Abstract
A new actinomycete strain Micromonospora sp. K310 was isolated from Ghanaian mangrove river sediment. Spectroscopy-guided fractionation led to the isolation of two new compounds from the fermentation culture. One of the compounds is butremycin (2) which is the (3-hydroxyl) derivative of the known Streptomyces metabolite ikarugamycin (1) and the other compound is a protonated aromatic tautomer of 5′-methylthioinosine (MTI) (3). Both new compounds were characterized by 1D, 2D NMR and MS data. Butremycin (2) displayed weak antibacterial activity against Gram-positive S. aureus ATCC 25923, the Gram-negative E. coli ATCC 25922 and a panel of clinical isolates of methicillin-resistant S. aureus (MRSA) strains while 3 did not show any antibacterial activity against these microbes.
Keywords: Micromonospora, macrolactam, tautomer, tetramic acid, mangroves
1. Introduction
Mangroves are unique woody plant communities that occur as a convergence place for both marine and terrestrial organisms around the intertidal coasts of tropical and sub-tropical regions. These habitats have been found to be a great assembly point of novel actinomycetes belonging to several genera including Actinomadura, Microbispora, Nonomuraea, Actinoplanes, Micromonospora, Verrucosispora, Arthrobacter, Isoptericola, Micrococcus, Microbacterium, Nocardia, Rhodococcus, Streptomyces and many more [1]. Novel metabolites with anti-infective, antitumour, anti-diabetic activity and anti-neurodegenerative activity have been isolated from these genera of actinomycetes obtained from different marine habitats. For example, Actinomadura sp. produce the anticancer agent IB-00208 and the chandrananimycins which possess antibacterial, anticancer and antifungal activity [2,3]. Verrucocispora sp. is noted for the abyssomicins which have antibacterial activity [4]. Caprolactones and chinikomycins are potent anticancer agents that have been isolated from Streptomyces sp. [5,6]. From Micromonospora sp. comes the diazepinomicin (ECO-4601) which is known to have antibacterial, anticancer and anti-inflammatory activities [7]. These examples are by no means a comprehensive list of all promising lead molecules isolated from actinomycetes, but underline that continued research of un- or underexplored mangrove ecosystems should yield many novel species of actinomycetes which as a result of their high genomic and metabolic diversity are most likely to produce new drug prototypes.
Similar ideas have driven the research in our laboratory to investigate mangroves in the Western Region of Ghana for possible isolation of new or novel marine actinomycetes. Several new species of Actinomyces, Streptomyces, Salinospora, Verrucocispora and Micromonospora have been isolated from the Western Region mangroves using casein starch with sea salts as culture media. One of these species Micromonospora sp. K310, was found to produce under the current growth media and conditions the [3-hydroxyl] derivative of ikarugamycin, named butremycin in recognition of the river from which the source organism was obtained and a protonated aromatic tautomer of 5′-methylthioinosine (3) complexed to sec-butoxide as a counter ion.
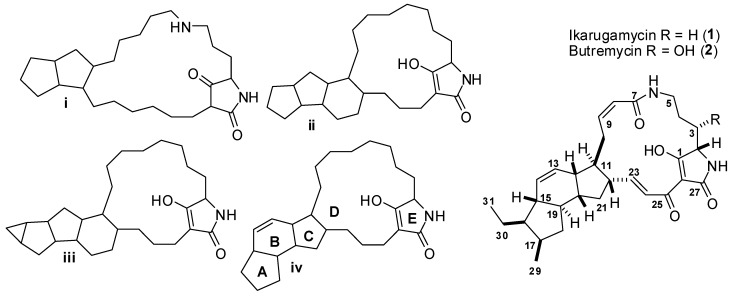
Butremycin belongs to the family of polycyclic tetramic acid macrolactams (PTM) that possess typical characteristic ring sequences as shown for the structure skeleton i–iv in Figure 1, where the last ring is normally the five membered ring of tetramic acid. Butremycin belongs to the category of compounds that possess the structure skeleton iv and in addition it has a 3-hydroxyl group which has been previously found in all PTMs reported so far with the exception of ikarugamycin [8,9]. Butremycin is the hydroxyl derivative of ikarugamycin and 3-hydroxylation is a feature common to some polycyclic tetramic acid macrolactams found in phylogenetically diverse organisms. The effect of 3-hydroxylation on the bioactivity of these tetramic acid macrolactams has been demonstrated by the work of Li et al. [8] but, to the best of our knowledge this is the first report of a PTM from a Micromonospora sp.
Figure 1.
Characteristic ring sequences for polycyclic tetramic acid macrolactams (PTM) compounds isolated to date including ikarugamycin and butremycin.
2. Results and Discussion
2.1. Sediment Sample Collection Sites
The Western Region of Ghana is particularly noted for large stretches of mangroves that have become characteristically embedded in the life of the natives. An important river, called the Butre, cuts across a large section of these mangroves before entering the sea. We collected sediment samples from the river at four different sites approximately 100m apart. Micromonospora sp. K310 was isolated from one of these sediments (coordinates: 4°49′43.73″N and 1°54′50.84″W).
