cellular expression and the released protein levels of LR11 in 11 leukemia cell lines of different origins other than THP-1, and among these, the B lymphoblastoid cell line CCRF-SB released a large amount of sLR11.9 We confirmed that CD9 was not expressed in this cell line; therefore, we chose CCRF-SB as the CD9-negative control cells (Figure 2a).
Figure 2.
LR11 and CD9 associate on the cell surface of PMA/THP-1. (a) The levels of sLR11 and the cellular expression of LR11 or CD9 in undifferentiated THP-1, differentiated PMA/THP-1 and CCRF-SB cells, as analyzed by western blot analysis. For analysis of the levels of cellular protein, the cells were cultured with serum-containing media. For analysis of sLR11 in the culture supernatant, the cells were cultured with fresh serum-free media for 24 h. The collected media were concentrated before analysis. (b) Confocal microscopy displaying the colocalization and interaction of LR11 and CD9 in PMA/THP-1. After THP-1 cells were incubated with 20 ng ml−1 PMA for 24 h, the attached cells were stained with anti-LR11 and anti-CD9 antibodies. Samples were analyzed by confocal microscopy.
LR11 and CD9 associate on the cell surface of PMA/THP-1
To assess the relationship between LR11 and CD9 in PMA/THP-1, we performed double immunofluorescence staining of these molecules followed by confocal microscopy. Although LR11 was expressed both on the cell surface and in the cytoplasm, partial colocalization of LR11 and CD9 was specifically seen on the cell surface (Figure 2b). As we hypothesized, CD9 may be associated with LR11 on the cell surface. To further confirm the relationship between LR11 and CD9, we performed immunoprecipitation of LR11 and CD9 in PMA/THP-1 or COS7 cells, as well as in 293T cells transiently co-transfected with expression plasmids encoding human LR11 and CD9 cDNAs. However, LR11 and CD9 did not co-precipitate in any of the cell lines tested (data not shown).
Expression of LR11 and CD9 in THP-1 cells stimulated with PMA at different time intervals
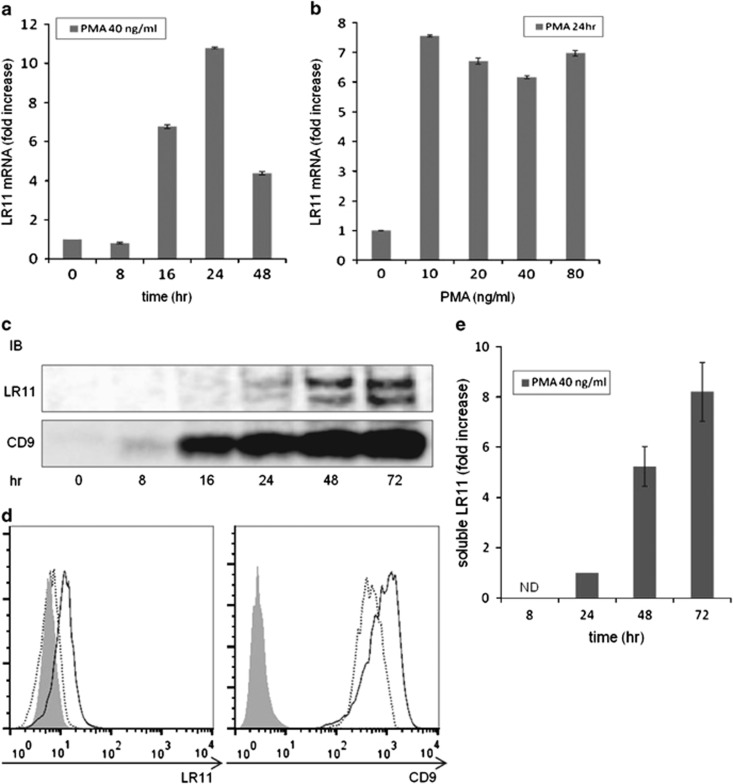
As shown in Figure 2a, LR11 was not expressed in undifferentiated THP-1 cells, but it was expressed and released in differentiated PMA/THP-1. We next examined the expression levels of LR11 and CD9 in THP-1 cells stimulated with PMA at different time intervals. Compared with unstimulated cells, when THP-1 cells were incubated with 40 ng ml−1 PMA, the levels of LR11 mRNA increased after 16 h, with a maximum 10-fold elevation after 24 h, followed by a decline toward baseline levels within 48 h (Figure 3a). There was no significant difference in the levels of LR11 mRNA