Abstract
Athletes engaged in strenuous training might experience transient immune suppression that could lead to greater incidence of upper respiratory tract infections (URTI). Since interleukin 21 (IL-21) stimulates immunoglobulin A (IgA) secreting cells and a low level of this immunoglobulin is associated with increased incidence of URTI, the aim of the present study was to investigate the effect of a basketball match on salivary cortisol (sC), salivary IL-21 (sIL-21) and salivary IgA (sIgA) levels. Twenty male basketball players participated in an official game in two teams (10 players in each team). The saliva samples were collected before the warm-up and approximately 10-15 min after the end of the match and were analysed by ELISA methods. sC concentration increased significantly after the match while sIL-21 level was reduced (p < 0.05). In opposition to the study's hypothesis, sIgA level did not change in response to the match. The present findings suggest that a basketball match is sufficiently stressful to elevate sC concentration and attenuates the sIL-21 output without compromising the sIgA level. It is reasonable to speculate that the stability of sIgA acute responses to the match, despite the decrement in sIL-21, indicates that other mechanisms rather than IL-21 stimulating B cell proliferation/differentiation might modulate IgA concentration and secretion rate.
Keywords: mucosal immunity, saliva, stress, interleukin, immunoglobulin
INTRODUCTION
It has been demonstrated that athletes engaged in strenuous training might have an increased risk of infection because of transient immune suppression [6, 19]. Probably, an insufficient period of recovery between successive training sessions may cause suppression of the immune system [2], increasing the risk of illness (e.g. greater incidence of upper respiratory tract infections – URTI). Furthermore, additional psychological stress could also affect the immune response of individuals [4].
In immunosuppressed athletes the mucosal surfaces are potential targets for pathogenic agents given their interface with the external environment. Such surfaces are normally protected by a network of organized structures located in the gut, urogenital tract, oral cavity and respiratory system, collectively known as the mucosal immune system (MIS) [9]. A low level of immunoglobulin A (IgA), particularly salivary IgA (sIgA), the major effector of the MIS [2], is associated with increased risk of URTI in athletes [6, 7]. Moreover, transient falls in sIgA are also related to increased risk of URTI [17].
The sIgA responses to acute exercise have been investigated during distinct designs, modes of exercise and sports. The results are still somewhat inconclusive and have produced conflicting findings. sIgA concentration is unchanged after moderate aerobic exercise lasting less than 1 h [2] as well as after a 70-minute soccer match [13]. On the other hand, some studies have reported decreased sIgA level following endurance sports such as swimming [27], kayaking [12], running [18] or in response to two futsal games in professional players [14].
A limitation to understanding the sIgA responses is that the potential mechanisms by which exercise influences salivary responses are poorly investigated. Recently, it has been shown that sIgA secreting cells are stimulated by interleukin (IL)-21, a potent inducer of human B cell proliferation/differentiation [11, 21, 24] which is produced by CD4+ T cells (especially T helper 17 (Th17)-producing cells) and natural killer T (NKT) cell [23]. Interestingly, IL-21 rapidly induced the generation of immunoglobulin-secreting cells and the secretion of vast quantities of sIgA from B cell subsets, and its effect exceeded that of IL-10 by up to 100-fold, highlighting the potency of IL-21 as a B cell differentiation factor [3]. Despite the well-established stimulatory role of IL-21 on sIgA secreting cells, no previous investigations have addressed the effect of sports competition on salivary IL-21 (sIL-21) and sIgA responses of athletes.
Therefore, the purpose of the present study was to investigate the effect of an official basketball match on sIL-21 and sIgA responses in both the first and second ranked teams in the under-19 state championship. It was hypothesized that cortisol concentration would be increased due to the great physiological and psychological stress. An additional hypothesis is that changes in salivary cortisol (sC) and sIL-21 could be associated with reduced sIgA level after the match.
MATERIALS AND METHODS
Subjects
Twenty male basketball players volunteered for this study (mean ± SD: age, 18.8 ± 0.4 years; height, 192 ± 10 cm; body mass, 87 ± 8). They played for two under-19 teams competing in the State Basketball Championship (São Paulo, Brazil). The two investigated teams were the first and the second ranked teams in the state championship at the time of the investigation. On a weekly basis, each player from both teams was typically training twice a day (90-120 minutes per session), five days a week, and played in one official match. The training sessions consisted of basketball drills, tactics, sprints, intermittent running exercises and specific conditioning work, as well as weight training and plyometrics. Participants provided informed consent before the study commenced. All procedures received local ethics committee approval.
