Abstract
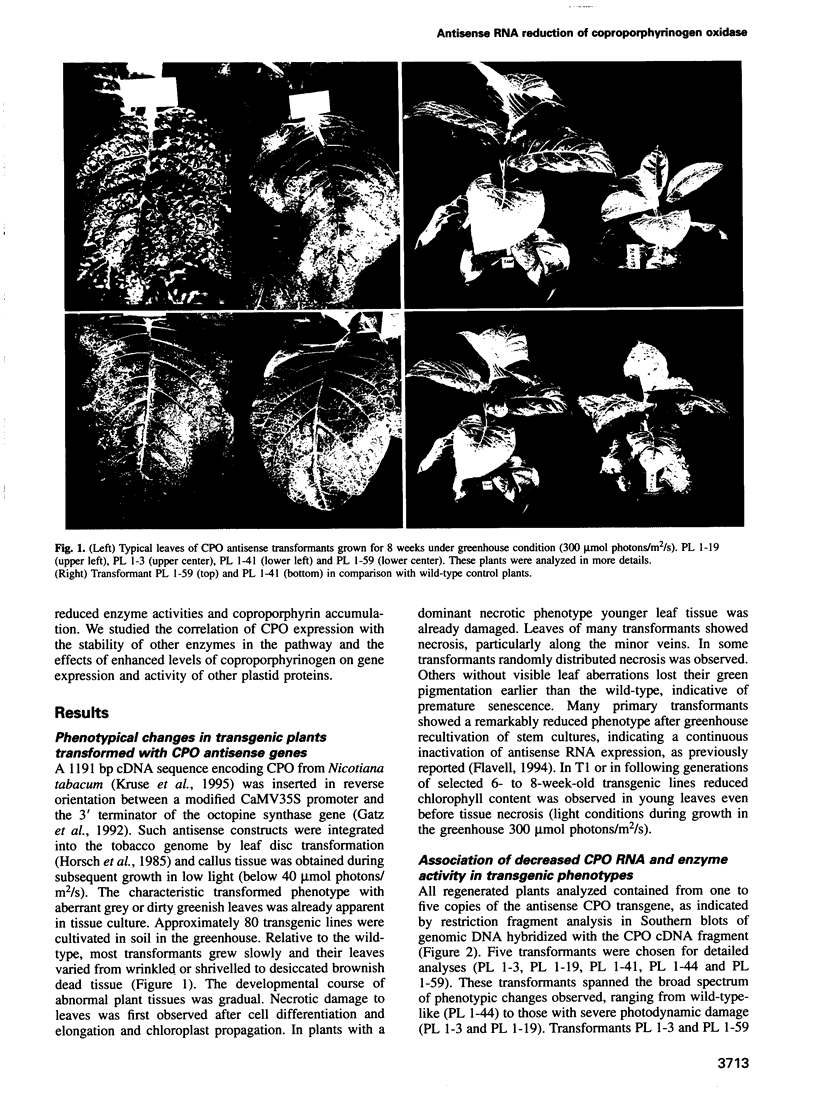
A full-length cDNA sequence encoding coproporphyrinogen oxidase was inserted in inverse orientation behind a CaMV promoter and transferred to tobacco (Nicotiana tabacum) by standard transformation techniques. Transformants showed reduced coproporphyrinogen oxidase activity and accumulation of photosensitive coproporphyrin(ogen), indicating antisense RNA expression. An inverse correlation was observed between the level of coproporphyrinogen oxidase and transformant phenotype. The latter is characterized by a broad range of growth retardation and necrosis, indicating oxidative leaf damage. Coproporphyrinogen is an apparent chromophore and its excitation finally leads to the production of reactive oxygen. Evidence is presented that indicates a direct correlation between the accumulation of non-metabolized coproporphyrinogen and oxidative damage to cellular structural components. Enzymatic and non-enzymatic antioxidants were investigated. Whereas superoxide dismutase activity increased in transgenic plants, catalase and ascorbate peroxidase activity remained constant. Tocopherol, rather than carotene or zeaxanthin, seemed to be involved in detoxification, indicating the putative localization and allocation of coproporphyrinogen. Expression of coproporphyrinogen oxidase antisense RNA did not significantly influence the level of other enzymes in the chlorophyll metabolic pathway, but deregulated gene expression of nuclear encoded plastid proteins. Accumulation of coproporphyrinogen and/or the resulting effects, such as oxidative stress, impairs a plastid/nuclear signal which may adapt gene expression to the plastid state.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamska I., Ohad I., Kloppstech K. Synthesis of the early light-inducible protein is controlled by blue light and related to light stress. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2610–2613. doi: 10.1073/pnas.89.7.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batschauer A., Mösinger E., Kreuz K., Dörr I., Apel K. The implication of a plastid-derived factor in the transcriptional control of nuclear genes encoding the light-harvesting chlorophyll a/b protein. Eur J Biochem. 1986 Feb 3;154(3):625–634. doi: 10.1111/j.1432-1033.1986.tb09444.x. [DOI] [PubMed] [Google Scholar]
- Becerril J. M., Duke S. O. Protoporphyrin IX Content Correlates with Activity of Photobleaching Herbicides. Plant Physiol. 1989 Jul;90(3):1175–1181. doi: 10.1104/pp.90.3.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer W. F., Jr, Fridovich I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem. 1987 Mar;161(2):559–566. doi: 10.1016/0003-2697(87)90489-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B., Adams W. W., 3rd The Xanthophyll Cycle, Protein Turnover, and the High Light Tolerance of Sun-Acclimated Leaves. Plant Physiol. 1993 Dec;103(4):1413–1420. doi: 10.1104/pp.103.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell R. B. Inactivation of gene expression in plants as a consequence of specific sequence duplication. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3490–3496. doi: 10.1073/pnas.91.9.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatz C., Frohberg C., Wendenburg R. Stringent repression and homogeneous de-repression by tetracycline of a modified CaMV 35S promoter in intact transgenic tobacco plants. Plant J. 1992 May;2(3):397–404. doi: 10.1111/j.1365-313x.1992.00397.x. [DOI] [PubMed] [Google Scholar]