2.2. Taxonomy of Strain K310 (Genbank Number KF803252)
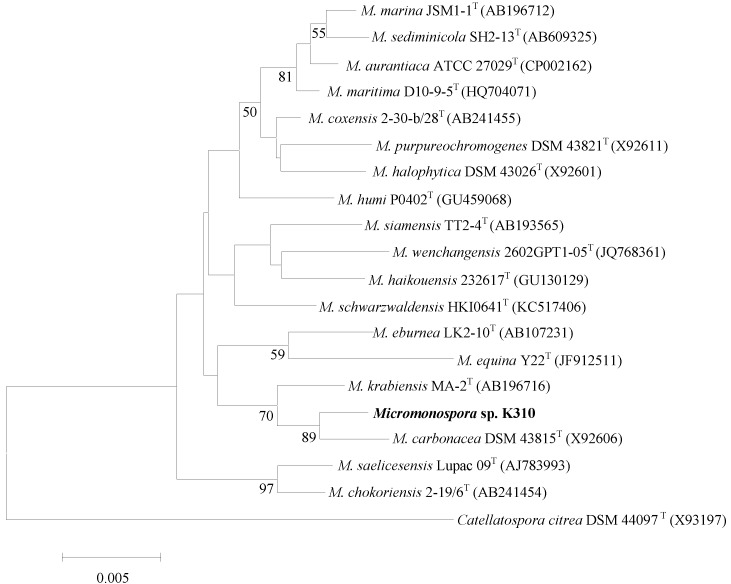
The organism exhibited a range of chemotaxonomic and phenotypic properties typical of members of the genus Micromonospora. An almost complete 16S rDNA gene sequence (1455 nt) was determined for the organism. Primary sequence analysis with the sequences of representatives of the family Micromonosporaceae confirmed that the unknown isolate was closely related to the species of the genus Micromonospora. The phylogenetic tree based on the neighbor-joining algorithm showed that the strain K310 formed a cluster with M. carbonacea DSM 43815T and M. krabiensis MA-2T among members of the genus Micromonospora (Figure 2). Strain K310 shared 16S rDNA gene sequence similarities of 99.45% (8 nt differences at 1449 locations), 99.03% (14 nt differences at 1436 locations) with M. carbonacea DSM and M. krabiensis MA-2T respectively. Sequence similarities with all other members of the genus Micromonospora were <98.90%.
Figure 2.
Neighbor-joining tree [10] based on almost complete 16 rDNA gene sequences (1455 nt) showing the position of Micromonospora sp. K310 (KF803252) amongst its phylogenetic neighbors. Catellatospora citrea DSM 44097T (GenBank accession no. X93197) was used as an out-group. Numbers at nodes indicate the levels of bootstrap support (%); only values >50% are shown. GenBank accession numbers are given in parentheses. Bar, 0.005 substitutions per site.
2.3. Structure Determination of Compound 2 (Butremycin)
A seven-day grown seed culture of Micromonospora sp. K310 was used in the inoculation of two L starch casein media in the presence of the Diaion HP-20 resin (50 g/L medium). After 28-day fermentation, the aqueous solution was filtered and HP20 resin was extracted with methanol (3 × 500 mL). The crude extract was concentrated under vacuum, followed by partition using a modified Kupchan method [11]. The purification was achieved through Sephadex LH-20 chromatography followed by semipreparative reversed-phase HPLC resulting in the isolation of 2 (46.6 mg) and 3 (57.0 mg).
Butremycin was isolated as pale yellow crystals soluble in CH3OH. The HRESIMS of this compound gave m/z = 495.2781 [M + H]+ corresponding to a molecular formula of C29H39N2O5 (Δ = −0.5 ppm) and 12 degrees of unsaturation. Molecular formulae for all fragments emanating from the molecular ion were obtained and used to confirm the subsequent structure obtained from NMR (Supplementary Figure S1).
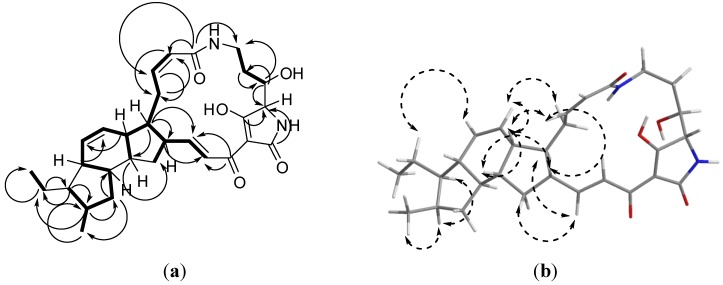
Analysis of the 1H, 13C and multiplicity edited gHSQCAD spectra suggested the presence of 5 quaternary, 16 methine, 6 methylene and 2 methyl carbons. Detailed analysis of the gCOSY spectrum provided substructures as illustrated in Figure 3a. The presence of six olefinic protons at δH 7.30 (1H, d, J = 15.2 Hz, H-24), 6.47 (1H, dd, J = 10.0, 15.1 Hz, H-23), 6.02 (1H, ddd, J = 2.6, 11.4, 11.4 Hz, H-9), 5.83 (1H, ddd, J = 2.1, 2.1, 10.0 Hz, H-14), 5.76 (1H, dd, J = 2.8, 11.4 Hz, H-8), 5.62 (1H, ddd, J = 2.8, 2.8, 9.9 Hz, H-13) was direct indication of the presence of three double bonds: H-8/H-9 (cis), H-13/H-14 (cis) and H-23/H-24 (trans). Subsequently, using these isolated olefins as starting points, sub-structure extensions were achieved by looking at gHMBCAD peaks from C-23 to H-22 and H-21 with confirmations from the gCOSY cross-peaks between H-21/H-22 and H-22/H-23. Similarly, gCOSY cross-peaks between H-11/H-12, H-12/H-13, H-13/H-14 and H-14/H-15 provided an extension of the C-13/C-14 olefin sub-structure. Also, sub-structure extensions for the C-8/C-9 olefin bond were obtained by inspecting the gCOSY correlations H-8/H-10, H-9/H-10, H-10/H-11 with confirmation from gHMBCAD correlations C-8 to H-10, C-9 to H-10, C-10 to H-8, and C-11 to both H-9 and H-10. From these substructures it became easier to follow the comprehensive spin system present in butremycin with the A5:B6:C5 ring structures (3a) obtained by 2D correlations H-11/H-22, C-11/H-22, H-12/H-20, H-15/H-19 and C-15/H-19.