Design
The investigated match was performed during the regular season and was played between 8.00 pm and 10.00 pm, under the current official basketball playing rules. The match was preceded by a 30-minute warm-up comprising light aerobic exercise, basketball and team drills, and dynamic stretching of the major muscle groups. The players were encouraged to drink water between break periods to maintain hydration status. Food intake was not strictly monitored during this study, but players were instructed to maintain their normal dietary intake during the week of testing, including the day of collections. Saliva samples were collected before the warm-up and approximately 10-15 min after each match at the same time from both teams. No weight management strategies were implemented and no differences in body mass from pre-match to post-match were observed for both teams.
Saliva Collection and Analysis
Athletes provided saliva samples before the pre-match warm-up (PRE) with post-match (POST) samples collected within 10-15 minutes of the completion of the game. The samples were collected into a pre-weighed sterile 15-ml centrifuge tube over a timed 5-minute period and stored at -80°C until assay. The tubes were re-weighed before analysis, so that saliva volume could be estimated. Saliva density was assumed to be 1.00 g · ml−1. Athletes abstained from food and caffeine products for at least 2 hours prior to the collection of saliva. After thawing saliva samples were centrifuged at 10,000 g for 10 minutes at 4°C. The samples were analysed for sC and sIgA in accordance with the manufacturer's recommendations (EIA kit; Salimetrics©, State College, PA, USA). The sIgA secretion rate (µg · min−1) was also calculated by multiplying the absolute sIgA concentration by salivary flow rate (ml · min−1). Salivary flow rate was determined by dividing the volume of saliva collected by the duration of the sampling period.
To perform the sIL-21 measurements, aliquots of each saliva sample were assayed by ELISA according to the manufacturer's recommendations (eBioscience©, San Diego, CA, USA). Briefly, 100 µl of sIL-21 capture antibody was added to all wells, except blank, mixed gently and incubated overnight (16-24 h) at 4°C. Plates were washed 3 times and standards and saliva were added in the respective wells. After 2 h, the plates were washed again and incubated with 100 µl of sIL-21 detection antibody for 60 minutes at room temperature. Plates were washed 3 times again and 100 µl of streptavidin-HRP was added and incubated for 30 minutes at room temperature in the dark followed by new washes and the addition of the substrate (tetramethylbenzidine – TMB). The reaction was stopped by the addition of 50 µl of stop solution, and colour was measured in an automated microplate spectrophotometer (Epoch, Biotek©, Winooski, VT, USA). Negative controls included the uncoated wells without saliva and/or primary antibody. The sIL-21 concentration was determined as pg · mL−1. Results were calculated using the standard curves created in each assay. Total protein (mg · ml−1) in the saliva was measured by using the BCA protein assay kit (Pierce Biotechnology©, Rockford, Illinois, USA) using bovine serum albumin as a standard. The coefficient of variation of the intra-assays was 4.8%, 6.0% and 3.2% for sC, sIgA and sIL-21, respectively.
Statistical Analysis
Data distribution was assessed using the Shapiro-Wilk normality test. Initially, a t test was used to compare each salivary parameter (sIL-21, sC and sIgA expressions (sIgAabs and sIgArate)) before and after the official match. Data are presented as mean ± SD. The accepted level of significance was p ≤ 0.05.
