Abstract
Lead is a highly neurotoxic agent that particularly affects the developing central nervous system. In the current study we investigated the neuroprotective effects of exercise training and/or diferuloyl methane (DM) supplement, which is known as curcumin, on lead acetate-induced neurotoxicity in the rat hippocampus. Sixty rats were randomly divided into six groups: 1) lead acetate, 2) DM supplement, 3) endurance training, 4) training+ DM supplement, 5) sham and 6) base. The rats in the training groups performed treadmill running consisting of 15 to 22 m · min-1 for 25 to 64 min, 5 times a week for 8 weeks. All groups except sham received lead acetate (20 mg · kg-1), whereas the sham group received DM solvent. In addition, the DM and training + DM groups received DM solution (30 mg · kg-1) intraperitoneally. Chronic administration of lead acetate resulted in a significant increase in the malondialdehyde (MDA) in plasma, but not in the hippocampus. In addition, it led to significantly decreased brain-derived neurotrophic factor (BDNF) in the hippocampus and total antioxidant capacity (TAC) levels, as compared to the sham group. Treadmill running, DM supplementation, or both resulted in a significant decrease in MDA levels and significantly increased BDNF and TAC levels, as compared to the lead acetate group. These results provide a rationale for an inhibitory role of DM supplement and regular exercise in the attenuation of lead-induced neurotoxicity.
Keywords: lead, endurance exercise, diferuloyl methane supplement, BDNF, oxidative stress
INTRODUCTION
Epidemiological studies have established a link between ambient air pollutants and health [17]. Lead has been detected in almost all phases of environmental and biological systems. It is related to a broad range of physiological, biochemical, and behavioural dysfunctions in humans and in experimental animals [3]. It has now become clear that high to moderate doses of lead exposure stimulate free radicals, resulting in oxidative damage to critical biomolecules, lipids, proteins and DNA, as well as adversely affecting the antioxidant defence systems of cells [18]. The depletion and changes in the activity of various antioxidant enzymes indicative of lipid peroxidation have been implicated in lead-induced oxidative tissue damage [3]. Brain tissue is particularly vulnerable to apoptosis due to oxidative stress of ROS related to various factors such as high-level utilization of oxygen in the presence of relatively weak antioxidant defence systems and complex chemical reactions for production of diverse neurotransmitters [8]. Studies on humans show that even blood lead levels of 10 µg · dL-1 may cause cognitive deficits [21]. The brain region, including the hippocampus, not only plays a major role in memory and learning, but produces neurons during the process of growth. Increased production of ROS has a high possibility of degrading neurons in the hippocampus and deteriorating cognitive and memory functions [8].
Lifestyle factors such as diet and exercise may provide beneficial effects on hippocampus function. Oiae et al. suggested that regular exercise training increased the production of neurotrophic factors such as brain-derived neurotrophic factor (BDNF), especially in the hippocampus [18], a major hub for learning and memory formation [22]. BDNF is one of the most versatile and important neurotrophic factors in the brain [8]. It has been reported that increased BDNF might be related to improved cognitive function such as memory and learning by elevating the rate of cellular survival and division as well as producing neurons [18]. It has been suggested that regular exercise could strengthen antioxidant protection of the hippocampus and BDNF mediates the protection of neurons, which in turn protects against oxidative stress of the hippocampus caused by ROS [8]. On the other hand, over thousands of years, diet, in conjunction with other lifestyle factors such as exercise, have had a crucial role in shaping cognitive capacity and brain evolution [15]. Polyphenols are natural substances that are present in plants, fruits and vegetables including olive oil and tea. The yellow pigment extracted from the rhizome of Curcuma longa, diferuloyl methane (DM), a polyphenolic non-flavanone compound, is the pharmacologically active substance of turmeric. DM is nontoxic and has antioxidant, anti-inflammatory and anti-proliferative activities. DM shows antioxidant activity equivalent to vitamins C and E [6].
