Abstract
Marine organisms produce secondary metabolites that may be valuable for the development of novel drug leads as such and can also provide structural scaffolds for the design and synthesis of novel bioactive compounds. The marine alkaloids, clathrodin and oroidin, which were originally isolated from sponges of the genus, Agelas, were prepared and evaluated for their antimicrobial activity against three bacterial strains (Enterococcus faecalis, Staphylococcus aureus and Escherichia coli) and one fungal strain (Candida albicans), and oroidin was found to possess promising Gram-positive antibacterial activity. Using oroidin as a scaffold, 34 new analogues were designed, prepared and screened for their antimicrobial properties. Of these compounds, 12 exhibited >80% inhibition of the growth of at least one microorganism at a concentration of 50 µM. The most active derivative was found to be 4-phenyl-2-aminoimidazole 6h, which exhibited MIC90 (minimum inhibitory concentration) values of 12.5 µM against the Gram-positive bacteria and 50 µM against E. coli. The selectivity index between S. aureus and mammalian cells, which is important to consider in the evaluation of a compound’s potential as an antimicrobial lead, was found to be 2.9 for compound 6h.
Keywords: marine alkaloid, Agelas, antimicrobial, antibacterial, antifungal, pyrrole-2-aminoimidazole, oroidin, clathrodin
1. Introduction
Marine natural products constitute a vital pool of biologically active compounds that are amenable to drug discovery. The marine ecosystem is a rich source of chemically and functionally diverse molecules that function in their native environment as offensive weapons to capture pray or for protection against predators [1]. Sponges, for example, have been shown to produce secondary metabolites with highly promising antimicrobial activities [2]. Marine organisms produce some of the most potent bioactive compounds discovered to date. However, because the concentrations of these compounds are usually very low, natural sources are unlikely to provide sufficient material for isolation and detailed biological evaluation, and chemical synthesis is often necessary to investigate their mode of action and their biological implications. This option is often hampered by the fact that natural compounds are also known for their high molecular weight, large number of chiral centers and complex 3D structures, which limit their synthetic availability and make them non-drug-like.
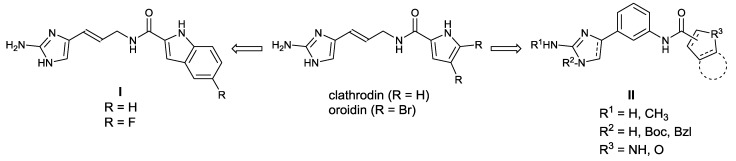
Alkaloids initially isolated from the sponges of the genus, Agelas, e.g., clathrodin and oroidin (Figure 1), belong to the pyrrole-2-aminoimidazole structural class of secondary metabolites and exhibit intriguing structural complexity and increasingly studied biological activities; thus, these compounds are attracting the attention of a growing number of researchers from numerous disciplines worldwide [3,4]. Apart from the genus, Agelas, several other genera of sponges, e.g., Hymeniacidon, Cymbaxinella and Axinella, have also been identified to produce these alkaloids [4,5]. Compared with many other pyrrole-2-aminoimidazoles, the structures of the oroidin class of alkaloids (the key precursor for this group) are relatively simple and are thus suitable candidates for optimization using established medicinal chemistry strategies. Because of its relatively low molecular mass and simple structure, oroidin offers several possibilities for chemical optimization through the introduction of additional side chains or functional groups.
Figure 1.

Structures of the marine alkaloids, clathrodin and oroidin.
Infectious diseases remain one of the leading global causes of death. The widespread occurrence of bacterial strains resistant to the currently used antimicrobials represents a significant health threat; therefore, novel structural classes of antimicrobials with novel mechanisms of action are urgently needed [6,7,8,9,10]. Marine alkaloids from Agelas sponges and their synthetic analogues have been extensively studied as inhibitors of bacterial biofilm formation [11,12,13,14,15,16,17,18,19,20] and as antibacterial [21,22,23], antifungal [24] and antiprotozoal [25,26] agents. Some mechanisms of antimicrobial and antibiofilm action have been proposed, and these include disruption of the bacterial cell membrane [27], targeting the response regulator protein, BfmR [11], and inhibition of enoyl reductases [26].
In our study, we first prepared two natural marine alkaloids, namely, clathrodin and oroidin, and evaluated their antimicrobial activity against a panel of laboratory strains of known pathogens, including Gram-positive (Enterococcus faecalis and Staphylococcus aureus) and Gram-negative bacteria (Escherichia coli) and fungi (Candida albicans). Our initial results revealed that clathrodin possessed almost no antimicrobial activity, but its dibromo analogue, oroidin, showed promising inhibition of the growth of the Gram-positive bacteria, S. aureus and E. faecalis. These results indicate that larger, lipophilic moieties, rather than the pyrrole ring found in the clathrodin molecule, are required for good antibacterial activity. The primary screen results stimulated us to prepare a series of oroidin analogues with pyrrol-replacing groups, such as indole and substituted indole rings. Using different substituents on the indole ring, we investigated the chemical properties required for biological activity. To further explore the structure-activity relationships, we also designed a series of conformationally restricted oroidin analogues. In addition to their antimicrobial evaluation, the in vitro cytotoxicity of these compounds on mammalian cells was determined to further assess the selectivity of the active compounds toward different prokaryotic and eukaryotic cells.
2. Results and Discussion
2.1. Design
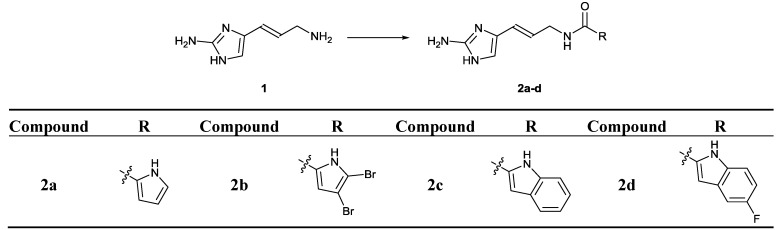
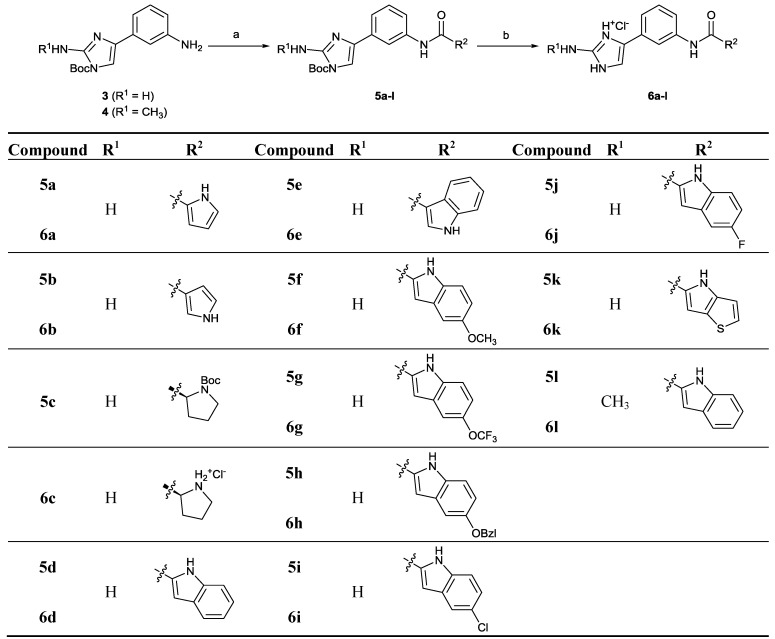
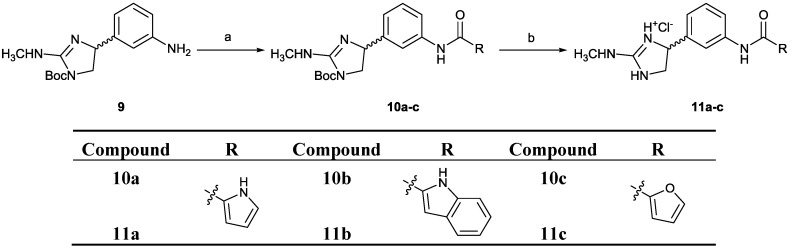
We designed two main classes of oroidin analogues, i.e., 4-(3-aminoprop-1-en-1-yl)-2-aminoimidazoles I and 4-phenyl-2-aminoimidazoles II (Figure 2). The structures of the class I compounds are closely related to natural alkaloids and were obtained through the replacement of the pyrrole or 2,3-dibromo-pyrrole rings with indole and 5-fluoro-indole moieties (Scheme 1, compounds 2c and 2d). With respect to the class II series, a set of 34 analogues was designed and prepared by introducing a phenyl ring into position 4 of the 2-aminoimidazole ring. In that way, a conformational constraint was introduced into the molecule to limit the flexibility of the compounds without altering the length of the molecule compared with the natural alkaloids. In addition to compounds with a pyrrol-2-yl substituent, which is present in clathrodin, analogues with pyrrol-3-yl, (R)-pyrrolidin-2-yl, indol-2-yl, indol-3-yl, thieno[3,2-b]pyrrol-5-yl and furan-2-yl substituents were prepared. With the introduction of these groups, we wanted to explore their optimal size and the hydrophobic/hydrophilic properties required for biological activity. In that respect, different 5-substituted indole rings were also studied. To assess the importance of the free primary amino group of the 2-aminoimidazole moiety, a set of 4-phenyl-(N-methylamino)-imidazoles, namely 5l and 6l (Scheme 2) and 10a–c and 11a–c (Scheme 3), was synthesized. Furthermore, the effects of different substituents on the imidazole N-1 nitrogen, such as Boc (tert-butyl-oxy-carbonyl) and benzyl (Scheme 4, compounds 13–16), were studied. Finally, a set of 4,5-dihydro-2-aminoimidazoles (Scheme 3, compounds 10a–c and 11a–c) with a reduced imidazole C=C bond was prepared and evaluated. With that modification, the effects of aromaticity and planarity of the imidazole ring on the biological activity were assessed.
Figure 2.
The design of 4-(3-aminoprop-1-en-1-yl)-2-aminoimidazoles (I) and 4-phenyl-2-aminoimidazoles (II) as clathrodin and oroidin analogues with potential antimicrobial activity.
Scheme 1.
Synthesis of clathrodin (2a), oroidin (2b) and their indole (2c) and 5-fluoro-indole (2d) analogues. Reagents and conditions: corresponding carboxylic acid, TBTU, NMM, DMF, rt, 6 h.
Scheme 2.
Synthesis of 4-phenyl-2-aminoimidazoles 5a–k and 6a–k and 4-phenyl-2-(N-methylamino)-imidazoles 5l and 6l. Reagents and conditions: (a) Corresponding carboxylic acid, TBTU, NMM, CH2Cl2, 35 °C, 24 h; (b) HCl(g), THF/EtOH, rt, 5 h.
Scheme 3.
Synthesis of 4-phenyl-4,5-dihydro-(N-methylamino)-imidazoles 10a–c and 11a–c. Reagents and conditions: (a) Corresponding carboxylic acid, TBTU, NMM, CH2Cl2, 35 °C, 24 h; (b) HCl(g), EtOH, rt, 5 h.
Scheme 4.
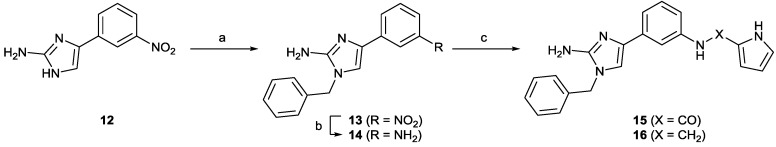
Synthesis of 1-benzyl-4-phenyl-2-aminoimidazoles 13 and 14. Reagents and conditions: (a) Benzyl bromide, K2CO3, CH3CN, 50 °C, 14 h; (b) H2/Pd-C, THF, rt, 5 h; (c) pyrrole-2-carboxylic acid, TBTU, Et3N, CH2Cl2, rt, 18 h (for the synthesis of 15); pyrrole-2-carbaldehyde, NaBH(OAc)3, CH3COOH, CH2Cl2, rt, 13 h (for the synthesis of 16).
