Abstract
In the present study we investigated the effect of two different exercise protocols on fibre composition and metabolism of two specific muscles of mice: the quadriceps and the gastrocnemius. Mice were run daily on a motorized treadmill, at a velocity corresponding to 60% or 90% of the maximal running velocity. Blood lactate and body weight were measured during exercise training. We found that at the end of training the body weight significantly increased in high-intensity exercise mice compared to the control group (P=0.0268), whereas it decreased in low-intensity exercise mice compared to controls (P=0.30). In contrast, the food intake was greater in both trained mice compared to controls (P < 0.0001 and P < 0.0001 for low-intensity and high-intensity exercise mice, respectively). These effects were accompanied by a progressive reduction in blood lactate levels at the end of training in both the exercised mice compared with controls (P=0.03 and P < 0.0001 for low-intensity and high-intensity exercise mice, respectively); in particular, blood lactate levels after high-intensity exercise were significantly lower than those measured in low-intensity exercise mice (P=0.0044). Immunoblotting analysis demonstrated that high-intensity exercise training produced a significant increase in the expression of mitochondrial enzymes contained within gastrocnemius and quadriceps muscles. These changes were associated with an increase in the amount of slow fibres in both these muscles of high-intensity exercise mice, as revealed by the counts of slow fibres stained with specific antibodies (P < 0.0001 for the gastrocnemius; P=0.0002 for the quadriceps). Our results demonstrate that high-intensity exercise, in addition to metabolic changes consisting of a decrease in blood lactate and body weight, induces an increase in the mitochondrial enzymes and slow fibres in different skeletal muscles of mice, which indicates an exercise-induced increase in the aerobic metabolism.
Keywords: skeletal muscles, training, morphology, electron microscopy
INTRODUCTION
Skeletal muscle, like other biological systems, has an elevated plasticity which is revealed by its capacity to produce a functional response in different conditions. In fact, when a specific pattern of muscle activity is repetitively applied, a series of biochemical and morphological changes occur, which allow a more appropriate functional response to be produced, depending on the specific stimulation [22]. This phenomenon is common in individuals who regularly practise sports. In fact, exercise training aimed at improving performance is always associated with several phenotypic modifications of the skeletal muscles [17]. These changes involve several components of the locomotor system, such as the contractile apparatus and/or the metabolism of the muscles [17], in order to modify muscular force, endurance and contractile capacity.
In mammals skeletal muscles can be generally classified into two major groups, slow-twitch and fast-twitch, based on their intrinsic contractile properties. Slow muscles, such as the soleus and the vastus intermedius, which play a strong antigravity function, predominantly express the slow type I myosin heavy chain (MHC) isoform with a variable percentage of type IIa MHC, the slowest of the fast MHCs [34]. Fast muscles, such as the gastrocnemius-plantaris complex and the vastus lateralis, predominantly express the two fast isoforms, IIx and IIb, in variable percentages, depending on the muscle, the region of the muscle, and the animal species [11]. Although the IIb MHC gene has been identified in the human genome [42], there is no evidence for the expression of the related protein in human muscles. Thus, in all mammals, including humans, the MHC slow isoform is type I and the two MHC fast isoforms, IIa and IIx, are present in humans whereas in rodents the MHC fast isoforms are IIa, IIb and IIx [40].
In rodents, acquisition of the adult profile of MHC gene expression in the skeletal muscles is a process that begins during the latter stages of fetal development and continues in the postnatal life [9]. In fact, at birth both slow and fast muscles are still in an undifferentiated state and during the first 3-4 weeks of neonatal development they rapidly grow and differentiate into their adult MHC phenotype [9]. This process occurs under the control of several factors. In fact, an intact innervation is needed to allow the correct muscle development [2], although environmental conditions seem to play an important role. In this respect, recent studies demonstrated that in rodents slow muscles are dependent on weight-bearing activity for both normal growth and the optimal expression of type I MHC [1].
Another factor which influences the fibre composition of skeletal muscles is exercise training. The type of training influences the presence of the different muscle fibres on the basis of specific motor activity. In fact, several studies have demonstrated the presence of different percentages in slow or fast fibres in specific muscles of individuals who performed endurance training, sprint or recreational activity [21, 25]. In fact, depending on the stimulus, skeletal muscle can increase in size [10], alter the composition in the type of muscle fibres [29], as well as increase enzyme activities [18]. For example, endurance trained individuals have a higher percentage of type I slow fibres (about 65% type I fibres in the gastrocnemius muscle), compared to recreationally active individuals (about 50% type I fibres in the gastrocnemius muscle) [21]. Slow and fast fibres differ considerably in maximum shortening velocity (type I about four to five times slower than type IIx) and power generating capacity [36]. In particular, type IIx fibres show an enzymatic profile involved in anaerobic metabolism [8] such as a high concentration of glycolytic enzymes [31]. In contrast, type I fibres have a high content of oxidative enzymes that favour aerobic metabolism [31]. Therefore, type II fibres, which depend for their energy supply on the anaerobic pathway, are more vulnerable to fatigue, due to fast energy depletion which impairs most cellular activities [16]. On the other hand, type I fibres confer to muscle elevated resistance to fatigue due to the elevated production of chemical energy derived from the enhanced aerobic metabolism [6].
It is well known that different exercise training influences the oxidative potential. In fact, repeated exercise requires an increase in the aerobic metabolism to sustain the prolonged muscle activity [6], whereas a brief burst of muscle exercise needs rapid production of energy obtained by anaerobic metabolism [41]. Therefore in repeated training, sustained by aerobic energy metabolism, an increase in muscle oxidative potential was reported [24, 28].
In the present study we investigated the effect of two different exercise protocols on fibre composition of specific skeletal muscles in mice. In particular, we used two models of prolonged exercise training, consisting of:
brief sequences of intense exercise (running at 90% of the maximal intensity) interspersed with recovery periods;
continuous exercise at moderate activity (running at 60% of maximal intensity).
Both training groups ran for a distance of 1,000 metres. This exercise was repeated 5 day per week, for a total of 8 weeks [4].
The two protocols represent two different endurance training methods which differ regarding the intensity of the activity. In a previous study we found that each experimental schedule modifies differently the levels of blood lactate measured at the end of the training period [4]. Therefore, the aim of the present study was to evaluate whether these changes in the levels of blood lactate might be related to modifications in the energy metabolism developed within the muscle fibres at the end of the training period. Since the two exercise schedules represent two different forms of prolonged, endurance exercise, we expected that an increase in the aerobic metabolism would occur. For this purpose, we analysed by immunoblotting and immunohistochemistry two different skeletal muscles, the gastrocnemius and the quadriceps, in order to evaluate the effect of each exercise schedule on the expression of specific enzymes involved in the oxidative metabolism as well as the slow fibre composition.