Despite the fact that lead can induce oxidative stress, these studies have only identified effects of exercise and/or antioxidants on mental health without considering air pollutants. Moreover, there is less information with respect to simultaneous effects of lifestyle, including aerobic regular training, antioxidant supplementation, or both, on the oxidant/antioxidant process and brain function during chronic exposure to lead acetate. Therefore, the present study was designed to study the effects of aerobic exercise, DM supplementation, or both, on BDNF in the hippocampus and also the significance of the malondialdehyde: total antioxidant capacity (MDA: TAC) ratio as a novel indicator of oxidative stress in the plasma and hippocampus of rats chronically exposed to lead acetate groups, which may be useful to optimize and monitor antioxidant therapy.
MATERIALS AND METHODS
Animals and experimental environment
The experimental protocol was approved by the Department of Physiology, University of Mazandaran, and was performed according to guiding procedures in the Care and Use of Animals, prepared by the Council of the American Physiological Society. Sixty male Wistar rats, 8 weeks of age (initial body weight of 240 ± 20 g), were obtained from the Laboratory of Animal Bearing and Multiplying at the Pasture Institute of Iran. Each rat was housed in a single standard cage of polycarbonate (20×15×15), made at the Pasture Institute of Iran, in a large airconditioned room with a controlled temperature of 22 ± 2°C, lightdark cycles 12:12 hours and humidity of 50 ± 5%. Measurements from the pollution determination station of the Iranian Meteorological Organization determined that the pollutant standard index (PSI) was in the normal range. Rats were fed with a standard rat chow provided by Pars Institute for Animal and Poultry with a daily regimen of 10 · 100-1 g body weight for each rat. Water was available ad libitum.
Experimental procedures
Rats were familiarized with the laboratory environment and running on the treadmill, and then were randomly assigned into six experimental groups of 10 rats as follows: Group 1 – lead acetate (Pb), exposed to lead at a concentration of 25 mg · kg-1 in the form of a water solution of lead acetate (for intraperitoneal injection), 3 days weekly for 8 wks; Group 2 – Pb + DM received DM supplement 30 mg · kg-1 5 days weekly for 8 wks (i.p.); Group 3 – endurance training (Pb + training); the rats in this group similarly received lead acetate and in addition they performed progressive running exercise of 15 to 22 m · min-1 for 25 to 64 min, 5 times a week; Group 4 – training and DM supplement (Pb + training + DM); rats in this group performed the same training protocol described above and in addition received lead acetate and DM supplementation; Group 5 – shamoperated group (sham); these rats received water and ethyl oleate, in the same manner and for the same duration as the other groups; and Group 6 – base (control); these rats received nothing.
Exercise training
All rats were acclimatized to ambient rearing conditions for 4–5 days in group housing conditions (four rats per cage) and then habituated to run on a treadmill (KN-73, Natsume Ltd., Japan), 5 sessions in the first week. Thus, the running speed and time were gradually increased from 15 to 22 m · min-1 and from 25 to 64 min. At the end of the belt were stationary wire loops, which were electrified. A mild shock (0.75 mA, 500 ms duration, 0.5 Hz rate) was delivered through these loops to motivate the rats to continuously walk on the moving belt and thus avoid foot shock. The wire loops were activated during all exercise sessions, and an experimenter monitored all treadmill sessions. Rats quickly learned to stay on the belt and avoid shock, except for one rat, which would not stay on the moving belt, and thus was quickly removed from the exercise group.
Lead acetate and DM supplementation
We are replicating a previously reported lead dosing regimen that caused oxidative stress; thus, the doses of DM supplement and lead acetate were 30 and 20 mg · kg-1, respectively [11]. Lead acetate (Sigma) was solubilised in Milli-Q water, and DM was solubilised in 50% ethanol. In order to perform intraperitoneal (i.p.) injections, DM was solubilised in ethyl oleate and was injected at a dose of 30 mg · kg-1. DM supplement was protected from light during the time of the experiment [11].
Sampling and tissue collection
After 8 weeks of treatment for each group followed by 24 hours of resting and after 12-14 hours overnight fasting, the animals were anaesthetized by intraperitoneal injection of a mixture of xylazine and ketamine (i.p). Cardiac blood samples were collected 24 h after the last dose of treatment. The blood samples were first centrifuged at 3,000 rpm for 15 minutes within 30 minutes of collection, and then stored at -80°C before assay and serum was separated for biochemical estimations of TAC and MDA. Brains were rapidly removed and the two hemispheres separated along the midline. The hippocampus from each hemisphere was then micro-dissected and frozen in liquid nitrogen and subsequently stored at -80°C for future analysis.