when other concentrations of PMA were tested (Figure 3b). The protein expression levels of LR11 were elevated after 16 h with 40 ng ml−1 PMA and gradually increased until 72 h. The protein expression levels of CD9 were elevated after 8 h with PMA and gradually increased until 72 h (Figure 3c). As shown in Figure 3e, an increase in the level of sLR11 released from the cells was detected after 24 h, but was much more evident after 48 h of PMA treatment. The cell surface expression levels of LR11 did not change after 24 h of PMA treatment but did after 72 h, whereas the cell surface expression levels of CD9 increased after 24 h of PMA treatment and even more so after 72 h (Figure 3d). Therefore, PMA induced a transient increase in the transcription level of the LR11 gene and subsequent production of LR11 cellular protein, accompanied by release of the cleaved form into the media.
Figure 3.
Expression of LR11 and CD9 in THP-1 cells stimulated with PMA at different time intervals. (a) The levels of LR11 mRNA in THP-1 cells stimulated with 40 ng ml−1 PMA at different time intervals. (b) The levels of LR11 mRNA in THP-1 cells stimulated with varying concentrations of PMA for 24 h. The gene expression levels of LR11 were determined by quantitative real-time PCR. Data are shown as the fold increase relative to the control (0 h or PMA 0 ng ml−1) and presented as the mean±s.d. (error bars, n=3). (c) The protein expression levels of LR11 or CD9 in THP-1 cells stimulated with 40 ng ml−1 PMA at different time intervals were analyzed by western blot analysis. (d) The cell surface expression levels of LR11 or CD9 in THP-1 cells stimulated with 40 ng ml−1 PMA for 24 h (dashed line) or 72 h (solid line) were evaluated by flow cytometry. Gray-colored histograms represent cells labeled with an isotype control antibody. (e) The concentrations of sLR11 released from THP-1 cells stimulated with 40 ng ml−1 PMA at different time intervals were determined by ELISA. Data are shown as the fold increase relative to the 24 h time point and presented as the mean±s.e. (error bars, n=3). ND, not detected. In all cases, the cells were cultured with fresh serum-free media.
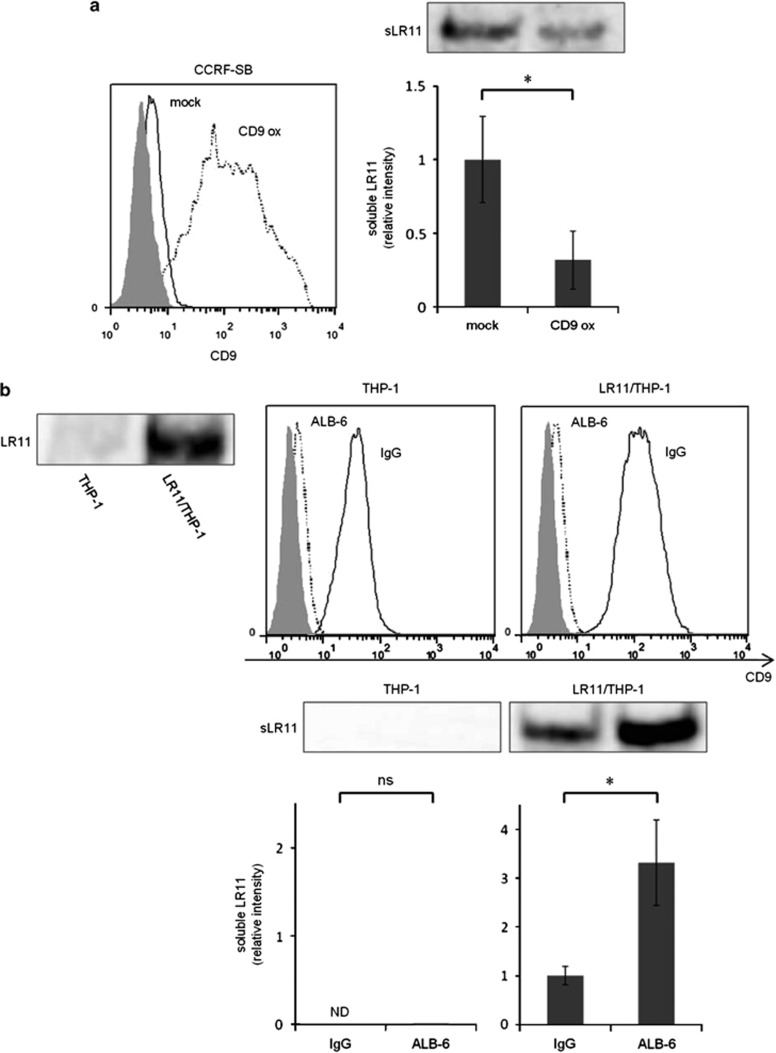
Effects of ectopic neo-expression of CD9 and neutralizing anti-CD9 mAbs on the shedding of LR11 in different cell types
