Abstract
Human kidney predominant protein, NCU-G1, is a highly conserved protein with an unknown biological function. Initially described as a nuclear protein, it was later shown to be a bona fide lysosomal integral membrane protein. To gain insight into the physiological function of NCU-G1, mice with no detectable expression of this gene were created using a gene-trap strategy, and Ncu-g1gt/gt mice were successfully characterized. Lysosomal disorders are mainly caused by lack of or malfunctioning of proteins in the endosomal-lysosomal pathway. The clinical symptoms vary, but often include liver dysfunction. Persistent liver damage activates fibrogenesis and, if unremedied, eventually leads to liver fibrosis/cirrhosis and death. We demonstrate that the disruption of Ncu-g1 results in spontaneous liver fibrosis in mice as the predominant phenotype. Evidence for an increased rate of hepatic cell death, oxidative stress and active fibrogenesis were detected in Ncu-g1gt/gt liver. In addition to collagen deposition, microscopic examination of liver sections revealed accumulation of autofluorescent lipofuscin and iron in Ncu-g1gt/gt Kupffer cells. Because only a few transgenic mouse models have been identified with chronic liver injury and spontaneous liver fibrosis development, we propose that the Ncu-g1gt/gt mouse could be a valuable new tool in the development of novel treatments for the attenuation of fibrosis due to chronic liver damage.
KEY WORDS: NCU-G1, Lysosome, Fibrosis
INTRODUCTION
Human kidney predominant protein, NCU-G1, is a protein whose function is only beginning to emerge. First reported in 1994 (Eskild et al., 1994), mouse NCU-G1 was cloned in 2001 (Kawamura et al., 2001). Initial studies described NCU-G1 as a nuclear protein with gene regulatory properties (Steffensen et al., 2007), whereas more recent reports have shown that NCU-G1 is a bona fide lysosomal type I integral membrane protein (Sardiello et al., 2009; Schieweck et al., 2009; Schröder et al., 2010). NCU-G1 is highly conserved, proline-rich and ubiquitously expressed; however, it shows no sequence homology to other known proteins.
The endosomal-lysosomal pathway is sensitive to protein dysfunctions, and alterations in protein composition and function can lead to a group of metabolic diseases categorized as lysosomal disorders (Futerman and van Meer, 2004; Parkinson-Lawrence et al., 2010). Intralysosomal accumulation of unmetabolized substrates can result from malfunction of any endosomal-lysosomal protein, but the severity and clinical manifestations vary owing to secondary alterations in other biochemical and cellular pathways (Futerman and van Meer, 2004), such as apoptosis and elevated levels of reactive oxygen species due to impaired lysosomal clearance of damaged organelles through autophagy (Tardy et al., 2004; Kiselyov et al., 2007; Kurz et al., 2008; Cox and Cachón-González, 2012). Certain cell types are more sensitive to lysosomal dysfunction than others, especially cells with low proliferation capacity (Kiselyov et al., 2007; Kurz et al., 2008; Cox and Cachón-González, 2012). This is reflected in the vast frequency of neurological symptoms in different lysosomal disorders (Parkinson-Lawrence et al., 2010; Cox and Cachón-González, 2012; Platt et al., 2012). For some disorders, however, other symptoms like liver damage are also a consistent clinical finding (Platt et al., 2012).
Persistent injury to the liver eventually leads to liver fibrosis and deposition of excess connective tissue (Bataller and Brenner, 2005; Friedman, 2008; Gieling et al., 2008; Kisseleva and Brenner, 2011; Ramachandran and Iredale, 2012). The development of liver fibrosis involves several liver cell types and immune cells, including hepatocytes, hepatic stellate cells (HSCs), Kupffer cells (KCs), endothelial cells and infiltrating leukocytes (Friedman, 2008; Pinzani and Macias-Barragan, 2010; Hernandez-Gea and Friedman, 2011). Dying cells secrete reactive oxygen species, and release necrotic and apoptotic fragments, leading to activation of KCs and recruitment of inflammatory cells to the site of injury (Bataller and Brenner, 2005; Friedman, 2008). Activated KCs and recruited leukocytes contribute to the activation of HSCs into myofibroblast-like cells by secreting pro-fibrogenic cytokines, including transforming growth factor-β (TGF-β) and platelet-derived growth factor (PDGF) (Bataller and Brenner, 2005; Friedman, 2008). When activated, HSCs start secreting high amounts of extracellular matrix (ECM) proteins that differ from those normally expressed, including fibrillar collagen, thus replacing dead parenchymal cells with fibrous ECM, ultimately compromising the organ function if untreated (Gressner et al., 2007; Mormone et al., 2011).
TRANSLATIONAL IMPACT.
Clinical issue
Lysosomal storage disorders are a group of inherited metabolic disorders that are caused by malfunction of the endosomal-lysosomal pathway, often with devastating consequences for patients. Most lysosomal disorders exhibit neurological symptoms, but internal organ injury and liver damage are consistent clinical findings in several lysosomal disorders. Indeed, recent research has uncovered a direct link between abnormal lysosomal function and liver fibrosis – scarring of the liver in response to chronic injury that eventually leads to liver cirrhosis. Currently, due to a shortage of more suitable models, the development of liver fibrosis is often studied in animal models that involve experimental induction of liver damage, which leads to acute fibrosis. An animal model of chronic fibrosis is needed to help improve our understanding of this condition.
Results
Transgenic mouse models of lysosomal disorders are valuable tools for characterization of the molecular mechanisms behind the broad spectrum of clinical symptoms, including liver fibrosis. In this study, the authors develop a novel mouse model with no detectable expression of the lysosomal membrane protein, NCU-G1, using a gene-trap strategy; NCU-G1 is highly expressed in liver, kidney, lung and prostate and might be crucial for normal lysosomal function. The authors report that disruption of Ncu-g1 in mice does not interfere with fertility, normal embryonic development or growth. They show that the predominant phenotype of Ncu-g1gt/gt mice is spontaneous development of liver fibrosis, accompanied by splenomegaly. Using gene expression analyses, the authors show that Ncu-g1gt/gt mice exhibit all the hallmarks of well-established liver fibrosis by the age of 6 months. Finally, they report that NCU-G1 deficiency leads to accumulation of lipofuscin and iron in Kupffer cells in the liver.
Implications and future directions
These results indicate that disruption of NCU-G1 function leads to chronic liver injury and activation of fibrogenesis in mice. Ncu-g1gt/gt mice therefore represent a potential animal model for studies of these conditions and for the development of treatments for liver fibrosis. Further studies using this model will enable a more extensive characterization of the biochemical and molecular pathways that are affected during chronic liver damage and fibrogenesis, and will determine whether abnormal storage of lipofuscin and iron in Kupffer cells is a primary or secondary effect of NCU-G1 ablation. Finally, these findings suggest that disruption of Ncu-g1 might be causative for a previously undescribed lysosomal disorder that has liver fibrosis as its predominant phenotype.
Several rodent models for inducible liver fibrosis are available (Weiler-Normann et al., 2007; Starkel and Leclercq, 2011), but only a few transgenic mouse models have been identified that develop spontaneous liver fibrosis (Fickert et al., 2002; Takehara et al., 2004; Wandzioch et al., 2004; Warskulat et al., 2006). Mouse models are widely used in the study of lysosomal disorders and the impact of lysosomal protein deficiency (Parkinson-Lawrence et al., 2010; Platt et al., 2012). However, the physiological consequences of loss of NCU-G1 function have never been characterized before. The lysosomal localization suggests some alterations in the endosomal-lysosomal pathway following Ncu-g1 gene disruption. In this article, we present a new mouse model, created by using a gene-trap strategy to introduce a transcriptional stop codon to intron 1 of the Ncu-g1 gene (Ncu-g1gt/gt). Ncu-g1gt/gt mice have no detectable expression of NCU-G1, display normal growth and fertility, and have a life expectancy up to at least 18 months. The predominant phenotype is a spontaneous development of liver fibrosis by the age of 6 months. Analyses of Ncu-g1gt/gt liver indicate that absence of this lysosomal membrane protein leads to an increased rate of hepatic cell death, oxidative stress, activation of the fibrogenic response, and accumulation of lipofuscin and iron in KCs.
RESULTS
Successful generation of Ncu-g1gt/gt mice
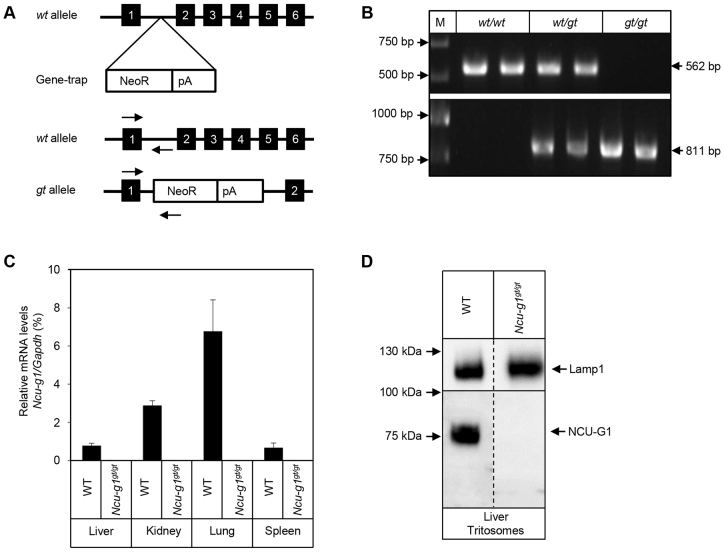
Breeding of heterozygous mice carrying a gene-trap cassette inserted into intron 1 of the Ncu-g1 gene (Fig. 1A) resulted in three expected genotypes (Ncu-g1wt/wt, Ncu-g1wt/gt and Ncu-g1gt/gt) (Fig. 1B). Expression of mRNA for Ncu-g1 was analyzed to assess any transcription leakage from the gene-trap. Fig. 1C shows a total elimination of Ncu-g1 mRNA in homozygous gene-trap mice compared with wild-type mice in all organs studied. Lysosome-enriched fractions from mouse liver after tyloxapol treatment (Schieweck et al., 2009) were used to confirm the loss of NCU-G1 at the protein level as shown in Fig. 1D. Genotype and sex distributions for a total of 364 pups generated by four heterozygous breeding pairs were analyzed and found to be in accordance with Mendelian distributions (P<0.001), indicating that NCU-G1 was not essential for mouse embryonic development (data not shown). Heterozygotes and homozygotes had similar growth rates as their wild-type siblings. When mated, homozygous Ncu-g1gt/gt mice produced litters at the same frequency and of a similar litter size as wild-type and heterozygote mating (data not shown).