Analyses of markers
Brain-derived neurotrophic factor (BDNF) assay
For protein extraction, the hippocampus was homogenized in a lysis buffer (18 ml · mg-1 tissue) containing 137 mM NaCl, 20 mM Tris–HCl (pH 8.0), 1% NP40, 10% glycerol, 1 mM PMSF, leupeptin (1 mg · ml-1), sodium vanadate (0.5 mM), and AEBSF (100 mg · ml-1) [1]. Samples were then centrifuged for 3 min (14 000 rpm, 3 min, 4°C) and supernatant collected and stored at -20°C. The BDNF protein level was determined by a BDNF ELISA kit according to the manufacturer's recommendations.
Malondialdehyde (MDA) assay
Lipid peroxidation levels in the tissue homogenate were measured with a thiobarbituric-acid reaction by the method of Ohkawa et al. [19]. Sample homogenates (1 ml) were incubated at 37°C in an oscillating water bath for one hour. At the end of the incubation period, 0.5 ml of BHT (0.5 mg/ml in absolute ethanol) and 1 ml of TCA (25%) were added. The tubes were sealed and heated for 10 minutes in a boiling water bath to release MDA (the end product of lipid peroxidation) from proteins. To avoid adsorption of MDA to insoluble proteins, the samples were cooled to 4°C and centrifuged at 2000 x g for 20 minutes. Following centrifugation, 2 ml of the protein-free supernatant was removed from each tube and 0.5 ml of TBA (butyl hydroxytoluene) (0.33%) was added to this fraction [11]. All tubes were heated for one hour at 95°C in a water bath. After cooling, the TBA-MDA complexes were extracted with 2 ml of butanol. The light absorbance was read at 532 nm on a spectrophotometer and MDA levels were determined from a standard curve that was generated from 1,1,1,3 tetramethoxypropane. The results are represented as nmol/mg tissue.
Total antioxidant capacity (TAC) assay
Serum TAC was measured using a commercially available kit (Randox Laboratories, Crumlin, UK) as previously described [12]. In this method, the most potent radical, hydroxyl radical, is produced. First, a ferrous ion solution is mixed with hydrogen peroxide. The sequentially produced radicals such as brown coloured dianisidinyl radical cations, produced by the hydroxyl radical, are potent radicals. The antioxidative effect of the sample against the potent free radical reactions is then measured. The assay has excellent precision values, which are lower than 3%. The results are expressed in µmol · ml-1. The concentration of lead was detected by means of atomic absorption spectrophotometry (AAS).
Statistical analysis
Statistical analysis was performed using a commercial software package (SPSS version 16.0 for Windows). Results are expressed as means ± SE. Data for BDNF and MDA: TAC ratio were normally distributed after log transformation. Prior to formal statistical testing, all data were assessed for assumptions of normality and the assumption for equality of variance. The assumption of sphericity for the ANOVA was tested using Mauchly's method. A one-way ANOVA was used to detect statistical difference between groups. Tukey tests were performed to assess differences in the mentioned markers between groups. The differences were considered significant at p < 0.05.
RESULTS
Mean values for body mass, brain mass and brain-body mass ratio levels in rats chronically exposed to lead acetate are shown in Table 1. An insignificant decrease in body mass and a trend for a decrease in brain mass was detected after lead acetate administration (20 mg · kg-1), as compared to the other groups. Aerobic training and DM supplementation protocols during chronic exposure to lead acetate caused preservation in body and brain mass (Table 1).
TABLE 1.