We next investigated the effects of ectopic neo-expression of CD9 in CD9-negative cells and of neutralizing anti-CD9 mAbs (clone ALB-6)19, 20 in CD9-positive cells on the shedding of LR11. When LR11-positive and CD9-negative CCRF-SB cells were transiently overexpressed with CD9, the amount of sLR11 released from CD9-overexpressing cells significantly decreased compared with that from the control cells (Figure 4a). To confirm the effects of neutralizing anti-CD9 mAbs, control THP-1 cells and LR11-transfected THP-1 cells (LR11/THP-1) were cultured for 4 h in the presence of ALB-6, or an isotype control antibody. As shown in Figure 4b (upper panel), ALB-6 altered the expression levels of CD9 in both normal THP-1 and LR11/THP-1 cells. Furthermore, the amount of sLR11 released from ALB-6-cultured LR11/THP-1 cells significantly increased compared with that from the cells cultured with an isotype control antibody (Figure 4b, lower panel).
Figure 4.
Effects of ectopic neo-expression of CD9 and neutralizing anti-CD9 mAbs on the shedding of LR11 in different cell types. (a) Effect of ectopic neo-expression of CD9 in CCRF-SB cells. CCRF-SB cells were transiently transfected with a CD9 expression plasmid or the empty vector (mock control) as described in Materials and methods. After 48 h, the media were changed to fresh serum-free media. Cells were cultured for 12 h, after which the culture media were collected for analysis of sLR11. The cell surface expression levels of CD9, evaluated by flow cytometry, in CD9-overexpressing CCRF-SB cells (CD9 ox; dashed line) or mock-transfected cells (solid line) 48 h after transfection are shown (left panel). The protein levels of sLR11 released from CD9-overexpressing cells or mock-transfected cells were analyzed by western blot analysis (right panel). (b) The effects of neutralizing anti-CD9 mAbs (ALB-6) in THP-1 cells. THP-1 cells were transiently transfected with the LR11 expression plasmid. After 24 h, the media were changed to fresh serum-free media supplemented with ALB-6 or an isotype control antibody (IgG). Cells were cultured for 4 h, followed by adding more fresh serum-free media. Finally, the media were collected for analysis of sLR11 48 h after transfection. The expression of LR11 in normal THP-1 and LR11-transfected THP-1 cells (LR11/THP-1) 48 h after transfection was analyzed by western blot analysis (inset). The cell surface expression levels of CD9 in THP-1 and LR11/THP-1 cells with ALB-6 (dashed line) or IgG (solid line) were determined by flow cytometry (upper panel). The protein levels of sLR11 released from these cells were analyzed by western blot analysis (lower panel). In the flow cytometry figures, gray-colored histograms represent cells labeled with the isotype control antibody. In the bar graph panels, data are shown as the fold increase relative to the control and presented as the mean±s.d. (error bars, n=3). *P<0.05; ns, not significant; ND, not detected.
