Abstract
Bronchiolar Clara cells play a critical role in lung homoeostasis. The main goal of this study was to evaluate the effects of chronic allergy on these cells and the efficacy of budesonide (BUD) and montelukast (MK) in restoring their typical phenotypes after ovalbumin-induced chronic allergy in mice. Chronic allergy induced extensive bronchiolar Alcian blue-periodic acid-Schiff (AB/PAS)-positive metaplasia. In addition, cells accumulated numerous big electron-lucent granules negative for Clara cell main secretory protein (CC16), and consequently, CC16 was significantly reduced in bronchoalveolar lavage. A concomitant reduction in SP-D and CYP2E1 content was observed. The phenotypic changes induced by allergy were pharmacologically reversed by both treatments; MK was more efficient than BUD in doing so. MK decreased AB/PAS reactivity to control levels whereas they remained persistently elevated after BUD. Moreover, most non-ciliated cells recovered their normal morphology after MK, whereas for BUD normal cells coexisted with ‘transitional’ cells that contained remnant mucous granules and stained strongly for CC16 and SP-D. Glucocorticoids were also less able to reduce inflammatory infiltration and maintained higher percentage of neutrophils, which may have contributed to prolonged mucin expression. These results show that chronic allergy-induced mucous metaplasia of Clara cells affects their defensive mechanisms. However, anti-inflammatory treatments were able to re-establish the normal phenotype of Clara cell, with MK being more efficient at restoring a normal profile than BUD. This study highlights the role of epithelial cells in lung injuries and their contribution to anti-inflammatory therapies.
Keywords: asthma treatment, CC16, chronic inflammation, Clara cells
The epithelium lining the respiratory tract is the first target for many environmental contaminants and micro-organisms. In the last decade, airway epithelial cells have been recognized as active components of host defence (Bals & Hiemstra 2004; Bartlett et al. 2008), with bronchiolar Clara cells representing key players in lung homoeostasis. These cells have a rich p450 cytochrome content that contributes to toxic compound detoxification (Forkert 1995), and secrete molecules which play a role in the defence against pathogens, such as surfactant protein D (SP-D), and in inflammatory response control such as Clara cell secretory protein (CCSP also known as CC10 or CC16). CC16 is the prototypic member of the subfamily of secretoglobins, having anti-inflammatory functions (Ni et al. 2000) mainly due to diverse biochemical and biological properties among which are the inhibition of phospholipase A2 (Levin et al. 1986) and chemotaxis of neutrophils and monocytes (Vasanthakumar et al. 1988). Furthermore, CC16 is an immunomodulator that interferes with the cytokine network by downregulating proinflammatory Th1 cytokines, including IFNγ, IL-1, IL-6 and TNFα (Dierynck et al. 1995). SP-D binds to the carbohydrate structures expressed by a wide variety of micro-organisms, allergens and apoptotic cells and thereby functions as an opsonin to enhance the uptake of these cells and particles (Crouch et al. 2000). In this way, SP-D thereby initiates effector mechanisms of innate immunity and modulates the inflammatory response in the lung.
Clara cells and their secretory products control Th2 cytokines and eosinophil infiltration in the inflammatory response induced in asthma, and SP-D modulates this allergic inflammation by several mechanisms, including the downregulation of eosinophilic infiltration, the skewing of cytokines towards a Th1 profile (Liu et al. 2005) and the modulation of the IgE-mediated mast cell functions (Malherbe et al. 2005). In addition, SP-D inhibits early histamine release from basophils after an allergen challenge (Erpenbeck et al. 2006). CC16 has been linked to asthma regulation, with studies on CC16-deficient mice showing an increase in Th2 cytokine levels, eosinophilia and lung mucous production after being challenged with ovalbumin (OVA) (Chen et al. 2001; Wang et al. 2001b). Moreover, CC16 was also shown to suppress Th2 cytokine expression (Hung et al. 2004), thus playing a direct role in the regulation of the T-cell-mediated inflammatory response and thereby complementing Clara cell strategies to maintain lung homeostasis. In addition, we previously reported that Clara cells show hypertrophy in response to an acute allergic OVA challenge, and they can also markedly increase their stored CC16 content in big secretory granules (Roth et al. 2007).
In many cases, however, chronic inflammation might reduce or even suppress the early defensive strategy triggered by Clara cells. Several works have demonstrated that mucous hypersecretion, which is a hallmark of chronic airway disease, is due in part to Clara cell metaplasia via EGFR signalling in proximal and distal airways (Shim et al. 2001; Kim et al. 2002; Reader et al. 2003; Evans et al. 2004). This phenotypic change may explain the reduction in CC16 levels reported in serum and bronchoalveolar lavage (BAL) (Van Vyve et al. 1995; Shijubo et al. 1999b), as well as the decreased number of Clara cells found in airways of patients with severe asthma (Shijubo et al. 1999a). However, the severity at which metaplasia alters the Clara cell population and whether treatment with anti-inflammatory agents can revert to a normal phenotype still remains to be determined.
Two of the main anti-inflammatory therapies widely used to treat asthma are inhaled glucocorticoids and leukotriene receptor antagonists. Their different mechanisms of action allow for the possibility of evaluating their putative differential effects on Clara cell metaplasia. Inhaled glucocorticoids, such as budesonide (BUD), are considered to be the most effective anti-inflammatory therapy currently available for the treatment of persistent asthma (Necela & Cidlowski 2004), whereas leukotriene receptor antagonists, such as montelukast (MK), have also been demonstrated to be efficient monotherapy controllers in children with mild asthma (Knorr et al. 2001; Wahn & Balachandra Dass 2008). Moreover, MK and other antagonists have the advantage of being orally administered drugs. Even though both glucocorticoids and leukotriene receptor antagonists have been shown to reduce goblet cell hyperplasia/metaplasia and mucous hypersecretion in experimental models (Kumar et al. 2003; Henderson et al. 2006), it has not yet been established whether they are effective in restoring non-mucous Clara cells and their normal secretory activity.
The main goals of this study were to evaluate the effects of chronic allergy on Clara cell biology and to investigate the efficacy of BUD and MK in restoring their typical phenotypes in mouse airways. To this end, we performed a detailed analysis of non-ciliated bronchiolar cells by electron microscopy and immunogold for CC16 and investigated metaplasia progression by AB/PAS and EGFR analysis in correlation with Clara cell expression of CC16, SPD and CYP2E, with the latter being the main P450 enzyme present in these cells.
Material and methods
Animals and tissue processing
Six- to eight-week-old female mice of Balb/c strain (6 animals/group in three different experiments) were maintained under controlled temperature (21 ± 3 °C) and lighting conditions (14 h light/10 h dark), with free access to tap water and OVA free commercial lab chow (Cargill, Argentina). Four different experimental groups were studied as following (Figure 1): Allergic group: animals were i.p inoculated with 0.1 ml of OVA grade VI (100 μg, Sigma-Aldrich, St. Louis, MO, USA) absorbed to 2 mg of alum adjuvant (Pierce, Rockford, IL, USA) (day 0). A boosting injection was then administered in the same manner 2 weeks later (day 14), and the mice were challenged with daily exposures to 1% OVA solution in saline (grade V Sigma-Aldrich) and nebulized for 30 min from days 24 to 54. This aerosol was generated by an ultrasonic nebulizer (Respirex, Córdoba, Argentina) and used 20 min/day for 7 consecutive days at a flow rate of 1 ml/min and a particle size of 2–8 μm. From day 55 to 61, the OVA solution was replaced by saline; Budesonide group (BUD group): animals were first treated as in the allergic group. However, after the last OVA nebulization on day 55, mice were treated with an aerosol of BUD solution (45 μg/ml) (Phoenix Labs, Buenos Aires, Argentina) for 30 min over 7 consecutive days (days 55 to 61); Montelukast group (MK group): animals were subjected to the same protocol as the allergic group, but from days 55 to 61, the mice were treated with oral MK (5 mg/Kg); Control group: OVA-sensitization between days 0 and 14, followed by exposure to saline solution from day 24 to 54 instead of the OVA challenge.
Figure 1.
Schematic representation of the experimental protocol for allergic lung inflammation and treatments.
Twenty-four hours after each group protocol, mice were anaesthetized with an i.p. injection of 10 mg of chloral hydrate (Merck, Darmstadt, Germany) and exsanguinated via the renal artery. The left lungs of three mice per group in three different experiments were fixed for morphological analysis by means of intratracheal perfusion as described below. Bronchoalveolar lavage was collected from the right lungs of three animals per group in three different experiments for Western blotting, and the lung tissue was then lysed on ice with the addition of 200 μl of cold phosphate buffer saline (PBS) containing 1.25% Igepal CA-630, 1 mM EDTA, 2 mM PMSF, 10 μg/ml leupeptin and 10 μg/ml aprotinin. The lysate was centrifuged at 14,000 g for 30 min at 4 °C to pellet the Igepal CA-630-insoluble material, and the supernatant was withdrawn and stored in aliquots frozen at −70 °C until required.