Figure 3.
(a) Selected COSY (▬) and gHMBCAD (→) data for butremycin; (b) Selected ROESY (↔) data for butremycin (2).
The tetramic acid structure was constructed by analyzing the gHMBCAD correlations from C-1 (δC 195.5) to H-2, C-27 (δC 179.9) to H-2 and C-3 (δC 72.5) to H-2 and comparison to reference shifts for comparable tetramic acids [12]. All the NMR data for this compound are summarized in Table 1 and raw data can be obtained from Supplementary Figures S2–S7.
Table 1.
1H and 13CNMR data of butremycin in CD3OD. δ in ppm, J in Hz.
| Position | δH mult (J Hz) | δC mult | HMBC |
|---|---|---|---|
| 1 | 195.5, C | 2 | |
| 2 | 3.70, d (2.4) | 68.4, CH | |
| 3 | 3.86, ddd (7.2, 1.9, 1.9) | 72.5, CH | 2, 4, 5 |
| 4 | 1.51, m; 1.24, m | 32.1, CH2 | 2, 3 |
| 5 | 3.42, m; 2.71, t (12.0) | 37.9, CH2 | 3 |
| 6-NH | 8.43, s | ||
| 7 | 168.9, C | 5, 8, 9 | |
| 8 | 5.76, dd (11.4, 2.8) | 124.8, CH | 10 |
| 9 | 6.02, ddd (11.4, 11.4, 2.6) | 141.8, CH | 10 |
| 10 | 3.53, m; 2.09, m | 25.9, CH2 | 8 |
| 11 | 1.35, m | 50.3, CH | 9, 10, 20, 22, 23 |
| 12 | 2.51, m | 43.4, CH | 10, 14 |
| 13 | 5.62, ddd (9.9, 2.8, 2.8) | 129.6, CH | |
| 14 | 5.83, dt (10.0, 2.1) | 131.9, CH | |
| 15 | 1.48, m | 48.4, CH | 13, 14, 19 |
| 16 | 1.29, m | 48.5, CH | 17, 30 |
| 17 | 2.20, m | 34.3, CH | 18, 29, 30 |
| 18 | 2.05, m; 0.63, ddd (11.9, 11.8, 6.8) | 39.9, CH2 | 19, 29 |
| 19 | 1.08, m | 50.4, CH | 14, 18, 21 |
| 20 | 2.00, m | 43.2, CH | 13, 18, 21 |
| 21 | 2.01, m; 1.10, m | 38.1, CH2 | 19, 22, 23 |
| 22 | 2.32, m | 49.6, CH | 21, 23, 24 |
| 23 | 6.47, dd (15.1, 10.0) | 147.8, CH | 21, 22 |
| 24 | 7.30, d (15.2) | 130.4, CH | 22 |
| 25 | 184.7, C | 23, 24 | |
| 26 | 102.8, C | ||
| 27 | 179.9, C | 2 | |
| 28-NH | 8.43, s | ||
| 29 | 0.81, d (6.0) | 18.1, CH3 | 17, 18 |
| 30 | 1.42, m; 1.30, m | 22.7, CH2 | 16, 31 |
| 31 | 0.87, t (7.1) | 13.7, CH3 | 30 |
The majority of the stereochemistry is identical to that reported for ikarugamycin, and the ROESY correlations observed confirm this proposal (Figure 3). The only remaining issue is the stereochemistry at C-3. The small coupling constant between H-2 and H-3 (ca. 2 Hz) is indicative of a nearly orthogonal relationship between C-2–H-2 and C-3–H-3. This is consistent with a local 2S*, 3S* stereochemistry. The alternative, a 2S*, 3R* gives rise to an anti-periplanar arrangement of C-2–H-2 and C-3–H-3 which should result in a large coupling constant between H-2 and H-3. This suggests that the overall relative stereochemistry for butremycin is 2S*, 3S*, 11S*, 12R*, 15S*, 16R*, 17R*, 19R*, 20R*, 22R*.