2.2. Chemistry
Clathrodin (2a) and its indole (2c) and 5-fluoro-indole (2d) analogues were prepared by coupling amine 1 [28] and the appropriate carboxylic acid (pyrrole-2-carboxylic acid, 4,5-dibromo-pyrrole-2-carboxylic acid, indole-2-carboxylic acid or 5-fluoro-indole-2-carboxylic acid), as depicted in Scheme 1.
The 4-phenyl-2-aminoimidazoles 5a–k and 6a–k and the 4-phenyl-2-(N-methylamino)-imidazoles 5l and 6l were synthesized according to Scheme 2. First, N-Boc-protected derivatives 5a–l were prepared in a TBTU-promoted coupling reaction between tert-butyl 2-amino-4-(3-aminophenyl)-1H-imidazole-1-carboxylate (3) or tert-butyl 4-(3-aminophenyl)-2-(methylamino)-1H-imidazole-1-carboxylate (4) and various carboxylic acids (pyrrole-2-carboxylic acid, pyrrole-3-carboxylic acid, N-Boc-d-proline, indole-2-carboxylic acid, indole-3-carboxylic acid, 5-substituted indole-2-carboxylic acids or 4H-thieno[3,2-b]pyrrole-5-carboxylic acid). Next, the N-Boc protecting groups of 5a–l were removed with gaseous hydrochloric acid to obtain the target compounds 6a–l. The detailed procedures for the syntheses of tert-butyl 2-amino-4-(3-aminophenyl)1H-imidazole-1-carboxylate (3) and tert-butyl 4-(3-aminophenyl)-2-(methylamino)-1H-imidazole-1-carboxylate (4) are described elsewhere [29].
Compound 8, which contains a 5-hydroxyl substituent on the indole ring, was prepared from the 5-benzyloxy-indol derivative 5h through the two-step procedure depicted in Scheme 5. After a palladium-catalyzed hydrogenation to remove the O-benzyl group, the obtained compound 7 was converted into the target 4-phenyl-2-aminoimidazole 8 upon cleavage of the Boc protecting group with gaseous hydrochloric acid.
Scheme 5.
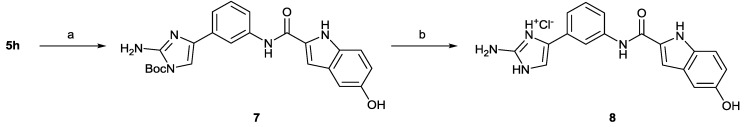
Synthesis of 4-phenyl-2-aminoimidazoles 7 and 8. Reagents and conditions: (a) H2/Pd-C, THF/MeOH, rt, 10 h; (b) HCl(g), THF/EtOH, rt, 5 h.
The 4-phenyl-4,5-dihydro-(N-methylamino)-imidazoles 11a–c were prepared using the above-described procedure for the syntheses of compounds 6a–l (Scheme 3). The TBTU-promoted coupling reactions between amine 9 and different carboxylic acids (pyrrole-2-carboxylic acid, indole-2-carboxylic acid or furan-2-carboxylic acid) yielded compounds 10a–c, which were converted into 11a–c upon cleavage of the N-Boc protecting group with gaseous hydrochloric acid. The synthesis of tert-butyl 4-(3-aminophenyl)-2-(methylamino)-4,5-dihydro-1H-imidazole-1-carboxylate (9) is reported elsewhere [29].
For the preparation of compounds 15 and 16, which contain a benzyl group on N-1 of the imidazole ring (Scheme 4), 4-(3-nitrophenyl)-2-aminoimidazole (12) was first reacted with benzyl bromide in the presence of potassium carbonate to obtain the 1-benzylated derivative 13. The nitro group of 13 was then reduced through catalytic hydrogenation, and the obtained amine 14 was coupled with pyrrole-2-carboxylic acid to afford the target compound 15. 1-Benzyl-4-phenyl-2-aminoimidazole 16, an analogue of 15 with a reduced amide bond, was obtained using sodium triacetoxyborohydride to achieve the reductive amination of 14 with pyrrole-2-carbaldehyde.
2.3. Biological Evaluation
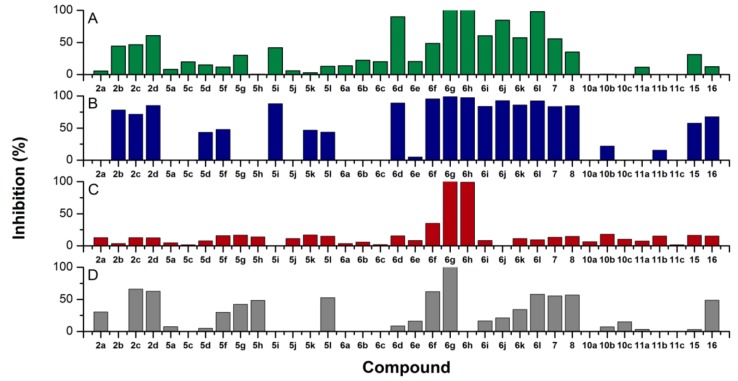
The compounds belonging to both structural classes, i.e., 4-(3-aminoprop-1-en-1-yl)-2-aminoimidazoles I (2a–d) and 4-phenyl-2-aminoimidazoles II (5a–c, 5d, 5f–l, 6a–l, 7, 8, 10a–c, 11a–c, 15 and 16) were evaluated for their antimicrobial activity against three bacterial strains (Gram-positive Enterococcus faecalis ATCC 29212 and Staphylococcus aureus ATCC 25923 and Gram-negative Escherichia coli ATCC 25922) and one fungal strain (Candida albicans ATCC 90028). The primary screening results at a concentration of 50 µM are presented in Figure 3. The antimicrobial screening assays were performed using broth microdilution method, as detailed in the Experimental Section. The minimum inhibitory concentrations (MIC50, MIC90) were further determined for those compounds that showed >80% inhibition of growth in the primary screen (Table 1). In addition, the selected compounds were also tested for mammalian cell cytotoxicity to determine the selectivity indices (SI) for their antimicrobial effects (Table 1 and Table 2).
Figure 3.
Primary antimicrobial screening results for clathrodin (2a), oroidin (2b) and their analogues at a concentration of 50 µM against: (A) Enterococcus faecalis (ATCC 29212); (B) Staphylococcus aureus (ATCC 25923); (C) Escherichia coli (ATCC 25922); and (D) Candida albicans (ATCC 90028). The results are based on the activity measured after 24 h (bacteria) or 48 h (C. albicans) of incubation (n = 3). Ciprofloxacin was used as a reference antibiotic in the antibacterial assays; MIC90 (minimum inhibitory concentration) values for E. faecalis, S. aureus and E. coli were 3, 1.5 and 0.048 µM (1, 0.5 and 0.016 µg/mL), respectively. And amphotericin B was used as a reference in the antifungal assay (MIC90 = 0.5 µM (0.5 µg/mL).
Table 1.
MIC90 and MIC50 values and the selectivity indices (SI) used to evaluate the antimicrobial activities of oroidin (2b) and selected oroidin analogues. The SI values were calculated as the ratio between the antimicrobial MIC50 and the Huh-7 cytotoxicity IC50 values.
| Cpd | Enterococcus faecalis (ATCC 29212) | Staphylococcus aureus (ATCC 25923) | Escherichia coli (ATCC 25922) | Candida albicans (ATCC 90028) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC90 (µM) | MIC50 (µM) | SI | MIC90 (µM) | MIC50 (µM) | SI | MIC90 (µM) | MIC50 (µM) | SI | MIC90 (µM) | MIC50 (µM) | SI | |
| 2b | 100 | 30.6 | >3.3 | |||||||||
| 5i | 75 | 11.7 | >8.6 | |||||||||
| 6d | 50 | 34.0 | 1.2 | 50 | 29.4 | 1.4 | ||||||
| 6f | 50 | 11.9 | 2.7 | |||||||||
| 6g | 25 | 11.4 | 2.7 | 25 | 9.0 | 3.5 | 25 | 17.7 | 1.8 | 50 | 30.5 | 1.0 |
| 6h | 12.5 | 8.0 | 2.7 | 12.5 | 7.3 | 2.9 | 50 | 30.4 | 0.7 | |||
| 6i | 50 | 7.2 | 3.0 | |||||||||
| 6j | 100 | 27.3 | 0.7 | 50 | 22.1 | 0.9 | ||||||
| 6k | 100 | 27.1 | 2.0 | |||||||||
| 6l | 100 | 54.2 | 1.0 | 75 | 10.8 | 4.9 | ||||||
| 7 | 100 | 11.6 | 8.1 | |||||||||
| 8 | 100 | 40.5 | >2.5 | |||||||||
Table 2.
Mammalian cell cytotoxicity of oroidin (2b) and selected oroidin analogues that showed >80% inhibition in the primary antimicrobial screening. The cytotoxicity IC50 values against a hepatocellular carcinoma cell line (Huh-7) were determined by an ATP assay after 24 h of exposure to the compound. The results are averages from two independent dose-response experiments.
| Compound | Cytotoxicity |
|---|---|
| IC50 (µM) | |
| 2b | >100 * |
| 5i | >100 * |
| 6d | 42.3 |
| 6f | 32.2 |
| 6g | 31.0 |
| 6h | 21.2 |
| 6i | 21.7 |
| 6j | 19.8 |
| 6k | 53.6 |
| 6l | 52.3 |
| 7 | 94.0 |
| 8 | >100 * |
* = highest concentration tested.
The parent compound, clathrodin (2a), exhibited activities below the hit threshold (>80% inhibition of growth at a concentration of 50 μM) against all of the microbial strains tested. Interestingly, its dibromo-pyrrole analogue, oroidin (2b), showed noticeably higher antibacterial activity against the Gram-positive bacteria, S. aureus (>90% inhibition of growth) and E. faecalis (approximately 50% inhibition of growth), but was also inactive against C. albicans and the Gram-negative bacteria, E. coli. Based on these results, a set of oroidin analogues was designed and prepared, i.e., the dibromo-pyrrole ring was substituted with other groups, such as indole and substituted indole rings with similar spatial and hydrophobic/hydrophilic properties to dibromo-pyrrole. Based on the primary screening results, the hit rates (>80% inhibition of growth) of all 36 tested compounds against S. aureus, E. faecalis, E. coli and C. albicans were 33, 14, six and 3%, respectively (Figure 3). Twelve compounds were active against S. aureus, and five of these were also active against the other Gram-positive bacterium, E. faecalis. The majority of the active compounds belonged to the 4-phenyl-2-aminoimidazole structural class, which is presented in Scheme 2 and Scheme 5. In general, the most active compounds were analogues containing an indol-2-yl, 5-substituted indol-2yl or thieno[3,2-b]pyrrol-5-yl group in the eastern part of the molecule and also possessing unsubstituted imidazole N-1 nitrogen (6d, 6f–l). Interestingly, compound 6e, which contains an indol-3-yl substituent, was inactive. Those compounds with smaller substituents in the eastern part, such as pyrrol-2-yl (5a, 6a), pyrrol-3-yl (6b) and (R)-pyrrolidin-2-yl (5c, 6c) and those compounds with Boc substituents on the imidazole N-1 nitrogen (5a, 5c–l, 7 and 10a–c), were less active. The only active compound containing a Boc substituent on the imidazole N-1 was compound 7. In general, compounds with Boc substituents on the imidazole N-1 nitrogen (5a, 5c–l, 7 and 10a–c) were less active than the products with free amino groups (6a, 6c–l, 8 and 11a–c); therefore, some Boc analogues, like compound 5b, were not tested, as no increase in the biological activity could be anticipated. According to these findings, the unsubstituted imidazole N-1 nitrogen plays an important role in enhancing the biological activity, possibly by forming direct or indirect interactions with the target. The most promising results were obtained for the 4-phenyl-2-aminoimidazoles, 6g and 6h, containing 5-O-substituted indole rings. Interestingly, compound 6g showed full inhibition of growth of all four microbes tested, whereas compound 6h, which contains a large, lipophilic 5-benzyloxy substituent on the indole ring, was active against all bacterial strains tested, but showed no activity against C. albicans. Compounds structurally similar to 6g have been identified as inhibitors of the biofilm formation in Gram-negative bacteria [18]. The 4-phenyl-4,5-dihydro-(N-methylamino)-imidazoles, 10a–c and 11a–c, which contain a reduced imidazole C=C bond, were not active, which indicates the importance of the aromaticity and planarity of the imidazole ring for antimicrobial activity.