Effect of CD9 silencing on the shedding of LR11 in THP-1 cells
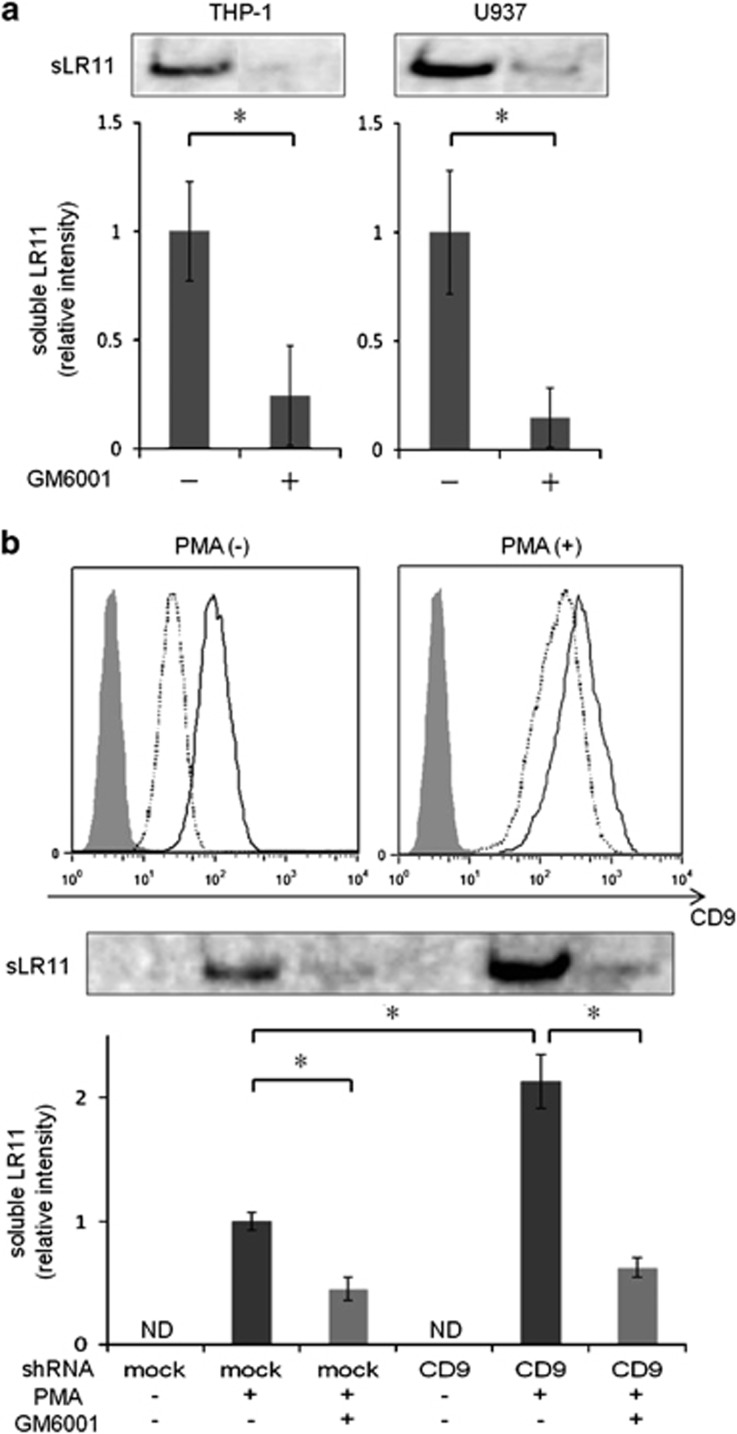
Previous studies have reported the constitutive and PMA-stimulated shedding of LR11 in various adherent cells,3, 15, 21 and the ADAM17-mediated shedding of LR11 was inhibited by the metalloproteinase inhibitor GM6001 in CHO-K1 cells transfected with LR11 cDNA.22 To confirm the effects of GM6001 in non-adherent hematopoietic cells, we assessed the PMA-stimulated shedding of LR11 in THP-1 and U937 cells. The amount of sLR11 released from these cells was significantly inhibited by treatment with GM6001 (Figure 5a), suggesting that LR11 is cleaved by ADAM17 not only in adherent cells but also non-adherent hematopoietic cells.