Ethical approval
All the experimental protocols were performed following the NIH guidelines for animal care and in compliance with federal, state and local laws on the ethical use of experimental animals.
Bronchoalveolar lavage collection, cell count and cytology
The trachea was cannulated with a 23-gauge blunt-tipped needle connected to surgical tubing, and BAL was obtained by three serial intratracheal instillations of 1 ml of cold (4 °C) PBS into the lung, with the aliquots being pooled. Cells were isolated by centrifugation at 200 g, and then the supernatant was withdrawn and stored at −70 °C for analysis of CC16 by Western blot. Pellets were resuspended in 500 μl of PBS and viable cells counted in a haemocytometer after vital staining with 0.5% trypan blue of the BAL suspension diluted 1:1. To characterize the inflammatory cell types, 5 × 104 cells were monolayered in a cytospin centrifuge (Shandon Southern Instruments Inc., Sewickely, PA, USA) and stained with May–Grünwald–Giemsa. The percentages of the different cell populations (eosinophils, neutrophils and macrophages) were determined for three cytospins gathered from each mouse. A total of 2000 cells per group were used to calculate the final percentages.
Western Blot analysis
The levels of CC16 were determined in BAL, while CYP2E1 and SP-D were evaluated in lung homogenates. Proteins from bronchoalveolar lavages (10 μg) and lung homogenates (50 μg) were run in 15% acrylamide gel according to Roth et al. (Roth et al. 2007). Proteins were transferred to a nitrocellulose membrane, and non-specific binding was blocked at RT with PBS containing 5% dried skimmed milk and 0.1% Tween-20 (blocking buffer). Membranes were rinsed and incubated for 2 h with one of the following antibodies: rabbit anti-CC16 diluted 1:5000 (R42, kindly provided by Dr. Jan Ryerse of the University of Saint Louis, USA), rabbit anti-SP-D diluted 1:1000 (Chemicon, Temecula, CA, USA) or goat anti-CYP2E1 diluted 1:1000 (PR32; Oxford Biomedical Research, Rochester Hills, MI, USA). Finally, blots were incubated with a Peroxidase–conjugated (HRP) goat anti-rabbit secondary antibody (Jackson Immunoresearch Labs Inc, West Grove, PA, USA), or using an HRP-conjugated bovine anti-goat IgG (sc-2352, Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted in blocking buffer (1:5000). Blots were thoroughly rinsed in PBS/0.1% Tween-20, and the HRP-coupled secondary antibody was exposed with ECL Western blot detection reagents (Amersham Biosciences, Bucks, UK) following the manufacturer's instructions. Emitted light was captured on Hyperfilm (Amersham-Pharmacia-Biotech, Bucks, UK). Semiquantitative signals were determined by densitometric analysis performed by applying the Scion Image software (V. beta 4.0.2, Scion Image Corp., Frederick, MD, USA), with the relative expression given in pixels2 (area units). The expression of β-actin, determined with a monoclonal anti β-actin (Sigma) (diluted 1:5000), was used as an internal control to confirm the equivalent total protein loading.
Light and electron microscopy
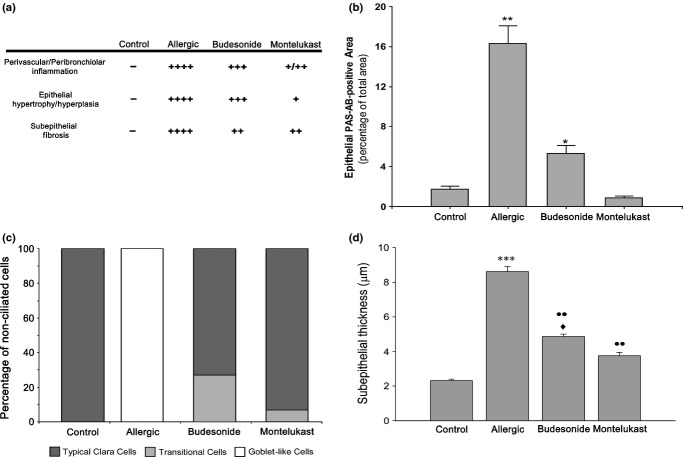
Lung blocks were formalin-fixed and processed for paraffin inclusion, and sections (5 μm) of the left lung lobe were obtained for staining with HE (haematoxylin–eosin) or AB/PAS. To identify mucous-secreting cells in the bronchiolar epithelium, these sections were first deparaffinized in xylene and hydrated in decreasing concentrations of ethanol, and then stained in AB for 10 min, washed in running water for 5 min, oxidized in 1% periodic acid for 10 min and washed again for another 5 min before being stained in Schiff's reagent for 10 min. Additionally, a histological scoring on HE sections was performed considering the grade of inflammation, the thickness of the epithelial layer and subepithelial fibrosis. To this end, three independent operators analysed at least five slides per animal and categorized those parameters as follows: scarce (+), mild (++), moderate (+++) or intense (++++). A total of three rats per group were evaluated, and the average of all observations was represented.
For semithin and ultrastructural analysis, the tracheal lumen of animals was perfused at 20-cm water pressure with a mixture of 1% (v/v) glutaraldehyde and 2% (w/v) formaldehyde in 0.1 M cacodylate buffer. Lungs were removed and then treated with 1% osmium tetroxide, before being dehydrated and embedded in Araldite. For light microscopy, 1-micron thick sections were cut serially and stained with a 1% toluidine blue and 1% borax solution. For ultrastructural studies and morphometric analysis, thin sections were cut with a diamond knife on a JEOL JUM-7 ultramicrotome and then examined using a Zeiss LEO 906E electron microscope.
Immunohistochemistry for CC16 and SP-D
Paraffin-embedded lung sections (5 μm) were deparaffinized, and antigen retrieval was performed in a microwave oven in 10 mM citric acid buffer, pH 6.0, for 14 min. Slides were allowed to cool to RT and then washed three times in PBS before being incubated with methanol-H2O2 (96:4, v/v) for 15 min to inhibit endogenous peroxidase. Non-specific binding was blocked with PBS-milk containing 5% (v/v) normal goat serum (Sigma) for 30 min. Then, the sections were incubated overnight at 4 °C with a SP-D polyclonal primary antibody (rabbit anti-mouse SP-D, which cross-reacts with rat SP-D, Chemicon, 1:1000), or with a rabbit anti-CC16 (Santa Cruz Biotechnology sc-25554) diluted 1:1000, both at 4 °C overnight. Bound antibodies were detected by an indirect technique using a biotin-labelled goat anti-rabbit antibody (Vector Laboratories, Burlingame, CA, USA) diluted 1:150 in PBS containing 1% normal goat serum. After incubation for 30 min at RT, the sections were treated with Vectastain Elite ABC complex (Vector), following the manufacturer's protocol. Staining was developed with 3,30-diaminobenzidine (Sigma) in a 0.1 M Tris buffer, pH 7.2, containing 0.03% H2O2. Slides were dehydrated, counterstained with Harris haematoxylin and mounted with Entellan (Merck).
Immunocytochemistry with gold complexes
After fixation, lungs were dehydrated, embedded in LR White (London Resin Corporation, Berkshire, UK) and polymerized at 50 °C.
To determine the CC16 expression and positive cell count, semithin sections of lung tissues were treated with PBS containing 1% bovine serum albumin (PBS–BSA) for 30 min and then incubated overnight at 4 °C with rabbit anti-CC16 antibody (R42) diluted 1:300, followed by an anti-rabbit gold complex (Prod. Number 15727; Ted Pella Inc., Redding, CA, USA) using a 1:20 dilution for 1 h at 37 °C. Slides were rinsed thoroughly with deionized water and incubated with a silver enhancement complex (Sigma) for 7 min.
For immunoelectron microscopy, thin sections about 60 nm thick were mounted on nickel grids and incubated overnight on the primary antibody rabbit anti-CC16 diluted 1:300 (R42, kindly provided by Dr. Jan Ryerse of The University of Saint Louis, USA), on goat anti-CYP2E1 (PR32; Oxford Biomedical Research) diluted 1:1000 or on mouse monoclonal anti-MUC5AC diluted 1:100 (sc-21701, Santa Cruz Biotechnology). Specific binding was examined by applying an anti-rabbit gold complex diluted 1:20 (Prod. Number 15727; Ted Pella Inc.), an anti-goat IgG-gold complex diluted 1:15 (25232; Electron Microscopy Sciences, Hatfield, PA, USA) or an anti-mouse IgG-gold complex diluted 1:20 (25133; Electron Microscopy Sciences).