The dose-response experiments conducted using the active compounds confirmed the primary screening results and demonstrated the potency differences between the analogues (Table 1). The lowest MIC90 values were obtained for derivative 6h with MIC90 values of 12.5 µM (5.7 µg/mL) against the Gram-positive bacteria and 50 µM against E. coli. The MIC90 values of the 5-trifluoromethoxy-indole derivative 6g were 25 µM (10.9 µg/mL) against all of the bacteria and 50 µM against C. albicans. For all the other derivatives, the MIC90s were ≥50 µM. For comparison, antibiotics currently used for treating Staphylococcus infections show MIC90 values in in vitro conditions typically in the range of 0.1–8 µg/mL [30]. However, the MIC value is not the only factor to be considered; the significance of the findings may also depend, for example, on the structural novelty and selectivity profile of the compound. Thus, in addition to the antimicrobial activity, the cellular impacts of the most promising compounds on a hepatocyte cell line, Huh-7, after 24 h of exposure were also evaluated to measure the selectivity of the effects between prokaryotic and eukaryotic cells. Clearly, most of the antimicrobially active derivatives showed a broad-spectrum effect, targeting both prokaryotic and eukaryotic cells (Table 2); however, in general, higher IC50 values were obtained for mammalian cell cytotoxicity than for antimicrobial activity (Table 1). The most active derivatives, 6g and 6h, displayed only modest selectivity towards S. aureus with selectivity index (SI) values of 3.5 and 2.9, respectively (Table 1).
The derivatives that were non-cytotoxic to mammalian cells at the highest tested concentration of 100 µM had only moderate antibacterial activity (MIC90 ≥ 75 µM and MIC50 ≥ 11.6 µM). The 4-phenyl-2-aminoimidazole derivative 7 exhibited an eight-fold selectivity between S. aureus and the mammalian cell line, but its antibacterial effect (MIC90 = 100 µM) was modest. The selectivity index of the 5-chloro-indole derivative 5i was >8.6, due to its non-cytotoxicity against mammalian cells at 100 µM (the highest tested concentration). An SI value greater than 10 can be regarded as a threshold for considering a compounds’ potential for further development [31]; thus, improving the selectivity of the most promising analogues found in this study through structural optimization would be justified.
3. Experimental Section
3.1. Determination of Antimicrobial Activity
3.1.1. Microbial Strains
Clinical control strains of Enterococcus faecalis (Gram-positive, ATCC 29212), Staphylococcus aureus (Gram-positive, ATCC 25923), Escherichia coli (Gram-negative, ATCC 25922) and a fungal strain, Candida albicans (ATCC 90028), were obtained from Microbiologics Inc. (St. Cloud, MN, USA) and used for the antimicrobial screening. Bacterial strains were grown on Mueller Hinton II agar (MHA, Becton Dickinson, Franklin Lakes, NJ, USA) slants and Mueller Hinton II broth (MHB, Becton Dickinson, Franklin Lakes, NJ, USA) and the Candida strain on Sabouraud dextrose agar (SDA, Becton Dickinson, Franklin Lakes, NJ, USA) plates. Media were prepared in MilliQ water, according to the manufacturer’s instructions, and autoclaved at 121 °C for 15 min. Prior to the assay, bacterial suspensions were prepared in MHB from fresh slant cultures and incubated at 37 °C for 16–20 h at 100 rpm. The Candida strain was grown on SDA plates at 28 °C for 18–24 h and suspended into sterile 0.9% saline for the assay.
3.1.2. Microdilution Assay
Antimicrobial assays were performed by the broth microdilution method following the guidelines of the Clinical and Laboratory Standards Institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST). Bacterial suspensions were prepared as described above and diluted with MHB to obtain a final inoculum of 5 × 105 colony-forming units (CFU)/milliliters in the assay (determined on the basis of absorbance values at 620 nm previously calibrated against plate counts). The Candida suspension was prepared in sterile 0.9% saline solution, and the suspension was diluted in RPMI-1640 media (with l-glutamine, without NaHCO3 and supplemented with 2% glucose and 0.165 M MOPS, buffered to pH 7; Lonza, Basel, Switzerland) to yield a final inoculum of 2.5 × 103 CFU/mL in the assay. Assays were carried out in clear 96-well microtiter plates and initiated by dispensing an equal volume of microbial suspension and sample solution diluted into the assay medium. The plates were incubated for 24 h at 37 °C (for Candida, the incubation was at 28 °C for 48 h) with agitation. Absorbance was measured at 620 nm with a plate reader at 0, 4 and 24 h with the bacteria and at 0, 24 and 48 h with Candida. The antimicrobial activity of the samples was calculated from the absorbance values by comparing to untreated controls and expressed as the percentage inhibition of growth. Reference antibiotics were used as positive controls on every assay plate (see Figure 2 for details). Compounds were initially assayed at a final concentration of 50 µM (n = 3), and those that showed >80% inhibition in the primary screen were tested further at several concentrations to confirm the activity and to determine the MIC90 and MIC50 values. The MIC90 was defined as the lowest concentrations that showed >90% inhibition of growth. MIC50 values were determined from the dose-response results by sigmoidal curve fitting with Origin software (OriginLab, Corp., Wellesley Hills, MA, USA).
3.2. Determination of Mammalian Cell Cytotoxicity
3.2.1. Cell Culture
Huh-7 cells (originating from human hepatocellular carcinoma) were kindly provided by Prof. Ralf Bartenschlager (University of Heidelberg, Heidelberg, Germany). The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS, Gibco, Grand Island, NY, USA), 100 µM non-essential amino acids, 2 mM l-glutamine and 100 µg/mL of streptomycin and 100 IU/mL of penicillin (Gibco). The cells were incubated at 37 °C in a humidified atmosphere with 5% CO2.
3.2.2. ATP Assay
The effect of the most promising compounds on the metabolic activity of Huh-7 hepatocytes was assessed by intracellular ATP quantitation (Promega’s CellTiter-Glo Cell Viability Assay, Madison, WI, USA). In brief, cells were seeded at 20,000 cells/well on white-walled 96-well microplates (ViewPlate, PerkinElmer Inc., Wellesley, MA, USA) and incubated at 37 °C, 5% CO2 and 95% humidity overnight and then exposed to the compounds for 24 h. Following the exposure, cells were washed with 100 µL PBS, and 50 µL of fresh assay media and 50 µL of the CellTiter-Glo reagent were added into the wells. After 2 min of shaking and 10 min incubation at rt, luminometric signal was measured using a Varioskan Flash plate reader (Thermo Fisher Scientific, Vantaa, Finland). Polymyxin-B sulfate (15,000 IU/mL, average cytotoxicity 88%) was used as a positive control on every assay plate. Compounds were primarily screened at 100 and 50 µM (n = 3) concentrations, and those that showed >50% cytotoxicity were further subjected to dose-response experiments to determine IC50 values (concentration ranging between 150 µM and 1.56 µM, depending on the potency of the compound). The IC50 values were calculated by fitting the data into sigmoidal dose-response curves with the Origin software.
3.3. Chemistry: General
Chemicals were obtained from Acros Organics (Geel, Belgium) and Sigma-Aldrich Corporation (St. Louis, MO, USA) and used without further purification. Analytical TLC was performed on silica gel Merck 60 F254 plates (0.25 mm), using visualization with UV light and ninhydrin. Column chromatography was carried out on silica gel 60 (particle size 240–400 mesh). HPLC analyses were performed on an Agilent Technologies 1100 instrument (Agilent Technologies, Santa Clara, CA, USA) with a G1365B UV-Vis detector, a G1316A thermostat and a G1313A autosampler using a Phenomenex Luna 5-μm C18 column (4.6 × 150 mm or 4.6 × 250 mm) (Phenomenex, Torrance, CA, USA) and a flow rate of 1.0 mL/min. The eluent consisted of trifluoroacetic acid (0.1% in water) or ammonia (0.1% in water) as solvent A and methanol as solvent B. Microwave-assisted reactions were performed using a CEM Discover microwave reactor (CEM Corp., Matthews, NC, USA). Melting points were determined on a Reichert hot stage microscope and are uncorrected. 1H, 13C and 19F NMR spectra were recorded at 400, 100 and 376 MHz, respectively, on a Bruker AVANCE III 400 spectrometer (Bruker Corporation, Billerica, MA, USA) in DMSO-d6, MeOH-d4 or acetone-d6 solutions, with TMS as the internal standard. IR spectra were recorded on a PerkinElmer Spectrum BX FT-IR spectrometer (PerkionElmer, Inc., Waltham, MA, USA) or Thermo Nicolet Nexus 470 ESP FT-IR spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Mass spectra were obtained using a VG Analytical Autospec Q mass spectrometer (Fisons, VG Analytical, Manchester, UK). The purity of the tested compounds was established to be ≥95%.
3.4. Synthetic Procedures
3.4.1. General Procedure A: Synthesis of compounds 2a–d
The corresponding carboxylic acid (0.36 mmol), TBTU (137 mg, 0.414 mmol) and N-methylmorpholine (0.08 mL, 0.72 mmol) were dissolved in dry dimethylformamide (2 mL) and stirred under argon at rt for 1 h. The prepared mixture was added dropwise to a stirred solution of compound 1 (50 mg, 0.36 mmol) and N-methylmorpholine (0.08 mL, 0.72 mmol) in dry dimethylformamide (1 mL) at 0 °C. After 1 h, the mixture was warmed to rt and stirred under argon for 5 h. The solvent was evaporated under reduced pressure, and the residue was purified by flash column chromatography using dichloromethane/methanol saturated with NH3 (6:1) as an eluent.
(E)-N-(3-(2-amino-1H-imidazol-4-yl)allyl)-1H-pyrrole-2-carboxamide (2a) (see Supplementary Information, Figure S1). Yield, 18%; brown solid; mp 95–98 °C; IR (KBr) ν = 3208 (N-H), 2927 (C-H), 1614 (C=O), 1558, 1520, 1406, 1323, 1197, 1113, 1040, 959, 884, 740 cm−1. 1H NMR (MeOH-d4) δ 4.05 (dd, 2H, J = 6.0 Hz, J = 1.2 Hz, -CH=CH-CH2-), 5.94 (dt,1H, J = 15.8 Hz, J = 6.0 Hz, -CH=CH-CH2-), 6.18 (dd, 1H, J = 3.7 Hz, J = 2.6 Hz, Ar-H4), 6.32 (td, 1H, J = 15.8 Hz, J = 1.2 Hz, -CH=CH-CH2-), 6.51 (s, 1H, imidazole-H), 6.82 (dd, 1H, J = 3.7 Hz, J = 1.4 Hz, Ar-H3), 6.93 (dd, 1H, J = 2.5 Hz, J = 1.4 Hz, Ar-H5); 13C NMR (MeOH-d4) δ 42.06, 110.22, 111.78, 117.01, 121.87, 122.66, 122.89, 126.87, 130.82, 151.66, 163.61; HRMS for C11H13N5O: calculated, 231.1120; found, 231.1189. HPLC: Phenomenex Luna 5 μm C18 column (4.6 mm × 150 mm); mobile phase: 10%–70% of MeOH in NH3(aq) (0.1%) in 20 min; flow rate: 1.0 mL/min; injection volume: 10 μL; retention time: 10.11 min (97.9% at 254 nm, 98.2% at 280 nm).