Fig. 1.
Verification of Ncu-g1 gene disruption. (A) Schematic representation of gene-trap vector insertion into the Ncu-g1 locus (top). The wild-type (wt) allele (middle) and the trapped (gt) allele (bottom) are shown. The targeting vector, consisting of a neomycin-resistance cassette (NeoR) and a polyadenylation site (pA), was inserted into intron 1 of the wt allele to produce a gene-trap (gt) allele. Arrows indicate the hybridization sites for genotyping primers (NCUG1 and FlpROSA, for wt and gt alleles, respectively). PCR products of 562 bp indicate the presence of Ncu-g1 wt allele and 811 bp the gt allele, respectively. (B) Genotyping of mice by PCR. The Ncu-g1 wt 562 bp PCR product was present in both homozygous wild-type (wt/wt) and heterozygous (wt/gt) samples, but not in homozygous gene-trap (gt/gt) samples. Conversely, the 811 bp gene-trap PCR product was present in gt/gt and wt/gt samples, but not in wt/wt samples. (C) The absence of mRNA transcripts in Ncu-g1gt/gt was verified in liver, kidney, lung and spleen, using TaqMan qPCR and Gapdh as a reference gene. (D) No NCU-G1 protein was detected by western blot analyses of Ncu-g1gt/gt liver tritosomes. Lamp1 served as a loading control.
Phenotype of Ncu-g1gt/gt mice
At birth, Ncu-g1gt/gt mice were indistinguishable from wild-type siblings, and displayed no obvious behavioral differences. At the age of 6 months, lack of NCU-G1 resulted in a fibrotic appearance of the liver, with large nodules on the surface (Fig. 2A). However, the liver:body weight ratio was unaffected (Fig. 2A). Splenomegaly was observed in Ncu-g1gt/gt mice with a nearly doubled spleen:body weight ratio (Fig. 2B). This is probably a result of portal vein hypertension caused by the liver fibrosis. Measurements of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities showed moderate but significant increases in Ncu-g1gt/gt mice compared with wild-type animals (Fig. 2C). Other organs (kidney, lung, heart, brain) appeared normal and were not further evaluated in this study. Interestingly, Ncu-g1gt/gt mice were kept until the age of 18 months before termination, indicating that the liver fibrosis does not progress to a lethal condition during this period.
Fig. 2.
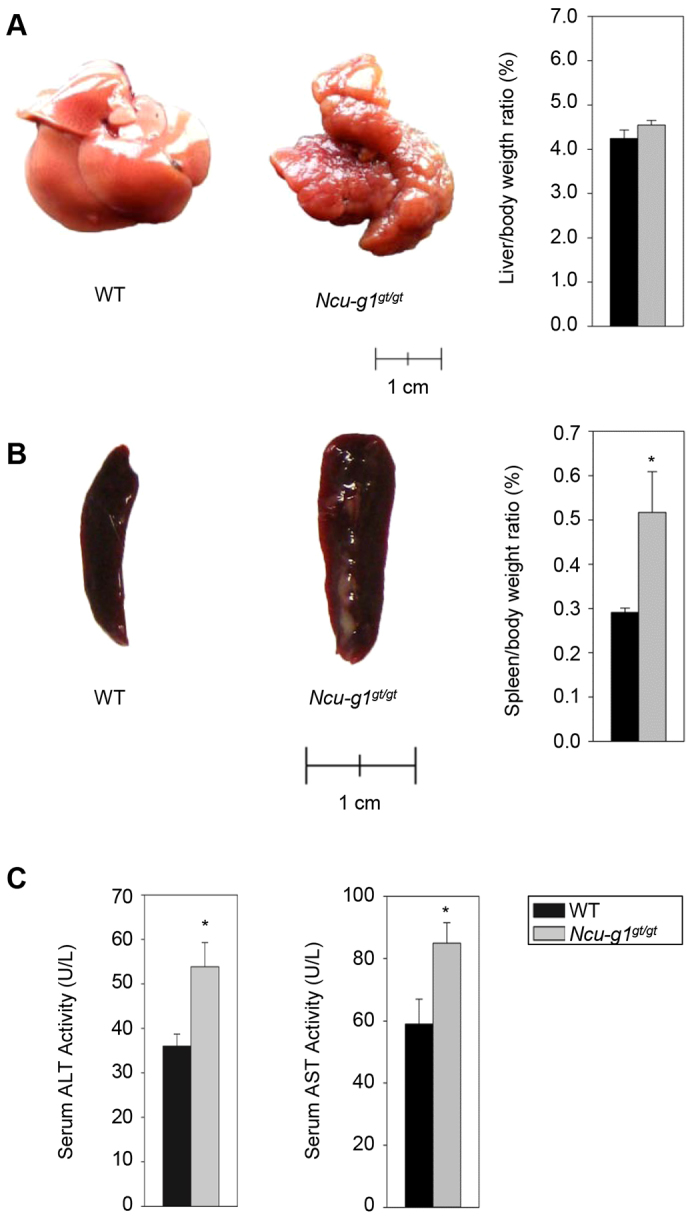
Loss of NCU-G1 leads to liver fibrosis and splenomegaly in 6-month-old mice. (A) Representative livers from wild-type (WT) and Ncu-g1gt/gt mice. Wild-type liver has a smooth surface with easily recognizable individual lobes, whereas the Ncu-g1gt/gt liver has a fibrotic appearance and a contracted, distorted and nodular appearance. Disruption of Ncu-g1 did not result in hepatomegaly, as indicated by the liver weight:body weight ratio (P=0.23, n=6–7). (B) Representative pictures of a wild-type (WT) and Ncu-g1gt/gt spleen. Spleen weight:body weight ratio of WT and Ncu-g1gt/gt spleens shows a significantly enlarged spleen in Ncu-g1gt/gt mice (n=6–7, *P=0.02). (C) Serum ALT and AST activity levels are significantly elevated in Ncu-g1gt/gt mice (n=4, *P<0.05). Values are expressed as mean±s.e.m.
Inflammation and excess collagen in Ncu-g1gt/gt liver
In order to gain insight into the underlying mechanisms behind the Ncu-g1gt/gt mouse phenotype, qPCR arrays were used to identify changes in gene expression in groups of genes known to be involved in liver fibrogenesis. This initial screening suggested that genes associated with fibrosis, hepatotoxicity and oxidative stress responses were regulated in Ncu-g1gt/gt liver (supplementary material Table S1). In addition, a screening of different signal transduction pathways suggested a strong upregulation of genes encoding chemokines and proteins involved in control of the cell cycle (supplementary material Table S1). A selection of these genes was verified by qPCR (see below).
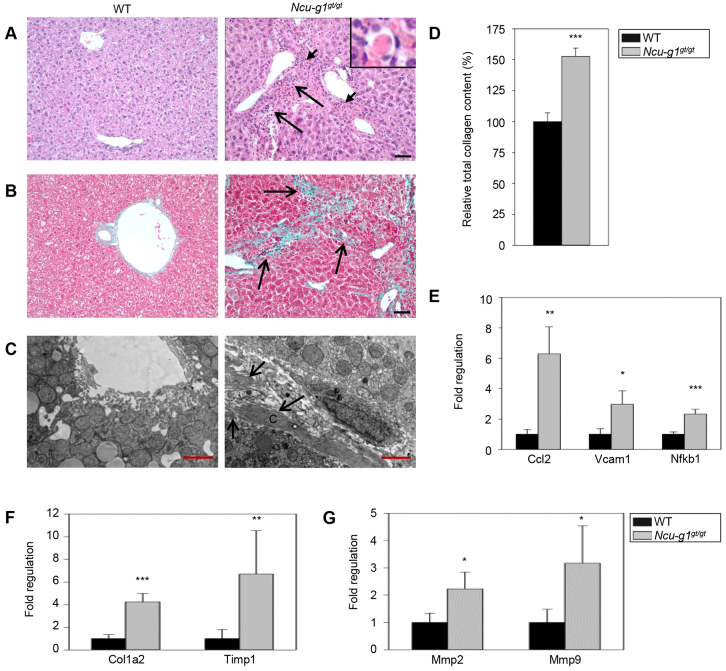
Light-microscopic examination of hematoxylin and eosin (H&E)-stained liver sections from Ncu-g1gt/gt mice showed infiltration of mononuclear leukocytes and granulocytes in the portal areas with some piecemeal necrosis (Fig. 3A). Widespread deposition of collagen was revealed in sections stained with Masson-Goldner’s trichrome (Fig. 3B). This observation was supported by electron microscopy, showing thick collagen fibers in the space of Disse (Fig. 3C) and sirius-red quantification of total collagen content in liver homogenates (Fig. 3D). In affected areas, loss of hepatocytes and bridging fibrosis resulted in disruption of the normal tissue architecture (Fig. 3A,C). Expression of genes such as Vcam1, Ccl2 and Nfkb1, involved in leukocyte recruitment (Fig. 3E), and Mmp2, Mmp9, Timp1 and Col1a2, involved in ECM remodeling (Fig. 3F,G), were found to be elevated in Ncu-g1gt/gt liver, as detected by qPCR analysis, supporting the microscopy findings.
Fig. 3.