To assess primary antibody specificity, additional slides were incubated in parallel, but the primary antibodies were replaced with 1% normal rabbit serum. To control non-specific binding of the secondary antibody, the primary antibody was replaced with PBS–BSA.
Morphometric analysis
All morphologic and morphometric evaluations were performed on large bronchioles (900–1700 μm diameter), obtained from a total of 3 animals per group. AB/PAS-positive cells were quantified in paraffin lung sections using pictures taken at 40×, by evaluating a total of 15 bronchioles per animal and calculating the percentage of positive area. The scoring of AB/PAS reactivity was performed with a computer-assisted imaging system (Image J, version 1.44p by NIH, Bethesda, MD, USA), based on the intensity and the stained area measured in each field of vision, and expressed as epithelial AB/PAS-positive area per total area.
CC16-positive cells were identified by performing gold immunostaining with silver enhancement on a total of 5 LR White semithin sections obtained at different lung levels and analysing a total of 2000 bronchiolar epithelial cells per animal. The immunoreactive cells were counted at 40× and expressed as the number of CC16 immunopositive cells per mm of basal membrane, using the Image J software. All positive cells were included even when the label was low.
To compare the effects of BUD and MK on the Clara cell phenotype, we performed an ultrastructural evaluation. For this purpose, thin sections were obtained from 3 different regions of Araldite-embedded lungs, and 30 pictures per group taken at 2300× were analysed. A total of 200 non-ciliated cells per group were evaluated, and these were classified as normal or mucous cells. When they were categorized as normal, they had recovered the typical cupola with abundant paranuclear mitochondria and small electron-dense secretory granules localized under the plasma membrane. In contrast, mucous cells included the goblet-like cells, with large electron-lucent fused granule and also the transitional cells that still exhibited a hypertrophied cytoplasm containing electron-lucent granules coexisting with the typical electron-dense granules from Clara cells.
Finally, thin Araldite lung sections were used to determine the bronchiolar subepithelial stroma thickness including the basal membrane and smooth muscle cell layers. For this purpose, thin sections were obtained from 3 different regions and mounted on nickel grids. A total of 30 pictures per group were taken by the electron microscope at a 2300× magnification, and the subepithelial thickness was measured at 3 different points for each picture using the MegaVision soft imaging system (v 3.2 Build 831).
Statistical analysis
Data obtained from Western blots, and cell counts were expressed as the mean ± SEM of three independent experiments (n = 3 per group) and analysed by a one-way anova, followed by post hoc comparison with the Tukey–Kramer test. For β-actin expression, a Fisher's post-test was applied. A significance level of P < 0.05 was used for all tests.
Results
Bronchiolar Clara cells undergo mucous transformation when submitted to chronic allergy
For the semithin sections of the control bronchioles, the typical secretory Clara cells stained strongly with toluidine blue and appeared to be protruding the apical cytoplasm towards the lumen (Figure 2a), with the epithelium being negative for AB/PAS (Figure 2b). After OVA challenges for 30 consecutive days, the bronchiolar epithelium appeared tall and highly hypertrophied, with stratified-like aspect. In addition, most typical Clara cells had changed to large hypertrophied secretory cells, characterized by a wide cupola that overwhelmed neighbouring ciliated cells and stained weaker with toluidine blue compared with controls (Figure 2c). Together with these morphological changes, the bronchiolar epithelium from allergic animals exhibited numerous AB/PAS-positive cells in the paraffin sections (Figure 2d).
Figure 2.
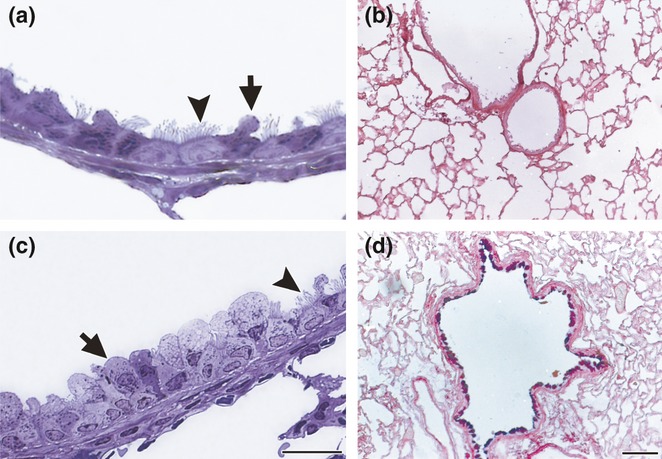
Bronchiolar epithelium in chronic allergy: semithin sections of lung embedded in araldite resin and stained with toluidine blue (a and c); paraffin sections stained with AB/PAS (b and d). In control lung, the epithelium was thin and the secretory Clara cells stained strongly with toluidine blue (arrows), which delineates in dark blue the secretory granules under the plasma membrane (a). Control lungs were negative after AB/PAS stain (b). After chronic exposure to OVA, the epithelium was tall, with non-ciliated cells appearing as a wide apical cytoplasm that stained pale with toluidine blue. Ciliated cells (arrowheads) appeared in a basal position in relation to non-ciliated cells. A thick stromal layer, composed of collagen, fibroblasts and smooth cells, underlay the epithelium (c). An intense AB/PAS stain was observed in bronchioles exposed to chronic inflammation (d). Bars represent 20 μm in (a) and (c), and 200 μm in (b) and (d).
When observed by electron microscopy, the allergic group showed that most non-ciliated cells had lost their typical profile characterized by the presence of numerous mitochondria and small apical electron-dense secretory granules (Figure 3a). These cells now exhibited a phenotype compatible with mucous-secreting cells, being tall cells with the nuclei confined to the basal compartment, high electron-dense cytoplasm and filled up with rough endoplasmic reticulum cisternae and scarce thin mitochondria. In addition, the apical cytoplasm contained large fused electron-lucent secretory granules (Figure 3c) that immunostained for mucin, as demonstrated by immunoelectron microscopy (inset Figure 3c). Collectively, these morphological, histochemical and immunocytochemical results indicated that the Clara cells underwent mucous metaplasia in the bronchioles after OVA challenges.
Figure 3.
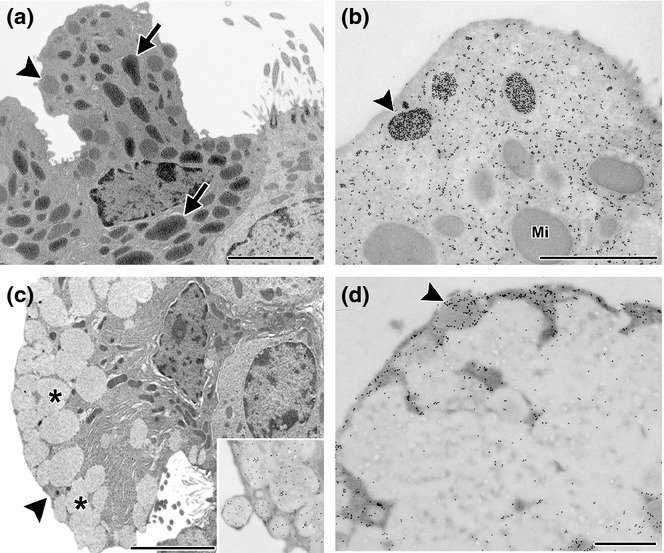
Clara cells in chronic allergy. Electron microscopy (a and c) and immunoelectron microscopy for CC16 (b and d). In control mice, cells typically contained numerous mitochondria (arrow) and a few small secretory granules with round profiles and a moderate electron density (arrowhead) appearing under the plasma membrane (a). By immunogold labelling, CC16 can be observed in the cytoplasm, being very intense on some electron-dense apical secretory granules (arrowhead in b). Mi, mitochondria. In OVA challenged animals, cells appeared deeply modified, especially for the numerous large and electron-lucent fused secretory granules (*) in the whole apical cytoplasm, with abundant RER in the basal cytoplasm intermingled with some slim mitochondria (b). The large fused electron-lucent granules of these mucous transformed cells were positive for mucin (inset Figure 2b) and negative for CC16, with only a few positive small granules appearing under the plasma membrane (arrowhead) (d). Bar represents 1 μm.
Next, we investigated the expression of EGFR, which has been shown to be associated with the differentiation process in lung and described as the last link in the cascade leading to metaplasia (Shim et al. 2001; Kim et al. 2002). In the present study, Western blot confirmed that this receptor increased significantly in the lung homogenate of allergic animals (Figure 4), causing striking phenotypic changes to occur in the Clara cells.