MATERIALS AND METHODS
Animals
Ten-week-old male C57BL mice (n=24) (Harlan Laboratories, San Pietro al Natisone, UD, Italy), weighing 25.6 ± 0.1 g, were used. Before the experiment, the animals were housed one per cage in a temperature-controlled room (20 ± 2°C) with a 12/12 h light/dark cycle and were allowed access to standard chow (Harlan Laboratories) and water ad libitum, except one hour before and during the experiment (see below). All experimental procedures were performed in accordance with the ethical committee of the University of Pisa and the Helsinki Declaration for the use and care of laboratory animals.
Experimental set-up
This experimental work used a motorized treadmill (Columbus Instruments, Columbus, OH, USA) to perform exercise training. The treadmill consisted of 6 single lines, and each mouse could individually run in a single line.
After a period of adaptation (1 week) to the motorized treadmill, mice underwent an incremental exercise test for five consecutive days (from Monday to Friday), modified from Ferreira et al. [15], in order to establish for each mouse the highest running velocity (maximal velocity). In detail, we started from a basal velocity of 6 m · min-1 for 3 min at 0% grade, which was progressively increased by 3 m · min-1 every 3 min, until mice were unable to maintain the required running intensity for 3 min [15]. At the end of this period, for each mouse we were able to determine the maximal velocity, as the highest velocity which was maintained for at least 3 min.
Considering all experimental mice, the values of maximal velocity ranged from 27 to 39 m · min-1. These values were used to divide the mice into three groups, each containing mice with similar values of maximal velocity (Table A). Mice belonging to each group were assigned to two different types of exercise training or used as controls, as reported below.
TABLE A.
VALUES OF MAXIMAL VELOCITY OBTAINED DURING THE INCREMENTAL TEST USED TO CREATE THE EXPERIMENTAL GROUPS
| CON | LOW | HIT |
|---|---|---|
| 30 | 30 | 39 |
| 27 | 27 | 36 |
| 30 | 30 | 36 |
| 27 | 30 | 39 |
| 36 | 30 | 36 |
| 36 | 27 | 39 |
| 30 | 27 | 39 |
| 36 | 27 | 36 |
Note: CON -Unexercised (sedentary) mice, LOW -Continuous submaximal running, HIT -High-intensity interval running
The two different training types were selected on the basis of different pattern of exercise:
a sequence of short periods (2 min) of intense effort running at 90% of the maximal running velocity followed by 1 min recovery (HIT);
continuous submaximal running at 60% of the maximal running velocity (LOW).
In particular, mice with the highest maximal velocity were considered HIT, mice with lower maximal velocity were considered LOW, whereas mice with more heterogeneous values of maximal velocity were considered CON (controls).
Therefore, we obtained three experimental groups:
High-intensity interval running (HIT) (n=8)
Continuous submaximal running (LOW) (n=8)
Unexercised (sedentary) mice (CON) (n=8).
To avoid potential bias derived from significant differences in the maximal velocity between mice belonging to the same experimental group, we created experimental groups composed of animals with a similar range of performance (Table A).
Since the experimental groups were composed of 8 animals, we could divide each group into 2 subgroups, each composed of 4 mice, with the same maximal velocity (Table A). Mice belonging to each subgroup could run in the same session. Therefore, two lines of the treadmill remained empty in all running sessions.
The distance to run was determined based on the incremental test, by considering the mean distance covered during 45–60 min of continuous moderate running at 15 m · min-1. The mean distance was fixed at 1,000 m, thus keeping constant the total workload in both types of exercise training.
Exercise training
The exercise training started at 10 a.m. each weekday (from Monday to Friday) for 8 weeks and for a total of 40 sessions.
We carried out the experiment starting always at the same time of the day for an overall training time of 3 hours (10 a.m. – 1 p.m.). This time interval allowed us to avoid diurnal effects on training performance [13], which were further minimized by varying for each group the starting time of training.
In each session, running mice (namely, LOW and HIT mice) were run for a distance of 1,000 metres, and the session ended when the whole distance was covered. Sedentary mice were placed for 30 minutes on the turned off treadmill.
For all animals food was stopped one hour before physical exercise.
To evaluate correlations between food consumption and weight increase, food intake was measured every other day, whereas mouse weight was measured once a week.
Blood lactate concentration
To evaluate the effect of exercise training on running performance before the 1st session and at the end of the 1st, 20th and 40th session, the blood lactate concentrations were measured. Capillary blood samples were taken at steady state 2 min before the 1st session of training and 3 min after the 1st, 20th and 40th session from the tail vein and transferred to 0.2 mL vials containing heparin. The samples were singly dropped on Bm-Lactate strips (Roche Diagnostics GmbH, Mannheim, Germany) and the blood lactate concentration was measured using an Accutrend/Accusport Lactate Portable Analyzer (Roche Diagnostics GmbH), according to Bishop [5]. The time frames for blood collection were kept constant for all mice.
Tissue preparation and immunohistochemical analysis
Mice were sacrificed by deep anaesthesia using chloral hydrate (450 mg/kg); for each mouse, gastrocnemius and quadriceps muscles were rapidly dissected out and immersed in Carnoy's fixative solution (60% ethanol absolute, 30% chloroform, 10% acetic acid) for 24 h and then transferred to 70% ethylic alcohol overnight at 4°C. Samples were dehydrated in increasing alcohol solutions (80%, 96% and 100% ethylic alcohol), immersed in xylene for 4 h, and finally embedded in paraffin. Tissue blocks were sectioned using a microtome in order to obtain 7 µm thick slices. Such a sample was completely cut and consecutive serial sections were collected in strict anatomical order on SuperFrost plus™ slides (Fisher Scientific SAS, Illkirch Cedex, France).
In each muscle, immunohistochemistry was done every 10th section for a total of about 250-300 sections.
Sections assigned to immunohistochemistry were dried at 37°C for about 12 h and immunolabelled with mouse primary antibody (AbI) against slow skeletal myosin (1:1000, Sigma Aldrich, St. Louis, MO USA). Briefly, de-paraffined and re-hydrated sections were immersed in 0.1% Triton X-100 (Sigma Aldrich) in TBS for 15 min. Then, sections were quenched for endogenous peroxidase activity (3% H2O2 for 10 min), incubated in a blocking solution of 10% normal goat serum (Vector Laboratories, Burlingame, CA USA) and TBS, for 1 h at room temperature and finally immersed in the above described antibody solutions also containing 2% normal goat serum in TBS overnight at 4°C.