Inflammation, dying hepatocytes and collagen deposition in Ncu-g1gt/gt liver. (A) Hematoxylin and eosin staining of liver sections from 6-month-old wild-type (WT) and Ncu-g1gt/gt mice with a distorted tissue structure, massive infiltration of mononuclear leucocytes and granulocytes (long arrows), and acidophilic hepatocytes (short arrows, inset) in portal areas of Ncu-g1gt/gt mice. Scale bar: 50 μm. (B) Masson-Goldner’s trichrome staining of liver sections from WT and Ncu-g1gt/gt mice shows fibrosis in periportal regions of Ncu-g1gt/gt mice (arrows), where connective tissue stains green. Scale bar: 50 μm. (C) Representative transmission electron microscopy micrographs of perisinusoidal areas in liver sections from WT and Ncu-g1gt/gt mice. Massive deposition of fibrous collagen is present in the space of Disse of the Ncu-g1gt/gt liver (arrows) (C, collagen). Scale bars: 2 μm. (D) Relative total collagen content in WT and Ncu-g1gt/gt liver as measured by sirius-red colorimetric plate assay. (E) qPCR analyses show elevated expression of genes involved in leukocyte recruitment (Ccl2, Vcam1, Nfkb1), and (F,G) extracellular matrix remodeling (Col1a1, Timp1, Mmp2, Mmp9) in Ncu-g1gt/gt liver (n=4, *P<0.05, **P<0.005, ***P<0.001). Values are expressed as mean±s.e.m.
Increased rate of cell death and oxidative stress in Ncu-g1gt/gt liver
In the Ncu-g1gt/gt livers, scattered acidophilic hepatocytes were seen (Fig. 3A, insert). Immunoreactivity for active caspase 3, a specific marker for apoptosis, showed an increased number of apoptotic cells in Ncu-g1gt/gt mouse liver (Fig. 4A). Increased levels of active caspase 3 were also confirmed by western blot (Fig. 4B). Leukocyte infiltration and damage to hepatocytes are often associated with increased oxidative stress (Friedman, 2008), as already indicated by qPCR array analyses. Fig. 4C shows the increased levels of expression of a selection of genes associated with oxidative stress: iNos2, Gpx3, Sod3 and Noxa1.
Fig. 4.
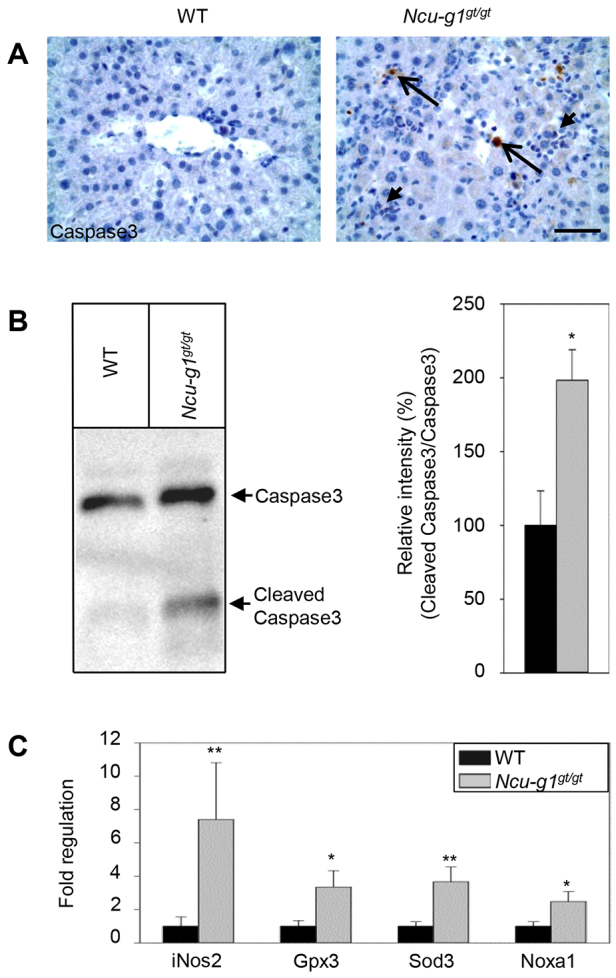
Increased rate of cell death and oxidative stress in Ncu-g1gt/gt liver. (A) Liver sections labeled for active caspase 3 and counterstained with Mayer’s hematoxylin indicate apoptosis (long arrows) and inflammation (short arrows) in Ncu-g1gt/gt mice. Scale bar: 50 μm. (B) Representative western blot showing caspase 3 expression in wild-type (WT) and Ncu-g1gt/gt liver. The levels of active caspase 3 were significantly higher in Ncu-g1gt/gt liver homogenates (n=4, *P<0.05). (C) Expression of genes involved in oxidative stress (iNos2, Gpx3, Sod3, Noxa1) were elevated in Ncu-g1gt/gt liver (n=4, *P<0.05, **P<0.005). Values are expressed as mean±s.e.m.
Active fibrogenesis in Ncu-g1gt/gt liver
Injury to hepatic cells and oxidative stress are inducers of liver fibrogenesis, a process that involves several cell types including KCs, HSCs and recruited leukocytes (Bataller and Brenner, 2005; Friedman, 2008; Pinzani and Macias-Barragan, 2010; Hernandez-Gea and Friedman, 2011). KCs can be activated upon liver injury by phagocytosis of apoptotic bodies (Canbay et al., 2003a). Analyses of frozen sections from wild-type and Ncu-g1gt/gt livers showed increased staining for CD68, a marker for activated KCs (Kinoshita et al., 2010), in Ncu-g1gt/gt livers (Fig. 5A). To further characterize the KCs, staining for F4/80 revealed swollen and hypertrophic macrophages (Fig. 5B), displaying a post-phagocytic phenotype. Because inflammatory macrophages have been shown to switch to a restorative subset after phagocytosis (Ramachandran et al., 2012), we investigated the expression of an inflammatory (Cd86) and a restorative (Cd81) marker (Ramachandran et al., 2012) by qPCR. Fig. 5C shows a significant increase in Cd86 expression, suggesting that the activated macrophages in Ncu-g1gt/gt livers belong to a pro-inflammatory subtype (Ramachandran et al., 2012).
Fig. 5.
Activated fibrogenic response in Ncu-g1gt/gt liver. (A,B) Immunofluorescence image shows CD68- and F4/80-stained KCs (green). Liver sections were counterstained with DAPI (blue). Infiltrating leukocytes can be seen in Ncu-g1gt/gt liver section (white arrow). CD68-positive cells seem to congregate at the site of leukocyte infiltration. In Ncu-g1gt/gt liver sections, F4/80-positive cells appear swollen and hypertrophic. Scale bars: 50 μm. (C) qPCR analyses of the restorative (Cd81) and the pro-inflammatory (Cd86) macrophage markers show elevated expression of only Cd86 in Ncu-g1gt/gt liver. (D,E) Activated KCs produce pro-fibrogenic cytokines. qPCR analyses show elevated expression of genes characteristic for activated fibrogenesis (Tnf, Pdgfb, Tgfb1, Tgfbr2). (F) Presence of activated HSCs was indicated by increased expression of genes characteristic for activated HSCs (α-Sma, Igfbp3, Vim). (G) This was supported by α-Sma staining in liver sections from wild-type (WT) and Ncu-g1gt/gt mice. Note the positive labeling of α-Sma indicating activated HSCs (arrows) in affected areas. Liver sections were counterstained with hematoxylin. Scale bar: 50 μm. (H) Immunofluorescence imaging shows increased levels of myeloperoxidase at the protein level in Ncu-g1gt/gt liver (green). Liver sections were counterstained with DAPI (blue). Note the granular staining pattern of myeloperoxidase, clustering of myeloperoxidase-positive cells and increased levels of invading polymorphonuclear leukocytes with small nuclei. Scale bar: 50 μm. (I) qPCR analyses supported the elevated myeloperoxidase (Mpo) expression in Ncu-g1gt/gt liver. (J) The expression of S100 calcium binding protein A8 (S100a8) was also elevated in Ncu-g1gt/gt liver. (C-F,I,J) n=4, *P<0.05, **P<0.005, ***P<0.001. Values are expressed as mean±s.e.m.
Activated KCs stimulate HSC activation by secreting reactive oxygen species and pro-fibrotic cytokines, such as TNF-α (Canbay et al., 2003a), TGF-β and PDGF (Bataller and Brenner, 2005; Friedman, 2008). Fig. 5D,E show increased expression of mRNA for all three cytokines in addition to a receptor for TGF-β, thus confirming activation of KCs. When HSCs are activated by pro-fibrotic cytokines, they evolve into myofibroblast-like cells and start expressing α-Sma, Vim, Igfbp3 and collagen (predominantly type I), characteristic for their activation (Boers et al., 2006; Friedman, 2008). This was demonstrated by the increased expression of α-Sma, Igfbp3 and Vim as shown in Fig. 5F and supported by immunostaining of liver sections from Ncu-g1gt/gt mice, showing the presence of star-shaped α-Sma-positive cells in the liver parenchyma (Fig. 5G). Taken together, these results point to activation of both KCs and HSCs in Ncu-g1gt/gt liver. When activated, these cells recruit white blood cells to sites of lesion, depicted in Fig. 3A and Fig. 4A (Maher, 2001; Bataller and Brenner, 2005; Zhan et al., 2006). The qPCR arrays suggested a massive increase in the mRNA levels for myeloperoxidase. This was confirmed at the protein level by immunofluorescence (Fig. 5H) and verified by qPCR (Fig. 5I). Myeloperoxidase is most probably produced by the infiltrating polymorphonuclear leukocytes (Ford, 2010; Amanzada et al., 2011). This enzyme was found in a clustered distribution in small cells, and showed a low degree of colocalization with KCs, another cell type capable of producing this enzyme (Brown et al., 2001) (supplementary material Fig. S1). In support of these findings, the mRNA level of S100 calcium binding protein A8 (S100A8), a protein known to be involved in chemotaxis of polymorphonuclear leukocytes (Ryckman et al., 2003), was significantly upregulated in Ncu-g1gt/gt liver (Fig. 5J).
