Abstract
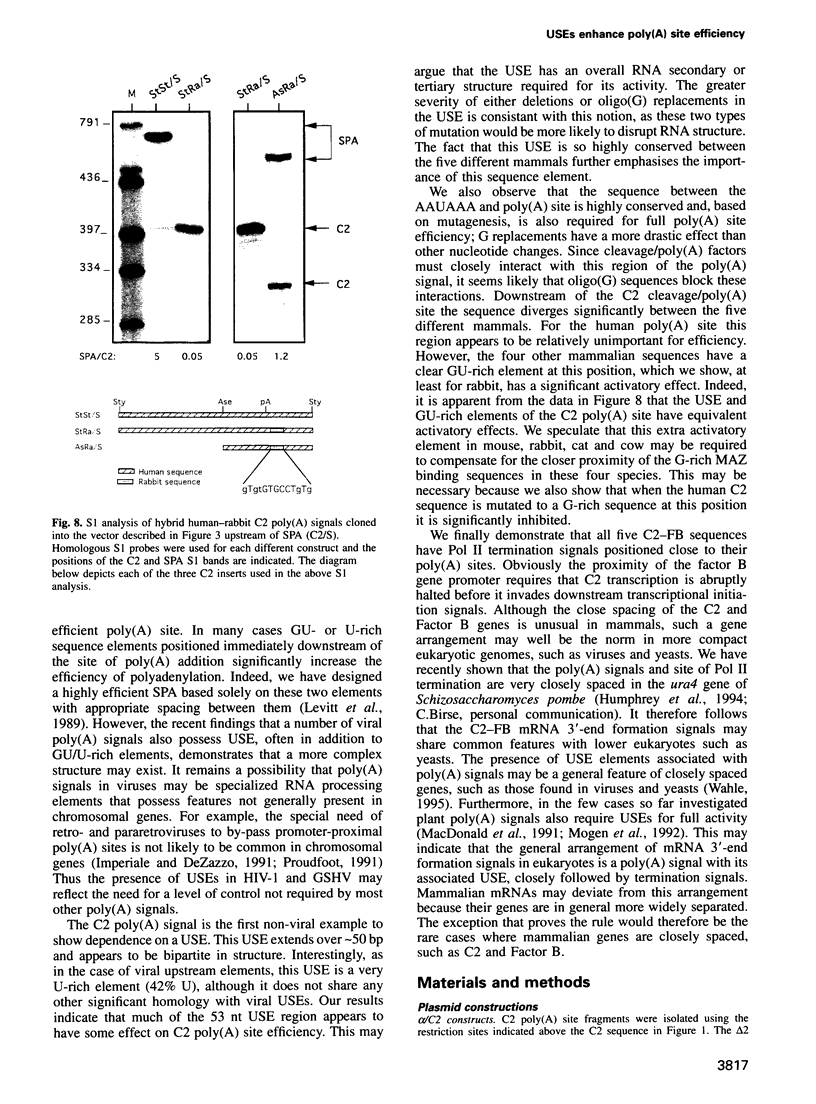
Poly(A) signals of mammalian pre-mRNA have been defined as an AAUAAA sequence 10-30 nt upstream of the cleavage/poly(A) site followed by a GU/U-rich element immediately downstream. However, a number of viral poly(A) signals have been shown to possess additional signals upstream of AAUAAA that increase poly(A) site efficiency. We describe the first non-viral example of such an upstream sequence element (USE) for the poly(A) site of the human C2 complement gene. As this gene is very closely spaced to the related Factor B gene [the C2 poly(A) site is only 421 bp from the transcription start site of Factor B] we have isolated this same intergenic sequence from four other mammals (mouse, cat, rabbit and cow). We show that the USE of the C2 poly(A) site is highly conserved between these five different mammals. Furthermore, extensive mutagenesis of the human USE indicates that most of the 53 nt sequence is required for full activity. The human C2 poly(A) site does not possess any obvious downstream GU/U-rich sequences, although sequences immediately 3' to AAUAAA as well as 13 nt of sequence following the cleavage site are both required for full activity. Interestingly the other mammalian C2 poly(A) sites do possess significant downstream GU/U-rich sequences. Finally we show that all five mammalian C2 poly(A) signals are immediately followed by conserved signals for transcriptional termination, consistent with the close proximity of the downstream Factor B gene.