(E)-N-(3-(2-amino-1H-imidazol-4-yl)allyl)-4,5-dibromo-1H-pyrrole-2-carboxamide (2b) (see Supplementary Information, Figure S2). Yield, 25%; yellow solid; mp 201–204 °C; IR (KBr) ν = 3117 (N-H), 2934 (C-H), 1611 (C=O), 1562, 1515, 1410, 1389, 1320, 1214, 1023, 955, 818, 754 cm−1. 1H NMR (MeOH-d4) δ 4.03 (d, 2H, J = 6.0 Hz, -CH=CH-CH2-), 5.91 (dt, 1H, J = 15.8 Hz, J = 6.0 Hz, -CH=CH-CH2-), 6.31 (d, 1H, J = 15.8 Hz, -CH=CH-CH2-), 6.51 (s, 1H, imidazole-H), 6.85 (s, 1H, Ar-H3); 13C NMR (MeOH-d4) δ 42.18, 99.96, 106.09, 114.29, 117.00, 122.12, 122.28, 128.88, 130.94, 151.72, 161.53; HRMS for C11H11Br2N5O: calculated, 386.9330; found, 386.9408. HPLC: Phenomenex Luna 5 μm C18 column (4.6 mm × 150 mm); mobile phase: 10%–70% of MeOH in NH3(aq) (0.1%) in 20 min; flow rate: 1.0 mL/min; injection volume: 10 μL; retention time: 13.69 min (98.7% at 254 nm, 98.5% at 280 nm).
(E)-N-(3-(2-amino-1H-imidazol-4-yl)allyl)-1H-indole-2-carboxamide (2c) (see Supplementary Information, Figure S3). Yield, 26%; white solid; mp ˃ 230 °C; IR (KBr) ν = 3205 (N-H), 2930 (C-H), 1618 (C=O), 1546, 1418, 1340, 1308, 1258, 1100, 958, 812, 745 cm−1. 1H NMR (MeOH-d4) δ 4.12 (dd, 2H, J = 6.2 Hz, J = 1.3 Hz, -CH=CH-CH2-), 5.96 (ddd, 1H, J = 15.8 Hz, J = 6.2 Hz, J = 5.8 Hz, -CH=CH-CH2-), 6.36 (td, 1H, J = 15.8 Hz, J = 1.3 Hz, -CH=CH-CH2-), 6.50 (s, 1H, imidazole-H), 7.07 (ddd, 1H, J = 8.0 Hz, J = 7.0 Hz, J = 1.0, Ar-H6), 7.11 (d, 1H, J = 0.9 Hz, Ar-H3), 7.22 (ddd, 1H, J = 8.3 Hz, J = 7.0 Hz, J = 1.1, Ar-H5), 7.45 (ddd, 1H, J = 8.3 Hz, J = 1.8 Hz, J = 0.9, Ar-H4), 7.61 (td, 1H, J = 8.1 Hz, J = 1.0 Hz, Ar-H7); 13C NMR (MeOH-d4) δ 41.01, 102.99, 111.64, 116.17, 119.74, 120.24, 121.27, 121.34, 123.61, 127.62, 129.83, 130.82, 136.90, 150.54, 162.56; HRMS for C15H15N5O: calculated, 281.1277; found, 281.1344. HPLC: Phenomenex Luna 5 μm C18 column (4.6 mm × 150 mm); mobile phase: 10%–70% of MeOH in NH3(aq) (0.1%) in 20 min; flow rate: 1.0 mL/min; injection volume: 10 μL; retention time: 17.29 min (99.2% at 254 nm, 99.4% at 280 nm).
(E)-N-(3-(2-amino-1H-imidazol-4-yl)allyl)-5-fluoro-1H-indole-2-carboxamide (2d) (see Supplementary Information, Figure S4). Yield, 27%; yellow solid; mp 153–156 °C; IR (KBr) ν = 3227 (N-H), 2928 (C-H), 1618 (C=O), 1544, 1485, 1420, 1326, 1258, 1226, 1158, 1109, 954, 856, 798, 757, 730 cm−1. 1H NMR (MeOH-d4) δ 4.11 (dd, 2H, J = 6.2 Hz, J = 1.3 Hz, -CH=CH-CH2-), 5.95 (td, 1H, J = 15.8 Hz, J = 6.2 Hz, -CH=CH-CH2-), 6.36 (td, 1H, J = 15.8 Hz, J = 1.3 Hz, -CH=CH-CH2-), 6.50 (s, 1H, imidazole-H), 7.02 (ddd, 1H, J = 8.0 Hz, J = 6.9 Hz, J = 0.9, Ar-H6), 7.08 (d, 1H, J = 0.9 Hz, Ar-H3), 7.28 (ddd, 1H, J = 9.6 Hz, J = 2.1 Hz, J = 0.4, Ar-H4), 7.43 (tdd, 1H, J = 9.0 Hz, J = 4.5 Hz, J = 0.7 Hz, Ar-H7); 13C NMR (MeOH-d4) δ 40.96, 102.82 (d, 4JC-F = 5.2 Hz, C-4), 105.28 (d, 2JC-F = 23.2 Hz, C-8), 112.26 (d, 2JC-F = 27.0 Hz, C-2), 112.76 (d, 3JC-F = 9.6 Hz, C-7), 115.93, 120.71, 120.96, 127.73 (d, 3JC-F = 10.3 Hz, C-3), 129.32, 132.58, 133.51, 150.19, 157.96 (d, 1JC-F =234.0 Hz, C-1), 162.19; HRMS for C15H14FN5O: calculated, 299.1182; found, 299.1194. HPLC: Phenomenex Luna 5 μm C18 column (4.6 mm × 150 mm); mobile phase: 10%–70% of MeOH in NH3(aq) (0.1%) in 20 min; flow rate: 1.0 mL/min; injection volume: 10 μL; retention time: 18.61 min (98.8% at 254 nm, 99.0% at 280 nm).
3.4.2. General Procedure B: Synthesis of Compounds 5a–l and 10a–c (with 5f as an Example)
To a suspension of 5-methoxy-indole-2-carboxylic acid (251 mg, 1.31 mmol) and TBTU (456 mg, 1.42 mmol) in dichloromethane (5 mL), N-methylmorpholine (0.601 mL, 5.47 mmol) was added and the mixture stirred at rt for 0.5 h upon which a clear solution formed. Compound 3 (300 mg, 1.09 mmol) was added and the mixture stirred at 35 °C for 24 h. The solvent was evaporated in vacuo, the residue dissolved in ethyl acetate (30 mL) and washed successively with water (2 × 10 mL), saturated aqueous NaHCO3 solution (2 × 10 mL) and brine (1 × 10 mL). The organic phase was dried over Na2SO4, filtered and the solvent evaporated under reduced pressure. The crude product was purified by flash column chromatography using ethyl acetate/petroleum ether or dichloromethane/methanol as an eluent, to afford 5f (305 mg, 62% yield) as a white solid. Analytical and spectroscopic data for compounds 5a–e, 5l and 10a–c are reported elsewhere [29].
tert-Butyl 2-amino-4-(3-(5-methoxy-1H-indole-2-carboxamido)phenyl)-1H-imidazole-1-carboxylate (5f) (see Supplementary Information, Figure S5). Yield, 62%; white solid; mp 173–177 °C; IR (KBr) ν = 3415 (N-H), 3299 (N-H), 3115 (C-H), 2991 (C-H), 2831 (C-H), 1739 (C=O), 1639, 1597, 1536, 1453, 1433, 1354, 1323, 1275, 1238, 1218, 1153, 1117, 1032, 976, 897, 847, 791, 758, 718 cm−1. 1H NMR (DMSO-d6) δ 1.60 (s, 9H, t-Bu), 3.79 (s, 3H, OCH3), 6.64 (s, 2H, NH2), 6.89 (dd, 1H, 3J = 9.2 Hz, 4J = 2.4 Hz, Ar-H), 7.14 (d, 1H, 4J = 2.4 Hz, Ar-H), 7.29–7.38 (m, 4H, 4 × Ar-H), 7.48 (dd, 1H, 3J = 7.6 Hz, 4J = 0.8 Hz, Ar-H), 7.72–7.75 (m, 1H, Ar-H), 8.13 (s, 1H, Ar-H), 10.19 (s, 1H, NH), 11.57 (s, 1H, NH); 13C NMR (DMSO-d6) δ 27.51 (CCH3), 55.25 (OCH3), 84.70 (CCH3), 102.04, 103.52, 106.07, 113.19, 115.04, 116.55, 118.81, 119.92, 127.35, 128.69, 131.74, 132.08, 133.80, 136.95, 139.12, 148.88, 150.43, 153.82, 159.61; MS (ESI) m/z (%) = 448.2 (MH+, 15), 392.1 ([MH-t-Bu]H+, 90), 348.1 ([MH-Boc]H+, 100). HRMS for C24H26N5O4: calculated, 448.1985; found, 448.1983. HPLC: Phenomenex Luna 5 μm C18 column (4.6 mm × 150 mm); mobile phase: 10%–90% of MeOH in TFA (0.1%) in 20 min; flow rate: 1.0 mL/min; injection volume: 10 μL; retention time: 16.515 min (98.2% at 254 nm, 98.9% at 280 nm).
tert-Butyl 2-amino-4-(3-(5-(trifluoromethoxy)-1H-indole-2-carboxamido)phenyl)-1H-imidazole-1-carboxylate (5g) (see Supplementary Information, Figure S6). Yield, 76%; white solid; mp 185–187 °C; IR (KBr) ν = 3434 (N-H), 3368 (N-H), 3100 (C-H), 3006 (C-H), 1721 (C=O), 1649, 1600, 1548, 1435, 1364, 1257, 1233, 1217, 1206, 1152, 1119, 1064, 977, 895, 877, 854, 794, 775, 760, 715 cm−1. 1H NMR (DMSO-d6) δ 1.60 (s, 9H, t-Bu), 6.64 (s, 2H, NH2), 7.22 (dd, 1H, 3J = 9.2 Hz, 4J = 1.6 Hz, Ar-H), 7.30 (s, 1H, Ar-H), 7.35 (t, 1H, 3J = 8.0 Hz, Ar-H), 7.49–7.57 (m, 3H, 3 × Ar-H), 7.74–7.76 (m, 2H, 2 × Ar-H), 8.14 (s, 1H, Ar-H), 10.36 (s, 1H, NH), 12.01 (s, 1H, NH); 13C NMR (DMSO-d6) δ 27.51 (CCH3), 84.70 (CCH3), 104.09, 106.13, 113.61, 113.96, 116.63, 117.63, 118.88, 120.15, 120.41 (q, 1C, 1JC-F = 253 Hz, CF3), 127.02, 128.73, 133.56, 133.85, 135.15, 136.89, 138.90, 142.20, 148.87, 150.43, 159.22; 19F NMR (DMSO-d6) δ −59.92 (s, 3F, CF3); MS (ESI) m/z (%) = 502.2 (MH+, 10), 446.1 ([MH-t-Bu]H+, 90), 402.1 ([MH-Boc]H+, 100). HRMS for C24H23N5O4F3: calculated, 502.1702; found, 502.1712. HPLC: Phenomenex Luna 5 μm C18 column (4.6 mm × 150 mm); mobile phase: 60%–90% of MeOH in TFA (0.1%) in 20 min; flow rate: 1.0 mL/min; injection volume: 10 μL; retention time: 6.886 min (99.5% at 254 nm, 99.2% at 280 nm).