EFFECTIVENESS OF THE 8-WEEK TRAINING AND SUPPLEMENTATION PROTOCOLS ON BODY MASS, BRAIN MASS, AND BRAIN-BODY MASS RATIO IN RATS CHRONICALLY EXPOSED TO LEAD ACETATE.
| Markers | B | SH | L | TL | DML. | DMTL. |
|---|---|---|---|---|---|---|
| Body mass (g) | 342 ± 25† | 341 ± 34† | 306 ± 33 | 328 ± 20† | 322 ± 22 | 343 ± 34† |
| Brain mass (g) | 1.738 ± 0.149 | 1.705 ± 0.149 | 1.618 ± 0.192 | 1.765 ± 0.209 | 1.723 ± 0.101 | 1.794 ± 0.185 |
| Brain/body mass ratio | 0.0053 ± 0.006 | 0.0051 ± 0.005 | 0.0053 ± 0.006 | 0.0049 ± 0.011 | 0.0054 ± 0.005 | 0.0052 ± 0.054 |
Note: Values are means ± SD for 8 rats; Abbreviations: B (base); SH (Sham); L (Lead); TL (Training + Lead); DML. (DM supplement + Lead); DMTL. (DM supplement + Training + Lead) groups.
Significant difference compared to lead acetate group (p < 0.05).
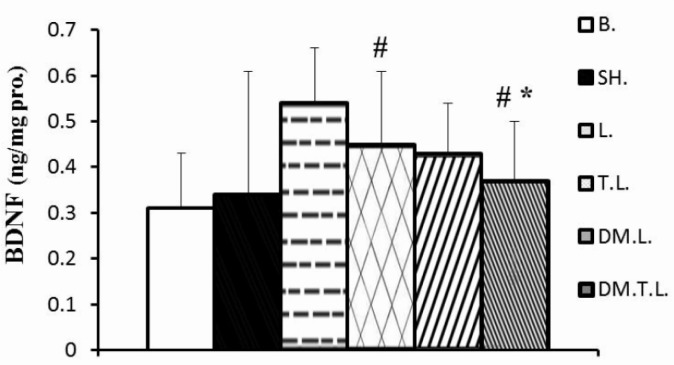
Changes in BDNF, MDA levels in the hippocampus and MDA: TAC ratio in plasma are shown in Figures 1–3. The administration of lead at the concentration of 20 mg · kg-1 for 8 weeks resulted in a decrease in BDNF levels by 16.5 and 17% as compared to the base and sham group, respectively. In contrast, DM supplement and/or exercise training significantly increased the BDNF in hippocampus (45% and 76%) levels, as compared to the lead acetate group. The mixture of training and DM supplement was more effective than DM + lead and/or training + lead alone treatment (Figure 1).
FIG. 1.
HIPPOCAMPUS BDNF CONCENTRATIONS IN EXPERIMENTAL ANIMALS
Note: Statistical significance p < 0.05; * Significant difference compared to the control groups (base and sham); # Significant difference compared to the lead group. Abbreviations: B (base); SH (Sham); L (Lead); TL (Training + Lead); DM.L. (DM supplement + Lead); DM.T.L. (DM supplement + Training + Lead) groups.
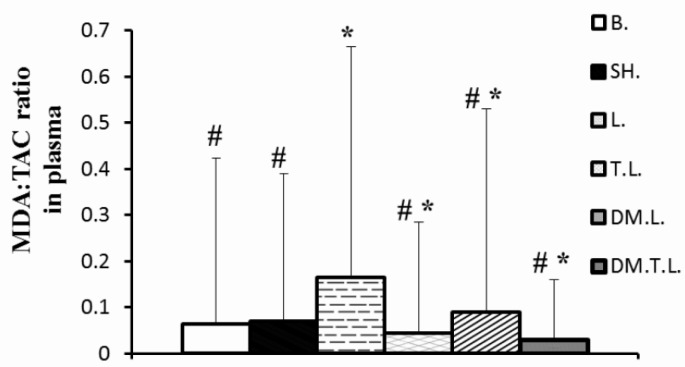
FIG. 3.