Full text
PDF



Images in this article
Selected References
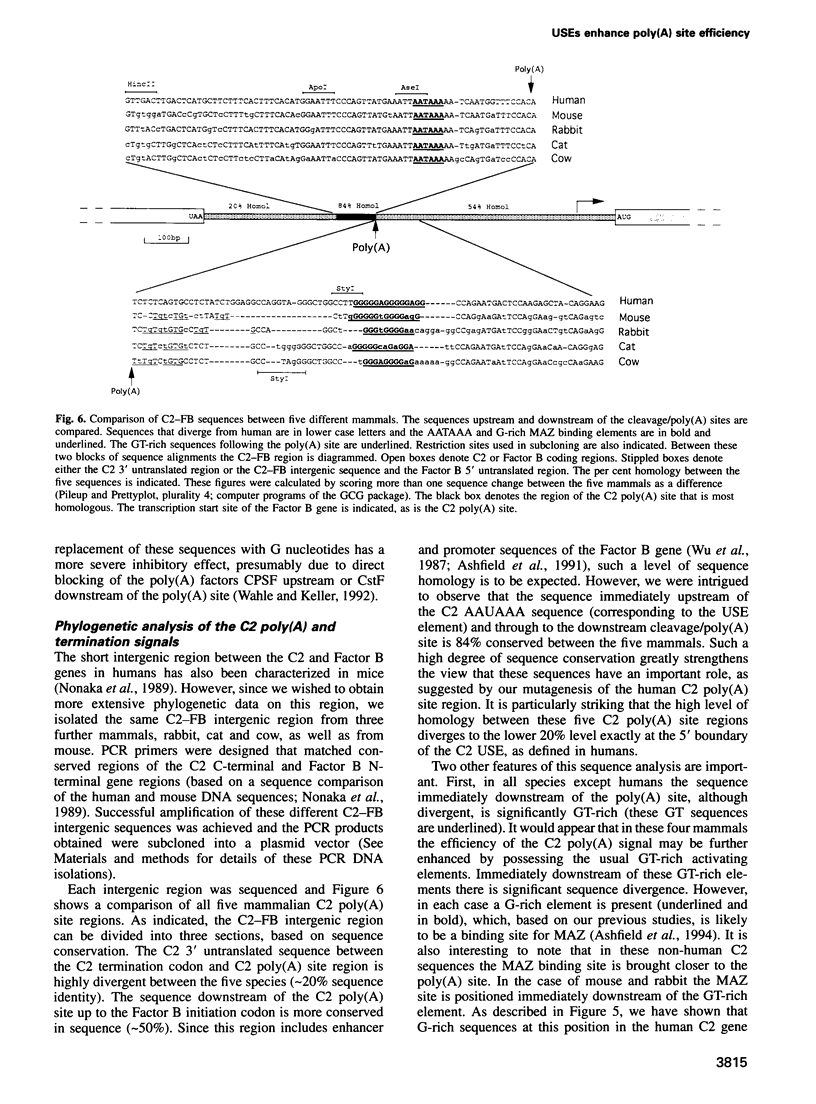
These references are in PubMed. This may not be the complete list of references from this article.
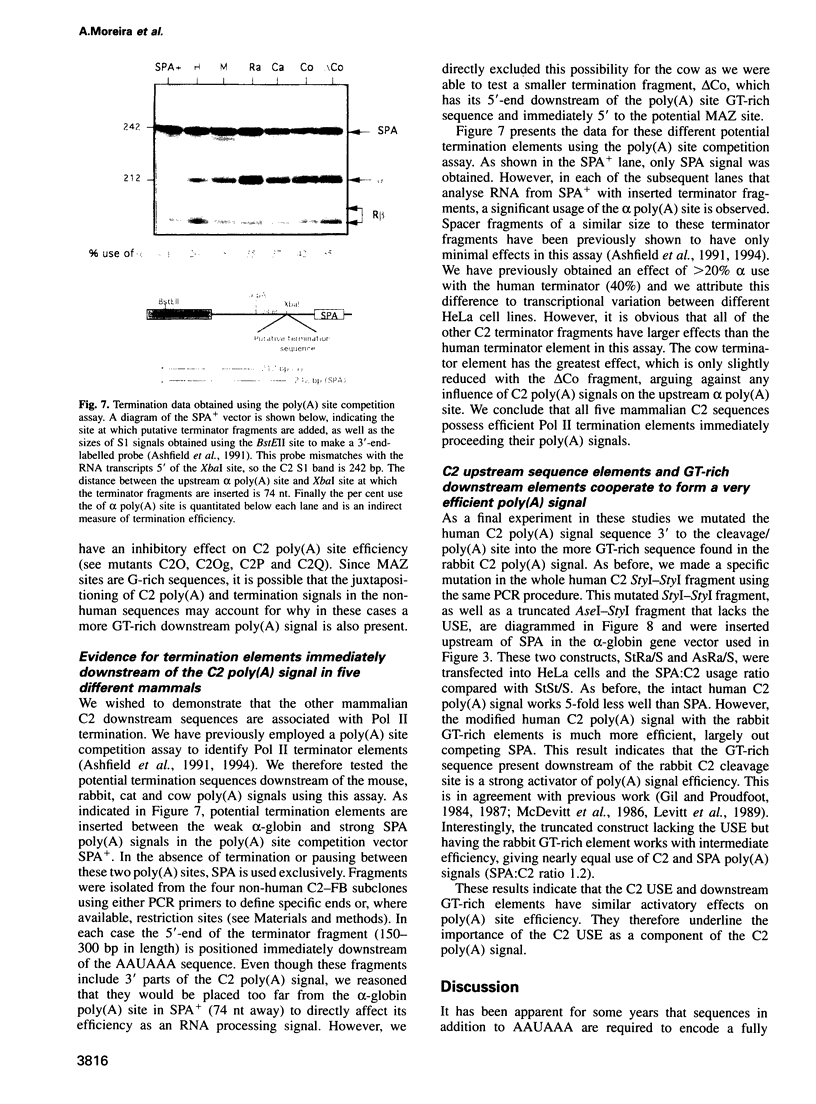
- Ashfield R., Enriquez-Harris P., Proudfoot N. J. Transcriptional termination between the closely linked human complement genes C2 and factor B: common termination factor for C2 and c-myc? EMBO J. 1991 Dec;10(13):4197–4207. doi: 10.1002/j.1460-2075.1991.tb04998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashfield R., Patel A. J., Bossone S. A., Brown H., Campbell R. D., Marcu K. B., Proudfoot N. J. MAZ-dependent termination between closely spaced human complement genes. EMBO J. 1994 Dec 1;13(23):5656–5667. doi: 10.1002/j.1460-2075.1994.tb06904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselin C., Nepveu A., Marcu K. B. Molecular requirements for transcriptional initiation of the murine c-myc gene. Oncogene. 1989 May;4(5):549–558. [PubMed] [Google Scholar]
- Bilger A., Fox C. A., Wahle E., Wickens M. Nuclear polyadenylation factors recognize cytoplasmic polyadenylation elements. Genes Dev. 1994 May 1;8(9):1106–1116. doi: 10.1101/gad.8.9.1106. [DOI] [PubMed] [Google Scholar]
- Bossone S. A., Asselin C., Patel A. J., Marcu K. B. MAZ, a zinc finger protein, binds to c-MYC and C2 gene sequences regulating transcriptional initiation and termination. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7452–7456. doi: 10.1073/pnas.89.16.7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. H., Tiley L. S., Cullen B. R. Efficient polyadenylation within the human immunodeficiency virus type 1 long terminal repeat requires flanking U3-specific sequences. J Virol. 1991 Jun;65(6):3340–3343. doi: 10.1128/jvi.65.6.3340-3343.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell S., Alwine J. C. Efficiency of utilization of the simian virus 40 late polyadenylation site: effects of upstream sequences. Mol Cell Biol. 1989 Oct;9(10):4248–4258. doi: 10.1128/mcb.9.10.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrington J., Ganem D. Regulation of polyadenylation in human immunodeficiency virus (HIV): contributions of promoter proximity and upstream sequences. EMBO J. 1992 Apr;11(4):1513–1524. doi: 10.1002/j.1460-2075.1992.tb05196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeZazzo J. D., Imperiale M. J. Sequences upstream of AAUAAA influence poly(A) site selection in a complex transcription unit. Mol Cell Biol. 1989 Nov;9(11):4951–4961. doi: 10.1128/mcb.9.11.