Abstract
The purpose of this study was to examine the effect of acute low-dose celecoxib administration on exercise-induced inflammation, muscle damage and lipid peroxidation. Twenty healthy untrained males (age: 25.5±4.5 yrs, weight: 72.7±7.9 kg, height: 177.3±7.2 cm) were randomly assigned to treatment (T) or placebo (P) groups. Blood samples were obtained before, immediately after, 3 h after and 24 h after exercise. Subjects ran for 30 min at 75% O2 max on a treadmill. Participants consumed 100 mg celecoxib or a placebo immediately after and 12 h after the immediately post-exercise blood sample. Total leukocytes, neutrophils, creatine kinase (CK), C-reactive protein (CRP) and malondialdehyde (MDA) were assessed at each time point. Significant increases in total leukocytes and neutrophils were observed 3 h after exercise in both groups (P < 0.05). CK and CRP levels were significantly increased immediately, 3 h and 24 h after exercise in both groups (P < 0.05). A significant increase in MDA was observed immediately after exercise in both groups (P < 0.05); however, no significant group differences were observed for MDA or CK. These findings suggest that inhibition of cyclo-oxygenase activity with low-dose celecoxib does not affect exercise-induced inflammation, muscle damage, or lipid peroxidation.
Keywords: celecoxib, inflammation, muscle damage, lipid peroxidation
INTRODUCTION
Routine exercise training of moderate intensity is known to enhance immune function [27] and reduce risk of mortality [11, 23], cardiovascular diseases, obesity, and type 2 diabetes [17]. However, an acute bout of prolonged (>1.5 h) and/or strenuous exercise (55-75% maximal O2 uptake) has also been shown to provoke muscle damage/soreness [4], and elicit an acute inflammatory [15], oxidative stress [7, 9], and immunosuppressive response [25] during the post-exercise recovery period. In order to alleviate these potential adverse responses following prolonged/strenuous exercise, the consumption of non-steroidal anti-inflammatory drugs (NSAIDs) has been strategically used to reduce soreness, muscle damage, and inflammation following exercise [13].
The original NSAIDs (e.g. ibuprofen) inhibited both the constitutive cyclooxygenase (COX)-1 and the more inducible COX-2 enzyme, which both catalyze the generation of prostanoids [prostaglandins (PGE2 and PGF2a), prostacyclins and thromboxanes]; which are involved in numerous inflammatory, anaphylactic, and cardiovascular physiological processes [14]. However, it has been postulated that COX-2 is the key enzyme which regulates the amount and duration of inflammatory cell accumulation [29] and lipid peroxidation [1] following exercise-induced muscle damage. Therefore, it seems plausible that the more recently developed selective COX-2 inhibitors may be more appropriate and specific to reduce excessive inflammatory reactions following exercise than the non-selective (COX-1 and COX-2) inhibitors [28].
Celecoxib, a sulfonamide, is a selective COX-2 inhibitor that has anti-inflammatory and analgesic effects comparable to non selective NSAIDs, and presents with significantly less gastrointestinal toxicity [28]. To date, several studies have demonstrated a lack of efficacy of NSAIDs treatment on muscle inflammation after exercise [12, 18, 26], while others have reported enhanced recovery and reduction of inflammatory markers following exercise [8]. Therefore, given the equivocal results regarding the effect of non-selective anti-inflammatory drugs on exercise-induced inflammation, muscle damage, and lipid peroxidation, this study was designed to investigate the influence of an acute low dose of the selective COX-2 inhibitor celecoxib on inflammation, muscle damage, and lipid peroxidation following an intense bout of aerobic exercise.