RESULTS
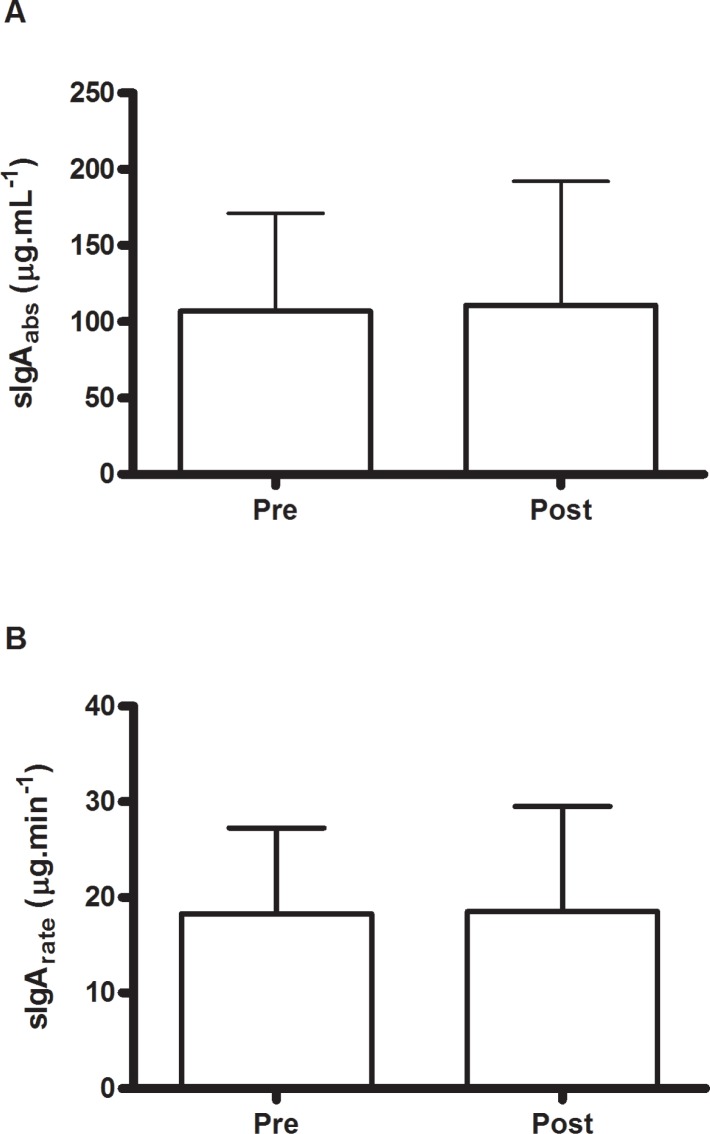
Figure 1A shows the sC response to the match. An increase was observed in post-match sC concentration compared with the pre-match value (p < 0.05). Figure 1B shows sIL-21 response to the match. There was a decrease in sIL-21 concentration after the match (p < 0.05). No change was observed for sIgA (absolute concentration and secretion rate) in response to the match (Figure 2A and 2B).
FIG. 1.
sC AND sIL-21 RESPONSES TO THE MATCH
Note: aDifferent from pre-game (p< 0.05); IL-21: Interleukin-21; sC: Salivary cortisol.
FIG. 2.
sIgAabs AND sIgArate RESPONSES TO THE MATCH.
Note: sIgAabs: Absolute concentration of secretory immunoglobulin A; sIgArate: Concentration of secretory immunoglobulin A relative to flow rate.
No significant difference (p = 0.7) was observed for total protein concentration from pre- (1.7±0.8 mg·ml−1) to post-match (1.9±0.9 mg·ml−1).
DISCUSSION
The main findings of the present study were: a) an increase in sC and b) a decrease in the sIL-21 level in response to an official basketball match. Despite the increment in sC and decrement in sIL-21, no change was observed for sIgA (absolute concentration and secretion rate). It has been previously reported that the additional level of stress associated with real competitions seems to maximize the sC response in professional basketball players [15]. Thus, the increase in sC concentration reported here supports the hypothesis that the psychological and physiological demands of a basketball match impose significant stress. Because sport competition has the potential to promote an excessive “danger” type of stress (signals of tissue damage and destruction) and inflammatory response [5], the acute cortisol increase could be important considering that endogenous glucocorticoids provide an essential restraint to limit the possible impairment of the immunological response [1].
IL-21 is an extremely important cytokine that can have both pro-inflammatory and anti-inflammatory effects on immune responses [26]. However, because IL-21 critically regulates immunoglobulin (Ig) production and drives the differentiation of B cells into antibody-producing plasma cells [25], it was hypothesized that this cytokine could influence sIgA responses to a basketball match. However, contrary to the initial study's hypothesis, there was not observed any significant change in sIgA after the match despite the decrease in sIL-21 post-match level.