tert-Butyl 2-amino-4-(3-(5-(benzyloxy)-1H-indole-2-carboxamido)phenyl)-1H-imidazole-1-carboxylate (5h) (see Supplementary Information, Figure S7). Yield, 42%; an off-white solid; mp 140–143 °C; IR (KBr) ν = 3439 (N-H), 3385 (N-H), 3116 (C-H), 3064 (C-H), 2984 (C-H), 1724 (C=O), 1642, 1624, 1596, 1539, 1433, 1361, 1274, 1246, 1232, 1210, 1156, 1116, 1067, 1015, 977, 841, 800, 758, 745, 732, 698 cm−1. 1H NMR (DMSO-d6) δ 1.60 (s, 9H, t-Bu), 5.13 (s, 2H, OCH2), 6.64 (s, 2H, NH2), 6.97 (dd, 1H, 3J = 8.8 Hz, 4J = 2.4 Hz, Ar-H), 7.25–7.51 (m, 11H, 11 × Ar-H), 7.72–7.75 (m, 1H, Ar-H), 8.14 (s, 1H, Ar-H), 10.18 (s, 1H, NH), 11.59 (s, 1H, NH); 13C NMR (DMSO-d6) δ 27.51 (CCH3), 69.61 (OCH2), 84.70 (CCH3), 103.54, 103.67, 106.07, 113.21, 115.53, 116.51, 118.77, 119.92, 127.30, 127.68, 128.36, 128.69, 131.84, 132.22, 133.80, 136.95, 137.54, 139.12, 148.88, 150.43, 152.81, 159.59 (signals for two C atoms overlap); MS (ESI) m/z (%) = 524.2 (MH+, 40), 468.2 ([MH-t-Bu]H+, 80), 424.2 ([MH-Boc]H+, 100). HRMS for C30H30N5O4: calculated, 524.2298; found, 524.2302. HPLC: Phenomenex Luna 5 μm C18 column (4.6 mm × 150 mm); mobile phase: 60%–90% of MeOH in TFA (0.1%) in 20 min; flow rate: 1.0 mL/min; injection volume: 10 μL; retention time: 7.723 min (96.8% at 254 nm, 98.4% at 280 nm).
tert-Butyl 2-amino-4-(3-(5-chloro-1H-indole-2-carboxamido)phenyl)-1H-imidazole-1-carboxylate (5i) (see Supplementary Information, Figure S8). Yield, 65%; white solid; mp 188–190 °C; IR (KBr) ν = 3,414 (N-H), 3,371 (N-H), 3,146 (C-H), 2,981 (C-H), 1,725 (C=O), 1,648, 1,600, 1,544, 1,434, 1,372, 1,360, 1,317, 1,283, 1,243, 1,223, 1,204, 1,157, 1,117, 1,059, 977, 915, 874, 855, 793, 759, 712 cm−1. 1H NMR (DMSO-d6) δ 1.60 (s, 9H, t-Bu), 6.64 (s, 2H, NH2), 7.24 (dd, 1H, 3J = 8.8 Hz, 4J = 2.0 Hz, Ar-H), 7.30 (s, 1H, Ar-H), 7.35 (t, 1H, 3J = 8.0 Hz, Ar-H), 7.45–7.50 (m, 3H, 3 × Ar-H), 7.72–7.75 (m, 1H, Ar-H), 7.79 (d, 1H, 4J = 2.0 Hz, Ar-H), 8.14 (t, 1H, 4J = 1.6 Hz, Ar-H), 10.32 (s, 1H, NH), 11.94 (s, 1H, NH); 13C NMR (DMSO-d6) δ 27.51 (CCH3), 84.70 (CCH3), 103.34, 106.11, 113.96, 116.58, 118.83, 120.11, 120.80, 123.85, 124.36, 128.07, 128.73, 132.97, 133.84, 135.14, 136.90, 138.95, 148.87, 150.44, 159.28; MS (ESI) m/z (%) = 452.1 (MH+, 15), 396.1 ([MH-t-Bu]H+, 100), 352.1 ([MH-Boc]H+, 40). HRMS for C23H23N5O3Cl: calculated, 452.1489; found, 452.1487. HPLC: Phenomenex Luna 5 μm C18 column (4.6 mm × 150 mm); mobile phase: 60%–90% of MeOH in TFA (0.1%) in 20 min; flow rate: 1.0 mL/min; injection volume: 10 μL; retention time: 5.299 min (98.3% at 254 nm, 98.6% at 280 nm).
tert-Butyl 2-amino-4-(3-(5-fluoro-1H-indole-2-carboxamido)phenyl)-1H-imidazole-1-carboxylate (5j) (see Supplementary Information, Figure S9). Yield, 76%; white solid; mp 189–192 °C; IR (KBr) ν = 3450 (N-H), 3409 (N-H), 3280 (N-H), 3113 (C-H), 2976 (C-H), 1726 (C=O), 1651, 1610, 1597, 1545, 1489, 1446, 1426, 1356, 1330, 1314, 1259, 1210, 1159, 1146, 1117, 1067, 955, 850, 791, 718 cm−1. 1H NMR (DMSO-d6) δ 1.60 (s, 9H, t-Bu), 6.64 (s, 2H, NH2), 7.10 (dt, 1H, 3J = 9.2 Hz, 4J = 2.4 Hz, Ar-H), 7.30 (s, 1H, Ar-H), 7.34 (t, 1H, 3J = 8.0 Hz, Ar-H), 7.45–7.50 (m, 4H, 4 × Ar-H), 7.72–7.75 (m, 1H, Ar-H), 8.14 (s, 1H, Ar-H), 10.29 (s, 1H, NH), 11.84 (s, 1H, NH); 13C NMR (DMSO-d6) δ 27.51 (CCH3), 84.70 (CCH3), 103.79 (d, 1C, 4JC-F = 5 Hz), 105.88 (d, 1C, 2JC-F = 23 Hz), 106.11, 112.51 (d, 1C, 2JC-F = 27 Hz), 113.57 (d, 1C, 3JC-F = 9 Hz), 116.58, 118.83, 120.08, 127.09 (d, 1C, 3JC-F = 10 Hz), 128.72, 133.17, 133.50, 133.84, 136.91, 138.98, 148.87, 150.43, 157.19 (d, 1C, 1JC-F = 231 Hz), 159.35; 19F NMR (DMSO-d6) δ −123.68 (s, 1F); MS (ESI) m/z (%) = 436.2 (MH+, 100). HRMS for C23H23N5O3F: calculated, 436.1785; found, 436.1780. HPLC: Phenomenex Luna 5 μm C18 column (4.6 mm × 150 mm); mobile phase: 10%–90% of MeOH in TFA (0.1%) in 20 min; flow rate: 1.0 mL/min; injection volume: 10 μL; retention time: 17.233 min (99.2% at 254 nm, 97.3% at 280 nm).
tert-Butyl 4-(3-(4H-thieno[3,2-b]pyrrole-5-carboxamido)phenyl)-2-amino-1H-imidazole-1-carboxylate (5k) (see Supplementary Information, Figure S10). Yield, 34%; off-white solid; mp 178–180 °C; IR (KBr) ν = 3,402 (N-H), 3,365 (N-H), 3,269 (N-H), 3154 (C-H), 2979 (C-H), 2933 (C-H), 1740 (C=O), 1635, 1596, 1541, 1519, 1460, 1428, 1352, 1311, 1256, 1239, 1207, 1153, 1118, 977, 895, 843, 827, 756, 717 cm−1. 1H NMR (acetone-d6) δ 1.67 (s, 9H, t-Bu), 6.44 (s, 2H, NH2), 7.09 (d, 1H, J = 5.2 Hz, Ar-H), 7.29–7.33 (m, 2H, 2 × Ar-H), 7.40–7.43 (m, 2H, 2 × Ar-H), 7.48–7.51 (m, 1H, Ar-H), 7.76–7.79 (m, 1H, Ar-H), 8.16 (s, 1H, Ar-H), 9.44 (s, 1H, NH), 11.07 (s, 1H, NH); 13C NMR (acetone-d6) δ 28.10 (CCH3), 85.61 (CCH3), 103.42, 107.05, 112.65, 117.25, 119.36, 120.84, 124.93, 128.62, 129.52, 132.06, 135.32, 138.56, 140.40, 142.30, 150.40, 151.58, 160.34; MS (ESI) m/z (%) = 424.2 (MH+, 10), 368.1 ([MH-t-Bu]H+, 100), 324.1 ([MH-Boc]H+, 50). HRMS for C21H22N5O3S: calculated, 424.1443; found, 424.1450. HPLC: Phenomenex Luna 5 μm C18 column (4.6 mm × 150 mm); mobile phase: 10%–90% of MeOH in TFA (0.1%) in 20 min; flow rate: 1.0 mL/min; injection volume: 10 μL; retention time: 16.043 min (95.3% at 254 nm, 95.8% at 280 nm).
3.4.3. tert-Butyl 2-Amino-4-(3-(5-hydroxy-1H-indole-2-carboxamido)phenyl)-1H-imidazole-1-carboxylate (7)
Compound 5h (496 mg, 0.947 mmol) was dissolved in a mixture of THF (10 mL) and MeOH (15 mL), Pd/C (100 mg) was added and the reaction mixture stirred under hydrogen atmosphere for 10 h. The catalyst was filtered off, the solvent removed under reduced pressure and the crude product purified by flash column chromatography using ethyl acetate/petroleum ether as an eluent, to afford 7 (see Supplementary Information, Figure S17) (275 mg, 67% yield) as an off-white solid; mp 192–196 °C; IR (KBr) ν = 3399 (N-H, O-H), 3272 (N-H, O-H), 3157 (C-H), 2979 (C-H), 1736 (C=O), 1625, 1597, 1538, 1432, 1391, 1353, 1319, 1276, 1212, 1154, 1119, 1062, 851, 786, 757, 717 cm−1. 1H NMR (DMSO-d6) δ 1.60 (s, 9H, t-Bu), 6.64 (br s, 2H, NH2), 6.78 (dd, 1H, 3J = 8.8 Hz, 4J = 2.4 Hz, Ar-H), 6.93 (d, 1H, 4J = 2.4 Hz, Ar-H), 7.26–7.35 (m, 4H, 4 × Ar-H), 7.47 (dd, 1H, 3J = 8.0 Hz, 4J = 1.6 Hz, Ar-H), 7.71–7.73 (m, 1H, Ar-H), 8.13–8.14 (m, 1H, Ar-H), 8.86 (s, 1H, OH), 10.12 (s, 1H, NH), 11.43 (s, 1H, NH); 13C NMR (DMSO-d6) δ 27.51 (CCH3), 84.69 (CCH3), 102.93, 104.35, 106.04, 112.85, 115.04, 116.45, 118.71, 119.85, 127.73, 128.68, 131.59, 131.60, 133.79, 136.97, 139.18, 148.88, 150.42, 151.19, 159.69; MS (ESI) m/z (%) = 434.2 (MH+, 10), 378.1 ([MH-t-Bu]H+, 100), 334.1 ([MH-Boc]H+, 50). HRMS for C23H24N5O4: calculated, 434.1828; found, 434.1823. HPLC: henomenex Luna 5 μm C18 column (4.6 mm × 150 mm); mobile phase: 10%–90% of MeOH in TFA (0.1%) in 20 min; flow rate: 1.0 mL/min; injection volume: 10 μL; retention time: 14.015 min (96.3% at 254 nm, 96.2% at 280 nm).
3.4.4. General Procedure C: Synthesis of Compounds 6a–l, 8 and 11a–c (with 6f as an Example)
A solution of compound 5f (160 mg, 0.358 mmol) in a mixture of THF and EtOH = 1:2 (15 mL) was saturated with gaseous HCl and stirred at rt for 5 h. The solvent was removed under reduced pressure, the solid filtered off and washed with diethyl ether and dichloromethane, to afford 6f (132 mg, 96% yield) as an off-white solid. Analytical and spectroscopic data for compounds 6a–e, 6l and 11a–c are reported elsewhere [29].