2.4. Structure Determination of Compound 3
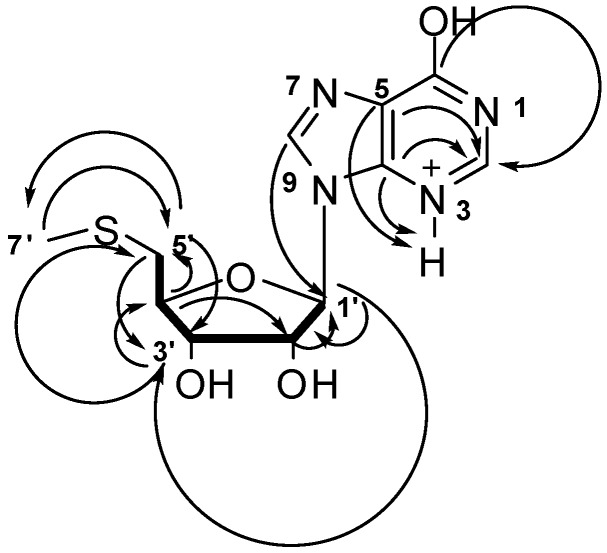
Compound 3 was isolated as a 1:1 complex with sec-butoxide which is most likely to come from the 2-butanol used in the solvent partition process. This compound 3/sec-butoxide complex was yellow and formed crystalline material soluble in CH3OH. The HRESIMS of this compound gave m/z = 299.0810 [M]+ corresponding to a molecular formula of C11H15N4O4S+ and 7 degrees of unsaturation. Analysis of the 1H, 13C and multiplicity edited gHSQCAD spectrum confirmed the presence of 3 quaternary, 6 methine, 1 methylene and 1 methyl carbons. The δC 90.23, 75.22, 73.98, 85.65 and 37.47 ppm corresponding to C-1′, C-2′, C-3′, C-4′ and C-5′ respectively, were very characteristic of a series of multiple oxygenations commonly found in sugars. Analysis of the 1H and 13C NMR provided proof of the presence of a five-membered ring sugar moiety, which could possibly be a furanose. Detailed analysis of the gCOSY spectrum provided the spin system encompassing H-1′ to H-5′ which was extended to include Me-7′ via the the gHMBC correlation from C-5′ to H-7′ (Figure 4). The characteristic 13C NMR chemical shifts δC C-6 (158.9), C-4 (150.2), C-5 (125.8) and C-8 (140.9) taken together with the gHMBCAD correlations C-6 to H-2, C-5 to H-2 and NH-3, C-4 to H-2, NH-3 suggested a protonated adenine ring. The ribose unit and the adenine ring were connected using two key gHMBCAD correlations, C-4 to H-1′ and C-8 to H1′. All NMR data obtained for this compound are summarized in Table 2 and raw data provided in Supplementary Figures S8–S12.
Figure 4.
Selected COSY (▬) and gHMBCAD (→) data for compound 3.
Table 2.
1H and 13C NMR data of compound 3 in CD3OD. δ in ppm, J in Hz.
| Position | δH mult (J Hz) | δC mult | HMBC |
|---|---|---|---|
| 1-N | |||
| 2 | 8.07, s | 146.8, CH | |
| 3-NH | 8.29, s | ||
| 4 | 150.2, C | 2, NH-3,1′ | |
| 5 | 125.8, C | 2, NH-3 | |
| 6 | 158.9, C | 2 | |
| 7-N | |||
| 8 | 140.9, CH | 1′ | |
| 9-N | |||
| 1′ | 6.02, d (4.8) | 90.2, CH | 2′,3′ |
| 2′ | 4.73, t (5.1) | 75.2, CH | 1′ |
| 3′ | 4.32, t (4.8) | 73.9, CH | 4′,5′ |
| 4′ | 4.22, q (5.4) | 85.6, CH | 2′,5′ |
| 5′ | 2.93, dd (14.1, 5.5) 2.873, dd (14.1, 5.9) | 37.5, CH2 | 3′,7′ |
| 6′-S | |||
| 7′ | 2.14, s | 16.5, CH3 | 5′ |
2.5. Possible Biosynthesis of Compound 3
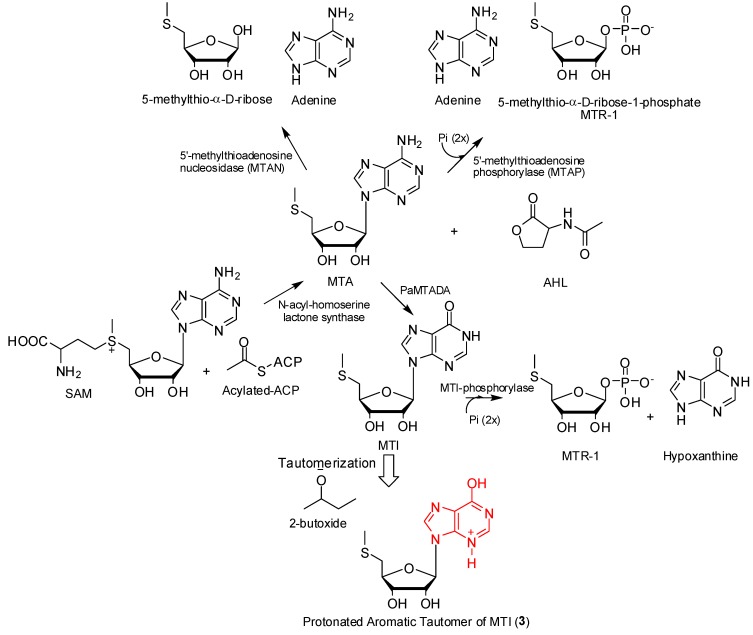
Organisms from the Phyla Bacteria, Eucarya and Archaea all require small molecular entities or signal molecules like N-acyl-homoserine lactone (AHL) for intercellular communication or quorum sensing [13]. In order to facilitate the perpetuation of complex community behaviors like the secretion of proteins, motility, virulence factor production, and plasmid transfer or biofilm maturation, these organisms rely mainly on quorum sensing [14]. AHL is biosynthesized from S-adenosyl methionine (SAM) and acylated-acyl carrier protein (A-ACP) in the presence of LuxI enzymes or N-acyl-homoserine lactone synthase with the production of 5′-methylthioadenosine (MTA) as by-product (Scheme 1) [13]. MTA negatively regulates the reactions leading to the formation of AHL through inhibition of AHL synthase [13]. Hence the efficient removal of MTA is essential in any sort of attempt to create a build-up of AHL. Typical one-step MTA degradation pathways are shown in Scheme 1 for Bacteria, Eucarya and Archaea [15]. Recently, Guan et al. [15] reported an unusual two-step MTA degradation