Figure 5.
Effect of CD9 silencing on the shedding of LR11 in THP-1 cells. (a) Effect of the metalloproteinase inhibitor GM6001 on the shedding of LR11 in non-adherent hematopoietic cells. PMA-stimulated (20 ng ml−1, 24 h) THP-1 and U937 cells were incubated with or without GM6001 (50 μM) for 24 h. The protein levels of sLR11 released from these cells were analyzed by western blot analysis. (b) The effect of shRNA-mediated CD9 silencing in THP-1 cells. THP-1 cells stably transfected with the CD9-targeted shRNA or with control shRNA (mock control) were generated as described in Materials and methods. The cell surface expression levels of CD9 in the CD9 shRNA-silenced-THP-1 cells (clone #8; dashed line) and mock controls (clone #1; solid line) with or without the stimulation of PMA (20 ng ml−1, 24 h) were evaluated by flow cytometry (upper panel). Gray-colored histograms represent the cells labeled with an isotype control antibody. Cells were either unstimulated or stimulated for 24 h with 20 ng ml−1 PMA in the presence or absence of GM6001 (50 μM). The protein levels of sLR11 released from the cells were analyzed by western blot analysis. In all cases, the cells were cultured with fresh serum-free media. Data are shown as the fold increase relative to the control and presented as the mean±s.d. (error bars, n=3). *P<0.05; ns, not significant; ND, not detected.
We next investigated the effect of CD9 silencing on the ADAM17-mediated shedding of LR11 from THP-1 cells treated with GM6001. As shown in Figure 5b (upper panel), a significant reduction in the cell surface expression of CD9 was achieved regardless of stimulation of PMA in CD9 shRNA-silenced THP-1 cells. The PMA-stimulated release of sLR11 significantly increased in CD9 shRNA-silenced THP-1 cells compared with that of the cells transfected with the control shRNA, and this increase was negated by treatment with GM6001 (Figure 5b, lower panel). These results suggest that CD9 inhibits the shedding of LR11 in leukemia cells, and this effect is mediated by ADAM17.
Discussion
In this study, we showed that neo-expression of CD9 inhibits the shedding of LR11, whereas treatment with neutralizing anti-CD9 mAb or CD9 silencing had the opposite effects for the ADAM17-mediated shedding of LR11 in various leukemia cell lines. This role of CD9 in the shedding of LR11 is in agreement with a recent report demonstrating the role of CD9 in the shedding of TNF-α and intercellular adhesion molecule-1.16 In that report, the PMA-stimulated shedding of TNF-α and intercellular adhesion molecule-1 proteins after CD9 silencing both in THP-1 cells and in CD9-positive Jurkat T-cell line was investigated. Therefore, we also examined the shedding of LR11 in Jurkat T cells. Although the LR11 mRNA and cellular LR11 protein were expressed, LR11 was not expressed on the cell surface, and the sLR11 released from the cells could not be detected in Jurkat T cells (data not shown). The difference between the expression of mRNA and cell surface expression of LR11 in Jurkat T cells was also observed in normal CD3+ T cells (Figure 1). In addition, sLR11 was not released even after the stimulation of PMA±ionomycin or after cross-linking CD3 receptors in CD9 shRNA-silenced Jurkat T cells. These results suggest that LR11 is only expressed in cytoplasm in CD3+ T cells and Jurkat T cells. Furthermore, the association between LR11 and CD9 on the cell surface may have an important role in the ADAM17-mediated shedding mechanism.