SHOWS MDA:TAC RATIO CONCENTRATIONS IN PLASMA IN EXPERIMENTAL ANIMALS
Note: * Significant difference compared to the control groups (base and sham); # more significant than in the lead group. Abbreviations;: B (base); SH (Sham); L (Lead); TL (Training + Lead); DM.L. (DM supplement + Lead); DM.T.L. (DM supplement + Training + Lead) groups
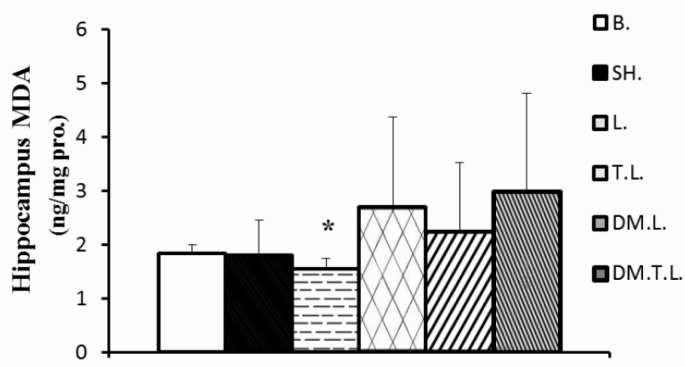
On the other hand, administration of lead acetate (20 mg · kg-1) caused an increase in the concentration of the hippocampus MDA by 59 and 0.31% and MDA: TAC ratio in plasma by 161.9 and 135.7% as compared to the base and sham group, respectively. In contrast, DM supplement and/or exercise training insignificantly decreased the MDA in the hippocampus (20%, 17%, respectively) and significantly decreased the MDA: TAC ratio in plasma (45.45 and 72.72%, respectively) as compared to lead acetate. However, co-treatment with DM supplement and training was more effective to decrease lead-induced oxidative stress than the other two treated groups (Figures 2 and 3).
FIG. 2.
HIPPOCAMPUS MDA CONCENTRATIONS IN EXPERIMENTAL ANIMALS
Note: * Significant difference compared to the control groups (base and sham); Abbreviation; s: B (base); SH (Sham); L (Lead); TL (Training + Lead); DM.L. (DM supplement + Lead); DM.T.L. (DM supplement + Training + Lead) groups.
DISCUSSION
Lead is a ubiquitous environmental and industrial pollutant that assaults the CNS through several cellular and molecular pathways to cause disease [7]. The present study demonstrates a protective effect of regular exercise and DM supplementation against lead neurotoxicity in the hippocampus of rats that were chronically exposed to lead acetate. Enhancement of MDA associated with reduced TAC and BDNF may lead to neurodegenerative conditions resulting from lead toxicity via disrupting oxidant/antioxidant balance, whereas lifestyle factors such as regular exercise and DM supplementation reverse this process through increasing BDNF and improving antioxidant defence systems. Other recent studies have linked environmental exposures causing oxidative stress with neurodegenerative diseases and aging [18]. Pb causes significant changes in oxidative stress in different brain regions, particularly in the hippocampus and cerebral cortex, which are found to be more vulnerable to Pb-induced neurotoxicity [20]. Several mechanisms including cholinergic dysfunction, glutamate receptor alterations, impaired antioxidant defence enzymes in the brain and enhanced oxidative stress have been suggested to be causative factors with lead neurotoxicity [21]. Others have suggested that oxidative stress caused by lead is associated with disrupted prooxidant/antioxidant balance [16]. Generation of highly reactive oxygen species in the aftermath of lead exposure may result in systematic mobilization and depletion of the cell's intrinsic antioxidant defences. Lipid peroxidation appears to be markedly enhanced in the brain of lead-treated rats [5], which concurs with our findings of decreased production of TAC and increased MDA.