Accumulation of autofluorescent material in Ncu-g1gt/gt Kupffer cells
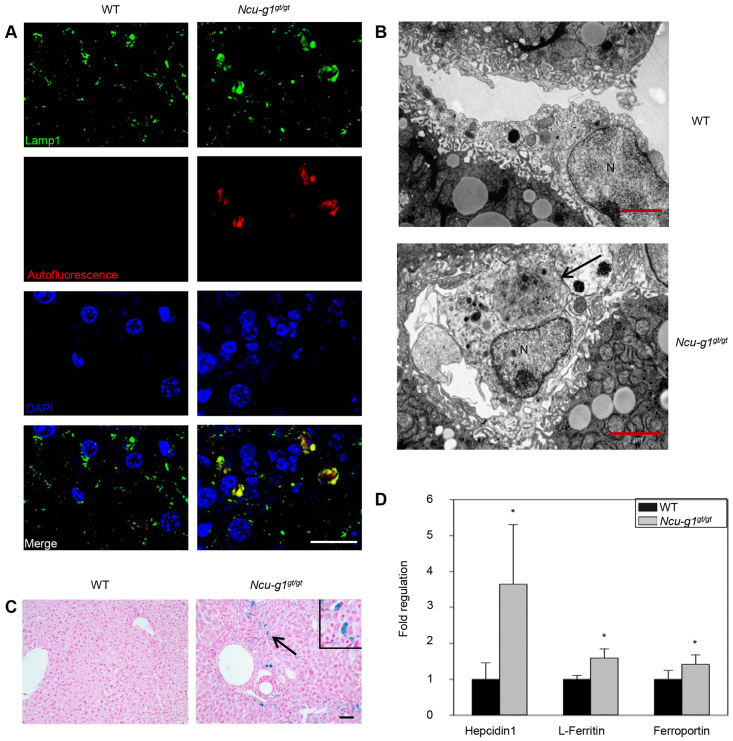
Dysfunction of lysosomal proteins is often associated with intralysosomal storage of undigested material due to impaired hydrolytic or transport activity (Futerman and van Meer, 2004). Hence, we investigated the possibility of storage in Ncu-g1gt/gt liver lysosomes. Fig. 6A shows immunofluorescence staining for Lamp1, a marker for lysosomes. Lamp1-positive vesicles were massively enlarged. Fluorescence examination revealed that autofluorescent material was present in these enlarged Lamp1-coated vesicles, and colocalized with CD68-positive staining, indicating activated KCs (supplementary material Fig. S2A). Storage in KCs was confirmed by electron microscopy, where large storage vacuoles were observed in this cell type in Ncu-g1gt/gt liver (Fig. 6B). Such structures were not obviously seen in other cell types (data not shown). Affected KCs were identified based on morphology after immunoreactivity for F4/80 (supplementary material Fig. S2B). These cells were also positively stained with Perls’ Prussian blue (Fig. 6C and supplementary material Fig. S2B), indicating storage of iron in Ncu-g1gt/gt liver KCs. Gene expression analyses showed that Ncu-g1 mRNA was expressed in isolated parenchymal as well as non-parenchymal cells from wild-type liver, ruling out the possibility that storage in KCs, but not hepatocytes, was a result of a cell-type-specific lack of Ncu-g1 expression (data not shown). In support of these findings, the mRNA levels of Hepcidin1 and L-Ferritin, proteins involved in intracellular iron accumulation (Ganz, 2011), were shown to be elevated in Ncu-g1gt/gt liver (Fig. 6D). In addition, the expression of the iron transporter Ferroportin was increased (Fig. 6D).
Fig. 6.
Intralysosomal accumulation of lipofuscin and iron in Ncu-g1gt/gt KCs. (A) Immunofluorescence image analyses showed colocalization of Lamp1-coated vesicles (green) and autofluorescence (red) in Ncu-g1gt/gt but not in wild-type (WT) liver. Liver sections were counterstained with DAPI (blue). Scale bar: 20 μm. (B) Representative transmission electron microscopy micrographs of KCs from WT and Ncu-g1gt/gt liver. Large storage vacuoles are visible inside Ncu-g1gt/gt KCs (arrow) (N, nucleus). Scale bars: 2 μm. (C) Perls’ Prussian blue staining (blue) of liver sections from WT and Ncu-g1gt/gt mice revealed accumulation of iron (arrow, insert) in Ncu-g1gt/gt KCs. Scale bar: 50 μm. (D) qPCR analyses show elevated expression of genes involved in iron accumulation (Hepcidin1, L-Ferritin) and iron transport (Ferroportin) in Ncu-g1gt/gt liver. n=4, *P<0.05. Values are expressed as mean±s.e.m.
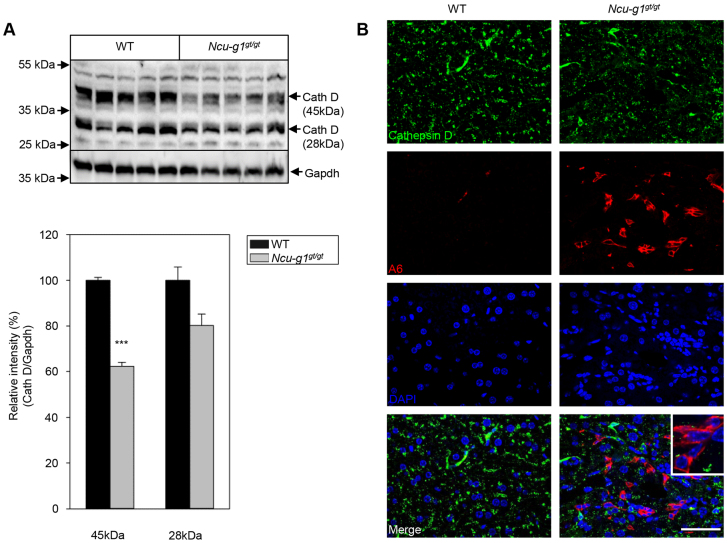
Next, we investigated the expression and activity of a selection of lysosomal enzymes known to be causative for lysosomal disorders. Immunofluorescence studies of cathepsin D expression showed colocalization with CD68 in liver sections from wild-type and Ncu-g1gt/gt mice (supplementary material Fig. S3). However, the overall expression of cathepsin D was reduced in Ncu-g1gt/gt liver (supplementary material Fig. S3). This finding was confirmed by western blot analyses where the expression of full-length cathepsin D was significantly reduced (Fig. 7A). Cathepsin D reduction has been shown during the process of tissue regeneration (Suleiman et al., 1980; Fernández et al., 2004). Chronic liver injury leads to activation of oval cells after hepatocytes have exhausted their proliferative capacity (Conigliaro et al., 2010), and might contribute to the observed increase in Mmp2 and Mmp9 expression as shown in Fig. 3G (Pham Van et al., 2008). We examined the oval cell compartment by immunofluorescence labeling for the specific marker, A6 (Engelhardt et al., 1993). Supplementary material Fig. S4 shows strongly increased staining for A6 in Ncu-g1gt/gt liver. Further fluorescence studies indicated that the reduced levels of cathepsin D in Ncu-g1gt/gt liver are partially due to the increased number of oval cells, which do not express cathepsin D (Fig. 7B).
Fig. 7.
Reduced expression of cathepsin D in Ncu-g1gt/gt liver. (A) Western blot analyses of whole liver homogenates show a significantly reduced protein expression of cathepsin D in Ncu-g1gt/gt liver (n=4, ***P<0.001). Values are expressed as mean±s.e.m. (B) Immunofluorescence image analyzing colocalization of cathepsin D (green) and the oval cell marker A6 (red). Liver sections were counterstained with DAPI (blue). Cathepsin D does not appear to colocalize with A6-positive cells in Ncu-g1gt/gt liver (inset). Scale bar: 50 μm.
No alterations of other lysosomal enzymes included in this study were detected (supplementary material Fig. S5).
DISCUSSION
In this study, we have shown that Ncu-g1gt/gt mice were viable and indistinguishable from wild-type siblings with regard to growth and fertility. However, lack of NCU-G1 expression led to spontaneous development of liver fibrosis within 6 months. The Ncu-g1gt/gt mice showed elevated serum ALT and AST activity levels, increased deposition of extracellular collagen, activated KCs and HSCs, infiltrating leukocytes and apoptotic cells. In support of these findings, the expression of marker genes for oxidative stress and fibrosis were increased in the Ncu-g1gt/gt liver. These phenotypic findings are all key elements in liver fibrogenesis (Bataller and Brenner, 2005; Friedman, 2008; Kisseleva and Brenner, 2008). Furthermore, autofluorescent lipofuscin and iron accumulated in Ncu-g1gt/gt liver KCs. Despite ubiquitous expression of Ncu-g1 (Steffensen et al., 2007; Schieweck et al., 2009) and the verified loss of transcript in all assayed organs, this study suggested that lack of NCU-G1 predominantly affects the liver. The observed splenomegaly is likely a secondary effect due to portal vein hypertension.
Lysosomes are present in nearly every cell of the body, but pathological manifestations of lysosomal disorders can be restricted to specific organs as shown for mice with disrupted lysosomal integral membrane protein 1 (Limp1). These animals are both fertile and viable and do not present any overt phenotype, but they suffer from a disturbed water balance, and hence a kidney pathology (Schröder et al., 2009). Limp1 is ubiquitously expressed, so the kidney pathology could be a result of an organ-specific function of Limp1 that cannot be compensated by other proteins (Schröder et al., 2009).