MATERIALS AND METHODS
Participants
Twenty untrained male volunteers took part in this study. All subjects were informed verbally and in writing about the nature and demands of the study, and subsequently completed a health history questionnaire. Qualifying volunteers were provided with and asked to sign an institutionally approved informed consent form. In addition to meeting the health criteria, individuals engaging in the following were also excluded from the study: smoking, alcohol intake, vitamin supplementation (e.g., vitamin A, C and E) or NSAIDs. Three-day diet records were used to estimate subjects’ average daily intake of vitamin A, C, and E prior to the beginning of the study. All subjects were untrained and had no known allergies to NSAIDs. Subjects were randomly assigned to treatment (T) (100 mg celecoxib) or placebo (P) groups. Each group was matched for height, weight, BMI, % fat and cardiovascular fitness (no significant group differences) (Table 1). The protocol of the study was approved by the university ethics committee in accordance with the Helsinki Declaration.
TABLE 1.
SUBJECTS’ CHARACTERISTICS IN TREATMENT (T) AND PLACEBO (P) GROUPS (N = 10)
| Group | VO2max (ml·kg-1·min-1) | BMI (kg·m-2) | Height (cm) | Body mass (kg) | Age (yrs) | Skin folds (mm) |
|---|---|---|---|---|---|---|
| T | 34.5 ± 2.8 | 23.3 ± 2.7 | 176.6 ± 7.7 | 72.4 ± 8.6 | 25.7 ± 3.9 | 45.1 ± 6.4 |
| P | 35.0 ± 5.2 | 23.0 ± 1.1 | 178.2 ± 6.8 | 73.1 ± 5.7 | 25.3 ± 5.2 | 40.2 ± 8.4 |
Note: Data represent mean ± SD.
Dietary records
Subjects were asked to keep a dietary record of their food intake 3 days prior to the exercise test session. Total daily Vitamin A, C, and E intake during the 3 days prior to testing were determined using the Dietary Manager Computer program (Food process-2). Dietary analysis revealed no statistically significant differences in vitamin A, C, and E intake between groups (Table 2).
TABLE 2.
MEAN TOTAL DIETARY INTAKE OF VITAMIN A, C, AND E OF SUBJECTS IN TREATMENT (T) AND PLACEBO (P) GROUPS
| Variable | group T | group P | P |
|---|---|---|---|
| Vitamin A (RE) | 529.4 ± 384.3 | 570.1 ± 320.2 | 0.58 |
| Vitamin C (mg) | 61.6 ± 26.3 | 45.9 ± 21.7 | 0.21 |
| Vitamin E (mg) | 4.4 ± 2.6 | 3.4 ± 1.7 | 0.41 |
Note: Data represent mean ± SD.
Preliminary testing
Cardiovascular fitness was determined by performing a maximal oxygen uptake test (O2max test). O2max was measured indirectly on a treadmill using the Bruce protocol. Briefly, subjects warmed up for 4 minutes at a speed of 3 mph on a 2.5% grade. Following warm-up, subjects ran on a treadmill beginning at a moderate pace; every 3 minutes the grade and intensity were increased until exhaustion [3]. Heart rate was monitored by a Polar Vantage XL heart rate monitor (Polar Beat, Port Washington, NY, USA). This was conducted at least 2 weeks prior to the scheduled exercise session.
Experimental design and procedures
On the morning of the trial, subjects arrived at the laboratory. They were instructed to have a standard breakfast consisting of two boiled eggs 2 h prior to the test. All subjects were required to avoid any strenuous exercise for 72 h prior to participation in the study. Participants performed a 10-min warm-up consisting of running at 50% O2 max (5 min) and stretching (5 min). Following the warm-up and light stretching, participants ran on a treadmill for 30 min at 75% O2 max. Blood samples were taken from an antecubital forearm vein before exercise, immediately after, 3 h after, and 24 h after exercise. Each sample was taken following 15 min of standing in a resting position (except for the post-exercise sample, which was taken immediately upon cessation of exercise). Subjects consumed 100 mg of celecoxib or placebo immediately following exercise and 12 h after exercise.