- Giannopolitis C. N., Ries S. K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977 Feb;59(2):309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havir E. A., McHale N. A. Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol. 1987 Jun;84(2):450–455. doi: 10.1104/pp.84.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z. H., Li J., Sundqvist C., Timko M. P. Leaf Developmental Age Controls Expression of Genes Encoding Enzymes of Chlorophyll and Heme Biosynthesis in Pea (Pisum sativum L.). Plant Physiol. 1994 Oct;106(2):537–546. doi: 10.1104/pp.106.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U., Santarius K. A. Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B. 1970 Jul;25(7):718–728. doi: 10.1515/znb-1970-0714. [DOI] [PubMed] [Google Scholar]
- Höfgen R., Axelsen K. B., Kannangara C. G., Schüttke I., Pohlenz H. D., Willmitzer L., Grimm B., von Wettstein D. A visible marker for antisense mRNA expression in plants: inhibition of chlorophyll synthesis with a glutamate-1-semialdehyde aminotransferase antisense gene. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1726–1730. doi: 10.1073/pnas.91.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanningmeier U. Possible control of transcript levels by chlorophyll precursors in Chlamydomonas. Eur J Biochem. 1988 Nov 1;177(2):417–424. doi: 10.1111/j.1432-1033.1988.tb14391.x. [DOI] [PubMed] [Google Scholar]
- Kruse E., Mock H. P., Grimm B. Coproporphyrinogen III oxidase from barley and tobacco--sequence analysis and initial expression studies. Planta. 1995;196(4):796–803. [PubMed] [Google Scholar]
- Mapleston R. E., Griffiths W. T. Light modulation of the activity of protochlorophyllide reductase. Biochem J. 1980 Jul 1;189(1):125–133. doi: 10.1042/bj1890125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo A. K., Pick U., Hoffman-Falk H., Edelman M. The rapidly metabolized 32,000-dalton polypeptide of the chloroplast is the "proteinaceous shield" regulating photosystem II electron transport and mediating diuron herbicide sensitivity. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1572–1576. doi: 10.1073/pnas.78.3.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield S. P., Taylor W. C. Carotenoid-deficient maize seedlings fail to accumulate light-harvesting chlorophyll a/b binding protein (LHCP) mRNA. Eur J Biochem. 1984 Oct 1;144(1):79–84. doi: 10.1111/j.1432-1033.1984.tb08433.x. [DOI] [PubMed] [Google Scholar]
- Meyer G., Kloppstech K. A rapidly light-induced chloroplast protein with a high turnover coded for by pea nuclear DNA. Eur J Biochem. 1984 Jan 2;138(1):201–207. doi: 10.1111/j.1432-1033.1984.tb07900.x. [DOI] [PubMed] [Google Scholar]
- Mock H. P., Trainotti L., Kruse E., Grimm B. Isolation, sequencing and expression of cDNA sequences encoding uroporphyrinogen decarboxylase from tobacco and barley. Plant Mol Biol. 1995 May;28(2):245–256. doi: 10.1007/BF00020244. [DOI] [PubMed] [Google Scholar]
- Smith A. G., Marsh O., Elder G. H. Investigation of the subcellular location of the tetrapyrrole-biosynthesis enzyme coproporphyrinogen oxidase in higher plants. Biochem J. 1993 Jun 1;292(Pt 2):503–508. doi: 10.1042/bj2920503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. Mechanisms of toxicity of photoactivated artificial porphyrins. Role of porphyrin-protein interactions. Ann N Y Acad Sci. 1987;514:309–322. doi: 10.1111/j.1749-6632.1987.tb48786.x. [DOI] [PubMed] [Google Scholar]
- Stamm C., Sarret Y., Schmitt D., Thivolet J. Les fibronectines. Pathol Biol (Paris) 1992 Jun;40(6):649–654. [PubMed] [Google Scholar]
- Willekens H., Langebartels C., Tiré C., Van Montagu M., Inzé D., Van Camp W. Differential expression of catalase genes in Nicotiana plumbaginifolia (L.). Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10450–10454. doi: 10.1073/pnas.91.22.10450. [DOI] [PMC free article] [PubMed] [Google Scholar]