2-Amino-4-(3-(5-methoxy-1H-indole-2-carboxamido)phenyl)-1H-imidazol-3-ium chloride (6f) (see Supplementary Information, Figure S11). Yield, 96%; off-white solid; mp 237–241 °C; IR (KBr) ν = 3301 (N-H), 3138 (C-H), 2955 (C-H), 2761 (C-H), 1673 (C=O), 1653, 1625, 1585, 1541, 1452, 1418, 1336, 1281, 1238, 1208, 1177, 1153, 1132, 1116, 1022, 883, 839, 788, 755 cm−1. 1H NMR (DMSO-d6) δ 3.79 (s, 3H, OCH3), 6.89 (dd, 1H, 3J = 9.2 Hz, 4J = 2.4 Hz, Ar-H), 7.15 (d, 1H, 4J = 2.4 Hz, Ar-H), 7.33 (s, 1H, Ar-H), 7.37–7.49 (m, 6H, 4 × Ar-H, NH2), 7.69–7.72 (m, 1H, Ar-H), 8.08 (s, 1H, Ar-H), 10.42 (s, 1H, NH), 11.70 (s, 1H, NH), 12.16 (s, 1H, NH), 12.85 (s, 1H, NH); 13C NMR (DMSO-d6) δ 55.25 (OCH3), 102.02, 104.14, 109.43, 113.23, 115.19, 116.46, 119.74, 120.33, 126.39, 127.26, 128.12, 129.30, 131.54, 132.16, 139.45, 147.82, 153.84, 159.72; MS (ESI) m/z (%) = 348.2 ([M − Cl]+, 100). HRMS for C19H18N5O2: calculated, 348.1461; found, 348.1459. HPLC: Phenomenex Luna 5 μm C18 column (4.6 mm × 150 mm); mobile phase: 60%–90% of MeOH in TFA (0.1%) in 20 min; flow rate: 1.0 mL/min; injection volume: 10 μL; retention time: 3.029 min (98.2% at 254 nm, 98.7% at 280 nm).
2-Amino-4-(3-(5-(trifluoromethoxy)-1H-indole-2-carboxamido)phenyl)-1H-imidazol-3-ium chloride (6g) (see Supplementary Information, Figure S12). Yield, 66%; off-white solid; mp 255–260 °C; IR (KBr) ν = 3291 (N-H), 3148 (C-H), 3061 (C-H), 1677 (C=O), 1660, 1605, 1545, 1494, 1449, 1406, 1333, 1319, 1251, 1242, 1214, 1199, 1178, 1158, 1128, 1108, 1096, 970, 897, 868, 800, 788, 733 cm−1. 1H NMR (DMSO-d6) δ 7.22–7.25 (m, 1H, Ar-H), 7.34 (s, 1H, Ar-H), 7.41–7.58 (m, 6H, 4 × Ar-H, NH2), 7.68–7.71 (m, 1H, Ar-H), 7.74 (s, 1H, Ar-H), 8.07 (t, 1H, 4J = 1.6 Hz, Ar-H), 10.55 (s, 1H, NH), 12.11 (s, 1H, NH), 12.13 (s, 1H, NH), 12.81 (s, 1H, NH); 13C NMR (DMSO-d6) δ 104.71, 109.50, 113.67, 113.95, 116.58, 117.75, 120.00, 120.40 (q, 1C, 1JC-F = 253 Hz, CF3), 120.44, 126.34, 126.92, 128.18, 129.35, 133.36, 135.21, 139.21, 142.21, 147.83, 159.34; 19F NMR (DMSO-d6) δ −56.93 (s, 3F, CF3); MS (ESI) m/z (%) = 402.1 ([M − Cl]+, 100). HRMS for C19H15N5O2F3: calculated, 402.1178; found, 402.1171. HPLC: Phenomenex Luna 5 μm C18 column (4.6 mm × 150 mm); mobile phase: 10%–90% of MeOH in TFA (0.1%) in 20 min; flow rate: 1.0 mL/min; injection volume: 10 μL; retention time: 19.439 min (96.3% at 254 nm, 97.0% at 280 nm).
2-Amino-4-(3-(5-(benzyloxy)-1H-indole-2-carboxamido)phenyl)-1H-imidazol-3-ium chloride (6h) (see Supplementary Information, Figure S13). Yield, 51%; off-white solid; mp 175–178 °C; IR (KBr) ν = 3253 (N-H), 3145 (C-H), 3032 (C-H), 2773 (C-H), 1678 (C=O), 1624, 1608, 1537, 1487, 1447, 1426, 1384, 1331, 1287, 1229, 1203, 1160, 1118, 1023, 940, 786, 757, 732 cm−1. 1H NMR (DMSO-d6) δ 5.13 (s, 2H, OCH2), 6.98 (dd, 1H, 3J = 9.2 Hz, 4J = 2.4 Hz, Ar-H), 7.26 (d, 1H, 4J = 2.4 Hz, Ar-H), 7.32–7.51 (m, 12H, 10 × Ar-H, NH2), 7.67–7.69 (m, 1H, Ar-H), 8.08 (t, 1H, 4J = 2.0 Hz, Ar-H), 10.37 (s, 1H, NH), 11.69 (s, 1H, NH), 12.12 (s, 1H, NH), 12.80 (s, 1H, NH); 13C NMR (DMSO-d6) δ 69.61 (OCH2), 103.67, 103.92, 109.59, 113.27, 115.69, 116.50, 119.85, 120.33, 126.49, 127.23, 127.68, 128.15, 128.37, 129.34, 131.59, 132.31, 137.52, 139.38, 147.74, 152.85, 159.72 (signals for two C atoms overlap); MS (ESI) m/z (%) = 424.2 ([M − Cl]+, 100). HRMS for C25H22N5O2: calculated, 424.1774; found, 424.1771. HPLC: Phenomenex Luna 5 μm C18 column (4.6 mm × 150 mm); mobile phase: 60%–90% of MeOH in TFA (0.1%) in 20 min; flow rate: 1.0 mL/min; injection volume: 10 μL; retention time: 7.803 min (95.3% at 254 nm, 95.1% at 280 nm).
2-Amino-4-(3-(5-chloro-1H-indole-2-carboxamido)phenyl)-1H-imidazol-3-ium chloride (6i) (see Supplementary Information, Figure S14). Yield, 71%; white solid; mp 201–204 °C; IR (KBr) ν = 3410 (N-H), 3260 (N-H), 3145 (C-H), 3032 (C-H), 2761 (C-H), 1693 (C=O), 1667, 1610, 1542, 1485, 1442, 1412, 1326, 1301, 1275, 1245, 1224, 1190, 1124, 1056, 914, 854, 798, 782, 754, 725 cm−1. 1H NMR (DMSO-d6) δ 7.25 (dd, 1H, 3J = 8.8 Hz, 4J = 2.0 Hz, Ar-H), 7.33 (s, 1H, Ar-H), 7.40–7.51 (m, 6H, 4 × Ar-H, NH2), 7.69–7.71 (m, 1H, Ar-H), 7.79 (d, 1H, 4J = 2.0 Hz, Ar-H), 8.07 (s, 1H, Ar-H), 10.53 (s, 1H, NH), 12.05 (s, 1H, NH), 12.14 (s, 1H, NH), 12.84 (s, 1H, NH); 13C NMR (DMSO-d6) δ 103.87, 109.52, 114.01, 116.53, 119.98, 120.40, 120.82, 123.98, 124.42, 126.37, 127.98, 128.18, 129.36, 132.75, 135.21, 139.23, 147.80, 159.41; MS (ESI) m/z (%) = 352.1 ([M − Cl]+, 100). HRMS for C18H15N5OCl: calculated, 352.0965; found, 352.0959. HPLC: Phenomenex Luna 5 μm C18 column (4.6 mm × 150 mm); mobile phase: 60%–90% of MeOH in TFA (0.1%) in 20 min; flow rate: 1.0 mL/min; injection volume: 10 μL; retention time: 5.338 min (98.4% at 254 nm, 98.8% at 280 nm).
2-Amino-4-(3-(5-fluoro-1H-indole-2-carboxamido)phenyl)-1H-imidazol-3-ium chloride (6j) (see Supplementary Information, Figure S15). Yield, 77%; off-white solid; mp 202–205 °C; IR (KBr) ν = 3443 (N-H), 3275 (N-H), 3145 (C-H), 2764 (C-H), 1662 (C=O), 1628, 1607, 1544, 1486, 1449, 1411, 1327, 1287, 1244, 1231, 1204, 1145, 1103, 954, 840, 780, 752, 727 cm−1. 1H NMR (DMSO-d6) δ 7.11 (dt, 1H, 3J = 9.2 Hz, 4J = 2.0 Hz, Ar-H), 7.33 (s, 1H, Ar-H), 7.40–7.50 (m, 7H, 5 × Ar-H, NH2), 7.69–7.71 (m, 1H, Ar-H), 8.08 (t, 1H, 4J = 1.6 Hz, Ar-H), 10.49 (s, 1H, NH), 11.95 (s, 1H, NH), 12.14 (s, 1H, NH), 12.83 (s, 1H, NH); 13C NMR (DMSO-d6) δ 104.34 (d, 1C, 4JC-F = 5 Hz), 105.89 (d, 1C, 2JC-F = 23 Hz), 109.51, 112.65 (d, 1C, 2JC-F = 26 Hz), 113.63 (d, 1C, 3JC-F = 9 Hz), 116.52, 119.93, 120.39, 126.37, 127.00 (d, 1C, 3JC-F = 9 Hz), 128.17, 129.35, 132.96, 133.57, 139.28, 147.81, 157.20 (d, 1C, 1JC-F = 231 Hz), 159.47; 19F NMR (DMSO-d6) δ −123.59 (s, 1F); MS (ESI) m/z (%) = 336.1 ([M − Cl]+, 100). HRMS for C18H15N5OF: calculated, 336.1261; found, 336.1264. HPLC: Phenomenex Luna 5 μm C18 column (4.6 mm × 150 mm); mobile phase: 60%–90% of MeOH in TFA (0.1%) in 20 min; flow rate: 1.0 mL/min; injection volume: 10 μL; retention time: 3.585 min (99.4% at 254 nm, 99.1% at 280 nm).
4-(3-(4H-Thieno[3,2-b]pyrrole-5-carboxamido)phenyl)-2-amino-1H-imidazol-3-ium chloride (6k) (see Supplementary Information, Figure S16). Yield, 78%; off-white solid; mp 198–202 °C; IR (KBr) ν = 3241 (N-H), 3135 (C-H), 3047 (C-H), 2763 (C-H), 1677 (C=O), 1625, 1541, 1488, 1460, 1385, 1348, 1308, 1231, 1191, 1115, 1084, 963, 877, 827, 748, 711 cm−1. 1H NMR (DMSO-d6) δ 7.03 (dd, 1H, 3J = 5.2 Hz, 4J = 0.8 Hz, Ar-H), 7.31 (s, 1H, Ar-H), 7.36–7.49 (m, 6H, 4 × Ar-H, NH2), 7.66–7.69 (m, 1H, Ar-H), 8.06 (t, 1H, 4J = 1.6 Hz, Ar-H), 10.24 (s, 1H, NH), 11.99 (s, 1H, NH), 12.14 (s, 1H, NH), 12.82 (s, 1H, NH); 13C NMR (DMSO-d6) δ 103.91, 109.43, 111.90, 116.27, 119.48, 120.15, 122.94, 126.45, 128.08, 128.28, 129.26, 130.49, 139.64, 141.32, 147.77, 159.41; MS (ESI) m/z (%) = 324.1 ([M − Cl]+, 100). HRMS for C16H14N5OS: calculated, 324.0919; found, 324.0911. HPLC: Phenomenex Luna 5 μm C18 column (4.6 mm × 150 mm); mobile phase: 60%–90% of MeOH in TFA (0.1%) in 20 min; flow rate: 1.0 mL/min; injection volume: 10 μL; retention time: 2.790 min (96.5% at 254 nm, 97.0% at 280 nm).