After washing out, slices were incubated with secondary anti-mouse biotinylated antibody (Vector Laboratories) diluted 1:200 in TBS for 1 h, followed by ABC kit (Vector Laboratories) and diaminobenzidine (DAB, Vector Laboratories). Finally, slices were dehydrated in increasing alcohol solutions, mounted with DPX (Sigma Aldrich) and observed at light microscope (Nikon Eclipse 80i, Japan) coupled to a colour video camera equipped with Nis Element Software (Nikon).
These sections were used to count the number of the slow fibres in both gastrocnemius and quadriceps muscles. Counts were carried out at 4x magnification by two different observers, unaware of the experimental groups.
In each mouse, contralateral muscles were dissected and subjected to immunoblotting (see below).
Transmission electron microscopy
Two mice for every group were anaesthetized with an intraperitoneal injection of chloral hydrate and then thoracotomized. Subsequently they were perfused with a fixing solution (2% paraformaldehyde and 0.1% glutaraldehyde in phosphate buffer solution) through the left ventricle. Both quadriceps and gastrocnemius muscles of each animal were dissected, gently stretched for 10 seconds and immersed in the same fixative solution for 3 h. Samples were then post-fixed in buffered 1% osmium tetroxide for 1.30 h, dehydrated in ethanol and embedded in Epon-araldite. To be sure to analyse the same area of each muscle in each examination group, we cut little blocks of about 5 mm3 in the central part and in the extremities of the muscles. These blocks were cut and non-serial ultrathin sections (leaving a space of a few micrometers from each other) were collected on grids. We obtained 20 grids for each sample. Since biological structures vary in appearance depending upon position and orientation, we used blocks in which the fibres of each sample were found in longitudinal orientation. This allowed us to observe the structures of interest always in the same placement. The grids were stained with uranyl acetate and lead citrate and examined with the Jeol Jem 100 SX transmission electron microscope. Contralateral muscles were immersed in 4% paraformaldehyde in PBS for 24 h and then subjected to the paraffin embedding procedure for light microscopy, as already described (see above).
Mitochondrial isolation and western blot assay
Isolation of the intact mitochondria from the muscles (gastrocnemius and quadriceps) was performed using a Mitochondria Isolation Kit purchased from MitoSciences (Eugene, Oregon, USA). Immediately after dissection, samples were washed twice with 1.5 ml of wash buffer solution, and placed in a pre-chilled Dounce homogenizer. The rupture of tissues was performed by 30-40 Dounce strokes with 2.0 ml of isolation buffer.
Homogenates were centrifuged at 1,000 g for 10 min at 4°C and the supernatant was subsequently centrifuged at 12,000 g for 15 min at 4°C.
After removing the supernatant, the pellet was re-suspended in 1 ml of isolation buffer supplemented with 10 µl of protease inhibitor cocktail. Two consecutive centrifugations at 12,000 g for 15 min at 4°C allowed us to obtain the mitochondrial fraction from each muscle sample. The pellet was then re-suspended in 500 µl of the isolation buffer supplemented with protease inhibitor cocktail.
An aliquot of supernatant obtained from the isolated mitochondria was used to determine the protein concentration by a protein assay kit (Sigma Aldrich). Samples containing 5 µg of total protein for quadriceps and gastrocnemius were solubilized and electrophoresed on 12% sodium dodecyl sulfate-polyacrylamide gel. Following electrophoresis, the proteins were transferred to PVDF membrane (GE Healthcare, Milan, Italy). The membrane was immersed in blocking solution (5% non-fat dried milk in PBS containing 0.05% Tween-20) overnight, at 4°C, and incubated with the primary antibody cocktail anti-Oxidative Phosphorylation Rodent complex (1:250) (MitoSciences) for 1.30 h at 4°C. The blot was probed with horseradish peroxidase-labelled anti-mouse secondary antibody (1:500) and the bands were visualized with enhanced chemiluminescence reagents (GE Healthcare). Films of Western blots were scanned and the optical density of the bands was measured using NIH Image 1.61 software. Each result was confirmed in triplicate.
Statistical analysis
Data related to food intake, body weight and blood lactate levels from each mouse were used to obtain the mean value ± SEM for each group. Concerning the morphological analysis, the counts performed from each observer for each mouse were used to obtain the mean values ± SEM of each group.
Finally, values related to the densitometric analysis represent the mean ± SEM of three independent measurements.
Comparisons among groups were made by using a one-way ANOVA combined with Scheffè's post hoc test. Comparison between blood lactate levels before and after training within the same group was made using Student's t-test for paired data.
The null hypothesis was rejected for P < 0.05.
All statistical analyses were performed between independent groups.
RESULTS
Body weight and food intake
Body weight did not differ among the groups before the beginning of the exercise protocol. At the end of training body weight gain was greater in the HIT group (18.2 ± 1.4%) compared to LOW (8.7 ± 0.6%, P < 0.0001) and CON (12.7 ± 0.5%, P < 0.005) groups, and it was lower in the LOW group compared to the CON group (P < 0.05) (see Table 1). In contrast, the food intake of both HIT and LOW mice was greater compared to the CON group (P < 0.0001). In order to compare the body weight increase in relation to the food intake, Table 2 shows that LOW mice, despite consumption of significantly more food compared to CON mice, underwent a smaller weight increase; in contrast, the weight increase of HIT mice, in accordance with a food consumption level significantly more elevated compared to both LOW (P < 0.0001) and CON groups, was significantly greater (see Table 2).
TABLE 1.
BODY WEIGHT EVALUATION PERFORMED AT T0 AND T40 AND BODY WEIGHT GAIN AT T40 FOR ALL EXPERIMENTAL GROUPS (CON, LOW AND HIT)
| T0 (gr) | T40 (gr) | Increase (%) | |
|---|---|---|---|
| CON | 25.5±0.3 | 28.8±0.4 | 12.7±0.5 |
| LOW | 25.8±0,3 | 28.0±0,3 | 8.7±0.6* |
| HIT | 25.5±0.3 | 30.1±0.2*,# | 18.2±1.4*,# |
Note:
P < 0.05 vs CON
P < 0.001 vs LOW
TABLE 2.