Blood sampling and analysis
Approximately 6 ml of whole blood was withdrawn at each time point. 1.5 ml from each sample was added to tubes containing ethylenediaminetetra-acetic acid (EDTA) for determination of leukocyte differentials using a cell counter (K-1000 Sysmax, Japan). Haemoglobin and haematocrit concentrations from whole blood samples were used to estimate plasma volume shifts. All post-exercise samples were corrected for plasma volume change according to the methods of Dill and Costill [5]. 4.5 ml of the blood was allowed to clot at 37.5°C, and centrifuged at 5,000 g for 30 min. Serum was prepared according to standard methods. Serum creatine kinase (CK) was determined using commercially available methods (Roche Hittachi-911 Chemistry Autoanalizer, Germany and Japan). Serum CRP was measured by a nephelometric procedure using commercially available kits (Minineph, ZK044. L.R, Birmingham, UK). For malondialdehyde (MDA) measurement, 0.05 ml serum was added to 0.25 ml of 0.1M TCA and 0.7 ml distilled water, vortexed in a 1.5 ml centrifuge tube for 10 s, centrifuged at 4500 g for 5 min and used for high performance liquid chromatography (HPLC) (Jasco, Japan) analysis [10].
Statistical analysis
All data are expressed as means ± SD. A two (groups) x four (time) analysis of variance (ANOVAs) with repeated measures on time was used to compare group, time, and group x time interactions for each variable. Significant interactions were further explored using a Bonferroni correction analysis. The significance level for this study was set at P < 0.05 for all tests.
RESULTS
Effect of exercise on markers of inflammation
Leukocyte, neutrophil, monocyte and lymphocyte counts, and CRP concentrations before, immediately after, 3 h after, and 24 h after exercise are shown in Table 3. Total leukocyte and neutrophil counts were significantly increased 3 h after exercise in both groups and returned to pre-exercise values 24 h after exercise (P < 0.05). Lymphocyte counts decreased significantly 3 h after exercise in both groups (P < 0.05). There were no significant changes in monocyte counts in either group (P > 0.05). Serum CRP concentration increased significantly immediately after exercise and remained elevated for 24 h after exercise in both groups (P < 0.05). However, no significant changes were found for total leukocyte, neutrophil, monocyte and lymphocyte counts, or CRP concentration between groups (P > 0.05).
TABLE 3.
TOTAL LEUKOCYTE, NEUTROPHIL AND MONOCYTE COUNT
| Parameters | Groups | Pre | Post | 3h | 24h |
|---|---|---|---|---|---|
| Total leukocyte count (n·L-1) | P | 6528 ± 993.1 | 7154 ± 1219.4 | 9720 ± 1353.5* | 6321 ± 1088.3 |
| T | 7071 ± 1003.2 | 7594 ± 1640.9 | 9167 ± 1780.9* | 6648 ± 1442.5 | |
| Blood neutrophil count (n·L-1) | P | 3540 ± 734.9 | 4161 ± 841.2 | 6660 ± 1492.7* | 3386 ±775.2 |
| T | 3798 ±951.3 | 4249 ± 1407.6 | 5935 ± 1605.5* | 3669 ± 971.7 | |
| Blood monocyte count (n·L-1) | P | 394 ± 102.3 | 460 ± 100.0 | 527 ± 81.65 | 385 ± 104.9 |
| T | 467 ± 128.3 | 459 ± 114.4 | 545 ± 130.3 | 464 ± 156.8 | |
| Blood lymphocyte count (n·L-1) | P | 2266 ± 589.2 | 2220 ± 631.2 | 2252 ± 478.8† | 2200 ± 567.5 |
| T | 2441 ± 401.9 | 2525 ± 687.1 | 2401 ± 646.3† | 2167 ± 615.3 | |
| CRP (mg·L-1) | P | 2.0 ± 0.7 | 2.68 ± 0.68* | 3.31 ± 1.03* | 4.19 ± 1.0* |
| T | 1.96 ± 0.6 | 2.51 ± 0.4* | 3.12 ± 0.6* | 4.17 ± 1.2* | |
Note: Data represent mean ± SD.
Indicates significant difference from pre-exercise in both groups (P < 0.05).
Indicates decrease of lymphocytes compared with pre-exercise in both groups (P < 0.05).