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeZazzo J. D., Kilpatrick J. E., Imperiale M. J. Involvement of long terminal repeat U3 sequences overlapping the transcription control region in human immunodeficiency virus type 1 mRNA 3' end formation. Mol Cell Biol. 1991 Mar;11(3):1624–1630. doi: 10.1128/mcb.11.3.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriquez-Harris P., Levitt N., Briggs D., Proudfoot N. J. A pause site for RNA polymerase II is associated with termination of transcription. EMBO J. 1991 Jul;10(7):1833–1842. doi: 10.1002/j.1460-2075.1991.tb07709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil A., Proudfoot N. J. A sequence downstream of AAUAAA is required for rabbit beta-globin mRNA 3'-end formation. 1984 Nov 29-Dec 5Nature. 312(5993):473–474. doi: 10.1038/312473a0. [DOI] [PubMed] [Google Scholar]
- Gil A., Proudfoot N. J. Position-dependent sequence elements downstream of AAUAAA are required for efficient rabbit beta-globin mRNA 3' end formation. Cell. 1987 May 8;49(3):399–406. doi: 10.1016/0092-8674(87)90292-3. [DOI] [PubMed] [Google Scholar]
- Gilmartin G. M., Fleming E. S., Oetjen J. Activation of HIV-1 pre-mRNA 3' processing in vitro requires both an upstream element and TAR. EMBO J. 1992 Dec;11(12):4419–4428. doi: 10.1002/j.1460-2075.1992.tb05542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin G. M., Fleming E. S., Oetjen J., Graveley B. R. CPSF recognition of an HIV-1 mRNA 3'-processing enhancer: multiple sequence contacts involved in poly(A) site definition. Genes Dev. 1995 Jan 1;9(1):72–83. doi: 10.1101/gad.9.1.72. [DOI] [PubMed] [Google Scholar]
- Grosveld G. C., de Boer E., Shewmaker C. K., Flavell R. A. DNA sequences necessary for transcription of the rabbit beta-globin gene in vivo. Nature. 1982 Jan 14;295(5845):120–126. doi: 10.1038/295120a0. [DOI] [PubMed] [Google Scholar]
- Hart R. P., McDevitt M. A., Nevins J. R. Poly(A) site cleavage in a HeLa nuclear extract is dependent on downstream sequences. Cell. 1985 Dec;43(3 Pt 2):677–683. doi: 10.1016/0092-8674(85)90240-5. [DOI] [PubMed] [Google Scholar]