CORRELATIONS BETWEEN FOOD CONSUMPTION AND WEIGHT INCREASE EVALUATED AT THE END OF THE EXERCISE PROTOCOL (T40) FOR EACH GROUP
| LOW | HIT | |
|---|---|---|
| Food intake (%)* | +10.9 † | +26.2 † |
| Weight increase (%)* | -30.8 † | +42.2 † |
| Weight increase compared to food intake (%)* | -37.6 † | +12.6 † |
Note: Data are expressed as percentage compared to CON.
p < 0.0001
Blood lactate
Before the first training session (T0) no difference in blood lactate concentration between the groups was found. At the end of the first training session (T1) blood lactate concentrations measured both in HIT (5.10±0.06, P < 0.0001) and LOW (4.93±0.13, P < 0.0001) were significantly higher compared with CON (4.09±0.12). These levels became similar between groups at the end of the twentieth training session (T20), whereas after the last training session (T40), blood lactate concentrations in both LOW and HIT (3.51±0.09, 2.95±0.12, respectively) were decreased. In particular, at T40 blood lactate measured in LOW and HIT mice appeared significantly lower compared with CON (P < 0.005, P < 0.0001, respectively) and also with the levels measured at T1 in the same groups, as expected from effective training. Moreover, blood lactate levels of HIT mice at the end of the training (T40) were significantly lower than those measured in LOW mice (P < 0.005) (see Table 3).
TABLE 3.
BLOOD LACTATE CONCENTRATIONS MEASURED BEFORE THE 1ST SESSION AND AT THE END OF THE 1ST, 20TH AND 40TH TRAINING SESSION
| T0 (mmol · L-1) | T1 (mmol · L-1) | T20 (mmol · L-1) | T40 (mmol · L-1) | |
|---|---|---|---|---|
| CON | 4.075±0.11 | 4.09±0.12 | 4.06±0.10 | 4.10±0.11 |
| LOW | 4.03±0.11 | 4.93±0.13* | 3.73±0.08 | 3.51±0.09*,§ |
| HIT | 4.06±0.09 | 5.10±0.06* | 4.09±0.13 | 2.95±0.12*,#,§ |
Note:
P < 0.0001 vs CON
P < 0.005 vs LOW
P < 0.0001 vs the same group at T1 and T20
Morphological analysis and immunohistochemistry
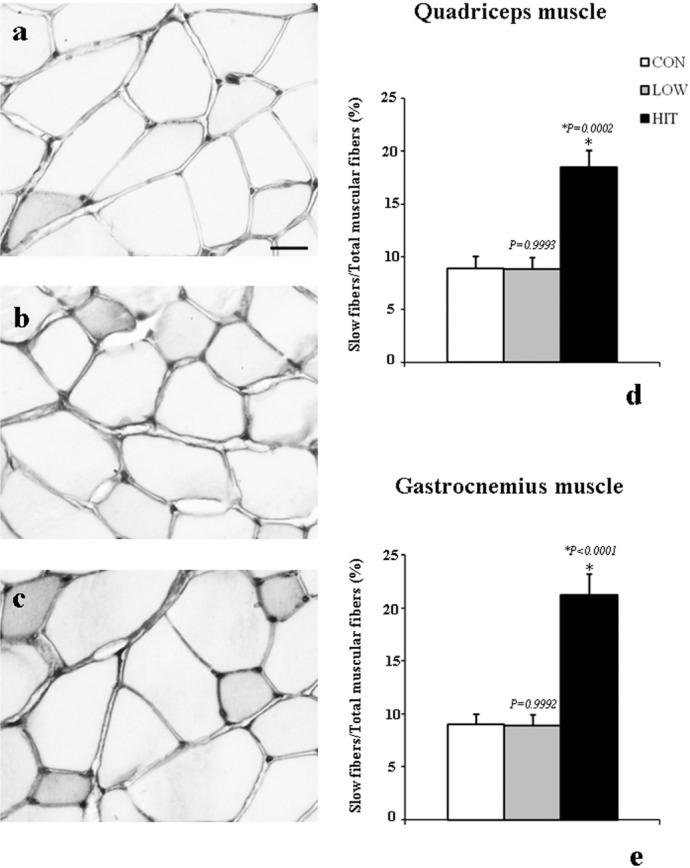
Immunohistochemistry shows that low-intensity training did not produce any change in the positivity for myosin skeletal slow protein within the quadriceps muscle compared with CON mice (Figs. 1b, 1a, respectively). In contrast, we found increased immunopositivity for this protein in the quadriceps muscle of HIT mice (Fig. 1c). Similar results were obtained for the gastrocnemius muscle (data not shown).
FIG. 1.
IMMUNOPOSITIVITY FOR SLOW SKELETAL MYOSIN IN THE QUADRICEPS AND GASTROCNEMIUS MUSCLES OF MOUSE AFTER EXERCISE TRAINING
Note: Representative immunoperoxidase stains for slow skeletal myosin in the quadriceps muscle from CON (a), LOW (b), and HIT mice (c) are shown. The immunopositivity for this protein is increased in mice after high-intensity exercise training (c), as confirmed by the histograms showing the percentage of slow fibres among total muscle fibres in the quadriceps muscle (d). The same result was observed in the gastrocnemius muscle (e).
*P < 0.001 versus CON and LOW
Scale bar= 33.48 µm
These qualitative results were confirmed by the count of the number of slow fibres (namely, those fibres immunopositive for the myosin skeletal slow protein) both in quadriceps and gastrocnemius muscles of all the experimental groups. In the CON group, the percentage of slow fibres counted in the quadriceps muscle was 8.88 ± 1.20%. The percentage of slow fibres counted at the end of the experiment in the LOW group was not different from CON (8.81 ± 1.13%), whereas in the HIT group it was significantly higher compared to both CON and LOW groups (18.46 ± 1.60%; P < 0.0005) (Fig. 1d).
A similar trend was found when the slow fibres of the gastrocnemius were counted. In fact, in the gastrocnemius of the HIT group the percentage of slow fibres (21.19 ± 2.03%) was found to be significantly higher compared to CON (8.98 ± 1.03%; P < 0.0001) and LOW (8.90 ± 1.06%; P < 0.0001) (Fig. 1e).
In electron microscopy no ultrastructural alterations were detected in LOW and HIT groups compared to CON mice. In fact, gastrocnemius muscle (Fig. 2) and quadriceps muscle (data not shown) belonging to all groups presented well-formed myofibres in which it is possible to recognize the sarcomere organization in dark and light bands and the typical morphology of the nucleus (Fig. 2a, b, c).
FIG. 2.