pathway for Pseudomonas aeruginosa (Scheme 1) that involves the deamination aided by a 5′-methylthioadenosine deaminase (PaMTADA) to 5′-methylthioinosine (MTI) followed by N-ribosyl phosphorolysis to hypoxanthine and 5-methylthio-α-d-ribose 1-phosphate (MTR-1) [15]. In the course of the current study, we have isolated and characterized a protonated aromatic tautomer of MTI (compound 3) from Micromonospora sp. K310 in considerable quantities. It is possible that this molecule may be an indicator of the presence of this unique two-step MTA degradation pathway previously reported for Pseudomonas aeruginosa in Micromonospora sp. K310 and hence further studies are being conducted.
Scheme 1.
Possible biosynthetic origin of compound (3) showing 5′-methylthioadenosine (MTA) degradation pathways in Phyla Bacteria, Eucarya and Archaea and Pseudomonas aeruginosa.
2.6. Antibacterial Activity of Butremycin
Butremycin was tested for antibacterial activity against the Gram-positive S. aureus ATCC 25923, the Gram-negative E. coli ATCC 25922 and a panel of clinical isolates of methicillin-resistant S. aureus (MRSA) strains. Butremycin was found to show very weak antibacterial activity against almost all strains while rifampicin was distinctly more active and Table 3 shows the MIC of both compounds.
Table 3.
Antibacterial activity of butremycin against Staphylococcus aureus ATCC25923, Escherichia coli ATCC 25922 and a selection of methicillin-resistant Staphylococcus aureus (MRSA) strains.
| Strain | Source of Isolate | MIC (µg mL−1) | |
|---|---|---|---|
| Butremycin | Rifampicin | ||
| S. aureus ATCC 25923 a | ATCC | 50 | 0.003 |
| E. coli ATCC 25922 a | ATCC | 50 | 0.01 |
| SMRSA 105 | Toe wound | >50 | 0.007 |
| SMRSA 124 | Open wound | >50 | 0.03 |
| SMRSA 116 | Knee abscess | >50 | 0.01–0.03 |
| EMRSA 15 | Urine infection | 50 | 0.001–0.003 |
a Laboratory strains; All clinical isolates were obtained from the NHS Grampian Microbiology Diagnostic Laboratory, Aberdeen Royal infirmary; Abbreviations: EMSRA = epidemic MRSA, SMRA = Scottish MRSA.
3. Experimental Section
3.1. General Experimental Procedures
NMR data were acquired on a Varian VNMRS 600 MHz spectrometer. High resolution mass spectrometric data were obtained using a Thermo Instruments MS system (LTQ XL/LTQ Orbitrap Discovery, Thermo Scientific, Bremen, Germany) coupled to a Thermo Instruments HPLC system (Accela PDA detector, Accela PDA autosampler and Accela pump). The following conditions were used: capillary voltage 45 V, capillary temperature 260 °C, auxillary gas flow rate 10–20 arbitrary units, sheath gas flow rate 40–50 arbitrary units, spray voltage 4.5 kV, mass range 100–2000 amu (maximum resolution 30,000). HPLC separations were carried out using a Phenomenex Luna reverse-phase (C18 250 × 10 mm, L × i.d.) column connected to a Waters 1525 Binary HPLC pump Chromatograph with a 2998 PDA detector, column heater and in-line degasser. Detection was achieved on-line through a scan of wavelengths from 200 to 400 nm. Diaion HP-20 was obtained from Resindion S.R.L., a subsidiary of Mitsubishi Chemical Co., Binasco, Italy. All solvents used throughout were HPLC-grade and purchased from Sigma-Aldrich (Munich, Germany) through a Ghana based agent (Huge Limited, Accra, Ghana). Sephadex LH-20 (25–100 μm) was purchased from GE Healthcare (Little Chalfont, UK). TLC Silica gel plates (60F254nm) were purchased from Merck KGaA (Darmstadt, Germany).