The majority of lysosomal disorders are characterized by intralysosomal accumulation of unmetabolized substrates; however, secondary alterations in other biochemical and cellular pathways contribute to a wide range of clinical symptoms (Futerman and van Meer, 2004). These vary from neurological and developmental problems to internal organ injury (Jevon and Dimmick, 1998; Futerman and van Meer, 2004; Parkinson-Lawrence et al., 2010). Liver fibrosis is a common finding in several lysosomal storage diseases, including Gaucher disease (James et al., 1981; Lachmann et al., 2000), cholesteryl ester storage disease (Desai et al., 1987; Bernstein et al., 2013), and Niemann Pick A/B and C disease (Kelly et al., 1993; Takahashi et al., 1997). Recent work by Moles et al. demonstrated a direct link between lysosomal dysfunction and liver fibrogenesis through the lysosomal enzyme acidic sphingomyelinase, deficient in Niemann Pick type A/B (Takahashi et al., 1997). This enzyme regulates fibrogenesis through control of HSC activation by the lysosomal proteases cathepsins B and D (Moles et al., 2009; Moles et al., 2010; Moles et al., 2012). Lysosomal cathepsins play a pivotal role in the execution of lysosomal cell death (reviewed in detail in Aits and Jäättelä, 2013). A direct link between hepatocyte cell death and cathepsin B and D leakage into the cytosol is well established (Roberg et al., 1999; Guicciardi et al., 2000; Kågedal et al., 2001; Canbay et al., 2003b). However, in Ncu-g1gt/gt liver the protein levels of cathepsin B and D are not elevated, indicating that the increased level of cell death and liver fibrosis observed in Ncu-g1gt/gt mice is not a result of an increased expression of these proteases. Decreased expression of cathepsin D has been reported for regenerating liver after partial hepatectomy, where the hepatocytes proliferate and restore liver volume within ~10 days (Suleiman et al., 1980; Fernández et al., 2004). In Ncu-g1gt/gt liver the reduced level of cathepsin D might result from the observed lack of cathepsin D expression in oval cells. Oval cells contribute to liver regeneration when the proliferative capacity of hepatocytes is exhausted or compromised (Conigliaro et al., 2010), as often seen in conjunction with chronic liver injury (Roskams et al., 2003; Conigliaro et al., 2010), and their activation has been shown to be closely related to inflammation and cytokine production (Knight et al., 2005). Activation of the oval cell compartment, as seen in the Ncu-g1gt/gt liver, indicates a suppressed hepatocyte proliferation at 6 months of age. The transgenic insults in the Ncu-g1gt/gt mouse are chronic and accompanied by massive infiltration of inflammatory cells, increased apoptosis and elevated expression of cytokines, yet these mice maintain their liver:body weight ratio and sufficient liver function to avoid progression to cirrhosis and death.
Spontaneous development of liver fibrosis in combination with a similar longevity as for the Ncu-g1gt/gt mice has been demonstrated in only a few transgenic mouse models. Among these are the Taut−/− mouse model and the hepatocyte-specific deletions of Bcl-xL or Mcl-1, all displaying elevated rates of hepatocyte apoptosis as the fibrogenic inducer (Takehara et al., 2004; Warskulat et al., 2006; Vick et al., 2009; Weber et al., 2010). Interestingly, the liver integrity in the Taut−/− mouse is maintained by oval cell proliferation (Warskulat et al., 2006). The ability of Ncu-g1gt/gt mice to maintain sufficient liver function and integrity with older age is currently under investigation.
With regard to putatively affected pathways in which NCU-G1 might be involved, the detection of autofluorescent lipofuscin might give some clues. Intralysosomal accumulation of unmetabolized substrates is often caused by deficiencies of soluble enzymes, but some are caused by dysfunction or absence of transmembrane proteins, some of which act as exporters of metabolites through the lysosomal membrane (Futerman and van Meer, 2004). Whether NCU-G1 with its single transmembrane region (Sardiello et al., 2009; Schieweck et al., 2009; Schröder et al., 2010) might serve any export function similar to the Lamp2-mediated cholesterol export from lysosomes remains to be determined (Schneede et al., 2011). Lipofuscin is an intralysosomal polymeric material, undegradable by lysosomal enzymes. It originates from oxidative damage to macromolecules, often associated with degradation of iron-containing proteins (Terman and Brunk, 2004). KCs contribute to the iron metabolism through phagocytosis of erythrocytes (Graham et al., 2007) and excess storage of heme in KCs has been shown to promote oxidative stress, increase hepatic inflammation, induce hepatocyte apoptosis and stimulate fibrogenesis (Otogawa et al., 2007). In support of the hypothesis that storage material in phagolysosomes of KCs can be causative for the inflammation observed in Ncu-g1gt/gt liver, the threefold increase of CD86 expression suggests that the Ncu-g1gt/gt hepatic macrophage subset is pro-inflammatory (Ramachandran et al., 2012). However, this result needs to be interpreted with caution, because liver sinusoidal endothelial cells are also known to express this marker (Knolle et al., 1998; Knolle and Limmer, 2003).
Ncu-g1gt/gt KCs also show accumulation of iron, as detected by Perls’ Prussian blue staining, coinciding with an elevated hepatic expression of the iron storage protein L-Ferritin (Recalcati et al., 2008). The plasma membrane iron exporter, Ferroportin, is expressed exclusively by KCs in liver (Knutson et al., 2003), and its expression is induced by erythrophagocytosis and iron loading (Knutson et al., 2003). During a state of inflammation, elevated Hepcidin1 levels target Ferroportin for degradation (Collins et al., 2008; Ganz, 2011). In agreement with the observed iron accumulation in KCs, the expression of Hepcidin1 and Ferroportin was increased in Ncu-g1gt/gt liver. These results also suggest a possible accumulation of erythrocyte-derived storage material in the phagolysosomes of Ncu-g1gt/gt KCs. An essential heme transporter from the phagolysosomes of macrophages (HRG1) has recently been described (White et al., 2013). Whether the glycosylated luminal tail of NCU-G1 might interact directly with HRG1 or offer protection against lysosomal proteases (Schieweck et al., 2009) remains to be elucidated. However, we cannot finally rule out that lipofuscin and iron deposition are rather a consequence than the cause of liver fibrosis.
In summary, we have created a viable Ncu-g1gt/gt mouse with spontaneous liver fibrosis as the predominant phenotype. The condition progresses slowly, but displays all hallmarks of fibrosis at 6 months of age. Elevated markers for oxidative stress and inflammation were detected in Ncu-g1gt/gt liver, in addition to increased accumulation of lipofuscin and iron in KCs. Fibrosis has many etiologies and consists of acute as well as chronic liver damage. It is well known that reversal of fibrosis is possible if the cause is remedied; however, that is often not possible. In the constant search for improved drugs to treat this condition, various animal models are essential tools. We propose that the Ncu-g1gt/gt mouse could be useful in the development of treatments for attenuation of fibrosis due to chronic liver damage.
MATERIALS AND METHODS
Generation of Ncu-g1gt/gt mice
Embryonic stem cells (ES cells 129S2, P084H04) carrying a gene-trap in the first intron of the Ncu-g1 gene (RIKEN cDNA 0610031J06 gene) were purchased from The German Gene-Trap Consortium (Neuherberg, Germany). The gene-trap leads to a stop in transcription at this point. In collaboration with the Norwegian Transgenic Centre (Oslo, Norway), ES cells were injected into C57BL/6 blastocysts and chimeric mice were obtained. Five chimeric males were mated with C57BL/6 females. The first litters consisted of 29 pups out of which nine were positive for neomycin and seven of these were shown to carry the gene-trap by PCR. Two females and one male were chosen for further breeding and pups were analyzed for presence of the gene-trap by PCR, using 200 ng of ear genomic DNA and the primer pairs shown in supplementary material Table S2 to differentiate between wild-type and gene-trap-carrying mice: FlpROSA, yielding a product of 811 bp when the gene-trap cassette is present; and NCUG1, yielding a 562 bp product when the gene-trap cassette is absent. Founders were selected and mated to yield the F1 generation. Mating resulted in mice heterozygous for the Ncu-g1 gene modification. Heterozygotes were mated, leading to the birth of homozygotes. Mice were maintained in an approved animal facility (National Lab Animal Center, The Norwegian Institute of Public Health, Oslo, Norway) with access to food and water ad libitum. Animal handling was according to national laws and regulations.
Analysis of gene expression
RNA extractions from mouse liver, kidney, lung and spleen (n=3) were carried out according to the manufacturer using RNeasy Plus kit from Qiagen (Hilden, Germany). Expression of Ncu-g1 mRNA was assessed using TaqMan gene expression assays for detection of mouse Ncu-g1: Mm00658309_g1, and mouse Gapdh: Mm99999915_g1 (Life Technologies, Carlsbad, CA, USA), and LightCycler® 480 Probes Master (Roche Applied Science, Mannheim, Germany). Signals below the threshold value (cp >40) were manually set to 40 in the calculations. RNA was extracted from livers of wild-type and Ncu-g1gt/gt mice (n=3), and gene expression was analyzed using the Fibrosis (PAMM-120A, Qiagen), Hepatotoxicity (PAMM-093F, Qiagen), Signal Transduction Pathway Finder (PAMM-014A, Qiagen) and Oxidative Stress and Antioxidant Defense RT2 Profiler™ Array (PAMM-065A, Qiagen), following the manufacturer’s instructions. To verify array results, and to further elucidate the fibrogenic response, RNA from livers of wild-type and Ncu-g1gt/gt mice (n=4, age 6 months) was analyzed for relative gene expression of selected mRNA transcripts by qPCR (supplementary material Table S3). Analysis was performed using LightCycler® 480 SYBR Green I Master Mix (Roche Applied Science). Relative gene expression was calculated using β-actin and eukaryotic translation elongation factor 2 as reference genes.
Isolation of mouse liver cells from wild-type mice was carried out by the two-step perfusion method as described (Hansen et al., 2002). Liver parenchymal and non-parenchymal cells were separated by differential centrifugation and Pronase E (Merck, Darmstadt, Germany) treatment as described elsewhere (Berg and Boman, 1973). The expression of Ncu-g1 was analyzed as described above.