No previous study has investigated the effect of acute exercise on sIL-21. However, several studies have demonstrated that this cytokine is critical for rapid immunoglobulin (Ig) production in response to acute infections. Furthermore, sustained Ig production by terminally differentiated plasma cells requires IL-10 signalling [10], and IL-10 production is stimulated by IL-21 [3]. Interestingly, it has been proposed that chronic regular exercise is able to induce an anti-inflammatory effect by increasing cytokines such as IL-10 [8] and that a negative consequence of this could be higher susceptibility to infection in athletes. Supporting this, recently it was demonstrated that a low level of sIgA secretion and exacerbated in vitro production of IL-10 by leukocytes in response to a multi-antigen challenge is associated with increased incidence of URTI in physically active individuals [7]. Interestingly, it has been proposed that intrinsic IL-21 signalling plays a critical role in CD8 T cell responses to an acute viral infection in vivo and may help in the design of effective vaccine strategies [20]. Furthermore, in another study, participants who developed elevated serum H1N1 antibody titres had significantly higher serum IL-21 level than those non-responder patients who failed to develop the vaccine-induced immunological responses [22].
Thus, the decrease in sIL-21 level observed in this study may not be able to impact sIgA due to the acute exercise (basketball match), but a consistent reduction of this cytokine concentration in long-term training might be responsible for greater risk of URTI or even higher incidence in athletes, due to a disturbance in sIgA secretion. Moreover, recently, Napimoga and coworkers [16] observed that patients with chronic periodontitis (gingival inflammation) presented increased sIgA level and this was associated with the up-regulation of sIL-21 and sIL-10 of these patients. Together, these studies seem to indicate the existence of a balance between sIL-21 and sIL-10 in healthy individuals and that any potentially stressful conditions (e.g. intensified training and competition) could be associated with imbalance of this equilibrium.
It could be argued that changes in the hydration status and their possible influence on saliva flow rate could have affected the present results, yet the alteration in saliva flow rate might induce change in saliva content. However, it is worth mentioning that besides no significant body weight changes observed from pre- to post-match, both saliva flow rate and total protein concentration also showed no differences after the match compared with before the match. Two studies conducted by Walsh and coworkers [28, 29] demonstrated that saliva flow rate and total protein concentration are potential markers of whole body hydration status in humans. Walsh and coworkers [28] showed that saliva osmolality, urine osmolality and saliva total protein concentration were strongly correlated with plasma osmolality during dehydration. In addition, the authors revealed that saliva total protein increased from pre- to post-exercise during a no-fluid-intake trial (prolonged exercise). The lack of changes in saliva flow rate and particularly in saliva total protein in the present study indicates that the athletes were able to maintain an appropriate hydration status due to their habitual procedure during an official match associated with drinking water ad libitum, as well as demonstrating that the present data were not affected by this factor.
It is important to recognize that the present study evaluated the short-term acute responses to the basketball match. The modulation of sC and sIL-21 on sIgA might require a longer time of follow-up since it is well known that certain hormonal and immunological responses occur with a delay.
CONCLUSIONS
The findings of the present study reinforce the assumption that an official basketball match can be considered a natural stress that elevates sC concentration. Additionally, the stress imposed by a basketball match decreased sIL-21 concentration; however, no change was observed in sIgA responses. It is reasonable to speculate that the stability of the sIgA acute responses to the match, despite the decrement in sIL-21, indicates that mechanisms other than IL-21 stimulating B cell proliferation/differentiation might regulate sIgA concentration and secretion rate. Future studies are required to elucidate the mechanisms involved in sIgA responses as well as the time course of such salivary measures after a single official basketball match or a period including a sequence of matches.
Acknowledgments
We would like to thank the FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo; São Paulo Research Foundation, Grant 2008/10404-3) for funding this research. We also wish to acknowledge all basketball players, coaches (Júlio Malfi and José Luiz Marcondes), and research support staff involved in this study for their committed participation. The results of the present study do not constitute endorsement of the product by the authors or the journal.