3.2. Identification of Micromonospora sp. K310
The Bacterial Genomic DNA Isolation Kit (Norgen Biotek, Ontario, Canada) was used for genomic DNA extraction from strain K310 according to the manufacturer’s protocol. PCR-mediated amplification of the 16S rDNA gene and purification of the PCR product were carried out following Chun and Goodfellow (1995) [16]. The almost complete (1455 bp) 16S rRNA gene sequence of strain K310 was determined using an ABI PRISM 3730 XL automatic sequencer. The identification of phylogenetic neighbors and calculation of pair-wise 16S rRNA gene sequence similarity were achieved using the EzTaxon-e server [17]. Multiple alignments with sequences from closely related species were performed by using the program CLUSTAL W in the MEGA5 software package [18]. Phylogenetic tree was constructed with the neighbor joining algorithm in MEGA5 [10,18]. Evolutionary distances were calculated using model of Jukes and Cantor (1969) [19]. Topologies of the resultant trees were evaluated by bootstrap analysis [20] based on 1000 re-samplings.
3.3. Isolation of Micromonospora sp. K310 and Preliminary Screening of the Secondary Metabolites
Sediment sample was collected from the Butre River in the Western Region of Ghana (coordinates: 4°49′43.73″N and 1°54′50.84″W). Micromonospora sp. K310 was isolated from the swampy mangrove sediment using the procedure outlined as follows. A small portion of the sediment was transferred into sterile 50 mL tubes, covered and the cap wrapped tightly with cellophane to prevent the entry of water or steam. The tube containing sample was then immersed in a water bath set at temperature 55 °C and heated for 3 h to eliminate non-sporulating bacteria. The sample was then suspended in 10 mL of sterile water under a clean bench and filtered using a previously autoclaved filter paper. The resultant filtrate was serially diluted to 10−1, 10−2 and 10−3 of the original volume. Subsequently, 50 μL of each of the three dilutions were transferred to previously prepared casein starch agar plates (agar, 15 g; soluble starch, 10 g; dibasic potassium phosphate, 2 g; potassium nitrate, 2 g; sodium chloride, 2 g; casein, 0.3 g; magnesium sulfate heptahydrate, 0.05 g; calcium carbonate, 0.02 g; iron sulfate heptahydrate, 0.01 g; sea salt, 33 g and 1 L of tap water) supplemented with nalidixic acid and nystatin at 2.5 μg/L each. Different colonies of Micromonospora sp. were observed after three weeks of incubation at 28 °C. Subsequently, each colony was repeatedly transferred and streaked on different fresh casein starch plates with the aid of autoclaved tooth picks until pure strains were obtained (Supplementary Figure S13). One individual bacteria colony from strain K310 was fermented in 50 mL of starch casein liquid media using previously autoclaved 250 mL Erlenmeyer flasks plugged with non-absorbent cotton wool. The culture was allowed to grow for ten days at 28 °C with continues agitation at 200 rpm. Diaion HP-20 (50 g/L) was added to the culture three days prior to harvesting. The culture was then filtered under suction through a piece of glass wool placed in a Buchner funnel and the filtrate was discarded. The Diaion HP-20 resins were then soaked repeatedly and alternatively in CH3OH and CH2Cl2 and the resultant extracts were combined and dried under vacuum to give the crude extract. The extract was then subjected to HPLC/HRESIMS analysis. Analysis of the resultant data from HPLC/HRESIMS revealed two very well resolved peaks with [M + H]+ and [M + Na]+ ions seen for the two compounds at m/z 299.0810 and 495.2781 respectively (Supplementary Figure S14). These masses were entered as independent queries in the Natural Products Identifier AntiBase 2013 software to check whether these molecules were new or novel. The search came with no relevant hits and the Micromonospora sp. K310 was tagged as one of the interesting strains for further investigation.
3.4. Large Scale Fermentation of K310
A pure colony of Micromonospora sp. K310 was used to inoculate a 250 mL Erlenmeyer flask containing 50 mL of liquid starch casein media as described above but without agar. After seven days of incubation at 28 °C with continuous agitation at 200 rpm, the culture was used to inoculate two 3 L conical flasks containing already autoclaved 1 L starch casein liquid media and plugged with non-absorbent cotton wool. The two 3 L flasks were incubated at 28 °C with continuous agitation at 250 rpm for three weeks. Diaion HP-20 resin (50 g/L) was added under sterile conditions using a serological pipette to both flasks after three weeks and returned back to the incubator set at 28 °C for another one week of continuous agitation at 250 rpm. After four weeks of incubation, the two 1 L cultures were harvested and filtered under pressure using a piece of glass wool placed in a Buchner funnel. The filtrate was discarded and the residue consisting mainly of the Diaion HP-20 resin with adsorbed organics was repeatedly and alternatively extracted with CH3OH and CH2Cl2. The CH3OH and CH2Cl2 extracts were combined and concentrated under reduced pressure to give 960 mg of a light yellow total crude extract (TCE).