Tetraspanins are not only expressed on the plasma membrane of cells but also within various types of intracellular vesicles involved in the endocytic pathway, particularly in exosomes.23 It has been reported that CD9 interacts with metalloprotease CD10 and enhances its release via exosomes.24 In that report, although the authors did not confirm CD9-dependent changes in the expression or peptidase activity of CD10 on the cell surface, both in exosomes were increased when CD9 was ectopically expressed in K562 CD10-positive cells. Meanwhile, our data suggest that CD9 has an inhibitory effect on the ADAM17-mediated shedding of LR11. Therefore, CD9 is predicted to have different roles on the cell surface and in exosomes.
We have demonstrated that high levels of sLR11 have a significant negative prognostic impact on the progression-free survival in FL patients.10 Because the immunohistological staining intensity of LR11 in lymph nodes of FL patients did not show significant association with the levels of serum sLR11, we hypothesized that the expression of CD9 in lymph nodes of FL patients may influence this lack of association. However, the expression of CD9 was not detected by immunohistology in lymph nodes of any FL patients although the number of samples tested was limited (data not shown). This result suggests that there may be another modulator of the shedding of LR11 other than CD9. In fact, it has been demonstrated that different types of tetraspanins, including CD81 and CD82, associate with ADAM10, the most closely-related family member to ADAM17, in its cleavage of TNF-α and epidermal growth factor.25 Therefore, there may be a number of molecules, of which CD9 may be one, which modulate the shedding mechanism of LR11.
We have also demonstrated that hypoxia induces the cellular expression and the released protein levels of LR11, and sLR11 regulates hypoxia-enhanced adhesion in immature hematological cells.26 Hypoxia also enhances the expression of TNF-α converting enzyme mRNA and activity of ADAM17, as shown by the increase in TNF-α shedding rate in synovial cells.27 On the other hand, it has been reported that CD9 is contained within secreted proteins and exosomes from tumor cells that have the potential to modulate the tumor's microenvironment to facilitate angiogenesis and metastasis under hypoxia.28 Although the regulation of ADAM17 and CD9 in hematopoietic cells under hypoxic conditions is not well-known, the LR11-ADAM17-CD9 association may affect the functions of hematopoietic cell, such as migration and adhesion, even in hypoxia.
In summary, we have shown that tetraspanin CD9 modulates the ADAM17-mediated shedding of LR11 in various leukemia cell lines. Because LR11 and CD9 colocalized on the cell surface of PMA/THP-1 and because CD9 had no effect on the shedding of LR11 in Jurkat T cells, in which LR11 is only expressed intracellularly, we conclude that the association between LR11 and CD9 on the cell surface has an important role in the ADAM17-mediated shedding mechanism.
Acknowledgments
This study was supported by Health and Labor Sciences Research Grants for Translational Research, Japan (HB) and grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology (NS).
The authors declare no conflict of interest.