Figure 4.
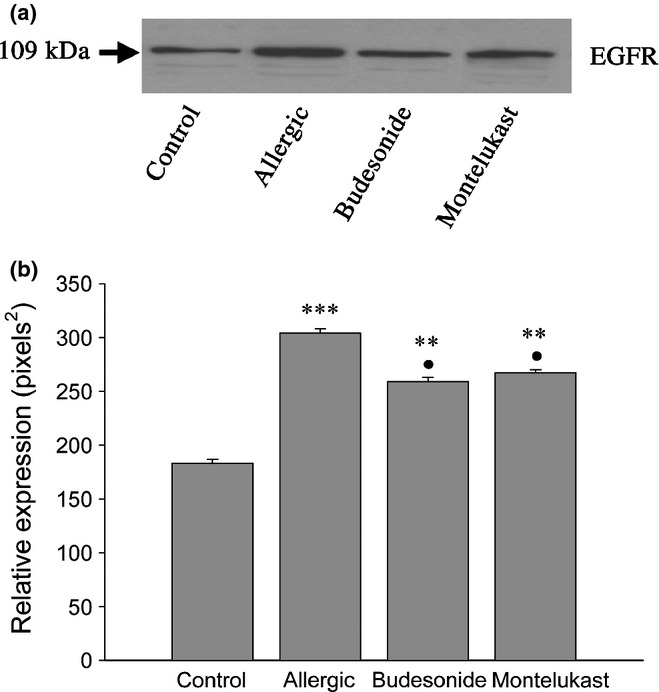
Analysis of EGFR expression by Western blot. The expression of this receptor was increased significantly in the allergic group (a and b, ***P < 0.001), with both anti-inflammatory treatments significantly reducing its level compared with the allergic group (•P < 0.05). However, expression still remained higher than control levels (**P < 0.01).
Mucous metaplasia impacts on Clara cell defensive molecules
We previously demonstrated that a hallmark of Clara cells exposed to acute allergy is overexpression of CC16 stored in big secretory granules (Roth et al. 2007). To evaluate whether the progress of mucous metaplasia can modify the capacity of Clara cells to produce proteins with key roles in lung homoeostasis, we performed immunohistochemistry and immunoelectron microscopy for CC16 and SP-D in lungs from animals chronically exposed to OVA.
Under normal conditions, the bronchiolar Clara cells exhibited strong cytoplasmic CC16, staining in the basal cytoplasm under the nuclei, which was occasionally widespread in the apical cytoplasm, as shown by immunohistochemistry (Figure 5c). In contrast, lungs from allergic mice exhibiting mainly mucous-like cells, showed a weak cytoplasmic staining, occasionally being more intense under the apical plasma membrane (Figure 5d). Moreover, although these modified cells secreted lower levels of CC16 than in the controls, as was observed in BAL when analysed by Western blot (Figure 5a, b), the total number of CC16-positive cells remained similar to that of controls (79.50 ± 1.90 vs. 74.00 ± 2.36, P < 0.05). Immunogold electron microscopy revealed that the large electron-lucent secretory granules had no CC16 expression, with the labelling being restricted to the scant cytoplasmic compartment. The occasional small remaining CC16-positive granules were localized under the plasma membrane (Figure 3d). This CC16 localization in allergic animals contrasted with controls where labelling was intense in Clara cells and localized at both the characteristic electron-dense granules under the plasma membrane, and decorating the abundant endoplasmic reticulum cisternae (Figure 3b).
Figure 5.
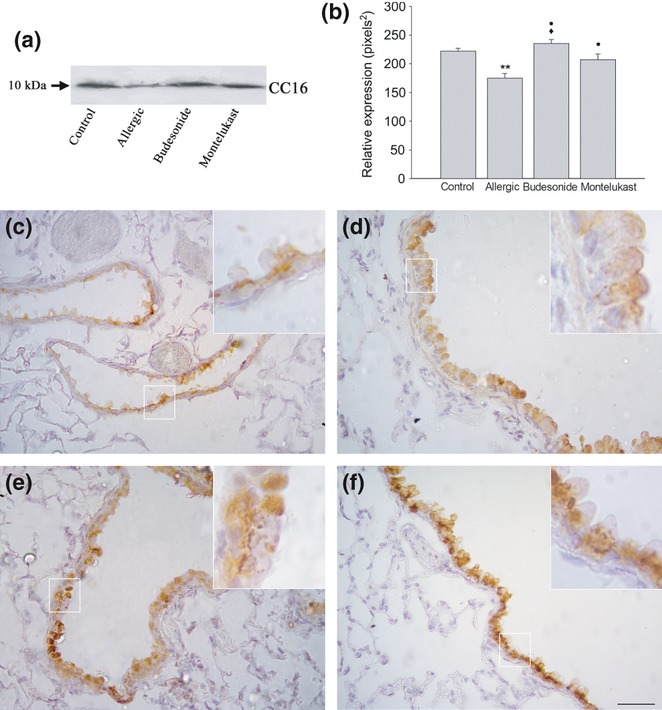
Analysis of CC16 content in chronic allergy: Western blot analysis (a and b) and paraffin sections immunolabelled with anti-CC16 antibody followed by peroxidase-DAB (c–f). A significant reduction in CC16 in bronchoalveolar lavage was noted by Western blot in comparison with control (**P < 0.01), with both anti-inflammatory treatments inducing a marked increase compared with allergic values (•P < 0.05); in budesonide (BUD), the increase was higher than in montelukast (MK) (♦P < 0.05). From immunohistochemistry, normal Clara cells exhibit CC16 labelling mainly localized under the nuclei in the basal cytoplasm and less intense in the apical cytoplasm (c); after inflammation, most cells showed a weak homogeneous stain (d). After BUD treatment, most cells exhibited homogeneous strong labelling (e), while after MK, cells with a normal profile exhibited strong labelling restricted to the basal cytoplasm (f). Bar represents 200 μm.
A reduction in the expression of SP-D in lung homogenate was also observed in the allergic group compared with the control group (Figure 6a, b). Immunohistochemical analysis verified the decrease in SP-D occurring in Clara cells, with labelling being restricted to the apical portion of the mucous cell cytoplasm in allergic animals (Figure 6d).
Figure 6.
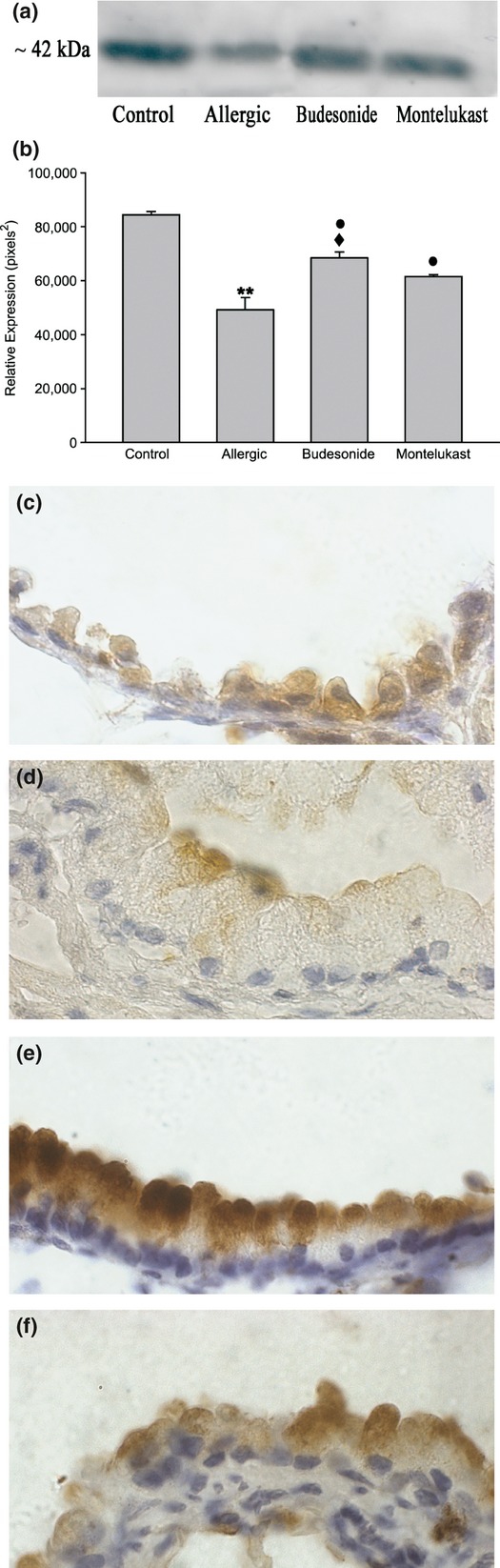
Analysis of SP-D content in chronic allergy: Western blot analysis (6a and b) and paraffin sections immunolabelled with anti-SP-D antibody followed by peroxidase-DAB (c–f). By Western blot, a significant reduction in SP-D in lung homogenate was noted in comparison with control (**P < 0.05), with both anti-inflammatory treatments inducing a marked increase compared with allergic values (•P < 0.05). By immunohistochemistry, normal Clara cells exhibited SP-D label in the whole cytoplasm, which was stronger delineating the plasma membrane (b); the hypertrophied mucous cells induced by chronic allergy expressed scarce SP-D content restricted to the apical portion under the plasma membrane (c). Budesonide (BUD) and montelukast (MK) anti-inflammatory treatments increased the antimicrobial content (d and e), which appeared to be particularly intense after BUD treatment (♦P < 0.05) (d). Bar represents 20 μm.