3.5. Extraction, Isolation and Purification of Compounds
The TCE was suspended in 150 mL of H2O and extracted three times with the same volume of CH2Cl2. The CH2Cl2 extract was concentrated under reduced pressure and transferred into a vial for subsequent analysis and separation while the water layer was extracted with the same volume of 2-butanol once. The 2-butanol layer was also concentrated under reduced pressure to give a water-butanol fraction WB (456 mg). This extract was subsequently loaded on a gravity column packed with Sephadex LH-20 and eluted with a 50/50 mixture of CH3CN and CH3OH. Seven fractions were collected from the Sephadex LH-20 run and labeled SF1-7. HPLC/HRESIMS showed that SF3 (120 mg) and SF5 (96 mg) contained the compounds of interest. These fractions were therefore subjected to HPLC separation and purification using a Phenomenex Luna C18 column (C18 250 × 10 mm, L × i.d.). Gradients of H2O:CH3CN (100% H2O to 100% CH3CN in 30 min and hold for 20 min) were used as eluent with column flow rates set at 1.5 mL/min to afford butremycin (46.6 mg) and compound 3 (57.0 mg).
Compound 2 (butremycin): Pale yellow crystals; UV (CH3OH/0.1%HCOOH) 227, 325 nm; IR (neat) νmax 3368, 2956, 2924, 2853, 1602, 1479, 1417, 1093, 1029 and 694 cm−1; For 1H, 13C NMR data, see Table 1; HRESIMS (positive mode) m/z 495.2781 [M + H]+; calcd. for C29H39N2O5.
Compound 3: Deep yellow crystals; UV (CH3OH/0.1%HCOOH) 248 nm; IR (neat) νmax 3392, 2926, 1698, 1589, 1548, 1418, 1377, 1344, 1219, 1127, 1093, 1050 cm−1; For 1H, 13C NMR data, see Table 2; HRESIMS (positive mode) m/z 299.0810 [M]+; calcd. for C11H15N4O4S+.
3.6. Antibacterial Activity
The antibacterial activity of butremycin was evaluated against S. aureus ATCC 25923, E. coli ATCC 25922 and a panel of methicillin-resistant S. aureus clinical isolates obtained from the NHS Grampian Microbiology Diagnostic Laboratory using slight modifications of the previously described method [21]. In brief, bacterial strains were grown in Müller-Hinton (MH) broth [22] to early stationary phase and then diluted to an OD620 = 0.1. The assays were performed in a 96-well micro titer plate format. Rifampicin and butremycin were dissolved in DMSO (Sigma, Munich, Germany), and the effect of different dilutions in broth on the growth was assessed after 18 h incubation at 37 °C using a Labsystems iEMS Reader MF plate reader at OD620. The MIC was determined as the lowest concentration showing no growth compared to the MH broth control. DMSO up to 10% was shown to have no antibacterial effect.
4. Conclusions
Butremycin, the (3-hydroxyl) derivative of ikarugamycin was isolated from Micromonospora sp. K310 isolated from a sediment collected from the Butre River which is situated in the Western Region mangroves of Ghana. This represents the first report of a PTM from a Micromonospora sp. The compound, butremycin was found to exhibit weak antibacterial activity against the Gram-positive S. aureus ATCC 25923, the Gram-negative E. coli ATCC 25922 and a panel of clinical isolates of methicillin-resistant S. aureus strains. In addition to this, the new species of Micromonospora was found interestingly to be actively involved in the synthesis of compound 3. This represents the first report of the isolation of a 5′-methylthioinosine derivative from a Micromonospora sp., notwithstanding the fact that recently this compound has been implicated in a two step 5′-methylthioinosine degradative pathway in Pseudomonas aeruginosa that is required for quorum sensing.
Acknowledgments
KK, HD and MJ acknowledge the Royal Society-Africa Award (AA090088). W.H is the recipient of the SULSA Leaders Award.