References
- Yamazaki H, Bujo H, Kusunoki J, Seimiya K, Kanaki T, Morisaki N, et al. Elements of neural adhesion molecules and a yeast vacuolar protein sorting receptor are present in a novel mammalian low density lipoprotein receptor family member. J Biol Chem. 1996;271:24761–24768. doi: 10.1074/jbc.271.40.24761. [DOI] [PubMed] [Google Scholar]
- Taira K, Bujo H, Hirayama S, Yamazaki H, Kanaki T, Takahashi K, et al. LR11, a mosaic LDL receptor family member, mediates the uptake of ApoE-rich lipoproteins in vitro. Arterioscler Thromb Vasc Biol. 2001;21:1501–1506. doi: 10.1161/hq0901.094500. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Bujo H, Yamazaki H, Ohwaki K, Jiang M, Hirayama S, et al. LR11, an LDL receptor gene family member, is a novel regulator of smooth muscle cell migration. Circ Res. 2004;94:752–758. doi: 10.1161/01.RES.0000120862.79154.0F. [DOI] [PubMed] [Google Scholar]
- Jiang M, Bujo H, Ohwaki K, Unoki H, Yamazaki H, Kanaki T, et al. Ang II-stimulated migration of vascular smooth muscle cells is dependent on LR11 in mice. J Clin Invest. 2008;118:2733–2746. doi: 10.1172/JCI32381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Bujo H, Shiba T, Jiang M, Maeno T, Shirai K. Enhanced circulating soluble LR11 in patients with diabetic retinopathy. Am J Ophthalmol. 2012;154:187–192. doi: 10.1016/j.ajo.2012.01.035. [DOI] [PubMed] [Google Scholar]
- Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, Zou F, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi T, Hirayama S, Miida T, Fukamachi I, Tokutake T, Ebinuma H, et al. Increased levels of soluble LR11 in cerebrospinal fluid of patients with Alzheimer disease. Dement Geriatr Cogn Disord. 2010;30:28–32. doi: 10.1159/000315539. [DOI] [PubMed] [Google Scholar]
- Guo LH, Westerteicher C, Wang XH, Kratzer M, Tsolakidou A, Jiang M, et al. SORL1 genetic variants and cerebrospinal fluid biomarkers of Alzheimer's disease. Eur Arch Psychiatry Clin Neurosci. 2012;262:529–534. doi: 10.1007/s00406-012-0295-x. [DOI] [PubMed] [Google Scholar]
- Sakai S, Nakaseko C, Takeuchi M, Ohwada C, Shimizu N, Tsukamoto S, et al. Circulating soluble LR11/SorLA levels are highly increased and ameliorated by chemotherapy in acute leukemias. Clin Chim Acta. 2012;413:1542–1548. doi: 10.1016/j.cca.2012.06.025. [DOI] [PubMed] [Google Scholar]
- Kawaguchi T, Ohwada C, Takeuchi M, Shimizu N, Sakaida E, Takeda Y, et al. LR11: a novel biomarker identified in follicular lymphoma. Br J Haematol. 2013;163:277–280. doi: 10.1111/bjh.12467. [DOI] [PubMed] [Google Scholar]
- Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Mol Aspects Med. 2008;29:258–289. doi: 10.1016/j.mam.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezyk R, Bzowska M, Bereta J. Structure and functions of tumor necrosis factor-alpha converting enzyme. Acta Biochim Pol. 2003;50:625–645. [PubMed] [Google Scholar]
- Scheller J, Chalaris A, Garbers C, Rose-John S. ADAM17: a molecular switch to control inflammation and tissue regeneration. Trends Immunol. 2011;32:380–387. doi: 10.1016/j.it.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Guo L, Eisenman JR, Mahimkar RM, Peschon JJ, Paxton RJ, Black RA, et al. A proteomic approach for the identification of cell-surface proteins shed by metalloproteases. Mol Cell Proteomics. 2002;1:30–36. doi: 10.1074/mcp.m100020-mcp200. [DOI] [PubMed] [Google Scholar]