Histochemistry
Livers from 6-month-old Ncu-g1gt/gt (n=6) and wild-type (n=3) mice were collected and fixed in 4% formaldehyde in 0.1 M PBS. Representative tissue blocks (5 mm thick) were embedded in paraffin, sliced in 4-μm sections and stained with H&E, Masson-Goldner’s trichrome staining for collagen, or Perls’ Prussian blue staining for iron according to standard procedures.
Immunohistochemistry
For labeling of α-Sma and active caspase 3, formalin-fixed paraffin-embedded sections were deparaffinized, rehydrated and demasked in a microwave oven for 24 minutes in Tris/EDTA buffer (pH 9.1). Rabbit polyclonal anti-α-Sma (1:500, ab5694, Abcam, Cambridge, MA, USA) and rabbit polyclonal anti-active caspase 3 (1:500, G7481, Promega, Madison, WI, USA) were used as antibodies. For labeling of F4/80, formalin-fixed paraffin-embedded sections were deparaffinized, rehydrated and demasked in a microwave oven for 24 minutes in Target retrieval solution (pH 6.0–6.2). Rat monoclonal anti-F4/80 (1:100, 14-4801, eBioscience, San Diego, CA, USA) and peroxidase-conjugated AffiniPure F(ab′)2 fragment mouse anti-rat IgG (H+L) (1:100, 212-036-168, Jackson ImmunoResearch Europe Ltd, Newmarket, Suffolk, UK) were used as primary and secondary antibodies, respectively.
The antigen-antibody complexes were visualized with Dako Cytomation Envision+ System-HRP (K4007, DAKO North America Inc., CA, USA) using 3,3′-diaminobenzidine as the chromogen. The sections were counterstained with Mayer’s hematoxylin solution.
Immunofluorescence
Formalin-fixed livers were sectioned using a Leica 9000s sliding microtome (Wetzlar, Germany) into 40-μm-thick free-floating sections. After blocking with 4% normal goat serum and permeabilization with 0.5% Triton X-100 in 0.1 M phosphate buffer (PB), sections were incubated with appropriate primary antibodies overnight. Antibodies used for immunofluorescence were: rat monoclonal anti-CD68 (1:500, FA-11, AbD Serotec, Oxford, UK); rabbit polyclonal anti-myeloperoxidase (1:300, Millipore, Billerica, MA, USA); rat monoclonal anti-F4/80 (1:25 cell culture supernatant). The monoclonal antibody against mouse Lamp1 (clone 1D4B) was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242. Rat monoclonal anti-A6 (cell culture supernatant; 1:25) was a kind gift from Valentina Factor and described previously (Engelhardt et al., 1990). Cathepsin D antibody was described previously (Claussen et al., 1997). After washing with 0.25% Triton X-100 in PB and incubation with Alexa-Fluor-488 or -633 secondary antibodies (Molecular Probes, Eugene, OR, USA), sections were counterstained with DAPI and mounted with Mowiol/DABCO. Confocal laser scanning microscopy was performed on a Leica TCS SP2 microscope (Wetzlar, Germany).
Transmission electron microscopy (TEM)
Ncu-g1gt/gt and wild-type mice (n=3, age 6 months) were subjected to perfusion fixation using HEPES buffer (0.1 M, pH 7.2–7.4) with 4% formaldehyde and 2.5% glutaraldehyde. Livers were cut into tissue blocks of 1 mm3, transferred to new fixative solution and kept at 4°C overnight. The samples were rinsed 2×10 minutes in 0.1 M sodium cacodylate buffer prior to post-fixation (2% OsO4 in 0.1 M sodium cacodylate buffer) for 1 hour, and rinsed 5×10 minutes in distilled water before bulk staining with 1.5% uranyl acetate [(CH3COO)2UO2·2H2O] in distilled water for 30 minutes in the dark. Fixed tissue samples were dehydrated in a graded series of ethanol for 10 minutes each (70%, 80%, 90%, 96%), 4×15 minutes in 100% ethanol and finally 2×10 minutes in propylene oxide. Following dehydration, tissue samples were infiltrated with epoxy:propylene oxide (1:1) for 30–60 minutes on a rotary shaker, evaporated overnight, and the following day incubated 1 hour in pure epoxy before embedding in plastic capsules and polymerization at 60°C. Five tissue blocks were selected per individual mouse. Ultrathin sections were obtained using a Leica Ultracut S microtome (Leica, Wetzlar, Germany) with diamond knife. The sections were collected on copper-coated grids and stained with lead citrate for 20 seconds. All electron micrographs were obtained with a CM200 transmission microscope (Philips, Amsterdam, The Netherlands) at 80 kV and at least 20 unique micrographs were analyzed in order to produce representative figures.
Sirius-red quantification of total liver collagen
Quantification of total collagen content from mouse liver tissue was carried out by a sirius-red colorimetric plate assay as described (Kliment et al., 2011). Briefly, livers were homogenized in 10 volumes of CHAPS buffer (50 mM Tris-HCl, pH 7.4, containing 150 mM NaCl, 10 mM CHAPS and protease inhibitors) using an Ultra-Turrax. The samples were diluted using PBS, dried at 37°C overnight on a microtiter plate, and total liver collagen content was determined colorimetrically using 0.1% sirius-red stain (Direct red 80, Sigma-Aldrich, St Louis, MO, USA) in saturated picric acid, and rat tail collagen (Sigma-Aldrich) as standards.
Western blotting
Liver tritosomes were isolated as described elsewhere (Schieweck et al., 2009). Tritosome preparations (20 μg protein) were electrophoresed on NuPAGE® 4–12% Bis-Tris Mini Gels (Life Technologies), and transferred onto PVDF membranes (Bio-Rad, Hercules, CA, USA). After blocking, membranes were incubated overnight at 4°C with rabbit anti-NCU-G1 serum (Schieweck et al., 2009) or rabbit anti-Lamp1 (1:1000, C54H11, Cell Signaling, Beverly, MA, USA). This was followed by 1 hour incubation with goat anti-rabbit secondary antibody conjugated to horseradish peroxidase (1:4000, 65-6120, Life Technologies). For liver homogenates, livers were homogenized in 20 volumes of lysis buffer (tris-buffered saline containing 1% Triton X-100 and protease inhibitors) by Potter Elvehjem homogenizer, incubated on ice for 30 minutes with continuous vortexing and cleared by centrifugation for 20 minutes at 14,000 g. The supernatants were used for western blotting after separation on 15% SDS-PAGE gels and transfer onto PVDF membranes (Bio-Rad). After incubation with primary antibodies overnight [caspase 3: 1:1000, Cell Signaling, Beverly, MA, USA; cathepsin D: 1:1000 (Claussen et al., 1997); cathepsin B and Gapdh: 1:300 each, Santa Cruz Antibodies] and washing, membranes were incubated for 1 hour with goat anti-rabbit secondary antibody conjugated to horseradish peroxidase (1:5000, Dianova). Protein bands were visualized using Amersham ECL Plus Western Blotting Detection Reagents (GE Healthcare, Pittsburgh, PA, USA).
Enzyme activity assays
Enzymatic activities of lysosomal hydrolases were determined from liver homogenates (see above) using standard procedures with colorimetric substrates (p-Nitrophenyl-α-D-mannopyranoside for α-mannosidase and p-Nitrophenyl N-acetyl-β-D-glucosaminide for β-hexosaminidase). Absorption of nitrophenolate was determined at 405 nm in a 96-well plate reader. For cathepsin B determination, protease inhibitors were omitted from the lysis buffer. Cathepsin B was determined using fluorescence substrate Z-Arg-Arg-AMC (Bachem, Weil am Rhein, Germany) according to Barrett (Barrett, 1980).
Serum analysis
Coagulated blood samples from wild-type and Ncu-g1gt/gt mice (n=4) were centrifuged at 1500 g for 20 minutes, and serum ALT and AST activities were measured at The Central Laboratory, Department of Basic Sciences and Aquatic Medicine, Norwegian School of Veterinary Science.
Statistical methods
All results are expressed as mean±s.e.m. Genotype distribution was analyzed using the Chi-square test (Microsoft Excel, Microsoft Corporation, Redmond, WA, USA). Other data were analyzed using two-tailed t-test (SigmaPlot™, Systat Software Inc., Chicago, IL, USA).
Supplementary Material
Acknowledgments
The A6 antibody was kindly provided by Dr Valentina Factor. We thank Camilla Schjalm for her contribution to the qPCR data, and Ingeborg Løstegaard Goverud, Tove Bakar, Hilde Letnes and Hilde Hyldmo for excellent technical assistance.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
All authors were involved in drafting the article, and all authors approved the final version to be published. X.Y.K., C.K.N., M.D., T.L. and W.E. conceived and designed the experiments. X.Y.K., C.K.N. and M.D. performed most of the experiments. W.E. generated the Ncu-g1gt/gt mice. P.I.L. performed genotyping. X.Y.K., C.K.N., M.D., E.-M.L. and N.R. analyzed most of the data. K.B.A. performed statistical analyses on genotype distribution. X.Y.K., C.K.N., M.D. and W.E. wrote the manuscript. E.-M.L., T.L., J.M., K.B.A., G.H.T., A.C.R. and E.T.K. corrected manuscript drafts.
Funding
This work was supported by South-Eastern Norway Regional Health Authority.