Supplementary Files
Supplementary Information (PDF, 1373 KB)
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hong K., Gao A.-H., Xie Q.-Y., Gao H., Zhuang L., Lin H.-P., Yu H.-P., Li J., Yao X.-S., Goodfellow M., et al. Actinomycetes for marine drug discovery isolated from mangrove soils and plants in China. Mar. Drugs. 2009;7:24–44. doi: 10.3390/md7010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maskey R.P., Li F.C., Qin S., Fiebig H.H., Laatsch H. Chandrananimycins A–C: Production of novel anticancer antibiotics from a marine Actinomadura sp. isolate M048 by variation of medium composition and growth conditions. J. Antibiot. (Tokyo) 2003;56:622–629. doi: 10.7164/antibiotics.56.622. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez J.C., Fernandez Puentes J.L., Perez Baz J., Canedo L.M. IB-00208, A new cytotoxic polycyclic xanthone produced by a marine-derived actinomadura. II. Isolation, physico-chemical properties and structure determination. J. Antibiot. (Tokyo) 2003;56:318–321. doi: 10.7164/antibiotics.56.318. [DOI] [PubMed] [Google Scholar]
- 4.Riedlinger J., Reicke A., Zahner H., Krismer B., Bull A.T., Maldonado L.A., Ward A.C., Goodfellow M., Bister B., Bischoff D. Abyssomicins, inhibitors of the para-aminobenzoic acid pathway produced by the marine Verrucosispora strain AB-18–032. J. Antibiot. (Tokyo) 2004;57:271–279. doi: 10.7164/antibiotics.57.271. [DOI] [PubMed] [Google Scholar]
- 5.Stritzke K., Schulz L., Laatsch H., Helmke E., Beil W. Novel caprolactones from a marine streptomycete. J. Nat. Prod. 2004;67:395–401. doi: 10.1021/np030321z. [DOI] [PubMed] [Google Scholar]
- 6.Li F., Maskey R.P., Qin S., Sattler I., Fiebig H.H., Maier A., Zeeck A., Laatsch H. Chinikomycins A and B: Isolation, structure elucidation, and biological activity of novel antibiotics from a marine Streptomyces sp. isolate M045. J. Nat. Prod. 2005;68:349–353. doi: 10.1021/np030518r. [DOI] [PubMed] [Google Scholar]
- 7.Charan R.D., Schlingmann G., Janso J., Bernan V., Feng X., Carter G.T. Diazepinomicin, a new antimicrobial alkaloid from marine Micromonospora sp. J. Nat. Prod. 2004;67:1431–1433. doi: 10.1021/np040042r. [DOI] [PubMed] [Google Scholar]
- 8.Li Y., Huffman J., Li Y., Du L., Shen Y. 3-Hydroxylation of the polycyclic tetramate macrolactam in the biosynthesis of antifungal HSAF from lysobacter enzymogenes C3. Med. Chem. Commun. 2012;3:982–986. doi: 10.1039/c2md20026k. [DOI] [Google Scholar]
- 9.Blodgetta J.A.V., Ohb D.-C., Caoa S., Curried C.R., Kolterc R., Clardya J. Common biosynthetic origins for polycyclic tetramate macrolactams from phylogenetically diverse bacteria. Proc. Natl. Acad. Sci. USA. 2010;107:11692–11697. doi: 10.1073/pnas.1001513107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saitou N., Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 11.Kupchan M.S., Britton R.W., Zeigler M.F., Sigel C.W. Bruceantin, a new potent antileukemic simaroubolide from Brucea antidysenterica. J. Org. Chem. 1973;38:178–179. doi: 10.1021/jo00941a049. [DOI] [PubMed] [Google Scholar]
- 12.Mikulaa H., Horkela E., Hansa P., Hametnera C., Fröhlicha J. Structure and tautomerism of tenuazonic acid—A synergetic computational and spectroscopic approach. J. Hazard. Mater. 2013;250–251:308–317. doi: 10.1016/j.jhazmat.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Parsek M.R., Val D.L., Hanzelka B.L., Cronan J.E., Jr., Greenberg E.P. Acyl homoserine-lactone quorum-sensing signal generation. Proc. Natl. Acad. Sci. USA. 1999;96:4360–4365. doi: 10.1073/pnas.96.8.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooley M., Chhabra S.R., Williams P. N-acylhomoserine lactone-mediated quorum sensing: A twist in the tail and a blow for host immunity. Chem. Biol. 2008;15:1141–1147. doi: 10.1016/j.chembiol.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Guan R., Meng-Chiao H., Fröhlich R.F.G., Tyler P.C., Almo S.C., Schramm V.L. Methylthioadenosine deaminase in an alternative quorum sensing pathway in Pseudomonas aeruginosa. Biochemistry. 2012;51:9094–9103. doi: 10.1021/bi301062y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chun J., Goodfellow M. A phylogenetic analysis of the genus Nocardia with 16S rRNA gene sequences. Int. J. Syst. Bacteriol. 1995;45:240–245. doi: 10.1099/00207713-45-2-240. [DOI] [PubMed] [Google Scholar]
- 17.Kim O.-S., Cho Y.-J., Lee K., Yoon S.-H., Kim M., Na H., Park S.-C., Jeon Y.S., Lee J.H., Yi H., et al. Introducing EzTaxon-e: A prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int. J. Syst. Evol. Microbiol. 2012;62:716–721. doi: 10.1099/ijs.0.038075-0. [DOI] [PubMed] [Google Scholar]
- 18.Tamura K., Peterson D., Peterson N., Stecher G., Nei M, Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jukes T.H., Cantor C.R. Evolution of Protein Molecules. In: Munro H.N., editor. Mammalian Protein Metabolism. Volume 3. Academic Press; New York, NY, USA: 1969. pp. 21–132. [Google Scholar]
- 20.Felsenstein J. Confidence limits on phylogeny: An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.2307/2408678. [DOI] [PubMed] [Google Scholar]
- 21.Domenech P., Kobayashi H., LeVier K., Walker G.C., Barry C.E. BacA, an ABC transporter involved in maintenance of chronic murine infections with Mycobacterium tuberculosis. J. Bacteriol. 2009;191:477–485. doi: 10.1128/JB.01132-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Müller H.J., Hinton J. A protein-free medium for primary isolation of the Gonococcus and Meningococcus. Proc. Soc. Exp. Biol. Med. 1941;48:330–333. doi: 10.3181/00379727-48-13311. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information (PDF, 1373 KB)