Changes in CYP2E1 cytochrome expression
CYP2E1, a cytochrome enzyme abundant in the Clara cells and mainly localized in mitochondria, (Roth et al. 2007) was revealed by Western blot and by the immunogold technique at the ultrastructural level. As shown in Figure 7a and b, the total lung cytochrome levels were reduced after OVA challenge. Similarly, mucous cells from allergic animals also showed reduced immunostaining for CYP2E1, which was restricted to the scant mitochondria intermingled with the mucous granules (Figure 7d) unlike in the control (Figure 7c).
Figure 7.
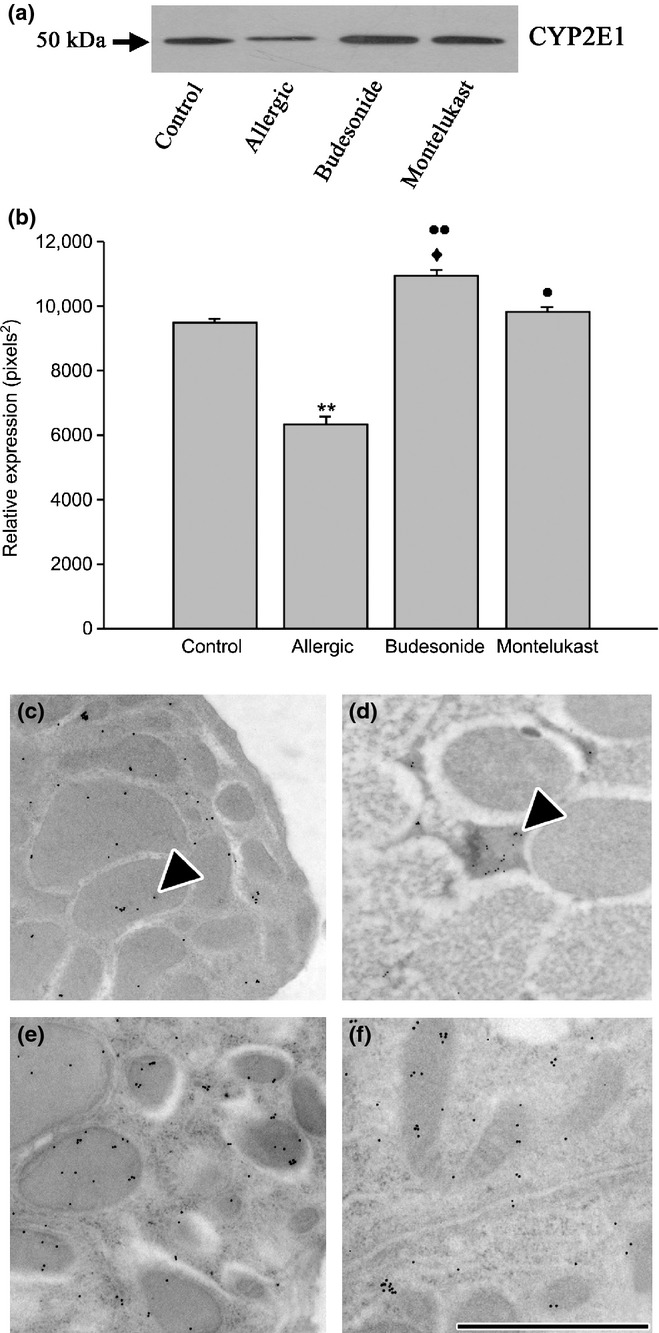
Analysis of CYP2E1 in chronic allergy: expression by Western blot (a and b) and by inmunoelectron microscopy (c–f). Western blot analysis of lung homogenate showed a decrease in CYP2E1 expression that was demonstrated to be significant (**P < 0.01). Analysis also evidenced that both budesonide (BUD) and montelukast (MK) treatments increased significantly the cytochrome expression (••P < 0.01 and •P < 0.05 respectively), but BUD reached values higher than MK (♦P < 0.05). CYP2E1 labelling by immunogold in control group showed abundant mitochondria and immunostained for the cytochrome (c); in contrast, chronic allergy produced few and small mitochondria, which were positive for CYP2E1 (d); while CYP2E1 immunostaining confirmed that the cells recovered their numerous mitochondria (arrowheads) immunolabelled for this cytochrome in BUD (e) and MK group (f). Bar represents 1 μm.
Recovery of Clara cell phenotype after anti-inflammatory treatment
We next analysed whether changes induced by allergic inflammation could be reversed by applying the two classical anti-inflammatory treatments BUD and MK. Although semithin lung sections showed that both treatments produced a remarkable decrease in the hypertrophy of the epithelium compared with allergic animals, the Clara cells still appeared to be enlarged and rounded after BUD treatment (Figure 8a), with the bronchioles exhibiting some AB/PAS-positive cells (Figure 8b). Other histological parameters were also differentially reversed by the treatments, with BUD being less efficient (Figure 9a). The morphometric analysis revealed that the total AB/PAS-positive staining was significantly higher in BUD than control (Figure 9b). For the MK group, the typical Clara cell profile was mostly recovered (Figure 8c), as corroborated by the AB/PAS morphometric analysis (Figure 9b).
Figure 8.
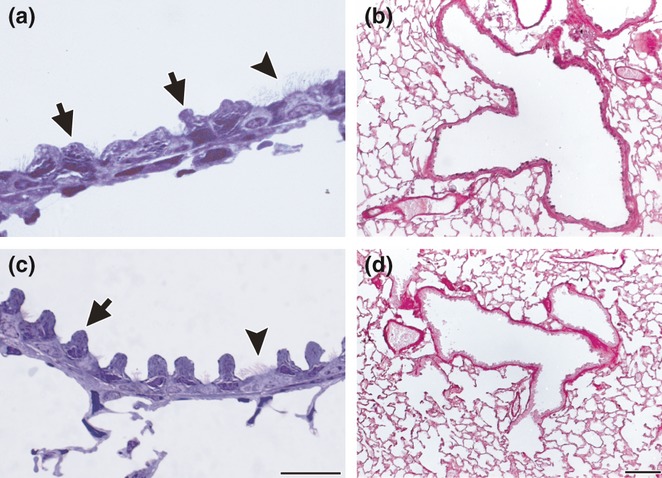
Bronchiolar epithelium after anti-inflammatory treatment: semithin sections of lung embedded in araldite and stained with toluidine blue (a and c); paraffin sections stained with AB/PAS (b and d). After budesonide (BUD) treatment for 7 days following OVA challenges, the hypertrophy was significantly reduced, but the non-ciliated Clara cells still appeared round and enlarged (a). AB/PAS staining remains strong after BUD (b). After montelukast treatment for 7 days, most cells recovered their characteristics of typical Clara cells (c) and AB/PAS staining was scarce (d). Arrows: non-ciliated cells, arrowheads: ciliated cells. Bars represent 20 μm in (a) and (c), and 200 μm in (b) and (d).
Figure 9.
Scoring of different parameters on lung histology of mice with allergic inflammation. (a) Inflammatory infiltration, epithelial hypertrophy and subepithelial fibrosis were evaluated by three different operators in HE sections. (b) Morphometric analysis of AB/PAS staining, which indicates that montelukast (MK) was more efficient than budesonide (BUD) to revert mucous metaplasia. (c) Distribution of non-ciliated bronchiolar cell phenotypes evaluated by electron microscopy evidencing the transformation of all non-ciliated Clara cells to goblet-like cells in allergic mice and the reversion after treatments. (d) subepithelial thickness; this parameter was analysed by electron microscopy and displayed a significant increase after allergy. Even though both therapies reduced the subepithelial layer, MK was more efficient than BUD. *P < 0.05,**P < 0.01, ***P < 0.001 vs. control; ••P < 0.01 vs. allergic; ♦P < 0.05 vs. budesonide.