- Humphrey T., Birse C. E., Proudfoot N. J. RNA 3' end signals of the S.pombe ura4 gene comprise a site determining and efficiency element. EMBO J. 1994 May 15;13(10):2441–2451. doi: 10.1002/j.1460-2075.1994.tb06529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperiale M. J., DeZazzo J. D. Poly(A) site choice in retroelements: deja vu all over again? New Biol. 1991 Jun;3(6):531–537. [PubMed] [Google Scholar]
- Levitt N., Briggs D., Gil A., Proudfoot N. J. Definition of an efficient synthetic poly(A) site. Genes Dev. 1989 Jul;3(7):1019–1025. doi: 10.1101/gad.3.7.1019. [DOI] [PubMed] [Google Scholar]
- Lutz C. S., Alwine J. C. Direct interaction of the U1 snRNP-A protein with the upstream efficiency element of the SV40 late polyadenylation signal. Genes Dev. 1994 Mar 1;8(5):576–586. doi: 10.1101/gad.8.5.576. [DOI] [PubMed] [Google Scholar]
- MacDonald M. H., Mogen B. D., Hunt A. G. Characterization of the polyadenylation signal from the T-DNA-encoded octopine synthase gene. Nucleic Acids Res. 1991 Oct 25;19(20):5575–5581. doi: 10.1093/nar/19.20.5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley J. L. Polyadenylation of mRNA precursors. Biochim Biophys Acta. 1988 May 6;950(1):1–12. doi: 10.1016/0167-4781(88)90067-x. [DOI] [PubMed] [Google Scholar]
- McDevitt M. A., Hart R. P., Wong W. W., Nevins J. R. Sequences capable of restoring poly(A) site function define two distinct downstream elements. EMBO J. 1986 Nov;5(11):2907–2913. doi: 10.1002/j.1460-2075.1986.tb04586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt M. A., Imperiale M. J., Ali H., Nevins J. R. Requirement of a downstream sequence for generation of a poly(A) addition site. Cell. 1984 Jul;37(3):993–999. doi: 10.1016/0092-8674(84)90433-1. [DOI] [PubMed] [Google Scholar]
- McLauchlan J., Gaffney D., Whitton J. L., Clements J. B. The consensus sequence YGTGTTYY located downstream from the AATAAA signal is required for efficient formation of mRNA 3' termini. Nucleic Acids Res. 1985 Feb 25;13(4):1347–1368. doi: 10.1093/nar/13.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogen B. D., MacDonald M. H., Leggewie G., Hunt A. G. Several distinct types of sequence elements are required for efficient mRNA 3' end formation in a pea rbcS gene. Mol Cell Biol. 1992 Dec;12(12):5406–5414. doi: 10.1128/mcb.12.12.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka M., Gitlin J. D., Colten H. R. Regulation of human and murine complement: comparison of 5' structural and functional elements regulating human and murine complement factor B gene expression. Mol Cell Biochem. 1989 Aug 15;89(1):1–14. doi: 10.1007/BF00228274. [DOI] [PubMed] [Google Scholar]