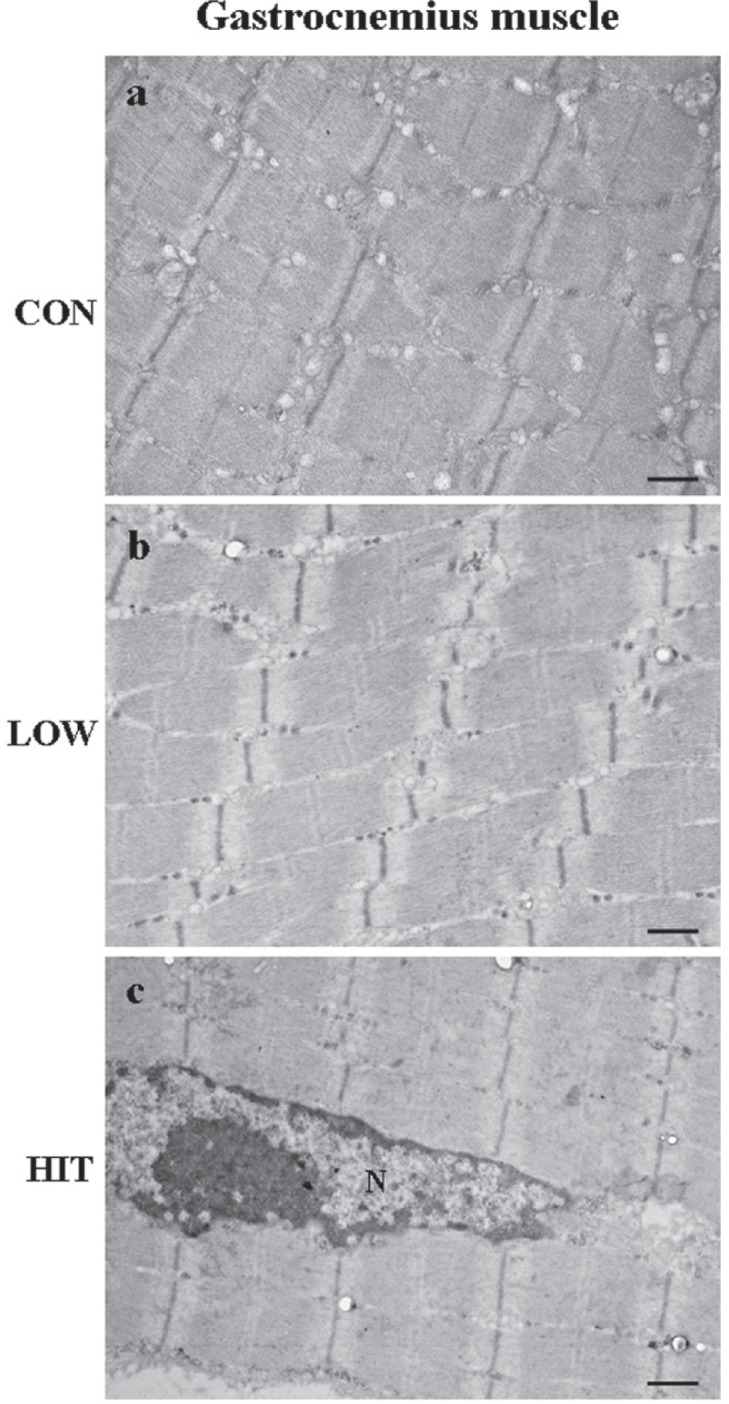
ULTRASTRUCTURAL ANALYSIS OF GASTROCNEMIUS MUSCLE OF MOUSE AFTER EXERCISE TRAINING
Note: Representative micrographs of gastrocnemius muscle from CON (a), LOW (b) and HIT mice (c). No ultrastructural alterations were detected in any of the mice groups. The myofibres are well formed, with repeating sections of dark and light bands.
In Fig. 2c the nucleus (N) of a myofibre.
Scale bars: a, b, c=0.62 m
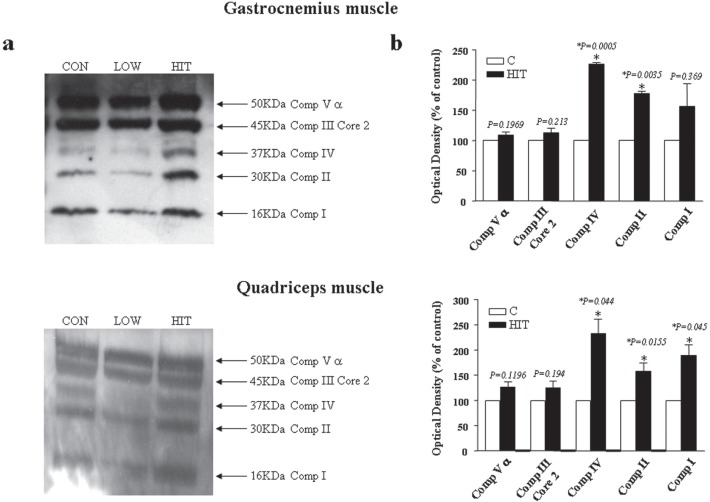
Western blot on mitochondrial complex enzymes
Immunoblotting analysis showed a significant increase in the expression of mitochondrial complex enzymes in HIT mice compared with CON mice (Fig. 3a), both in gastrocnemius and quadriceps muscle. In particular, this increase is significant for the enzymes corresponding to Complex IV, II and I of the mitochondrial chain, as presented in the graphs (Fig. 3b). In contrast, despite the prolonged exercise, no change in the expression of mitochondrial enzymes was found in LOW mice (Fig. 3a, b).
FIG. 3.
EFFECTS OF EXERCISE TRAINING ON MITOCHONDRIAL COMPLEX ENZYME EXPRESSION
Western blotting analysis for mitochondrial chain proteins in the gastrocnemius and quadriceps muscles of mice after exercise training (a). The expression of the enzymes corresponding to Complex IV, II and I of the mitochondrial chain is significantly increased in HIT mice compared with CON mice in both muscles. No difference was observed between CON and LOW mice. (b) Densitometric analysis of protein expression for HIT mice compared to CON is expressed in arbitrary units. Data represent the mean ± SEM of three independent experiments. *P < 0.001 VERSUS CON
DISCUSSION
The present study demonstrates that exercise training is a powerful inducer of muscle plasticity, by producing specific changes in muscle fibre composition.
We show that prolonged high-intensity exercise training produces specific biochemical and morphological changes in the quadriceps and the gastrocnemius muscles of mice, consisting of changes in the expression of specific mitochondrial enzymes and in the amount of slow-twitch muscle fibres. These effects are accompanied by progressive reduction in blood lactate levels during training and increase in body weight at the end of the training period.
In contrast, a different type of exercise training, consisting of continuous activity at moderate intensity, is unable to modify either mitochondrial enzyme expression or muscle fibre composition in the quadriceps and gastrocnemius of trained mice. In these mice, lactate levels appear slightly decreased at the end of the training, whereas body weight is unchanged.