Analysis by electron microscopy confirmed differences between both treatments, with MK being more effective than BUD in reversing the metaplasia induced by the allergic process. In the MK group, most cells reverted to their normal phenotype, as judged by the recovery of the typical cupola and the presence of small electron-dense granules under the plasma membrane (Figure 10a, b), with only 9% of non-ciliated cells still exhibiting mucin-like granules (Figure 9c). In contrast, after BUD exposure, 25% of the non-ciliated cells were still identified as transitional mucous phenotypes containing large electron-lucent granules. Moreover, unlike mucous cells in the allergic group, these cells contained mucin-positive granules that also stained strongly for CC16 (Figure 10f), a characteristic compatible with pregoblet cells. Although most of the remaining Clara cells after BUD exhibited features of a normal phenotype (Figure 10c), some of these still showed some remaining features of allergic-induced stimulation such as scant mucin-like intermingled granules (Figure 10e and inset), a marked development of the RER and Golgi complex, and numerous electron-dense granules that stained positive for CC16 (Figure 10d). Finally, both pharmacological treatments were observed to normalize EGFR expression, as can be concluded from the Western blot analysis (Figure 4a, b).
Figure 10.
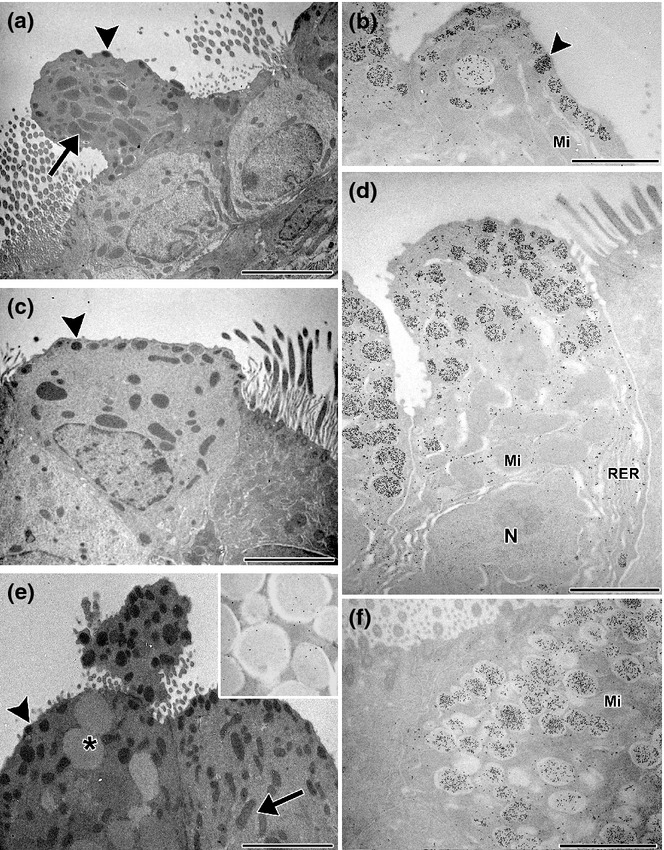
Clara cells after anti-inflammatory treatments: electron microscopy (a, c and e) and immunoelectron microscopy for CC16 (b, d and f). After montelukast (MK) treatment, non-ciliated cells were very similar to those of control, containing numerous mitochondria (arrows) and electron-dense secretory granules (arrowheads) (a), which were positive for CC16 (b); scarce electron-lucent big secretory granules remained after MK (b). After budesonide treatment, numerous non-ciliated cells lost most electron-lucent granules and recovered their typical big mitochondria as characteristics of the normal profile (c); significantly, these cells exhibited well-developed rough endoplasmic reticulum cisternae (RER) and numerous secretory granules, both of which were CC16-positive (d). Some non-ciliated cells still contained varied quantity of the big mucous-like secretory granules (e) (transitional cells) that stained strongly with anti-CC16 antibody (f). Inset: mucin labelling in granules. Mi, mitochondria; N, nucleus; *electron-lucent granules. Bars represent 1 μm.
CC16 and SP-D expression
After both BUD and MK treatments, the expression of CC16 increased in Clara cells as revealed by immunohistochemistry (Figure 5e, f), with cells completely stained in BUD-treated animals, but restricted to the basal portion in the MK treatment and in controls. Furthermore, the number of CC16-positive cells after BUD was significantly higher than after MK (92.00 ± 4.65 vs. 80.00 ± 4.21 respectively) (P < 0.05). The CC16 levels in BAL also increased compared with allergic group, but while MK post-treatment recovered the normal levels, CC16 was higher than normal after BUD (Figure. 5a, b).
SP-D expression exhibited similar differences in bronchiolar cells as revealed by immunohistochemistry with cells intensely stained after BUD (Figure 6e) but with a normal intensity after MK (Figure 6f). Using Western blot, the SP-D expression in lung homogenate was also observed to increase as compared to allergic group, also with levels being significantly higher for BUD than after MK post-treatment (Figure 6a, b).
CYP2E1 cytochrome expression
Concurrent with the recovery of the normal morphology, the pharmacological post-treatments provoked an increase in the CYP2E1 levels compared with the allergic group, as demonstrated by both immunohistochemistry (Figure 7d, e) and Western blot in lung homogenate (Figure 7a, b). However, while MK treatment normalized the CYP2E1 values, BUD showed significantly higher levels when compared with MK and control group.
Reversal of inflammatory parameters
In allergic animals, BAL cells consisted of 30% eosinophils and approximately 7% neutrophils (Figure 11). The thickness of the subepithelial stroma, evaluated by electron microscopy, was also significantly higher in this group (Figure 9d).
Figure 11.
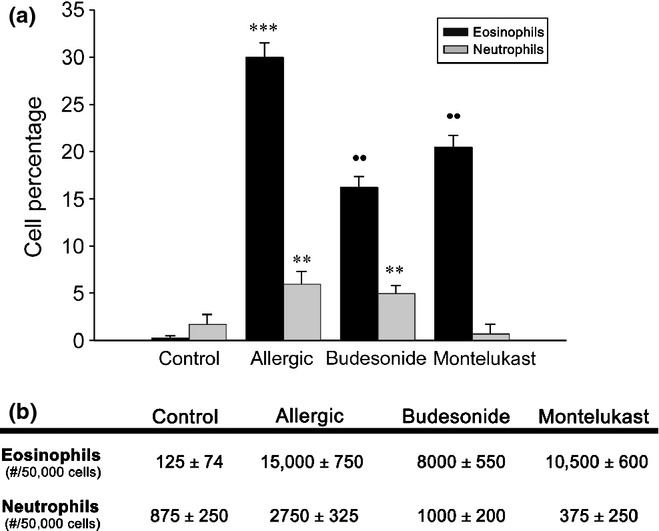
Inflammatory cells in bronchoalveolar lavage. (a) The allergic group exhibited an increased percentage of eosinophils (***P < 0.001) and neutrophils (**P < 0.01) in comparison with controls. (b) Both anti-inflammatory treatments significantly reduced the number of eosinophils in comparison with the allergic group (••P < 0.01), but values were still greater than in controls. Montelukast normalized neutrophils, in contrast with budesonide, which maintained high levels (**P < 0.01).
Even though both treatments reversed the inflammatory and remodelling parameters observed in the allergic group, these values frequently remained high compared with the control group. In addition, both the BUD and MK treatments also produced different results with regard to inflammatory parameters. MK also reduced the peribronchiolar infiltration better than BUD (Figure 9a). In addition, the neutrophils in BAL were normalized by MK, whereas after BUD, they remained higher than controls (Figure 11). Although eosinophils remained high for both treatments, BUD was more effective in reducing these inflammatory cells. Finally, differential reversal rates were observed when analysing the airway remodelling, with MK being more effective than BUD at reducing the subepithelial stroma thickness (Figure 9d).
Discussion
Clara cells are essential components of lung homeostasis due to their secretion of host defence molecules and their rich detoxifying P450 enzyme content. In this study, we provide evidence that chronic allergic inflammation significantly depresses CC16 and SP-D production in these cells, as a consequence of mucous metaplasia, which also affects the mitochondrial CYP2E1 (P450) content. In addition, we present results revealing that metaplasia can be reversed and that the Clara cell normal morphology and activity are recovered after BUD and MK treatments.