- Peterson M. L. Regulated immunoglobulin (Ig) RNA processing does not require specific cis-acting sequences: non-Ig RNA can be alternatively processed in B cells and plasma cells. Mol Cell Biol. 1994 Dec;14(12):7891–7898. doi: 10.1128/mcb.14.12.7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Lee B. A., Monks J. Multiple SP1 binding sites confer enhancer-independent, replication-activated transcription of HIV-1 and globin gene promoters. New Biol. 1992 Apr;4(4):369–381. [PubMed] [Google Scholar]
- Proudfoot N. Poly(A) signals. Cell. 1991 Feb 22;64(4):671–674. doi: 10.1016/0092-8674(91)90495-k. [DOI] [PubMed] [Google Scholar]
- Rothnie H. M., Reid J., Hohn T. The contribution of AAUAAA and the upstream element UUUGUA to the efficiency of mRNA 3'-end formation in plants. EMBO J. 1994 May 1;13(9):2200–2210. doi: 10.1002/j.1460-2075.1994.tb06497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russnak R. H. Regulation of polyadenylation in hepatitis B viruses: stimulation by the upstream activating signal PS1 is orientation-dependent, distance-independent, and additive. Nucleic Acids Res. 1991 Dec 11;19(23):6449–6456. doi: 10.1093/nar/19.23.6449. [DOI] [PMC free article] [PubMed] [Google Scholar]
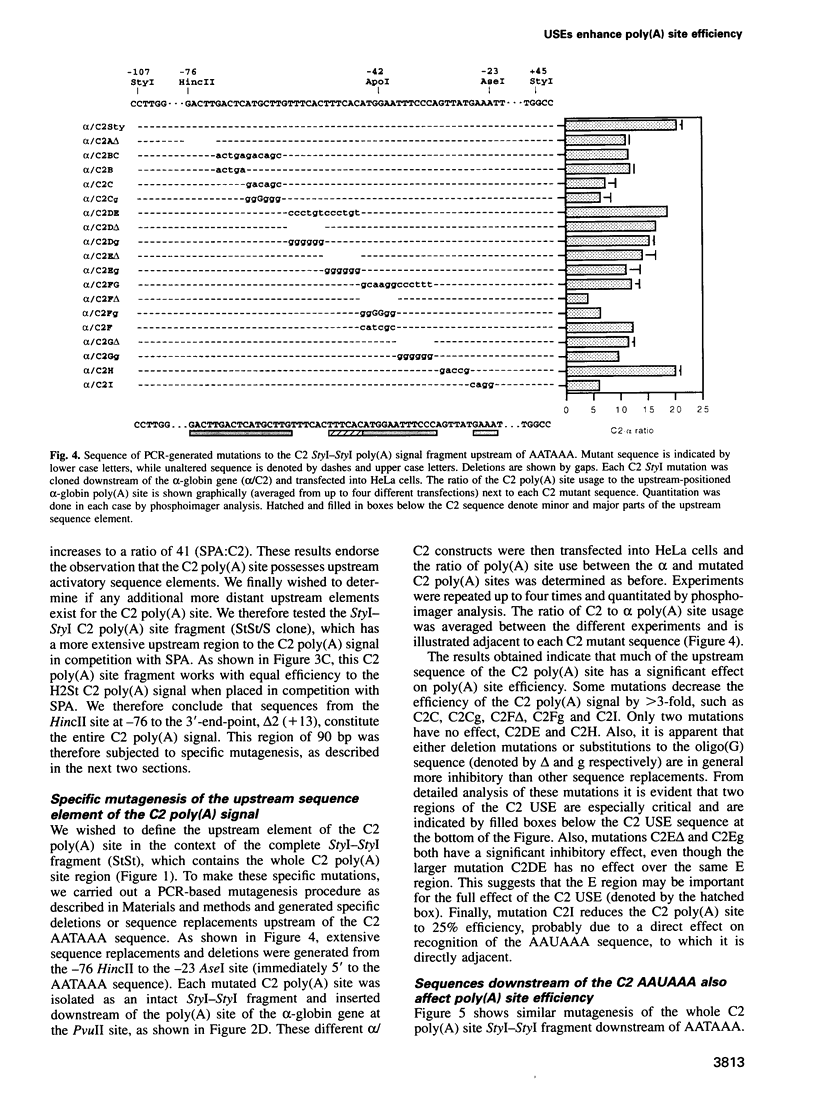
- Russnak R., Ganem D. Sequences 5' to the polyadenylation signal mediate differential poly(A) site use in hepatitis B viruses. Genes Dev. 1990 May;4(5):764–776. doi: 10.1101/gad.4.5.764. [DOI] [PubMed] [Google Scholar]
- Sanfaçon H., Brodmann P., Hohn T. A dissection of the cauliflower mosaic virus polyadenylation signal. Genes Dev. 1991 Jan;5(1):141–149. doi: 10.1101/gad.5.1.141. [DOI] [PubMed] [Google Scholar]
- Schek N., Cooke C., Alwine J. C. Definition of the upstream efficiency element of the simian virus 40 late polyadenylation signal by using in vitro analyses. Mol Cell Biol. 1992 Dec;12(12):5386–5393. doi: 10.1128/mcb.12.12.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagaki Y., Manley J. L., MacDonald C. C., Wilusz J., Shenk T. A multisubunit factor, CstF, is required for polyadenylation of mammalian pre-mRNAs. Genes Dev. 1990 Dec;4(12A):2112–2120. doi: 10.1101/gad.4.12a.2112. [DOI] [PubMed] [Google Scholar]