2-Amino-4-(3-(5-hydroxy-1H-indole-2-carboxamido)phenyl)-1H-imidazol-3-ium chloride (8) (see Supplementary Information, Figure S18). Yield, 85%; red solid; mp 248–252 °C; IR (KBr) ν = 3262 (N-H, O-H), 3151 (C-H), 3027 (C-H), 2758 (C-H), 1681 (C=O), 1651, 1632, 1586, 1540, 1446, 1419, 1340, 1316, 1281, 1233, 1204, 1152, 1123, 952, 850, 788, 753 cm−1. 1H NMR (DMSO-d6) δ 6.79 (dd, 1H, 3J = 8.8 Hz, 4J = 2.0 Hz, Ar-H), 6.94 (d, 1H, 4J = 2.0 Hz, Ar-H), 7.26–7.48 (m, 7H, 5 × Ar-H, NH2), 7.67–7.69 (m, 1H, Ar-H), 8.08 (s, 1H, Ar-H), 8.91 (s, 1H, OH), 10.32 (s, 1H, NH), 11.52 (s, 1H, NH), 12.12 (s, 1H, NH), 12.80 (s, 1H, NH); 13C NMR (DMSO-d6) δ 103.40, 104.34, 109.51, 112.89, 115.22, 116.39, 119.73, 120.24, 126.46, 127.65, 128.12, 129.31, 131.35, 131.66, 139.48, 147.77, 151.26, 159.82; MS (ESI) m/z (%) = 334.1 ([M − Cl]+, 100). HRMS for C18H16N5O2: calculated, 334.1304; found, 334.1296. HPLC: Phenomenex Luna 5 μm C18 column (4.6 mm × 150 mm); mobile phase: 60%–90% of MeOH in TFA (0.1%) in 20 min; flow rate: 1.0 mL/min; injection volume: 10 μL; retention time: 1.923 min (98.0% at 254 nm, 98.3% at 280 nm).
3.4.5. 1-Benzyl-4-(3-nitrophenyl)-1H-imidazol-2-amine (13)
To the suspension of compound 12 (0.864 g, 4.00 mmol) in CH3CN (30 mL) were added K2CO3 (1.38 g, 6.00 mmol) and benzyl bromide (0.713 mL, 6.00 mmol). The mixture was stirred at 50 °C for 14 h. The solvent was removed under reduced pressure, the brown residue was suspended in ethyl acetate (80 mL) and washed with water (2 × 40 mL) and brine (1 × 40 mL). The organic phase was dried over Na2SO4, filtered and the solvent removed in vacuo. The crude product was purified by column chromatography with dichloromethane/methanol (20:1) as an eluent to afford 13 (see Supplementary Information, Figure S19) (539 mg, 44% yield) as a yellow solid; mp 189–193 °C; IR (KBr) ν = 3404 (N-H), 3126 (C-H), 1649, 1585, 1523, 1546, 1440, 1342, 1211, 1068, 886, 802, 759, 717 cm−1. 1H NMR (DMSO-d6) δ 5.02 (s, 2H, CH2), 5.84 (s, 2H, NH2), 7.26–7.32 (m, 3H, 3 × Ar-H), 7.35–7.39 (m, 2H, 2 × Ar-H), 7.40 (s, 1H, Ar-H), 7.55 (t, 1H, 3J = 8.0 Hz, Ar-H), 7.92–7.95 (m, 1H, Ar-H), 7.99–8.03 (m, 1H, Ar-H), 8.41–8.43 (m, 1H, Ar-H); 13C NMR (DMSO-d6) δ 47.30, 113.02, 117.51, 119.60, 127.37, 127.40, 128.54, 129.57, 129.74, 133.49, 137.04, 137.53, 148.23, 150.14; MS (ESI) m/z (%) = 295.1 (MH+, 100). HRMS for C16H15N4O2: calculated, 295.1195; found, 295.1199. HPLC: Phenomenex Luna 5 μm C18 column (4.6 mm × 150 mm); mobile phase: 10%–90% of MeOH in TFA (0.1%) in 20 min; flow rate: 1.0 mL/min; injection volume: 10 μL; retention time: 16.113 min (99.8% at 254 nm, 98.5% at 280 nm).
3.4.6. 4-(3-Aminophenyl)-1-benzyl-1H-imidazol-2-amine (14)
Compound 13 (432 mg, 1.41 mmol) was dissolved in THF (50 mL), Pd/C (44 mg) was added and the reaction mixture was stirred under hydrogen atmosphere for 5 h. The catalyst was filtered off and the solvent removed under reduced pressure to yield 14 (see Supplementary Information, Figure S20) (379 mg, 97% yield) as an off-white solid; mp 192–195 °C; IR (KBr) ν = 3370 (N-H), 3067 (C-H), 1647, 1616, 1,569, 1545, 1481, 1438, 1363, 1338, 1271, 1202, 1129, 1067, 993, 976, 884, 819, 789, 732 cm−1. 1H NMR (DMSO-d6) δ 4.90 (s, 2H, NH2), 4.96 (s, 2H, CH2), 5.57 (s, 2H, NH2), 6.30–6.34 (m, 1H, Ar-H), 6.74–6.78 (m, 1H, Ar-H), 6.86 (t, 1H, 4J = 2.0 Hz, Ar-H), 6.89 (s, 1H, Ar-H), 6.90 (t, 1H, 3J = 7.8 Hz, Ar-H),7.23–7.30 (m, 3H, 3 × Ar-H), 7.33–7.38 (m, 2H, 2 × Ar-H); 13C NMR (DMSO-d6) δ 47.12, 109.56, 110.11, 111.41, 111.86, 127.28, 127.37, 128.47, 128.56, 135.66, 136.26, 137.89, 148.37, 149.25; MS (ESI) m/z (%) = 265.1 (MH+, 100). HRMS for C16H17N4: calculated, 265.1453; found, 265.1459. HPLC: Phenomenex Luna 5 μm C18 column (4.6 mm × 150 mm); mobile phase: 10%–90% of MeOH in TFA (0.1%) in 20 min; flow rate: 1.0 mL/min; injection volume: 10 μL; retention time: 11.334 min (95.3% at 254 nm, 96.6% at 280 nm).
3.4.7. N-(3-(2-Amino-1-benzyl-1H-imidazol-4-yl)phenyl)-1H-pyrrole-2-carboxamide (15)
To a suspension of pyrrole-2-carboxylic acid (77 mg, 0.69 mmol) in dichloromethane (30 mL) were added triethylamine (0.192 mL, 1.38 mmol) and TBTU (244 mg, 0.76 mmol) and the mixture stirred at rt for 0.5 h upon which an opalescent solution formed. Compound 14 (190 mg, 0.69 mmol) was added and the mixture stirred at rt for 18 h. The reaction mixture was diluted with dichloromethane (20 mL) and washed with saturated aqueous NaHCO3 solution (2 × 30 mL) and brine (1 × 40 mL). The organic phase was dried over Na2SO4, filtered and concentrated in vacuo. The crude product was purified by flash column chromatography using dichloromethane/methanol as an eluent, to afford 15 (see Supplementary Information, Figure S21) (105 mg, 41% yield) as an off-white solid; mp 229–232 °C; IR (KBr) ν = 3370 (N-H), 3066 (C-H), 1646 (C=O), 1606, 1570, 1545, 1496, 1454, 1437, 1364, 1308, 1202, 1169, 1130, 1068, 993, 896, 884, 823, 790, 725 cm−1. 1H NMR (DMSO-d6) δ 5.02 (s, 2H, CH2), 5.69 (s, 2H, NH2), 6.15–6.18 (m, 1H, Ar-H), 6.95–6.98 (m, 1H, Ar-H), 7.03 (s, 1H, Ar-H), 7.08–7.12 (m, 1H, Ar-H), 7.21 (t, 1H, 3J = 7.8 Hz, Ar-H), 7.26–7.32 (m, 4H, 4 × Ar-H), 7.35–7.40 (m, 2H, 2 × Ar-H), 7.54–7.58 (m, 1H, Ar-H), 7.94–7.97 (m, 1H, Ar-H), 9.70 (s, 1H, NH) 11.60 (br s, 1H, NH); 13C NMR (DMSO-d6) δ 47.20, 108.84, 110.73, 111.29, 115.37, 117.14, 118.45, 122.30, 126.18, 127.35, 127.44, 128.34, 128.52, 135.40, 135.49, 137.76, 139.34, 149.52, 158.99; MS (ESI) m/z (%) = 358.2 (MH+, 100). HRMS for C21H20N5O: calculated, 358.1668; found, 358.1661. HPLC: Phenomenex Luna 5 μm C18 column (4.6 mm × 150 mm); mobile phase: 10%–90% of MeOH in TFA (0.1%) in 20 min; flow rate: 1.0 mL/min; injection volume: 10 μL; retention time: 16.868 min (97.4% at 254 nm, 96.9% at 280 nm).
3.4.8. 4-(3-(((1H-Pyrrol-2-yl)methyl)amino)phenyl)-1-benzyl-1H-imidazol-2-amine (16)
To a suspension of compound 14 (190 mg, 0.69 mmol) in dichloromethane (30 mL) were added pyrrole-2-carboxaldehyde (208 mg, 1.04 mmol) and glacial acetic acid (40 μL, 0.69 mmol) upon which the mixture became clear. NaBH(OAc)3 (208 mg, 1.04 mmol) was added and the mixture stirred at rt for 13 h. Red opalescent solution was diluted with dichloromethane (20 mL) and washed with saturated aqueous NaHCO3 solution (2 × 30 mL) and brine (1 × 30 mL). The organic phase was dried over Na2SO4, filtered and concentrated in vacuo. Crude product was recrystallized from ethyl acetate to give 16 (see Supplementary Information, Figure S22)¸(120 mg, 49% yield) as red crystals; mp 165–168 °C; IR (KBr) ν = 3370 (N-H), 3066 (C-H), 1646, 1602, 1570, 1495, 1454, 1364, 1271, 1201, 1183, 1130, 1096, 993, 860, 790, 729 cm−1. 1H NMR (DMSO-d6) δ 4.14 (d, 2H, 3J = 5.5 Hz, CH2) 4.97 (s, 2H, CH2), 5.56–5.62 (m, 3H, NH, NH2), 5.91–5.96 (m, 2H, 2 × Ar-H), 6.40–6.44 (m, 1H, Ar-H), 6.62–6.65 (m, 1H, Ar-H), 6.80–6.84 (m, 1H, Ar-H), 6.93–6.98 (m, 3H, 3 × Ar-H), 7.23–7.31 (m, 3H, 3 × Ar-H), 7.33–7.39 (m, 2H, 2 × Ar-H), 10.70 (br s, 1H, NH); 13C NMR (DMSO-d6) δ 40.44, 47.12, 105.71, 107.11, 107.66, 110.20, 110.33, 112.09, 116.70, 127.27, 127.32, 128.47 (2 signals overlapped), 128.68, 135.62, 136.30, 137.90, 148.70, 149.29; MS (ESI) m/z (%) = 344.2 (MH+, 100). HRMS for C21H22N5: calculated, 344.1875; found, 344.1873. HPLC: Phenomenex Luna 5 μm C18 column (4.6 mm × 150 mm); mobile phase: 10%–90% of MeOH in TFA (0.1%) in 20 min; flow rate: 1.0 mL/min; injection volume: 10 μL; retention time: 11.474 min (97.9% at 254 nm, 97.4% at 280 nm).
4. Conclusions
In the present study, we have prepared the natural pyrrole-2-aminoimidazole alkaloids, clathrodin and oroidin, originally isolated from Agelas sponges and four families of their synthetic analogues. In total, 36 compounds were screened against a panel of laboratory strains, representing Gram-positive (Enterococcus faecalis and Staphylococcus aureus) and Gram-negative bacteria (Escherichia coli) and fungi (Candida albicans). Starting from the molecule of oroidin, with structural optimization using medicinal chemistry strategies, we have succeeded to prepare analogues with improved antimicrobial activities against all of the microbial strains tested. Twelve active compounds were selected, and their minimum inhibitory concentrations (MIC50, MIC90), as well as selectivities against mammalian cells were further determined. The most promising results were obtained for indole-based derivatives 6h with MIC90 of 12.5 µM against the Gram-positive bacteria and 50 µM against E. coli and 6g with MIC90 of 25 µM against all the bacteria and 50 µM against C. albicans. Although the effects were shown to be mostly broad-spectrum, targeting both prokaryotic and eukaryotic cells, in general, the IC50 values for mammalian cell cytotoxicity were slightly higher. Our results provide valuable information for future optimization towards a more selective antimicrobial compound.