In a previous report, we described that Clara cells became highly hypertrophied after a short exposure to OVA, with CC16 synthesis being exacerbated in an attempt to modulate the inflammatory process. In addition, Clara cells have been shown to express mucin colocalizing with CC16 in large secretory granules, consistent with an increase in EGFR (Roth et al. 2007). In the current work, we showed that prolonged exposure to the allergen completed the transformation of pregoblet non-ciliated bronchiolar cells into goblet-like cells, which was correlated with high levels of EGFR and the exclusion of CC16 from the secretory granules, thus favouring mucous secretion over CC16 upregulation as a protective mechanism. Similar changes in CC16 content were also found in a previous study induced by IL-13 instillation in vivo (Kim et al. 2002), where authors described that CC16 is expressed early in pregoblet cells in response to EGFR signalling. In contrast, MUC5AC appeared later and the mature terminally differentiated goblet cells no longer expressed CC16. The loss of stored CC16 in secretory granules shown here and the resultant reduction in the protein in BAL are in agreement with the decrease in CC16 found in patients with chronic asthma (Van Vyve et al. 1995; Shijubo et al. 1999a,b). This was interpreted as a cause for the further aggravation of the inflammatory response.
In our experimental model, chronic allergic inflammation also affected the SP-D expression in lung, particularly in the mucous transformed Clara cells, where this collectin was restricted to a small area under the plasma membrane. A marked reduction in SP-D and SP-A levels was also previously observed in BAL and lung in a dust mite allergen-induced experimental asthma model (Wang et al. 2001a). Our results provide evidence that Clara cell metaplasia is implicated in the chronic allergy-induced reduction in SP-D, thereby preventing an upregulatory response that has been previously described to occur as a part of the epithelial defensive mechanism induced by other injuries (Barbaro et al. 2002). In fact, SP-D has been demonstrated to be not only an antimicrobial molecule but also a modulator of the allergic inflammation in asthma (Hohlfeld et al. 2002; Liu et al. 2005; Malherbe et al. 2005; Erpenbeck et al. 2006), suggesting that alterations in the functions or levels of SP-A and SP-D may contribute to the persistence of chronic inflammation.
In the present study, the expression of CYP2E1 was found to be drastically reduced by chronic allergy. As CYP2E1 is a particularly abundant isoform in Clara cells (Forkert 1995), occurring mainly in their mitochondria (Roth et al. 2007), its decrease in chronic asthma is a consequence of the phenotypic changes in Clara cells with secretory activity predominating over the xenobiotic detoxification that is characteristic of the typical cells. In this way, chronic allergy may contribute to amplify the effects of dangerous molecules from the environment on the airways.
The recovery of the Clara cells after treatment with MK revealed the high potential of these cells to change according to the microenvironmental conditions. This effective asthma therapy controls inflammation by blocking CysLT1, which is responsible for most of the pathophysiological effects of CysLTs in asthma (Montuschi & Peters-Golden 2010). In the current work, most Clara cells exhibited a normal morphology after MK treatment, with CC16, SP-D and cytochrome expression recovering their normal levels. However, after BUD post-treatment, the re-establishment of the typical non-mucous Clara cells was slower, with there still being a considerable amount of AB/PAS material induced by chronic allergy. In addition, after BUD, the number of neutrophils in BAL was still high, which is in agreement with the well-known effect of the glucocorticoids in enhancing neutrophil survival (Stankova et al. 2002). Electron microscopy showed the remaining AB/PAS-positive cells appearing as an intermediate stage between the metaplastic mucous and the normal Clara cells, as indicated by the coexistence of MUC5AC with a high CC16 content. This CC16-hypersecreting transitional phenotype was also seen to occur after a short exposure to OVA as an early defensive mechanism (Roth et al. 2007), and its presence during anti-inflammatory treatment with BUD correlate with the persistence of a higher inflammatory microenvironment that also contributed to maintaining increased SP-D expression.
Differences between corticoids and CysLT receptor inhibitors as treatments of asthma have been previously reported. Established airway smooth muscle cell layer thickening and subepithelial fibrosis were seen to be reversible by CysLT1 receptor blockade therapy but not by corticosteroids (Henderson et al. 2006). It is striking, however, that both treatments reduced the EGFR levels by similar amounts, implying that the glucocorticoids could have induced an EGRF-binding increase without an actual rise in EGFR, as was demonstrated for human airway smooth muscle cells (Kassel et al. 2009). The persistence of neutrophil infiltration found after BUD treatment may also have contributed to a prolonged activation of the EGFR cascade and mucin expression in Clara cells, a mechanism already demonstrated for neutrophils in lung oxidative stress (Takeyama et al. 2000).
In agreement with our results, Kibe et al. reported that glucocorticoids are not sufficient to suppress the airway hyperresponsiveness or goblet cell hyperplasia induced by IL-13 in mice, although they were effective in inhibiting the expression of eotaxin and eosinophil accumulation (Kibe et al. 2003). Similarly, Trifilieff et al. demonstrated that the administration of dexamethasone after the last allergen challenge decreased the airway mucous-producing epithelial cell proliferation, but did not completely reverse the mucous hypersecretory phenotype in a murine asthma model (Trifilieff et al. 2000). Recently, it was also reported that the IL13-induced MUC5AC production by human bronchial epithelial cells was not inhibited by dexamethasone (Kanoh et al. 2011).
Taken together, the morphological and molecular evidence support the concept that allergic inflammation can severely affect the role of epithelial cells in the preservation of lung homoeostasis. Furthermore, the epithelium seems to be a sensible sensor of the effects of anti-inflammatory therapies, which can restore Clara cell function in innate immunity as well as control inflammation. Thus, the epithelium emerges as an attractive target for new therapeutic strategies in lung diseases.
Acknowledgments
The authors are thankful to Dr. Jorge Mukdsi and Dra Elsa Orgnero for revising the manuscript and to Mrs. Mercedes Guevara and Elena Pereyra for their technical assistance. We also want to thank Dr. Jan Ryerse of The University of Saint Louis, USA for kindly providing the antiserum anti-CC16. We thank Dr. Paul Hobson, native speaker, for revision of the manuscript. This study was supported by the PIP 5779/05 Grant from the Consejo National de Investigaciones Científicas y Tecnológicas (CONICET), by a PICT Grant from FONCyT-ANPCyT and by a grant from Secyt, Córdoba National University. AAQ, AT and CM are members of the scientific career from CONICET. CL and LNG are fellows of CONICET. FDR was a fellow of FONCyT-ANPCyT and CONICET.