The protocol of prolonged, intermittent, high-intensity exercise training was selected in order to produce strong bursts of anaerobic exercise interspersed with short resting periods, whereas prolonged, continuous activity at moderate intensity is mainly sustained by aerobic effort [6, 41]. In this study HIT mice show a significant decrease in blood levels of lactate at the end of the whole training period. This effect suggests the occurrence of a metabolic “switch”, leading the high-intensity trained skeletal muscle to reduce the anaerobic consumption of glucose and to increase the aerobic metabolism. In contrast, LOW mice, trained at moderate activity, show a mild decrease in the blood lactate at the end of the training period, suggesting only a slight increase in the rate of aerobic muscle activity.
On the other hand, comparing the increase in body weight between the two trained groups after the training period appears to produce contradictory results. In fact, the lack of physiological increase in body weight found in LOW mice at the end of training is consistent with the hypothesis that prolonged, continuous but moderate exercise induces aerobic energy consumption which leads to an increased basic metabolism [6]. For instance, aerobic exercise stimulates protein turnover by increasing protein breakdown and synthesis during the recovery in human skeletal muscle, thus increasing the energy demand [7]. These general metabolic effects produce a net decrease in body mass [20].
To solve this point, i.e. whether HIT or LOW mice undergo an effective increase in aerobic metabolism, we investigated the effect of each training protocol on slow-twitch muscle fibre composition as well as the content of mitochondrial enzymes in two skeletal muscles, the gastrocnemius and the quadriceps.
We found that in HIT mice both the quadriceps and the gastrocnemius show increased immunopositivity for the slow type I MHC protein isoform. In particular, the number of slow type I immunopositive fibres shows a more than two-fold increase in the quadriceps and gastrocnemius from HIT mice compared to CON. In contrast, no change in immunopositivity for the slow type I MHC fibres was found in either muscles from LOW mice compared to CON.
Slow and fast fibres of skeletal muscles are characterized by a different modality of energy consumption, prevalently anaerobic in fast fibres and aerobic in slow fibres [8, 31]. In fact, slow-twitch type I muscle fibres are rich in mitochondria and possess high oxidative capacity, which makes them resistant to fatigue, while fast-twitch type II muscle fibres are characterized by a prevalently anaerobic metabolism and low resistance to fatigue [41].
On the other hand, our data suggest that brief sequences of highly intense exercise, instead of enhancing “anaerobic-linked” fast fibres, induce an increase in the “aerobic-linked” slow fibres within specific skeletal muscles. To substantiate this hypothesis, we evaluated whether changes in the fibre composition are accompanied by specific modulation of mitochondrial enzymes. Western blot and related densitometric analysis in isolated mitochondria from HIT mice show increased expression of Complex IV and II of the mitochondrial respiratory chain in the gastrocnemius and Complex IV, II and I in the quadriceps, whereas no change in these mitochondrial enzymes was found in LOW mice.
These results indicate that the increase in the slow fibres observed in HIT mice at the end of the training is effectively associated with an increase in the aerobic metabolism, as suggested by the increase in the mitochondrial enzymes and the decrease in the blood lactate levels.
We are not able to establish the origin of these slow type I-positive fibres. It remains obscure whether these changes in muscle fibre composition are due to generation of new fibres from an undifferentiated cellular pool or induction of a new pattern of gene expression leading to a fast-to-slow transition of muscle fibres. Transitions between MHC isoforms are described in a sequential, reversible order from type I type II A type IIX and vice versa [32, 38].
Recent studies showed that transcriptional events underlie muscular plasticity after exercise, supporting the concept that the structural and functional changes induced by training on skeletal muscle might reflect the transient modulation in gene expression produced by the exercise [17]. In fact, highly repetitive, low-load exercise induces differentiation of muscle fibres towards a fatigue-resistance phenotype to sustain a high number of slow contractions, and increased levels of mRNAs encoding mitochondrial proteins are produced by endurance exercise training [33]. Nonetheless, occurrence of hybrid fibres in both developing and adult skeletal muscles, where a polymorphism of MHC gene expression leads each single myofibre to co-express more than one MHC isoform in various combinations [37], suggests that exogenous stimuli, such as exercise, might act through modulation of the expression of a specific MHC gene in hybrid fibres.
In order to translate our results to the human condition, it is important to note that mice have a muscle mitochondrial density higher than humans, and consequently, an elevated basal O2max, which is comparable to trained humans [23]. However, in highly trained humans, as in mice, low intensity continuous training is unable to further improve the performance through increased oxygen consumption, which is already elevated. Increasing the exercise intensity might be effective in improving aerobic fitness [26].
Various studies have reported an increase in citrate synthase maximal activity, as well as peak oxygen uptake (O2), after only 2 weeks of daily sprint training [40], indicating that improvements in aerobic energy metabolism can be rapidly stimulated by brief bouts of very intense exercise [35].
In line with this, endurance athletes have higher oxygen uptake during a repeated sprint test, indicating that sprint generates a greater contribution of aerobic metabolism to energy supply [19].
In our experimental protocol, we found that in HIT mice blood lactate is not significantly reduced at the intermediate time period (T20), and it is increased after the first exercise session (T1) compared to CON, suggesting that repeated bouts of high-intensity activity require a progressively reduced amount of anaerobically derived energy, and, if the training is long lasting (in our protocol, at least 8 weeks), a significant increase in the aerobic metabolism occurs at the end of the training period.
Therefore, our study indicates that two variables are equally fundamental to obtain an improvement in the aerobic performance: the intensity and the time interval of the training. In fact, only by using a training protocol with long-lasting and repeated high-intensity exercise did we obtain a significant increase in the aerobic metabolism.
Our training protocol, consisting of long-lasting, repeated brief periods of high-intensity activity, appears to be able to increase the aerobic metabolism to an extent which is higher than that produced by prolonged, continuous, moderate activity.
This leads us to speculate that insertion of brief bursts of highintensity activity within a typical aerobic exercise might improve the performance due to the high efficacy in stimulating aerobic metabolism.
Finally, the identification of the molecular mechanisms responsible for this muscular plasticity is a stimulating challenge to understand the compensatory processes induced by exercise in normal conditions as well as the exercise-induced motor recovery in several movement disorders.