Markers of Muscle Damage and Oxidative Stress Following Exercise:
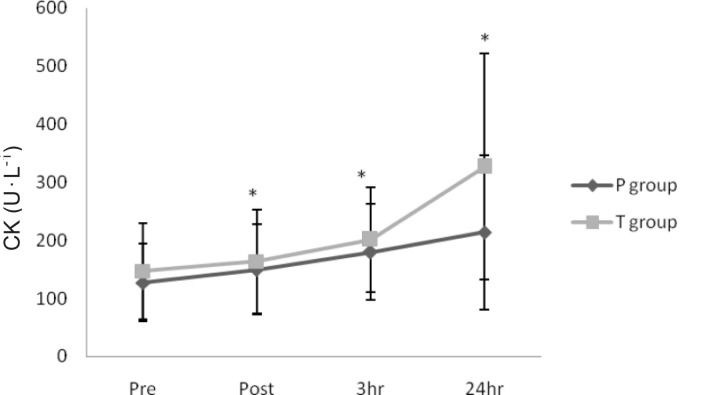
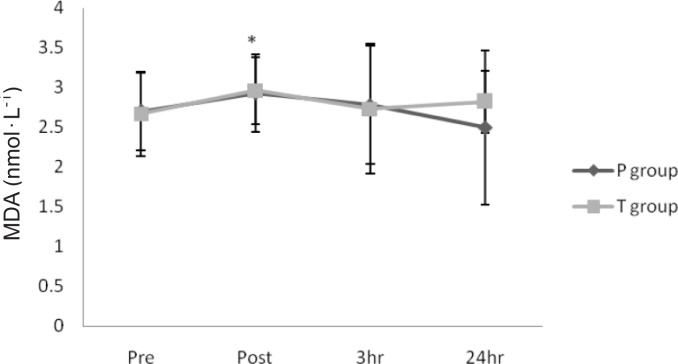
Serum CK activity were significantly increased immediately after, 3 h after, and 24 h after exercise in both groups (P < 0.05) (Figure 1). Serum MDA concentrations were significantly elevated immediately after exercise in both groups (P < 0.05) (Figure 2). However, there were no significant differences in CK or MDA concentration between groups (P > 0.05) (Figures 1 and 2).
FIG. 1.
SERUM CK ACTIVITY BEFORE AND AFTER EXERCISE
Note: * Indicates significant increase compared with pre-exercise in both groups (P < 0.05). Values represent means ± SD (n = 10). P: placebo, T: treatment.
FIG. 2.
SERUM MDA CONCENTRATION BEFORE AND AFTER EXERCISE
Note: * Indicates significant increase compared with pre-exercise in both groups (P < 0.05). Values represent means ± SD (n = 10). P: placebo, T: treatment.
DISCUSSION
The purpose of this study was to assess the effect of acute low-dose celecoxib administration on exercise-induced inflammation, muscle damage and lipid peroxidation markers following intensive aerobic exercise. To date, the efficacy of NSAIDs treatment on muscle damage, inflammation, and lipid peroxidation remains unclear. The equivocal results between studies, some demonstrating no effect [12, 18, 26], and others reporting a reduction in inflammatory markers following exercise [8], make it difficult to determine if NSAIDs provide protection against exercise-induced muscle damage and inflammation. In the present study, we used a selective COX-2 inhibitor and found no significant differences in all inflammatory markers between groups and each time period. These findings suggest that post-exercise administration of celecoxib does not prevent or alleviate exercise-induced inflammation.