Conflict of interest
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Bhattacharyya S, Zhao Y, Kay T.W.H, Muglia L.J. Glucocorticoids target suppressor of cytokine signaling 1 (SOCS1) and type 1 interferons to regulated Toll-like receptor-induced STAT1 activation. Proc. Natl. Acad. Sci. U. S. A. 2011;7:9554–9559. doi: 10.1073/pnas.1017296108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bishop N.C, Gleeson M. Acute and chronic effects on markers of mucosal immunity. Front. Biosci. 2009;14:4444–4456. doi: 10.2741/3540. [DOI] [PubMed] [Google Scholar]
- 3.Bryant V.L, Ma C.S, Avery D.T, Li Y, Good K.L, Corcoran L.M, Malefyt R.W, Tangye S.G. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells:predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J. Immunol. 2007;179:8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- 4.Clow A, Hucklebridge F. The impact of psychological stress on immune function in the athletic population. Exerc. Immunol. Rev. 2001;7:5–17. [PubMed] [Google Scholar]
- 5.Cooper D.M, Radom-Aizik S, Schwindt C, Zaldivar F., Jr Dangerous exercise:lessons learned from dysregulated inflammatory responses to physical activity. J. Appl. Physiol. 2007;103:700–709. doi: 10.1152/japplphysiol.00225.2007. [DOI] [PubMed] [Google Scholar]
- 6.Fahlman M.M, Engels H. Mucosal IgA and URTI in american college football players. A year longitudinal study. Med. Sci. Sports Exerc. 2005;37:374–386. doi: 10.1249/01.mss.0000155432.67020.88. [DOI] [PubMed] [Google Scholar]
- 7.Gleeson M, Bishop N, Oliveira M, McCauly T, Tauler P, Muhamad A.S. Respiratory infection risk in athletes:association with antigen-stimulated IL-10 production and salivary IgA secretion. Scand. J. Med. Sci Sports. 2011;22:410–417. doi: 10.1111/j.1600-0838.2010.01272.x. [DOI] [PubMed] [Google Scholar]
- 8.Gleeson M, Bishop N.C, Stensel D.J, Lindley M.R, Mastana S.S, Nimmo M.A. The anti-inflammatory effects of exercise:mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011;11:607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 9.Gleeson M, Pyne D.B. Exercise effects on mucosal immunity. Immunol. Cell. Biol. 2000;78:536–544. doi: 10.1111/j.1440-1711.2000.t01-8-.x. [DOI] [PubMed] [Google Scholar]
- 10.Good K.L, Bryant V.L, Tangue S.G. Kinetics of human B cell behavior and amplification of proliferative responses following stimulation with IL-21. J. Immunol. 2006;177:5236–5247. doi: 10.4049/jimmunol.177.8.5236. [DOI] [PubMed] [Google Scholar]
- 11.Kuchen S, Robbins R, Sims G.P, Sheng C, Phillips T.M, Lipsky P.E, Ettinger R. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell-B cell collaboration. J. Immunol. 2007;179:5886–5896. doi: 10.4049/jimmunol.179.9.5886. [DOI] [PubMed] [Google Scholar]
- 12.Mackinon L.T, Ginn E, Seymour G.J. Decreased salivary immunoglobulin A secretion rate after intense interval exercise in elite kayakers. Eur. J. Appl. Physiol. Occup. Physiol. 1993;67:180–184. doi: 10.1007/BF00376664. [DOI] [PubMed] [Google Scholar]
- 13.Moreira A, Arsati F, Cury P.R, Franciscon C, de Oliveira P.R, de Araújo V.C. Salivary immunoglobulin A response to a match in top-level brazilian soccer players. J. Strength. Cond. Res. 2009;23:1968–1973. doi: 10.1519/JSC.0b013e3181b3dd7a. [DOI] [PubMed] [Google Scholar]
- 14.Moreira A, Arsati F, de Oliveira Lima-Arsati Y.B, Freitas C.G, Araujo V.C. Salivary immunoglobulin A responses in Professional top-level futsal players. J. Strength. Cond. Res. 2011;25:1932–1936. doi: 10.1519/JSC.0b013e3181e7fbc0. [DOI] [PubMed] [Google Scholar]
- 15.Moreira A, McGuigan M.R, Arruda A.F.S, Freitas C.G, Aoki M.S. Monitoring internal load parameters during simulated and official basketball matches. J. Strength. Cond. Res. 2012;26:861–866. doi: 10.1519/JSC.0b013e31822645e9. [DOI] [PubMed] [Google Scholar]