CONCLUSIONS
Our study demonstrates that prolonged, repeated high-intensity exercise (HIT) produces biochemical and morphological changes within specific peripheral muscles of mice. We previously reported that the very same exercise training produces morphological changes in the adrenal gland of mice, which are suggestive of functional modifications of the adrenal activity [4]. In this paper we demonstrated that HIT mice show increased expression of specific mitochondrial enzymes involved in the respiratory chain and an increase in the number of slow-twitch type I-immunopositive muscle fibres. This occurs concomitantly with decreased production of blood lactate. We demonstrated that such plastic modifications are dependent on the specific training protocol, being absent in LOW mice that underwent prolonged, continuous moderate activity. Our results suggest that our protocol of prolonged training characterized by brief periods of high-intensity exercise might be promising in further improving aerobic performance of highly trained subjects.
Authors’ declaration
There are no conflicts of interest for any author.
REFERENCES
- 1.Adams G.R, Haddad F, McCue S.A, Bodell P.W, Zeng M, Qin L, Qin A.X, Baldwin K.M. Effects of spaceflight and thyroid deficiency on rat hindlimb development. II. Expression of MHC isoforms. J. Appl. Physiol. 2000;88:904–916. doi: 10.1152/jappl.2000.88.3.904. [DOI] [PubMed] [Google Scholar]
- 2.Adams G.R, McCue S.A, Zeng M, Baldwin K.M. Time course of myosin heavy chain transitions in neonatal rats: importance of innervation and thyroid state. Am. J. Physiol. 1999;276:R954–961. doi: 10.1152/ajpregu.1999.276.4.r954. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin K.M, Haddad F. Effects of different activity and inactivity paradigms on myosin heavy chain gene expression in striated muscle. J. Appl. Physiol. 2001;90:345–357. doi: 10.1152/jappl.2001.90.1.345. [DOI] [PubMed] [Google Scholar]
- 4.Bartalucci A, Ferrucci M, Fulceri F, Lazzeri G, Lenzi P, Toti L, Serpiello F.R, La Torre A, Gesi M. High-intensity exercise training produces morphological and biochemical changes in adrenal gland of mice. Histol. Histopathol. 2012;27:753–769. doi: 10.14670/HH-27.753. [DOI] [PubMed] [Google Scholar]
- 5.Bishop D. Evaluation of the Accusport Lactate Analyzer. Int. J. Sports Med. 2001;22:525–530. doi: 10.1055/s-2001-17611. [DOI] [PubMed] [Google Scholar]
- 6.Bogdanis G.C, Nevill M.E, Boobis L.H, Lakomy H.K. Contribution of phosphocreatine and aerobic metabolism to energy supply during repeated sprint exercise. J. Appl. Physiol. 1996;80:876–884. doi: 10.1152/jappl.1996.80.3.876. [DOI] [PubMed] [Google Scholar]
- 7.Carraro F, Stuart C.A, Hartl W.H, Rosenblatt J, Wolfe R.R. Effect of exercise and recovery on muscle protein synthesis in human subjects. Am. J. Physiol. 1990;259:E470–476. doi: 10.1152/ajpendo.1990.259.4.E470. [DOI] [PubMed] [Google Scholar]
- 8.Casey A, Constantin-Teodosiu D, Howell S, Hultman E, Green-haff P.L. Metabolic response of typeI and II muscle fibres during repeated bouts of maximal exercise in humans. Am. J. Physiol. 1996;271:E38–E43. doi: 10.1152/ajpendo.1996.271.1.E38. [DOI] [PubMed] [Google Scholar]
- 9.D'Albis A, Couteaux R, Janmot C, Roulet A. Specific programs of myosin expression in the postnatal development of rat muscles. Eur. J. Biochem. 1989;183:583–590. doi: 10.1111/j.1432-1033.1989.tb21087.x. [DOI] [PubMed] [Google Scholar]
- 10.D'Antona G, Lanfranconi F, Pellegrino M.A, Brocca L, Adami R, Rossi R, Moro G, Miotti D, Canepari M, Bottinelli R. Skeletalmusclehypertrophyandstructureandfunction of skeletalmusclefibresinmale bodybuilders. J. Physiol. (Lond.) 2006;570:611–627. doi: 10.1113/jphysiol.2005.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demirel H.A, Powers S.K, Naito H, Hughes M, Coombes J.S. Exerciseinduced alterations in skeletal muscle myosin heavy chain phenotype: dose-response relationship. J. Appl. Physiol. 1999;86:1002–1008. doi: 10.1152/jappl.1999.86.3.1002. [DOI] [PubMed] [Google Scholar]
- 12.De Palo E.F, Gatti R, Cappellin E, Schiraldi C, De Palo C.B, Spinella P. Plasma lactate, GH and GH-binding protein levels in exercise following BCAA supplementation in athletes. Amino Acids. 2001;20:1–11. doi: 10.1007/s007260170061. [DOI] [PubMed] [Google Scholar]
- 13.Djawdan M.T, Garland T., Jr Maximal running speeds of bipedal and quadrupedal rodents. J. Mammal. 1988;69:765–772. [Google Scholar]
- 14.Dumke C.L, Rhodes J.S, Garland T, Jr, Maslowski E, Swallow J.G, Wetter A.C, Cartee G.D.J. Genetic selection of mice for high voluntary wheel running: effect on skeletal muscle glucose uptake. J. Appl. Physiol. 2001;91:1289–1297. doi: 10.1152/jappl.2001.91.3.1289. [DOI] [PubMed] [Google Scholar]
- 15.Ferreira J.C.B, Rolim N.P.L, Bartholomeu J.B, Gobatto C.A, Kokubun E, Brum P.C. Maximal lactate steady state in running mice: effect of exercise training. Clin. Exp. Pharmacol. 2007;34:760–765. doi: 10.1111/j.1440-1681.2007.04635.x. [DOI] [PubMed] [Google Scholar]
- 16.Fitts R.H. The cross-bridge cycle and skeletal muscle fatigue. J. Appl. Physiol. 2008;104:551–558. doi: 10.1152/japplphysiol.01200.2007. [DOI] [PubMed] [Google Scholar]
- 17.Flück M, Hoppeler H. Molecular basis of skeletal muscle plasticity--from gene to form and function. Rev. Physiol. Biochem. Pharmacol. 2003;146:159–216. doi: 10.1007/s10254-002-0004-7. [DOI] [PubMed] [Google Scholar]