- Valsamakis A., Schek N., Alwine J. C. Elements upstream of the AAUAAA within the human immunodeficiency virus polyadenylation signal are required for efficient polyadenylation in vitro. Mol Cell Biol. 1992 Sep;12(9):3699–3705. doi: 10.1128/mcb.12.9.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsamakis A., Zeichner S., Carswell S., Alwine J. C. The human immunodeficiency virus type 1 polyadenylylation signal: a 3' long terminal repeat element upstream of the AAUAAA necessary for efficient polyadenylylation. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2108–2112. doi: 10.1073/pnas.88.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahle E. 3'-end cleavage and polyadenylation of mRNA precursors. Biochim Biophys Acta. 1995 Apr 4;1261(2):183–194. doi: 10.1016/0167-4781(94)00248-2. [DOI] [PubMed] [Google Scholar]
- Wahle E., Keller W. The biochemistry of 3'-end cleavage and polyadenylation of messenger RNA precursors. Annu Rev Biochem. 1992;61:419–440. doi: 10.1146/annurev.bi.61.070192.002223. [DOI] [PubMed] [Google Scholar]
- Weiss E. A., Gilmartin G. M., Nevins J. R. Poly(A) site efficiency reflects the stability of complex formation involving the downstream element. EMBO J. 1991 Jan;10(1):215–219. doi: 10.1002/j.1460-2075.1991.tb07938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw E., Proudfoot N. Alpha-thalassaemia caused by a poly(A) site mutation reveals that transcriptional termination is linked to 3' end processing in the human alpha 2 globin gene. EMBO J. 1986 Nov;5(11):2915–2922. doi: 10.1002/j.1460-2075.1986.tb04587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M. How the messenger got its tail: addition of poly(A) in the nucleus. Trends Biochem Sci. 1990 Jul;15(7):277–281. doi: 10.1016/0968-0004(90)90054-f. [DOI] [PubMed] [Google Scholar]
- Wilusz J., Shenk T., Takagaki Y., Manley J. L. A multicomponent complex is required for the AAUAAA-dependent cross-linking of a 64-kilodalton protein to polyadenylation substrates. Mol Cell Biol. 1990 Mar;10(3):1244–1248. doi: 10.1128/mcb.10.3.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. C., Morley B. J., Campbell R. D. Cell-specific expression of the human complement protein factor B gene: evidence for the role of two distinct 5'-flanking elements. Cell. 1987 Jan 30;48(2):331–342. doi: 10.1016/0092-8674(87)90436-3. [DOI] [PubMed] [Google Scholar]