There are several possible explanations for the lack of efficacy of celecoxib administration on inflammation markers in the present study compared with other studies: Celecoxib is a single-action NSAID blocking the COX-2 pathway and has no effect on COX-1 and lipoxygenase (LIPOX) pathways of arachidonic acid (AA) metabolism. Therefore, it has been proposed that celecoxib may not prevent the delayed COX-1 or the LIPOX induced inflammation and drift of AA metabolism towards this pathway, leading to muscle damage and inflammation [22]. Secondly, a typical sign of acute inflammation is an increase in total leukocyte count, which occurs within a few hours after muscle damage [27]. Evidence suggests that the inflammatory response following exercise is distinctly different from those accompanying infection or tissue injury [16]. Therefore, physical exercise can be regarded as a prototype of physical stress which induces a pattern of hormonal and immunological responses that is similar to, yet distinctly different from, clinical physical stressors (e.g. surgery, trauma and sepsis). Finally, it is also possible that the concentration of celecoxib at the site of the injury in this study was insufficient to have caused a significant anti-inflammatory effect following the exercise protocol.
In the present study, CK activity, an indirect qualitative index of muscle damage, was significantly elevated immediately after, 3 h after, and 24 h after exercise in both groups. There was no significant difference between the two groups, which suggests a lack of efficacy of celecoxib administration on CK induced from exercise. In agreement with the present study, Hasson et al. [8] and Donnelly et al. [6] reported that NSAIDs had no effect on CK levels following exercise. In contrast, Pizza et al. [19] and Tokmakidis et al. [26] found significantly lower post-exercise CK concentration in individuals consuming NSAIDs. These discrepant findings between studies highlight the need for further research to understand the underlying physiological mechanisms between NSAID consumption and exerciseinduced muscle damage. Future work should incorporate rigorously controlled dietary and NSAID intake prior to and following exercise; this would enable better comparisons between studies.
It has been well documented that both prolonged and intense exercise cause a marked rise in oxidative stress biomarkers [7, 21]. Potential sources of elevated reactive oxygen species (ROS) during exercise include mitochondria, NADPH oxidases, and xanthine oxidases derived from both skeletal muscle and phagocytic cells [20]. Similar to the aforementioned studies, we demonstrated a significant increase in circulating MDA immediately after exercise. We predicted that celecoxib administration would diminish both the immune and oxidative stress response; however, this did not occur. Current exercise and non-exercise data have not provided a clear explanation regarding the efficacy of both selective COX-2 and non-selective COX-2 inhibitors for preventing or attenuating oxidative stress [12]. Exercise and non-exercise studies have shown the ability of the nonselective COX-2 inhibitor aspirin (acetylsalicylic acid) to reduce the post-exercise oxidative stress response, decrease vascular (O2 -.) production, and attenuate angiotensin II-induced oxidative stress [24], whereas selective COX-2 inhibition has been shown to attenuate oxidative stress in the non-exercise setting but not following exercise [12, 13]. In fact, McAnulty et al. demonstrated an increased post-exercise oxidative stress response following NSAID intake compared to controls [12, 13]. While the potential mechanisms remain to be determined, it has been hypothesized that NSAID use along with strenuous exercise may actually increase gut permeability and enhance endotoxin leakage from the gut into the blood, resulting in inflammation and oxidative stress [2]. Given the equivocal findings regarding selective and non-selective COX-2 inhibition on exerciseinduced inflammation and oxidative stress, it is premature to decide whether NSAIDs should be used during exercise as a therapy to combat the inflammatory and oxidative stress response to exercise. Further research should be performed to provide a mechanistic explanation as to why results differ between studies.
CONCLUSIONS
In conclusion, our results indicate that celecoxib use following exercise does not alleviate inflammation, muscle damage, or oxidative stress. The relationship between selective and non-selective COX-2 inhibitors and muscle damage, inflammation, and oxidative stress should be further explored in future studies.
Acknowledgements
The authors wish to thank the Ardabil University of Medical Sciences for their support.