Acknowledgments
This work was supported by the Slovenian Research Agency (grant no. P1-0208 and grant no. Z1-5458) and by the European Union Seventh Framework Programme FP7 under grant agreement no. 245137.
Abbreviations
- ATCC
American Type Culture Collection
- d
doublet
- dd
doublet of doublets
- ddd
doublet of doublet of doublets
- DMF
N,N-dimethylformamide
- DMSO
dimethylsulfoxide
- dt
doublet of triplets
- ESI
electrospray ionization
- EtOH
ethanol
- FT-IR
fourier transform infrared
- HRMS
high resolution mass spectrometry
- Huh-7
human hepatocellular carcinoma cell line
- IR
infrared
- MOPS
3-(N-morpholino)propanesulfonic acid
- mp
melting point
- NMM
N-methylmorpholine
- R
radical
- RPMI-1640
Roswell Park Memorial Institute medium 1640
- rt
room temperature
- s
singlet
- TBTU
N,N,N′,N′-tetramethyl-O-(benzotriazol-1-yl)uronium tetrafluoroborate
- td
triplet of doublets
- THF
tetrahydrofuran
- TLC
thin layer chromatography
- TMS
tetramethylsilane
- Vis
visible light
Supplementary Files
Supplementary Information (PDF, 3708 KB)
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Al-Sabi A., McArthur J., Ostroumov V., French R.J. Marine toxins that target voltage-gated sodium channels. Mar. Drugs. 2006;4:157–192. doi: 10.3390/md403157. [DOI] [Google Scholar]
- 2.Doshi G.M., Aggarwal G.V., Martins E.A., Shanbhag P.P. Novel antibiotics from marine sources. Int. J. Pharm. Sci. Nanotech. 2011;4:1446–1461. [Google Scholar]
- 3.Al-Mourabit A., Zancanella M.A., Tilvi S., Romo D. Biosynthesis, asymmetric synthesis, and pharmacology, including cellular targets, of the pyrrole-2-aminoimidazole marine alkaloids. Nat. Prod. Rep. 2011;28:1229–1260. doi: 10.1039/c0np00013b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forte B., Malgesini B., Piutti C., Quartieri F., Scolaro A., Papeo G. A submarine journey: The pyrrole-imidazole alkaloids. Mar. Drugs. 2009;7:705–753. doi: 10.3390/md7040705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al Mourabit A., Potier P. Sponge’s molecular diversity through the ambivalent reactivity of 2-aminoimidazole: A universal chemical pathway to the oroidin-based pyrrole-imidazole alkaloids and their palau’amine congeners. Eur. J. Org. Chem. 2001:237–243. doi: 10.1002/1099-0690(200101)2001:2<237::AID-EJOC237>3.0.CO;2-V. [DOI] [Google Scholar]
- 6.Silver L.L. Challenges of antibacterial discovery. Clin. Microbiol. Rev. 2011;24:71–109. doi: 10.1128/CMR.00030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Payne D.J., Gwynn M.N., Holmes D.J., Pompliano D.L. Drugs for bad bugs: Confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 2007;6:29–40. doi: 10.1038/nrd2201. [DOI] [PubMed] [Google Scholar]
- 8.Chopra I., Schofield C., Everett M., O’Neill A., Miller K., Wilcox M., Frere J.M., Dawson M., Czapiewski L., Urleb U., et al. Treatment of health-care-associated infections caused by gram-negative bacteria: A consensus statement. Lancet Infect. Dis. 2008;8:133–139. doi: 10.1016/S1473-3099(08)70018-5. [DOI] [PubMed] [Google Scholar]
- 9.Theuretzbacher U. Accelerating resistance, inadequate antibacterial drug pipelines and international responses. Int. J. Antimicrob. Agents. 2012;39:295–299. doi: 10.1016/j.ijantimicag.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Walsh T.R., Toleman M.A. The emergence of pan-resistant gram-negative pathogens merits a rapid global political response. J. Antimicrob. Chemoth. 2012;67:1–3. doi: 10.1093/jac/dkr378. [DOI] [PubMed] [Google Scholar]
- 11.Thompson R.J., Bobay B.G., Stowe S.D., Olson A.L., Peng L.L., Su Z.M., Actis L.A., Melander C., Cavanagh J. Identification of BfmR, a response regulator involved in biofilm development, as a target for a 2-aminoimidazole-based antibiofilm agent. Biochemistry. 2012;51:9776–9778. doi: 10.1021/bi3015289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steenackers H.P.L., Ermolat’ev D.S., Savaliya B., De Weerdt A., De Coster D., Shah A., Van der Eycken E.V., De Vos D.E., Vanderleyden J., De Keersmaecker S.C.J. Structure-activity relationship of 2-hydroxy-2-aryl-2,3-dihydro-imidazo[1,2-a]pyrimidinium salts and 2N-substituted 4(5)-aryl-2-amino-1H-imidazoles as inhibitors of biofilm formation by Salmonella typhimurium and Pseudomonas aeruginosa. Bioorg. Med. Chem. 2011;19:3462–3473. doi: 10.1016/j.bmc.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 13.Steenackers H.P.L., Ermolat’ev D.S., Savaliya B., De Weerdt A., De Coster D., Shah A., Van der Eycken E.V., De Vos D.E., Vanderleyden J., De Keersmaecker S.C.J. Structure-activity relationship of 4(5)-aryl-2-amino-1H-imidazoles, N1-substituted 2-aminoimidazoles and imidazo[1,2-a]pyrimidinium salts as inhibitors of biofilm formation by Salmonella typhimurium and Pseudomonas aeruginosa. J. Med. Chem. 2011;54:472–484. doi: 10.1021/jm1011148. [DOI] [PubMed] [Google Scholar]
- 14.Rogers S.A., Huigens R.W., Cavanagh J., Melander C. Synergistic effects between conventional antibiotics and 2-aminoimidazole-derived antibiofilm agents. Antimicrob. Agents Chemother. 2010;54:2112–2118. doi: 10.1128/AAC.01418-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogers S.A., Huigens R.W., Melander C. A 2-aminobenzimidazole that inhibits and disperses gram-positive biofilms through a zinc-dependent mechanism. J. Am. Chem. Soc. 2009;131:9868–9869. doi: 10.1021/ja9024676. [DOI] [PubMed] [Google Scholar]
- 16.Richards J.J., Reyes S., Stowe S.D., Tucker A.T., Ballard T.E., Mathies L.D., Cavanagh J., Melander C. Amide isosteres of oroidin: Assessment of antibiofilm activity and C. elegans toxicity. J. Med. Chem. 2009;52:4582–4585. doi: 10.1021/jm900378s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richards J.J., Reed C.S., Melander C. Effects of N-pyrrole substitution on the anti-biofilm activities of oroidin derivatives against Acinetobacter baumannii. Bioorg. Med. Chem. Lett. 2008;18:4325–4327. doi: 10.1016/j.bmcl.2008.06.089. [DOI] [PubMed] [Google Scholar]
- 18.Bunders C.A., Richards J.J., Melander C. Identification of aryl 2-aminoimidazoles as biofilm inhibitors in gram-negative bacteria. Bioorg. Med. Chem. Lett. 2010;20:3797–3800. doi: 10.1016/j.bmcl.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 19.Ballard T.E., Richards J.J., Aquino A., Reed C.S., Melander C. Antibiofilm activity of a diverse oroidin library generated through reductive acylation. J. Org. Chem. 2009;74:1755–1758. doi: 10.1021/jo802260t. [DOI] [PubMed] [Google Scholar]
- 20.Stowe S.D., Richards J.J., Tucker A.T., Thompson R., Melander C., Cavanagh J. Anti-biofilm compounds derived from marine sponges. Mar. Drugs. 2011;9:2010–2035. doi: 10.3390/md9102010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huigens R.W., Reyes S., Reed C.S., Bunders C., Rogers S.A., Steinhauer A.T., Melander C. The chemical synthesis and antibiotic activity of a diverse library of 2-aminobenzimidazole small molecules against MRSA and multidrug-resistant A. baumannii. Bioorg. Med. Chem. 2010;18:663–674. doi: 10.1016/j.bmc.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Harris T.L., Worthington R.J., Melander C. A facile synthesis of 1,5-disubstituted-2-amino imidazoles: Antibiotic activity of a first generation library. Bioorg. Med. Chem. Lett. 2011;21:4516–4519. doi: 10.1016/j.bmcl.2011.05.123. [DOI] [PubMed] [Google Scholar]
- 23.Rogers S.A., Lindsey E.A., Whitehead D.C., Mullikin T., Melander C. Synthesis and biological evaluation of 2-aminoimidazole/carbamate hybrid anti-biofilm and anti-microbial agents. Bioorg. Med. Chem. Lett. 2011;21:1257–1260. doi: 10.1016/j.bmcl.2010.12.057. [DOI] [PubMed] [Google Scholar]
- 24.Hammami S., Bergaoui A., Boughalleb N., Romdhane A., Khoja I., Kamel M.B., Mighri Z. Antifungal effects of secondary metabolites isolated from marine organisms collected from the Tunisian coast. C. R. Chim. 2010;13:1397–1400. doi: 10.1016/j.crci.2010.04.022. [DOI] [Google Scholar]
- 25.Scala F., Fattorusso E., Menna M., Taglialatela-Scafati O., Tierney M., Kaiser M., Tasdemir D. Bromopyrrole alkaloids as lead compounds against protozoan parasites. Mar. Drugs. 2010;8:2162–2174. doi: 10.3390/md8072162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tasdemir D., Topaloglu B., Perozzo R., Brun R., O’Neill R., Carballeira N.M., Zhang X.J., Tonge P.J., Linden A., Ruedi P. Marine natural products from the Turkish sponge Agelas oroides that inhibit the enoyl reductases from Plasmodium falciparum, Mycobacterium tuberculosis and Escherichia coli. Bioorg. Med. Chem. 2007;15:6834–6845. doi: 10.1016/j.bmc.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 27.Bernan V.S., Roll D.M., Ireland C.M., Greenstein M., Maiese W.M., Steinberg D.A. A study on the mechanism of action of sceptrin, an antimicrobial agent isolated from the south-pacific sponge Agelas mauritiana. J. Antimicrob. Chemother. 1993;32:539–550. doi: 10.1093/jac/32.4.539. [DOI] [PubMed] [Google Scholar]
- 28.Little T.L., Webber S.E. A simple and practical synthesis of 2-aminoimidazoles. J. Org. Chem. 1994;59:7299–7305. doi: 10.1021/jo00103a021. [DOI] [Google Scholar]
- 29.Zidar N., Jakopi Ž., Madge D.J., Chan F., Tytgat J., Peigneur S., Sollner-Dolenc M., Tomasić T., Ilaš J., Peterlin Mašič L., et al. Substituted 4-phenly-2-aminoimidazoles and 4-phenyl-4,5-dihydro-2-aminoimidazoles as volzage-gated sodium channel modulators. Eur. J. Med. Chem. 2014;74:23–30. doi: 10.1016/j.ejmech.2013.12.034. [DOI] [PubMed] [Google Scholar]
- 30.CLSI (Clinical and Laboratory Standards Institute) Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Third Informational Supplement. CLSI; Wayne, PA, USA: 2013. pp. 1–206. [Google Scholar]
- 31.Vicente E., Perez-Silanes S., Lima L.M., Ancizu S., Burguete A., Solano B., Villar R., Aldana I., Monge A. Selective activity against Mycobacterium tuberculosis of new quinoxaline 1,4-di-N-oxides. Bioorg. Med. Chem. 2009;17:385–389. doi: 10.1016/j.bmc.2008.10.086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information (PDF, 3708 KB)