Supplementary material
Supplementary material available online at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.014050/-/DC1
References
- Aits S., Jäättelä M. (2013). Lysosomal cell death at a glance. J. Cell Sci. 126, 1905–1912 [DOI] [PubMed] [Google Scholar]
- Amanzada A., Malik I. A., Nischwitz M., Sultan S., Naz N., Ramadori G. (2011). Myeloperoxidase and elastase are only expressed by neutrophils in normal and in inflamed liver. Histochem. Cell Biol. 135, 305–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. (1980). Fluorimetric assays for cathepsin B and cathepsin H with methylcoumarylamide substrates. Biochem. J. 187, 909–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataller R., Brenner D. A. (2005). Liver fibrosis. J. Clin. Invest. 115, 209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg T., Boman D. (1973). Distribution of lysosomal enzymes between parenchymal and Kupffer cells of rat liver. Biochim. Biophys. Acta 321, 585–596 [DOI] [PubMed] [Google Scholar]
- Bernstein D. L., Hülkova H., Bialer M. G., Desnick R. J. (2013). Cholesteryl ester storage disease: review of the findings in 135 reported patients with an underdiagnosed disease. J. Hepatol. 58, 1230–1243 [DOI] [PubMed] [Google Scholar]
- Boers W., Aarrass S., Linthorst C., Pinzani M., Elferink R. O., Bosma P. (2006). Transcriptional profiling reveals novel markers of liver fibrogenesis: gremlin and insulin-like growth factor-binding proteins. J. Biol. Chem. 281, 16289–16295 [DOI] [PubMed] [Google Scholar]
- Brown K. E., Brunt E. M., Heinecke J. W. (2001). Immunohistochemical detection of myeloperoxidase and its oxidation products in Kupffer cells of human liver. Am. J. Pathol. 159, 2081–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canbay A., Feldstein A. E., Higuchi H., Werneburg N., Grambihler A., Bronk S. F., Gores G. J. (2003a). Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology 38, 1188–1198 [DOI] [PubMed] [Google Scholar]
- Canbay A., Guicciardi M. E., Higuchi H., Feldstein A., Bronk S. F., Rydzewski R., Taniai M., Gores G. J. (2003b). Cathepsin B inactivation attenuates hepatic injury and fibrosis during cholestasis. J. Clin. Invest. 112, 152–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claussen M., Kübler B., Wendland M., Neifer K., Schmidt B., Zapf J., Braulke T. (1997). Proteolysis of insulin-like growth factors (IGF) and IGF binding proteins by cathepsin D. Endocrinology 138, 3797–3803 [DOI] [PubMed] [Google Scholar]
- Collins J. F., Wessling-Resnick M., Knutson M. D. (2008). Hepcidin regulation of iron transport. J. Nutr. 138, 2284–2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conigliaro A., Brenner D. A., Kisseleva T. (2010). Hepatic progenitors for liver disease: current position. Stem Cells Cloning 3, 39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox T. M., Cachón-González M. B. (2012). The cellular pathology of lysosomal diseases. J. Pathol. 226, 241–254 [DOI] [PubMed] [Google Scholar]
- Desai P. K., Astrin K. H., Thung S. N., Gordon R. E., Short M. P., Coates P. M., Desnick R. J., Reynolds J. F. (1987). Cholesteryl ester storage disease: pathologic changes in an affected fetus. Am. J. Med. Genet. 26, 689–698 [DOI] [PubMed] [Google Scholar]
- Engelhardt N. V., Factor V. M., Yasova A. K., Poltoranina V. S., Baranov V. N., Lasareva M. N. (1990). Common antigens of mouse oval and biliary epithelial cells. Expression on newly formed hepatocytes. Differentiation 45, 29–37 [DOI] [PubMed] [Google Scholar]
- Engelhardt N. V., Factor V. M., Medvinsky A. L., Baranov V. N., Lazareva M. N., Poltoranina V. S. (1993). Common antigen of oval and biliary epithelial cells (A6) is a differentiation marker of epithelial and erythroid cell lineages in early development of the mouse. Differentiation 55, 19–26 [DOI] [PubMed] [Google Scholar]
- Eskild W., Simard J., Hansson V., Guérin S. L. (1994). Binding of a member of the NF1 family of transcription factors to two distinct cis-acting elements in the promoter and 5′-flanking region of the human cellular retinol binding protein 1 gene. Mol. Endocrinol. 8, 732–745 [DOI] [PubMed] [Google Scholar]
- Fernández M. A., Turró S., Ingelmo-Torres M., Enrich C., Pol A. (2004). Intracellular trafficking during liver regeneration. Alterations in late endocytic and transcytotic pathways. J. Hepatol. 40, 132–139 [DOI] [PubMed] [Google Scholar]
- Fickert P., Zollner G., Fuchsbichler A., Stumptner C., Weiglein A. H., Lammert F., Marschall H. U., Tsybrovskyy O., Zatloukal K., Denk H., et al. (2002). Ursodeoxycholic acid aggravates bile infarcts in bile duct-ligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterology 123, 1238–1251 [DOI] [PubMed] [Google Scholar]
- Ford D. A. (2010). Lipid oxidation by hypochlorous acid: chlorinated lipids in atherosclerosis and myocardial ischemia. Clin. Lipidol. 5, 835–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. L. (2008). Hepatic fibrosis – overview. Toxicology 254, 120–129 [DOI] [PubMed] [Google Scholar]
- Futerman A. H., van Meer G. (2004). The cell biology of lysosomal storage disorders. Nat. Rev. Mol. Cell Biol. 5, 554–565 [DOI] [PubMed] [Google Scholar]
- Ganz T. (2011). Hepcidin and iron regulation, 10 years later. Blood 117, 4425–4433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieling R. G., Burt A. D., Mann D. A. (2008). Fibrosis and cirrhosis reversibility-molecular mechanisms. Clin. Liver Dis. 12, 915–937, xi [DOI] [PubMed] [Google Scholar]
- Graham R. M., Chua A. C. G., Herbison C. E., Olynyk J. K., Trinder D. (2007). Liver iron transport. World J. Gastroenterol. 13, 4725–4736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressner O. A., Weiskirchen R., Gressner A. M. (2007). Biomarkers of hepatic fibrosis, fibrogenesis and genetic pre-disposition pending between fiction and reality. J. Cell. Mol. Med. 11, 1031–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guicciardi M. E., Deussing J., Miyoshi H., Bronk S. F., Svingen P. A., Peters C., Kaufmann S. H., Gores G. J. (2000). Cathepsin B contributes to TNF-α-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J. Clin. Invest. 106, 1127–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen B., Arteta B., Smedsrød B. (2002). The physiological scavenger receptor function of hepatic sinusoidal endothelial and Kupffer cells is independent of scavenger receptor class A type I and II. Mol. Cell. Biochem. 240, 1–8 [DOI] [PubMed] [Google Scholar]
- Hernandez-Gea V., Friedman S. L. (2011). Pathogenesis of liver fibrosis. Annu. Rev. Pathol. 6, 425–456 [DOI] [PubMed] [Google Scholar]
- James S. P., Stromeyer F. W., Chang C., Barranger J. A. (1981). LIver abnormalities in patients with Gaucher’s disease. Gastroenterology 80, 126–133 [PubMed] [Google Scholar]
- Jevon G. P., Dimmick J. E. (1998). Histopathologic approach to metabolic liver disease: Part 1. Pediatr. Dev. Pathol. 1, 179–199 [DOI] [PubMed] [Google Scholar]
- Kågedal K., Johansson U., Ollinger K. (2001). The lysosomal protease cathepsin D mediates apoptosis induced by oxidative stress. FASEB J. 15, 1592–1594 [DOI] [PubMed] [Google Scholar]
- Kawamura T., Kuroda N., Kimura Y., Lazoura E., Okada N., Okada H. (2001). cDNA of a novel mRNA expressed predominantly in mouse kidney. Biochem. Genet. 39, 33–42 [DOI] [PubMed] [Google Scholar]
- Kelly D. A., Portmann B., Mowat A. P., Sherlock S., Lake B. D. (1993). Niemann-Pick disease type C: diagnosis and outcome in children, with particular reference to liver disease. J. Pediatr. 123, 242–247 [DOI] [PubMed] [Google Scholar]
- Kinoshita M., Uchida T., Sato A., Nakashima M., Nakashima H., Shono S., Habu Y., Miyazaki H., Hiroi S., Seki S. (2010). Characterization of two F4/80-positive Kupffer cell subsets by their function and phenotype in mice. J. Hepatol. 53, 903–910 [DOI] [PubMed] [Google Scholar]
- Kiselyov K., Jennigs J. J., Jr, Rbaibi Y., Chu C. T. (2007). Autophagy, mitochondria and cell death in lysosomal storage diseases. Autophagy 3, 259–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisseleva T., Brenner D. A. (2008). Mechanisms of fibrogenesis. Exp. Biol. Med. 233, 109–122 [DOI] [PubMed] [Google Scholar]
- Kisseleva T., Brenner D. A. (2011). Anti-fibrogenic strategies and the regression of fibrosis. Best Pract. Res Clin. Gastroenterol. 25, 305–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliment C. R., Englert J. M., Crum L. P., Oury T. D. (2011). A novel method for accurate collagen and biochemical assessment of pulmonary tissue utilizing one animal. Int. J. Clin. Exp. Pathol. 4, 349–355 [PMC free article] [PubMed] [Google Scholar]
- Knight B., Matthews V. B., Akhurst B., Croager E. J., Klinken E., Abraham L. J., Olynyk J. K., Yeoh G. (2005). Liver inflammation and cytokine production, but not acute phase protein synthesis, accompany the adult liver progenitor (oval) cell response to chronic liver injury. Immunol. Cell Biol. 83, 364–374 [DOI] [PubMed] [Google Scholar]
- Knolle P. A., Limmer A. (2003). Control of immune responses by savenger liver endothelial cells. Swiss Med. Wkly. 133, 501–506 [DOI] [PubMed] [Google Scholar]