- 16.Napimoga M.H, Nunes L.H.A.C, Maciel A.A.B, Demasi A.P.D, Benatti B.B, Santos V.R, Bastos M.F, de Miranda T.S, Duarte P.M. Possible involvement of il-21 and il-10 on salivary IgA levels in chronic periodontitis subjects. Scand. J. Immunol. 2011;74:596–602. doi: 10.1111/j.1365-3083.2011.02605.x. [DOI] [PubMed] [Google Scholar]
- 17.Neville V, Gleeson M, Folland J.P. Salivary IgA as a risk factor for upper respiratory infections in elite Professional athletes. Med. Sci Sports Exerc. 2008;40:1228–1236. doi: 10.1249/MSS.0b013e31816be9c3. [DOI] [PubMed] [Google Scholar]
- 18.Nieman D.C, Henson D.A, Fagoaga O.R, Utter A.C, Vinci D.M, Davis J.M, Nehlsen-Cannarella S.L. Change in salivary IgA following a competitive marathon race. Int. J. Sports Med. 2002;23:69–75. doi: 10.1055/s-2002-19375. [DOI] [PubMed] [Google Scholar]
- 19.Nieman D.C. Is infection risk linked to exercise workload? Med. Sci. Sports Exerc. 2000;32:S406–S411. doi: 10.1097/00005768-200007001-00005. [DOI] [PubMed] [Google Scholar]
- 20.Novy P, Huang X, Leonard W.J, Yang Y. Intrinsic IL-21 signaling is critical for CD8 T cell survival and memory formation in response to vaccinia viral infection. J. Immunol. 2011;186:2729–38. doi: 10.4049/jimmunol.1003009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozaki K, Spolski R, Feng C.G, Qi C.F, Cheng J, Sher A, Morse H.C, Liu C, Schwartzberg P.L, Leonard W.J. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 22.Pallikkuth S, Pilakka Kanthikeel S, Silva S.Y, Fischl M, Pahwa R, Pahwa S. Upregulation of IL-21 receptor on B cells and IL-21 secretion distinguishes novel 2009 H1N1 vaccine responders from nonresponders among HIV-infected persons on combination antiretroviral therapy. J. Immunol. 2009;186:6173–6181. doi: 10.4049/jimmunol.1100264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parrish-Novak J, Dillon S.R, Nelson A, Hammond A, Sprecher C, Gross J.A, Johnston J, Madden K, Xu W, West J, Schrader S, Burkhead S, Heipel M, Brandt C, Kuijper J.L, Kramer J, Conklin D, Presnell S.R, Berry J, Shiota F, Bort S, Hambly K, Mudri S, Clegg C, Moore M, Grant F.J, Lofton-Day C, Gilbert T, Raymond F, Ching A, Yao L, Smith D, Webster P, Whitmore T, Maurer M, Kaushansky K, Holly R.D, Foster D. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 24.Pene J, Guglielmi L, Gauchat J.F, Drouet E, Guglielmi P, Boulay V, Delwail A, Foster D, Lecron J.C, Yssel H. IFN-mediated inhibition of human IgE synthesis by IL-21 is associated with a polymorphism in the IL-21R gene. J. Immunol. 2006;177:5006–5013. doi: 10.4049/jimmunol.177.8.5006. [DOI] [PubMed] [Google Scholar]
- 25.Spolski R, Kim H.P, Zhu W, Levy D.E, Leonard W.J. IL-21 mediates suppressive effects via tis induction of IL-10. J. Immunol. 2009;182:2859–2867. doi: 10.4049/jimmunol.0802978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spolski R, Leonard W.J. IL-21 is an immune activator that also mediates suppression via IL-10. Crit. Rev. Immunol. 2010;30:559–570. doi: 10.1615/critrevimmunol.v30.i6.50. [DOI] [PubMed] [Google Scholar]
- 27.Tharp G.D, Barnes M.W. Reduction of saliva immunoglobulin levels by swim training. Eur. J. Appl. Physiol. Occup. Physiol. 1990;60:61–64. doi: 10.1007/BF00572187. [DOI] [PubMed] [Google Scholar]
- 28.Walsh N.P, Laing S.J, Oliver S.J, Montague J.C, Walters R, Bilzon J.L.J. Saliva parameters as potential indices of hydration status during acute dehydration. Med. Sci. Sports Exerc. 2004;36:1535–1542. doi: 10.1249/01.mss.0000139797.26760.06. [DOI] [PubMed] [Google Scholar]
- 29.Walsh N.P, Montague J.C, Callow N, Rowlands A.V. Saliva flow rate, total protein concentration and osmolality as potential markers of whole body hydration status during progressive acute dehydration in humans. Arch. Oral Biol. 2004;49:149–154. doi: 10.1016/j.archoralbio.2003.08.001. [DOI] [PubMed] [Google Scholar]