- Hampe W, Riedel IB, Lintzel J, Bader CO, Franke I, Schaller HC. Ectodomain shedding, translocation and synthesis of SorLA are stimulated by its ligand head activator. J Cell Sci. 2000;113:4475–4485. doi: 10.1242/jcs.113.24.4475. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Lopez MD, Gilsanz A, Yanez-Mo M, Ovalle S, Lafuente EM, Dominguez C, et al. The sheddase activity of ADAM17/TACE is regulated by the tetraspanin CD9. Cell Mol Life Sci. 2011;68:3275–3292. doi: 10.1007/s00018-011-0639-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo M, Ebinuma H, Fukamachi I, Jiang M, Bujo H, Saito Y. Development of an immunoassay for the quantification of soluble LR11, a circulating marker of atherosclerosis. Clin Chem. 2009;55:1801–1808. doi: 10.1373/clinchem.2009.127027. [DOI] [PubMed] [Google Scholar]
- Ohwaki K, Bujo H, Jiang M, Yamazaki H, Schneider WJ, Saito Y. A secreted soluble form of LR11, specifically expressed in intimal smooth muscle cells, accelerates formation of lipid-laden macrophages. Arterioscler Thromb Vasc Biol. 2007;27:1050–1056. doi: 10.1161/ATVBAHA.106.137091. [DOI] [PubMed] [Google Scholar]
- Leung KT, Chan KY, Ng PC, Lau TK, Chiu WM, Tsang KS, et al. The tetraspanin CD9 regulates migration, adhesion, and homing of human cord blood CD34+ hematopoietic stem and progenitor cells. Blood. 2011;117:1840–1850. doi: 10.1182/blood-2010-04-281329. [DOI] [PubMed] [Google Scholar]
- Huang CL, Liu D, Masuya D, Kameyama K, Nakashima T, Yokomise H, et al. MRP-1/CD9 gene transduction downregulates Wnt signal pathways. Oncogene. 2004;23:7475–7483. doi: 10.1038/sj.onc.1208063. [DOI] [PubMed] [Google Scholar]
- Gliemann J, Hermey G, Nykjaer A, Petersen CM, Jacobsen C, Andreasen PA. The mosaic receptor sorLA/LR11 binds components of the plasminogen-activating system and platelet-derived growth factor-BB similarly to LRP1 (low-density lipoprotein receptor-related protein), but mediates slow internalization of bound ligand. Biochem J. 2004;381:203–212. doi: 10.1042/BJ20040149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermey G, Sjogaard SS, Petersen CM, Nykjaer A, Gliemann J. Tumour necrosis factor alpha-converting enzyme mediates ectodomain shedding of Vps10p-domain receptor family members. Biochem J. 2006;395:285–293. doi: 10.1042/BJ20051364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanez-Mo M, Gutierrez-Lopez MD, Cabanas C. Functional interplay between tetraspanins and proteases. Cell Mol Life Sci. 2011;68:3323–3335. doi: 10.1007/s00018-011-0746-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurov D, Barbashova L, Filatov A. Tetraspanin protein CD9 interacts with metalloprotease CD10 and enhances its release via exosomes. FEBS J. 2013;280:1200–1213. doi: 10.1111/febs.12110. [DOI] [PubMed] [Google Scholar]
- Arduise C, Abache T, Li L, Billard M, Chabanon A, Ludwig A, et al. Tetraspanins regulate ADAM10-mediated cleavage of TNF-alpha and epidermal growth factor. J Immunol. 2008;181:7002–7013. doi: 10.4049/jimmunol.181.10.7002. [DOI] [PubMed] [Google Scholar]
- Nishii K, Nakaseko C, Jiang M, Shimizu N, Takeuchi M, Schneider WJ, et al. The soluble form of LR11 protein is a regulator of hypoxia-induced, urokinase-type plasminogen activator receptor (uPAR)-mediated adhesion of immature hematological cells. J Biol Chem. 2013;288:11877–11886. doi: 10.1074/jbc.M112.442491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau M, Harper K, Grondin F, Pelmus M, McDonald PP, Dubois CM. Hypoxia-inducible factor mediates hypoxic and tumor necrosis factor alpha-induced increases in tumor necrosis factor-alpha converting enzyme/ADAM17 expression by synovial cells. J Biol Chem. 2007;282:33714–33724. doi: 10.1074/jbc.M704041200. [DOI] [PubMed] [Google Scholar]
- Park JE, Tan HS, Datta A, Lai RC, Zhang H, Meng W, et al. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol Cell Proteomics. 2010;9:1085–1099. doi: 10.1074/mcp.M900381-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]