REFERENCES
- 1.Ajith T, Subin J.P, Jacob J, Sanjay P.S, Babitha N.V. Antimutagenic and anti-oxidant activities of the non-steroidal anti-inflammatory drug celecoxib. Clin. Exp. Pharmacol. Physiol. 2005;32:893–888. doi: 10.1111/j.1440-1681.2010.04280.x. [DOI] [PubMed] [Google Scholar]
- 2.Bosenberg A.T, Brock-Utne J.G, Gaffin S.L, Wells M.T, Blake G.T. Strenuous exercise causes systemic endotoxemia. J. Appl. Physiol. 1988;65:106–108. doi: 10.1152/jappl.1988.65.1.106. [DOI] [PubMed] [Google Scholar]
- 3.Bruce R.A, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am. Heart J. 1973;85:546–562. doi: 10.1016/0002-8703(73)90502-4. [DOI] [PubMed] [Google Scholar]
- 4.Clarkson P.M, Hubal M.J. Exerciseinduced muscle damage in humans. Am. J. Phys. Med. Rehabil. 2002;81(Suppl.):S52–69. doi: 10.1097/00002060-200211001-00007. [DOI] [PubMed] [Google Scholar]
- 5.Dill D.B, Costill D.L. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J. Appl. Physiol. 1974;37:247–248. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- 6.Donnelly A.E, Maughan R.J, Whiting P.H. Effects of ibuprofen on exercise-induced muscle soreness and indices of muscle damage. Br. J. Sports Med. 1990;24:91–195. doi: 10.1136/bjsm.24.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher G, Schwartz D.D, Quindry J, Barberio M.D, Foster E.B, Jones K.W, Pascoe D.D. Lymphocyte enzymatic antioxidant responses to oxidative stress following high-intensity interval exercise. J. Appl. Physiol. 2011;110:730–737. doi: 10.1152/japplphysiol.00575.2010. [DOI] [PubMed] [Google Scholar]
- 8.Hasson S.M, Daniels J.C, Divine J.G, Niebuhr B.R, Richmond S, Stein P.G, Williams J.H. Effect of ibuprofen use on muscle soreness, damage, and performance: a preliminary investigation. Med. Sci. Sports. Exerc. 1993;25:9–17. doi: 10.1249/00005768-199301000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Ji L.L. Exercise and oxidative stress: role of the cellular antioxidant systems. Exerc. Sport Sci. Rev. 1995;23:135–166. [PubMed] [Google Scholar]
- 10.Karatas F, Karatepe M, Baysar A. Determination of free malondialdehyde in human serum by high-performance liquid chromatography. Anal. Biochem. 2002;311:76–79. doi: 10.1016/s0003-2697(02)00387-1. [DOI] [PubMed] [Google Scholar]
- 11.Liu R, Sui X, Laditka J.N, Church T.S, Colabianchi N, Hussey J, Blair S.N. Cardiorespiratory Fitness as a Predictor of Dementia Mortality in Men and Women. Med. Sci. Sports Exerc. 2011;44:253–9. doi: 10.1249/MSS.0b013e31822cf717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAnulty S, McAnulty L, Nieman D, Morrow J, Dumke C, Henson D. Effect of NSAID on muscle injury and oxidative stress. Int. J. Sports Med. 2007;28:915–909. doi: 10.1055/s-2007-964966. [DOI] [PubMed] [Google Scholar]
- 13.McAnulty S.R, Owens J.T, McAnulty L.S, Nieman D.C, Morrow J.D, Dumke C. L, Milne G.L. Ibuprofen use during extreme exercise: effects on oxidative stress and PGE2. Med. Sci. Sports Exerc. 2007;39:1075–1079. doi: 10.1249/mss.0b13e31804a8611. [DOI] [PubMed] [Google Scholar]
- 14.Miller S.B. Prostaglandins in health and disease: an overview. Semin. Arthritis Rheum. 2006;36:37–49. doi: 10.1016/j.semarthrit.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Nieman D.C, Konrad M, Henson D.A, Kennerly K, Shanely R.A, Wallner- Liebmann S.J. Variance in the Acute Inflammatory Response to Prolonged Cycling Is Linked to Exercise Intensity. J. Interferon Cytokine Res. 2011;32:12–7. doi: 10.1089/jir.2011.0038. [DOI] [PubMed] [Google Scholar]