- Knolle P. A., Uhrig A., Hegenbarth S., Löser E., Schmitt E., Gerken G., Lohse A. W. (1998). IL-10 down-regulates T cell activation by antigen-presenting liver sinusoidal endothelial cells through decreased antigen uptake via the mannose receptor and lowered surface expression of accessory molecules. Clin. Exp. Immunol. 114, 427–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson M. D., Vafa M. R., Haile D. J., Wessling-Resnick M. (2003). Iron loading and erythrophagocytosis increase ferroportin 1 (FPN1) expression in J774 macrophages. Blood 102, 4191–4197 [DOI] [PubMed] [Google Scholar]
- Kurz T., Terman A., Gustafsson B., Brunk U. T. (2008). Lysosomes and oxidative stress in aging and apoptosis. Biochim. Biophys. Acta 1780, 1291–1303 [DOI] [PubMed] [Google Scholar]
- Lachmann R. H., Wight D. G. D., Lomas D. J., Fisher N. C., Schofield J. P., Elias E., Cox T. M. (2000). Massive hepatic fibrosis in Gaucher’s disease: clinico-pathological and radiological features. QJM 93, 237–244 [DOI] [PubMed] [Google Scholar]
- Maher J. J. (2001). Interactions between hepatic stellate cells and the immune system. Semin. Liver Dis. 21, 417–426 [DOI] [PubMed] [Google Scholar]
- Moles A., Tarrats N., Fernández-Checa J. C., Marí M. (2009). Cathepsins B and D drive hepatic stellate cell proliferation and promote their fibrogenic potential. Hepatology 49, 1297–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles A., Tarrats N., Morales A., Domínguez M., Bataller R., Caballería J., García-Ruiz C., Fernández-Checa J. C., Marí M. (2010). Acidic sphingomyelinase controls hepatic stellate cell activation and in vivo liver fibrogenesis. Am. J. Pathol. 177, 1214–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles A., Tarrats N., Fernández-Checa J. C., Marí M. (2012). Cathepsin B overexpression due to acid sphingomyelinase ablation promotes liver fibrosis in Niemann-Pick disease. J. Biol. Chem. 287, 1178–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormone E., George J., Nieto N. (2011). Molecular pathogenesis of hepatic fibrosis and current therapeutic approaches. Chem. Biol. Interact. 193, 225–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otogawa K., Kinoshita K., Fujii H., Sakabe M., Shiga R., Nakatani K., Ikeda K., Nakajima Y., Ikura Y., Ueda M., et al. (2007). Erythrophagocytosis by liver macrophages (Kupffer cells) promotes oxidative stress, inflammation, and fibrosis in a rabbit model of steatohepatitis: implications for the pathogenesis of human nonalcoholic steatohepatitis. Am. J. Pathol. 170, 967–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson-Lawrence E. J., Shandala T., Prodoehl M., Plew R., Borlace G. N., Brooks D. A. (2010). Lysosomal storage disease: revealing lysosomal function and physiology. Physiology (Bethesda) 25, 102–115 [DOI] [PubMed] [Google Scholar]
- Pham Van T., Couchie D., Martin-Garcia N., Laperche Y., Zafrani E. S., Mavier P. (2008). Expression of matrix metalloproteinase-2 and -9 and of tissue inhibitor of matrix metalloproteinase-1 in liver regeneration from oval cells in rat. Matrix Biol. 27, 674–681 [DOI] [PubMed] [Google Scholar]
- Pinzani M., Macias-Barragan J. (2010). Update on the pathophysiology of liver fibrosis. Expert Rev. Gastroenterol. Hepatol. 4, 459–472 [DOI] [PubMed] [Google Scholar]
- Platt F. M., Boland B., van der Spoel A. C. (2012). The cell biology of disease: lysosomal storage disorders: the cellular impact of lysosomal dysfunction. J. Cell Biol. 199, 723–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran P., Iredale J. P. (2012). Liver fibrosis: a bidirectional model of fibrogenesis and resolution. QJM 105, 813–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran P., Pellicoro A., Vernon M. A., Boulter L., Aucott R. L., Ali A., Hartland S. N., Snowdon V. K., Cappon A., Gordon-Walker T. T., et al. (2012). Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc. Natl. Acad. Sci. USA 109, E3186–E3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recalcati S., Invernizzi P., Arosio P., Cairo G. (2008). New functions for an iron storage protein: the role of ferritin in immunity and autoimmunity. J. Autoimmun. 30, 84–89 [DOI] [PubMed] [Google Scholar]
- Roberg K., Johansson U., Öllinger, K. (1999). Lysosomal release of cathepsin D precedes relocation of cytochrome c and loss of mitochondrial transmembrane potential during apoptosis induced by oxidative stress. Free Radic. Biol. Med. 27, 1228–1237 [DOI] [PubMed] [Google Scholar]
- Roskams T. A., Libbrecht L., Desmet V. J. (2003). Progenitor cells in diseased human liver. Semin. Liver Dis. 23, 385–396 [DOI] [PubMed] [Google Scholar]
- Ryckman C., Vandal K., Rouleau P., Talbot M., Tessier P. A. (2003). Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J. Immunol. 170, 3233–3242 [DOI] [PubMed] [Google Scholar]
- Sardiello M., Palmieri M., di Ronza A., Medina D. L., Valenza M., Gennarino V. A., Di Malta C., Donaudy F., Embrione V., Polishchuk R. S., et al. (2009). A gene network regulating lysosomal biogenesis and function. Science 325, 473–477 [DOI] [PubMed] [Google Scholar]
- Schieweck O., Damme M., Schröder B., Hasilik A., Schmidt B., Lübke T. (2009). NCU-G1 is a highly glycosylated integral membrane protein of the lysosome. Biochem. J. 422, 83–90 [DOI] [PubMed] [Google Scholar]
- Schneede A., Schmidt C. K., Hölttä-Vuori M., Heeren J., Willenborg M., Blanz J., Domanskyy M., Breiden B., Brodesser S., Landgrebe J., et al. (2011). Role for LAMP-2 in endosomal cholesterol transport. J. Cell. Mol. Med. 15, 280–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder J., Lüllmann-Rauch R., Himmerkus N., Pleines I., Nieswandt B., Orinska Z., Koch-Nolte F., Schröder B., Bleich M., Saftig P. (2009). Deficiency of the tetraspanin CD63 associated with kidney pathology but normal lysosomal function. Mol. Cell. Biol. 29, 1083–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder B. A., Wrocklage C., Hasilik A., Saftig P. (2010). The proteome of lysosomes. Proteomics 10, 4053–4076 [DOI] [PubMed] [Google Scholar]
- Starkel P., Leclercq I. A. (2011). Animal models for the study of hepatic fibrosis. Best Pract. Res. Clin. Gastroenterol. 25, 319–333 [DOI] [PubMed] [Google Scholar]
- Steffensen K. R., Bouzga M., Skjeldal F., Kasi C., Karahasan A., Matre V., Bakke O., Guérin S., Eskild W. (2007). Human NCU-G1 can function as a transcription factor and as a nuclear receptor co-activator. BMC Mol. Biol. 8, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suleiman S. A., Jones G. L., Singh H., Labrecque D. R. (1980). Changes in lysosomal cathepsins during liver regeneration. Biochim. Biophys. Acta 627, 17–22 [DOI] [PubMed] [Google Scholar]
- Takahashi T., Akiyama K., Tomihara M., Tokudome T., Nishinomiya F., Tazawa Y., Horinouchi K., Sakiyama T., Takada G. (1997). Heterogeneity of liver disorder in type B Niemann-Pick disease. Hum. Pathol. 28, 385–388 [DOI] [PubMed] [Google Scholar]
- Takehara T., Tatsumi T., Suzuki T., Rucker E. B., I, Hennighausen L., Jinushi M., Miyagi T., Kanazawa Y., Hayashi N. (2004). Hepatocyte-specific disruption of Bcl-xL leads to continuous hepatocyte apoptosis and liver fibrotic responses. Gastroenterology 127, 1189–1197 [DOI] [PubMed] [Google Scholar]
- Tardy C., Andrieu-Abadie N., Salvayre R., Levade T. (2004). Lysosomal storage diseases: is impaired apoptosis a pathogenic mechanism? Neurochem. Res. 29, 871–880 [DOI] [PubMed] [Google Scholar]
- Terman A., Brunk U. T. (2004). Lipofuscin. Int. J. Biochem. Cell Biol. 36, 1400–1404 [DOI] [PubMed] [Google Scholar]
- Vick B., Weber A., Urbanik T., Maass T., Teufel A., Krammer P. H., Opferman J. T., Schuchmann M., Galle P. R., Schulze-Bergkamen H. (2009). Knockout of myeloid cell leukemia-1 induces liver damage and increases apoptosis susceptibility of murine hepatocytes. Hepatology 49, 627–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandzioch E., Kolterud A., Jacobsson M., Friedman S. L., Carlsson L. (2004). Lhx2−/−mice develop liver fibrosis. Proc. Natl. Acad. Sci. USA 101, 16549–16554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warskulat U., Borsch E., Reinehr R., Heller-Stilb B., Mönnighoff I., Buchczyk D., Donner M., Flögel U., Kappert G., Soboll S., et al. (2006). Chronic liver disease is triggered by taurine transporter knockout in the mouse. FASEB J. 20, 574–576 [DOI] [PubMed] [Google Scholar]
- Weber A., Boger R., Vick B., Urbanik T., Haybaeck J., Zoller S., Teufel A., Krammer P. H., Opferman J. T., Galle P. R., et al. (2010). Hepatocyte-specific deletion of the antiapoptotic protein myeloid cell leukemia-1 triggers proliferation and hepatocarcinogenesis in mice. Hepatology 51, 1226–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler-Normann C., Herkel J., Lohse A. W. (2007). Mouse models of liver fibrosis. Z. Gastroenterol. 45, 43–50 [DOI] [PubMed] [Google Scholar]
- White C., Yuan X., Schmidt P. J., Bresciani E., Samuel T. K., Campagna D., Hall C., Bishop K., Calicchio M. L., Lapierre A., et al. (2013). HRG1 is essential for heme transport from the phagolysosome of macrophages during erythrophagocytosis. Cell Metab. 17, 261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan S.-S., Jiang J. X., Wu J., Halsted C., Friedman S. L., Zern M. A., Torok N. J. (2006). Phagocytosis of apoptotic bodies by hepatic stellate cells induces NADPH oxidase and is associated with liver fibrosis in vivo. Hepatology 43, 435–443 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.