The ability of specific aspects of lifestyle such as diet, exercise and other factors that modulate mental function is becoming increasingly recognized. There is evidence to suggest that regular exercise improves brain function and leads to structural, biochemical, and physiological adaptations through a variety of pathways. Exercise counteracts deteriorative effects on the central nervous system caused by inactivity. High levels of ROS exceed the adaptive tolerance of cells, resulting in significant oxidative damage, apoptosis, and necrosis. Based on the theory of active oxygen, the aging process of an organic system and the subsequent decline of its functions can be primarily attributed to excessive expression of active oxygen, but can be reduced by strengthening the antioxidant system [8]. This notion was consistently demonstrated in this study, where regular exercise and antioxidant supplementation during chronic exposure to lead acetate showed an increased level of TAC. On the other hand, particularly the combined treatments of exercise and supplementation had a beneficial effect on reduction of oxidative stress by ROS suppression. Although apoptosis in the neurons of the hippocampus was not examined by the TUNEL assay in the present study, the study results of Chang-Hun Oiae and Sok Park indicated that regular exercise and lipoic acid supplementation could lead to strengthening of the antioxidant enzymatic system in the body [8]. However, further research is necessary to determine the effect of exercise and DM supplementation on detailed mechanisms of apoptosis and to demonstrate the antioxidant benefits of BDNF using scientifically sound research schemes. Studies have shown exercise-induced regulation of BDNF transcripts in the rat hippocampus and postulated that it may help to increase the brain's resistance to damage and neurodegeneration that occurs with aging [23]. Exercise training likely results in an increase in antioxidant defences such as TAC and thus increased resistance and tolerance to oxidative challenges. Although some studies suggest that exercise training enhances antioxidant capacity, the causal mechanisms are not known. It has also been shown that high intensity endurance exercise increases susceptibility to oxidation; a training regimen at 65% O2max has also been reported to decrease plasma TAC in rats [4]. Interestingly, a dose–response relationship between exercise duration/intensity and health-related quality of life has been reported, in which the best outcomes are associated with moderate exercise. This variability is probably related to differences in the exercise regimen (voluntary versus forced), in combination with the intensity (in forced exercise models) and duration of exercise exposure. In addition, although some studies show improvements after 1 week of exercise, most benefits have been associated with longer-term exercise (3–12 weeks) [10].
Dietary factors can affect multiple brain processes by regulating neurotransmitter pathways, synaptic transmission, membrane fluidity and signal-transduction pathways. The curry spice DM supplement, a traditional food preservative and medicinal herb in India, is relatively non-toxic and has few side effects at doses greater than the low doses that have been tested in mice. Despite concerns about poor oral bioavailability, the DM supplement has at least 10 known neuroprotective actions. Accumulating cell culture and animal model data show that the dietary DM supplement is a strong candidate for use in the prevention or treatment of major disabling age-related neurodegenerative diseases such as Alzheimer's, Parkinson's, and stroke [9]. It is a strong antioxidant that seems to protect the brain from lipid peroxidation and nitric-oxide-based radicals [14]. Interestingly, DM not only exhibits antioxidative and free radical scavenging properties, but also increases the activities of other antioxidant enzymes, such as SOD, CAT and GPx [2]. An increasing number of studies have shown that DM may be neuroprotective. Gomez-Pinilla recently found that DM, as a dietary supplement, counteracts cognitive dysfunction resulting from elevated ROS after brain trauma [13]. DM also produces marked increases in BDNF levels in the brain. Accordingly, when dietary supplementation and exercise are combined, inhibition of lead-induced antioxidant/oxidant imbalance, increases in BDNF and decreases in hippocampus oxidative stress appear to be more pronounced than when either intervention is implemented by itself.
CONCLUSIONS
In summary, our study provides a rationale for the inhibitory role of DM supplement and regular exercise in attenuating lead-induced neurotoxicity. Treatment with exercise and/or DM supplement reduced the accumulation of lead-induced oxidative stress in rat hippocampus. Moreover, the combination of training and DM supplement can make the brain more resistant to oxidative damage than DM + lead and/or training + lead alone treatment.