- 16.Nosaka K, Clarkson P.M. Variability in serum creatine kinase response after eccentric exercise of the elbow flexors. Int. J. Sports Med. 1996;17:120–127. doi: 10.1055/s-2007-972819. [DOI] [PubMed] [Google Scholar]
- 17.Pedersen B.K. The anti-inflammatory effect of exercise: its role in diabetes and cardiovascular disease control. Essays Biochem. 2006;42:105–117. doi: 10.1042/bse0420105. [DOI] [PubMed] [Google Scholar]
- 18.Peterson J.M, Trappe T.A, Mylona E, White F, Lambert C.P, Evans W.J, Pizza F.X. Ibuprofen and acetaminophen: effect on muscle inflammation after eccentric exercise. Med. Sci. Sports Exerc. 2003;35:892–896. doi: 10.1249/01.MSS.0000069917.51742.98. [DOI] [PubMed] [Google Scholar]
- 19.Pizza F.X, Cavender D, Stockard A, Baylies H, Beighle A. Anti-inflammatory doses of ibuprofen: effect on neutrophils and exercise-induced muscle injury. Int. J. Sports Med. 1999;20:98–102. doi: 10.1055/s-2007-971100. [DOI] [PubMed] [Google Scholar]
- 20.Powers S.K, Jackson M.J. Exercise induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quindry J, Stone W, King J, Broeder C. The effects of acute exercise on neutrophils and plasma oxidative stress. Med. Sci. Sports Exerc. 2003;35:1145–1139. doi: 10.1249/01.MSS.0000074568.82597.0B. [DOI] [PubMed] [Google Scholar]
- 22.Revathi S, Gupta A.K, Soni L.K, Kavitha S, Wagh R, Kaskhedikar S.G. Rationalization of physicochemical characters of 1,5-diarylpyrazole analogs as dual (COX-2/LOX-5) inhibitors: a QSAR approach. J. Pharm. Biomed. Anal. 2006;42:283–289. doi: 10.1016/j.jpba.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 23.Sabia S, Dugravot A, Kivimaki M, Brunner E, Shipley M.J, Singh-Manoux A. Effect of Intensity and Type of Physical Activity on Mortality: Results From the Whitehall II Cohort Study. Am. J. Public Health. 2011;102:698–704. doi: 10.2105/AJPH.2011.300257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinberg J, Gainnier M, Michel F, Faucher M, Arnaud C, Jammes Y. The post-exercise oxidative stress is depressed by acetylsalicylic acid. Respir. Physiol. Neurobiol. 2002;130:189–199. doi: 10.1016/s0034-5687(02)00002-6. [DOI] [PubMed] [Google Scholar]
- 25.Tauler P, Aguiló A, Gimeno I, Guix P, Tur J, Pons A. Different effects of exercise tests on the antioxidant enzyme activities in lymphocytes and neutrophils. J. Nutr. Biochem. 2004;15:479–484. doi: 10.1016/j.jnutbio.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Tokmakidis S.P, Kokkinidis E.A, Smilios I, Douda H. The effects of ibuprofen on delayed muscle soreness and muscular performance after eccentric exercise. J. Strength Cond. Res. 2003;17:53–59. doi: 10.1519/1533-4287(2003)017<0053:teoiod>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.Walsh N. P, Gleeson M, Shephard R.J, Woods J.A, Bishop N.C, Fleshner M, Simon P. Position statement. Part one: Immune function and exercise. Exerc. Immunol. Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- 28.Warden S.J. Cyclo-oxygenase-2 inhibitors: beneficial or detrimental for athletes with acute musculoskeletal injuries? Sports Med. 2005;35:271–283. doi: 10.2165/00007256-200535040-00001. [DOI] [PubMed] [Google Scholar]
- 29.Zhao S.P, Deng P, Huang H.G, Xu Z.M, Dai H.Y, Hong S.C, Zhou H.N. Expression of COX-2 mRNA in peripheral blood monocytes from patients with acute myocardial infarction and its significance. Clin. Chem. 2005;51:2170–2173. doi: 10.1373/clinchem.2005.054288. [DOI] [PubMed] [Google Scholar]