References
- Bals R, Hiemstra PS. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur. Respir. J. 2004;23:327–333. doi: 10.1183/09031936.03.00098803. [DOI] [PubMed] [Google Scholar]
- Barbaro M, Cutroneo G, Costa C, et al. Early events of experimental exposure to amorphous and crystalline silica in the rat: time course of surfactant protein d. Ital. J. Anat. Embryol. 2002;107:243–256. [PubMed] [Google Scholar]
- Bartlett JA, Fischer AJ, McCray PB., Jr Innate immune functions of the airway epithelium. Contrib. Microbiol. 2008;15:147–163. doi: 10.1159/000136349. [DOI] [PubMed] [Google Scholar]
- Chen LC, Zhang Z, Myers AC, Huang SK. Cutting edge: altered pulmonary eosinophilic inflammation in mice deficient for clara cell secretory 10-kda protein. J. Immunol. 2001;167:3025–3028. doi: 10.4049/jimmunol.167.6.3025. [DOI] [PubMed] [Google Scholar]
- Crouch E, Hartshorn K, Ofek I. Collectins and pulmonary innate immunity. Immunol. Rev. 2000;173:52–65. doi: 10.1034/j.1600-065x.2000.917311.x. [DOI] [PubMed] [Google Scholar]
- Dierynck I, Bernard A, Roels H, De Ley M. Potent inhibition of both human interferon-gamma production and biologic activity by the clara cell protein cc16. Am. J. Respir. Cell Mol. Biol. 1995;12:205–210. doi: 10.1165/ajrcmb.12.2.7865218. [DOI] [PubMed] [Google Scholar]
- Erpenbeck VJ, Ziegert M, Cavalet-Blanco D, et al. Surfactant protein d inhibits early airway response in aspergillus fumigatus-sensitized mice. Clin. Exp. Allergy. 2006;36:930–940. doi: 10.1111/j.1365-2222.2006.02524.x. [DOI] [PubMed] [Google Scholar]
- Evans CM, Williams OW, Tuvim MJ, et al. Mucin is produced by clara cells in the proximal airways of antigen-challenged mice. Am. J. Respir. Cell Mol. Biol. 2004;31:382–394. doi: 10.1165/rcmb.2004-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forkert PG. Cyp2e1 is preferentially expressed in clara cells of murine lung: localization by in situ hybridization and immunohistochemical methods. Am. J. Respir. Cell Mol. Biol. 1995;12:589–596. doi: 10.1165/ajrcmb.12.6.7766423. [DOI] [PubMed] [Google Scholar]
- Henderson WR, Jr, Chiang GK, Tien YT, Chi EY. Reversal of allergen-induced airway remodeling by cyslt1 receptor blockade. Am. J. Respir. Crit. Care Med. 2006;173:718–728. doi: 10.1164/rccm.200501-088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohlfeld JM, Erpenbeck VJ, Krug N. Surfactant proteins sp-a and sp-d as modulators of the allergic inflammation in asthma. Pathobiology. 2002;70:287–292. doi: 10.1159/000070744. [DOI] [PubMed] [Google Scholar]
- Hung CH, Chen LC, Zhang Z, et al. Regulation of th2 responses by the pulmonary clara cell secretory 10-kd protein. J. Allergy Clin. Immunol. 2004;114:664–670. doi: 10.1016/j.jaci.2004.05.042. [DOI] [PubMed] [Google Scholar]
- Kanoh S, Tanabe T, Rubin BK. Il-13-induced muc5ac production and goblet cell differentiation is steroid resistant in human airway cells. Clin. Exp. Allergy. 2011;41:1747–1756. doi: 10.1111/j.1365-2222.2011.03852.x. [DOI] [PubMed] [Google Scholar]
- Kassel KM, Schulte NA, Toews ML. Modulation of epidermal growth factor receptor binding to human airway smooth muscle cells by glucocorticoids and beta2-adrenergic receptor agonists. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;296:L693–L699. doi: 10.1152/ajplung.90446.2008. [DOI] [PubMed] [Google Scholar]
- Kibe A, Inoue H, Fukuyama S, et al. Differential regulation by glucocorticoid of interleukin-13-induced eosinophilia, hyperresponsiveness, and goblet cell hyperplasia in mouse airways. Am. J. Respir. Crit. Care Med. 2003;167:50–56. doi: 10.1164/rccm.2110084. [DOI] [PubMed] [Google Scholar]
- Kim S, Shim JJ, Burgel PR, et al. Il-13-induced clara cell secretory protein expression in airway epithelium: role of egfr signaling pathway. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;283:L67–L75. doi: 10.1152/ajplung.00404.2001. [DOI] [PubMed] [Google Scholar]
- Knorr B, Franchi LM, Bisgaard H, et al. Montelukast, a leukotriene receptor antagonist, for the treatment of persistent asthma in children aged 2 to 5 years. Pediatrics. 2001;108:E48. doi: 10.1542/peds.108.3.e48. [DOI] [PubMed] [Google Scholar]
- Kumar RK, Herbert C, Thomas PS, et al. Inhibition of inflammation and remodeling by roflumilast and dexamethasone in murine chronic asthma. J. Pharmacol. Exp. Ther. 2003;307:349–355. doi: 10.1124/jpet.103.053819. [DOI] [PubMed] [Google Scholar]
- Levin SW, Butler JD, Schumacher UK, Wightman PD, Mukherjee AB. Uteroglobin inhibits phospholipase a2 activity. Life Sci. 1986;38:1813–1819. doi: 10.1016/0024-3205(86)90135-9. [DOI] [PubMed] [Google Scholar]
- Liu CF, Chen YL, Shieh CC, Yu CK, Reid KB, Wang JY. Therapeutic effect of surfactant protein d in allergic inflammation of mite-sensitized mice. Clin. Exp. Allergy. 2005;35:515–521. doi: 10.1111/j.1365-2222.2005.02205.x. [DOI] [PubMed] [Google Scholar]
- Malherbe DC, Erpenbeck VJ, Abraham SN, Crouch EC, Hohlfeld JM, Wright JR. Surfactant protein d decreases pollen-induced ige-dependent mast cell degranulation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;289:L856–L866. doi: 10.1152/ajplung.00009.2005. [DOI] [PubMed] [Google Scholar]
- Montuschi P, Peters-Golden ML. Leukotriene modifiers for asthma treatment. Clin. Exp. Allergy. 2010;40:1732–1741. doi: 10.1111/j.1365-2222.2010.03630.x. [DOI] [PubMed] [Google Scholar]
- Necela BM, Cidlowski JA. Mechanisms of glucocorticoid receptor action in noninflammatory and inflammatory cells. Proc. Am. Thorac. Soc. 2004;1:239–246. doi: 10.1513/pats.200402-005MS. [DOI] [PubMed] [Google Scholar]
- Ni J, Kalff-Suske M, Gentz R, Schageman J, Beato M, Klug J. All human genes of the uteroglobin family are localized on chromosome 11q12.2 and form a dense cluster. Ann. N. Y. Acad. Sci. 2000;923:25–42. doi: 10.1111/j.1749-6632.2000.tb05517.x. [DOI] [PubMed] [Google Scholar]
- Reader JR, Tepper JS, Schelegle ES, et al. Pathogenesis of mucous cell metaplasia in a murine asthma model. Am. J. Pathol. 2003;162:2069–2078. doi: 10.1016/S0002-9440(10)64338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth FD, Quintar AA, Uribe Echevarria EM, Torres AI, Aoki A, Maldonado CA. Budesonide effects on clara cell under normal and allergic inflammatory condition. Histochem. Cell Biol. 2007;127:55–68. doi: 10.1007/s00418-006-0220-3. [DOI] [PubMed] [Google Scholar]
- Shijubo N, Itoh Y, Yamaguchi T, et al. Clara cell protein-positive epithelial cells are reduced in small airways of asthmatics. Am. J. Respir. Crit. Care Med. 1999a;160:930–933. doi: 10.1164/ajrccm.160.3.9803113. [DOI] [PubMed] [Google Scholar]
- Shijubo N, Itoh Y, Yamaguchi T, et al. Serum levels of clara cell 10-kda protein are decreased in patients with asthma. Lung. 1999b;177:45–52. doi: 10.1007/pl00007626. [DOI] [PubMed] [Google Scholar]
- Shim JJ, Dabbagh K, Ueki IF, et al. Il-13 induces mucin production by stimulating epidermal growth factor receptors and by activating neutrophils. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;280:L134–L140. doi: 10.1152/ajplung.2001.280.1.L134. [DOI] [PubMed] [Google Scholar]
- Stankova J, Turcotte S, Harris J, Rola-Pleszczynski M. Modulation of leukotriene b4 receptor-1 expression by dexamethasone: potential mechanism for enhanced neutrophil survival. J. Immunol. 2002;168:3570–3576. doi: 10.4049/jimmunol.168.7.3570. [DOI] [PubMed] [Google Scholar]
- Takeyama K, Dabbagh K, Jeong Shim J, Dao-Pick T, Ueki IF, Nadel JA. Oxidative stress causes mucin synthesis via transactivation of epidermal growth factor receptor: role of neutrophils. J. Immunol. 2000;164:1546–1552. doi: 10.4049/jimmunol.164.3.1546. [DOI] [PubMed] [Google Scholar]
- Trifilieff A, El-Hashim A, Bertrand C. Time course of inflammatory and remodeling events in a murine model of asthma: effect of steroid treatment. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L1120–L1128. doi: 10.1152/ajplung.2000.279.6.L1120. [DOI] [PubMed] [Google Scholar]
- Van Vyve T, Chanez P, Bernard A, et al. Protein content in bronchoalveolar lavage fluid of patients with asthma and control subjects. J. Allergy Clin. Immunol. 1995;95:60–68. doi: 10.1016/s0091-6749(95)70153-2. [DOI] [PubMed] [Google Scholar]
- Vasanthakumar G, Manjunath R, Mukherjee AB, Warabi H, Schiffmann E. Inhibition of phagocyte chemotaxis by uteroglobin, an inhibitor of blastocyst rejection. Biochem. Pharmacol. 1988;37:389–394. doi: 10.1016/0006-2952(88)90204-3. [DOI] [PubMed] [Google Scholar]
- Wahn U, Balachandra Dass S. Review of recent results of montelukast use as a monotherapy in children with mild asthma. Clin. Ther. 2008;30:1026–1035. doi: 10.1016/j.clinthera.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Wang JY, Shieh CC, Yu CK, Lei HY. Allergen-induced bronchial inflammation is associated with decreased levels of surfactant proteins a and d in a murine model of asthma. Clin. Exp. Allergy. 2001a;31:652–662. doi: 10.1046/j.1365-2222.2001.01031.x. [DOI] [PubMed] [Google Scholar]
- Wang SZ, Rosenberger CL, Espindola TM, et al. Ccsp modulates airway dysfunction and host responses in an ova-challenged mouse model. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001b;281:L1303–L1311. doi: 10.1152/ajplung.2001.281.5.L1303. [DOI] [PubMed] [Google Scholar]