REFERENCES
- 1.Adlard P.A, Perreau V.M, Cottnan C.W. The exercise-induced expression of BDNF within the hippocampus varies across life-span. Neurobiol. Aging. 2005;26:511–520. doi: 10.1016/j.neurobiolaging.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal R, Goel S.K, Behari J.R. Detoxification and antioxidant effects of curcumin in rats experimentally exposed to mercury. J. Appl. Toxicol. 2010;30:457–468. doi: 10.1002/jat.1517. [DOI] [PubMed] [Google Scholar]
- 3.Ahamed M, Siddiqui M.K. Low level lead exposure and oxidative stress: current opinions. Clin. Chim. Acta. 2007;383:57–64. doi: 10.1016/j.cca.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 4.Alipour M, Mohammadi M, Zarghami N, Ahmadias N. Influence of chronic exercise on red cell antioxidant defense, plasma malondialdehyde and total antioxidant capacity in hypercholesterolemic rabbits. J. Sports Sci. Med. 2006;5:682–691. [PMC free article] [PubMed] [Google Scholar]
- 5.Antonio-García M.T, Massó-Gonzalez E.L. Toxic effects of perinatal lead exposure on the brain of rats: involvement of oxidative stress and the beneficial role of antioxidants. Food Chem. Toxicol. 2008;46:2089–2095. doi: 10.1016/j.fct.2008.01.053. [DOI] [PubMed] [Google Scholar]
- 6.Ataie A, Sabetkasaei M, Haghparast A, Hajizadeh Moghaddam A, Ataie R, Nasiraei Moghaddam S.H. An investigation of the neuroprotective effects of curcumin in a model of homocysteine - induced oxidative stress in the rat's brain. DARU. 2010;18:128–136. [PMC free article] [PubMed] [Google Scholar]
- 7.Block M.L, Calderón-Garcidueñas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32:506–516. doi: 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole G.M, Teter B, Frautschy S.A. Neuroprotective effects of curcumin. Adv. Exp. Med. Biol. 2007;595:197–212. doi: 10.1007/978-0-387-46401-5_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cotman C.W, Berchtold N.C, Christie L.A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004;37:277–285. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Gomez-Pinilla F, Kostenkova K. The influence of diet and physical activity on brain repair and neurosurgical outcome. Surg. Neurol. 2008;70:333–336. doi: 10.1016/j.surneu.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez-Pinilla F. The influences of diet and exercise on mental health through hormesis. Ageing Res. Rev. 2008;7:49–62. doi: 10.1016/j.arr.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gómez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat. Rev. Neurosci. 2008;9:568–578. doi: 10.1038/nrn2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu P.C, Guo Y.L. Antioxidant nutrients and lead toxicity. Toxicology. 2002;180:33–44. doi: 10.1016/s0300-483x(02)00380-3. [DOI] [PubMed] [Google Scholar]
- 15.Makri A, Stilianakis N.I. Vulnerability to air pollution health effects. Int. J. Hyg. Environ. Health. 2008;211:326–336. doi: 10.1016/j.ijheh.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Migliore L, Coppedè F. Environmental- induced oxidative stress in neurodegenerative disorders and aging. Mutat. Res. 2009;674:73–84. doi: 10.1016/j.mrgentox.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 18.Oiae C.H, Park S. Effect of Regular Exercise and Dl-a-Iipoic Acid Supplementation on BDNF, Caspase-3 Proteins and Apoptosis in Aging-Induced Rate Hippocampus. Int. J. Applied Sports Sci. 2008;20:78–95. [Google Scholar]
- 19.Prasanthi R.P, Devi C.B, Basha D.C, Reddy N.S, Reddy G.R. Calcium and zinc supplementation protects lead (Pb)-induced perturbations in antioxidant enzymes and lipid peroxidation in developing mouse brain. Int. J. Dev. Neurosci. 2010;28:161–167. doi: 10.1016/j.ijdevneu.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Daniel S, Limson J.L, Dairam A, Watkins G.M, Daya S. Through mental binding, curcumin protects against lead- and cadmiuminduced lipid peroxidation in rat brain homogenates and against lead-induced tissue damage in rat brain. J. Inorg. Biochem. 2004;98:266–275. doi: 10.1016/j.jinorgbio.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Shukla P.K, Khanna V.K, Khan M.Y, Srimal R.C. Protective effect of curcumin against lead neurotoxicity in rat. Hum. Exp. Toxicol. 2003;22:653–658. doi: 10.1191/0960327103ht411oa. [DOI] [PubMed] [Google Scholar]
- 22.Soya H, Nakamura T, Deocaris C.C, Kimpara A, Iimura M, Fujikawa T, Chang H, McEwen B.S, Nishijima T. BDNF induction with mild exercise in the rat hippocampus. Biochem. Biophys. Res. Commun. 2007;358:961–967. doi: 10.1016/j.bbrc.2007.04.173. [DOI] [PubMed] [Google Scholar]
- 23.Strasser A, Skalicky M, Hansalik M, Viidik A. The Impact of Environment in Comparison with Moderate Physical Exercise and Dietary Restriction on BDNF in the Cerebral Parietotemporal Cortex of Aged Sprague-Dawley Rats. Gerontology. 2006;52:377–381. doi: 10.1159/000095117. [DOI] [PubMed] [Google Scholar]