- 18.Green H.J, Pette D. Early metabolicadaptationsofrab-bit fast-twitchmuscletochronic low-frequencystimulation. Eur. J. Appl. Physiol. 1997;75:418–424. doi: 10.1007/s004210050182. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton A.L, Nevill M.E, Brooks S, Williams C. Physiological responses to maximal intermittent exercise: differences between endurance-trained runners and games players. J Sports Sci. 1991;9(Winter):371–382. doi: 10.1080/02640419108729897. [DOI] [PubMed] [Google Scholar]
- 20.Hansen D, Dendale P, Berger J, van Loon L.J.C, Meeusen R. The Effects of Exercise Training on Fat-Mass Loss in Obese Patients During Energy Intake Restriction. Sports Med. 2007;37:31–46. doi: 10.2165/00007256-200737010-00003. [DOI] [PubMed] [Google Scholar]
- 21.Harber M, Trappe S. Single muscle fiber contractile properties of young competitive distance runners. J. Appl. Physiol. 2008;105:629–636. doi: 10.1152/japplphysiol.00995.2007. [DOI] [PubMed] [Google Scholar]
- 22.Harridge SD. Plasticity of human skeletal muscle: gene expression to in vivo function. Exp. Physiol. 2007;92:783–797. doi: 10.1113/expphysiol.2006.036525. [DOI] [PubMed] [Google Scholar]
- 23.Hoppeler H, Weibel E.R. Structural and functional limits for oxygen supply to muscle. Acta Physiol. Scand. 2000;168:445–456. doi: 10.1046/j.1365-201x.2000.00696.x. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs I, Esbjornsson M, Sylven C, Holm I, Jansson E. Sprint training effects on muscle myoglobin, enzymes, fiber types and blood lactate. Med. Sci. Sports Exerc. 1987;19:368–374. [PubMed] [Google Scholar]
- 25.Korhonen M.T, Cristea A, Alen M, Hakkinen K, Sipila S, Mero A, Viitasalo J.T, Larsson L, Suominen H. Aging, muscle fiber type, and contractile function in sprint-trained athletes. J. Appl. Physiol. 2006;101:906–917. doi: 10.1152/japplphysiol.00299.2006. [DOI] [PubMed] [Google Scholar]
- 26.Laursen P.B, Jenkins D.G. The scientific basis for high-intensity interval training: optimising training programmes and maximising performance in highly trained endurance athletes. Sports Med. 2002;32:53–73. doi: 10.2165/00007256-200232010-00003. [DOI] [PubMed] [Google Scholar]
- 27.LeBrasseur N.K, Walsh K, Arany Z. Metabolic benefits of resistance training and fast glycolytic skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2010;300:E3–E10. doi: 10.1152/ajpendo.00512.2010. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacDougall J.D, Hicks A.L, MacDonald J.R, McKelvie R.S, Green H.J, Smith K.M. Muscle performance and enzymatic adaptations to sprint interval training. J Appl Physiol. 1998;84:2138–2142. doi: 10.1152/jappl.1998.84.6.2138. [DOI] [PubMed] [Google Scholar]
- 29.Malisoux L, Francaux M, Theisen D. Whatdosingle-fibrestudiestellusaboutexercise training? Med. Sci. Sports Exerc. 2007;39:1051–1060. doi: 10.1249/mss.0b13e318057aeb. [DOI] [PubMed] [Google Scholar]
- 30.Peterson M.D, Sen A, Gordon P.M. Influence of resistance exercise on lean body mass in aging adults: a meta-analysis. Med. Sci. Sports Exerc. 2011;43:249–258. doi: 10.1249/MSS.0b013e3181eb6265. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pette D. Metabolic heterogeneity of muscle fibres. J. Exp. Biol. 1985;115:179–189. doi: 10.1242/jeb.115.1.179. [DOI] [PubMed] [Google Scholar]
- 32.Pette D, Staron R.S. Mammalian skeletal muscle fiber type transitions. Int. Rev. Cytol. 1997;170:143–223. doi: 10.1016/s0074-7696(08)61622-8. [DOI] [PubMed] [Google Scholar]
- 33.Puntschart A, Claassen H, Jostarndt K, Hoppeler H, Billeter R. mRNAs of enzymes involved in energy metabolism and mtDNA are increased in endurancetrained athletes. Am. J. Physiol. 1995;269:C619–625. doi: 10.1152/ajpcell.1995.269.3.C619. [DOI] [PubMed] [Google Scholar]
- 34.Rivero J.L, Talmadge R.J, Edgerton V.R. Interrelationships of myofibrillar ATPase activity and metabolic properties of myosin heavy chain-based fibre types in rat skeletal muscle. Histochem. Cell Biol. 1999;111:277–287. doi: 10.1007/s004180050358. [DOI] [PubMed] [Google Scholar]
- 35.Rodas G, Ventura J.L, Cadefau J.A, Cusso R, Parra J. A short training programme for the rapid improvement of both aerobic and anaerobic metabolism. Eur. J. Appl. Physiol. 2000;82:480–486. doi: 10.1007/s004210000223. [DOI] [PubMed] [Google Scholar]
- 36.Sargeant A.J. Structural and functional determinants of human muscle power. Exp. Physiol. 2007;92:323–331. doi: 10.1113/expphysiol.2006.034322. [DOI] [PubMed] [Google Scholar]
- 37.Staron R.S, Pette D. The multiplicity of combinations of myosin light chains and heavy chains in histochemically typed single fibres. Rabbit soleus muscle. Biochem. J. 1987;243:687–693. doi: 10.1042/bj2430687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevens L, Sultan K.R, Peuker H, Gohlsch B, Mounier Y, Pette D. Time-dependent changes in myosin heavy chain mRNA and protein isoforms in unloaded soleus muscle of rat. Am. J. Physiol. 1999;277(6 Pt 1):C1044–1049. doi: 10.1152/ajpcell.1999.277.6.C1044. [DOI] [PubMed] [Google Scholar]
- 39.Svedahl K, MacIntosh B.R. Anaerobic threshold: The concept and methods of measurement. Can. J. Appl. Physiol. 2003;28:299–323. doi: 10.1139/h03-023. [DOI] [PubMed] [Google Scholar]
- 40.Talmadge R.J. Myosin heavy chain isoform expression following reduced neuromuscular activity: potential regulatory mechanisms. Muscle Nerve. 2000;23:661–679. doi: 10.1002/(sici)1097-4598(200005)23:5<661::aid-mus3>3.0.co;2-j. Review. [DOI] [PubMed] [Google Scholar]
- 41.Van Praagh E, Doré E. Short-term muscle power during growth and maturation. Sports Med. 2002;32:701–728. doi: 10.2165/00007256-200232110-00003. [DOI] [PubMed] [Google Scholar]
- 42.Weiss A, Schiaffino S, Leinwand L. Comparative sequence analysis of the complete human sarcomeric myosin heavy chain family:implications for functional diversity. J. Mol. Biol. 1999;290:61–65. doi: 10.1006/jmbi.1999.2865. [DOI] [PubMed] [Google Scholar]