Abstract
Plasma gelsolin (pGSN) produced by muscle is an abundant protein of extracellular fluids capable of severing actin filaments and eliminating actin from the circulation. Additionally, pGSN modulates the cellular effects of some bioactive lipids. In this study we test the hypothesis that hormonal and metabolic adaptations to exercise are associated with changes in gelsolin concentration in blood. Plasma samples were collected from twenty healthy males recruited from untrained (UT, n=10) and endurance trained (ET, n=10) groups that performed 30-60 minutes of exercise on a cycloergometer at a workload corresponding to 70% of VO2max. Gelsolin concentration was determined by quantitative Western blot analysis with an anti-human gelsolin antibody. The gelsolin concentration in UT and ET subjects before starting exercise ranged from 104 to 330 and 163 to 337 µg · ml-1 respectively. After 30 minutes of exercise we observed a significant decrease of plasma gelsolin in the UT group (p<0.05) while the gelsolin concentration in the ET group rose on average from 244 to 271 µg · ml-1. However, this increase did not reach statistical significance. Endurance training might increase the ability of muscle tissue to express plasma gelsolin as part of an adaptive mechanism.
Keywords: extracellular gelsolin, blood, physical exercise
INTRODUCTION
Plasma gelsolin (pGSN) represents one of the three currently described isoforms of gelsolin [26, 28]. This PIP2- and calcium-regulated protein circulates in blood plasma at a concentration of ∼250 µg · ml-1 and is primarily involved in the rapid severing and removal of actin filaments released into the bloodstream from dead and injured cells [17]. This function prevents several toxic effects of circulating F-actin, which activates platelets, slows down fibrinolysis by binding to plasmin [3, 17], increases blood viscosity [11], and compromises the viability of endothelial cells [9]. Additionally, direct interaction of gelsolin with bioactive lipids such as lipopolysaccharide [1], lipoteichoic acid [2, 4], lysophosphatidic acid [19], platelet-activating factor [23] and sphingosine 1-phosphate [6] reduces the ability of these molecules to induce cell activation and gelsolin's ability to sever F-actin. All gelsolin isoforms are encoded by a single gene located on chromosome 9 in humans [15]. Skeletal, cardiac, and smooth muscles have large amounts of pGSN mRNA, account for a large fraction of body mass and total protein synthesis, and they are the major source of plasma gelsolin [15]. So far, the factors that regulate pGSN expression in muscles and pGSN concentration in blood are not defined [5, 18]. The observed decrease of blood gelsolin concentration in patients with a variety of conditions, including acute respiratory distress syndrome (ARDS) [21], acute liver injury [12], sepsis [16], malaria [25], subarachnoid hemorrhage [7], traumatic brain injury [27] and neuroinfection [13], lacks clear pathophysiological mechanisms. In this study we aim to evaluate changes in pGSN concentration in male volunteers occurring during 30–60 minutes of a workload corresponding to 70% of O2 max.
MATERIALS AND METHODS
Recruitment of subjects
Healthy males (n=20) were recruited from students of the Faculty of Physical Education and Sport at the Józef Piłsudski University of Physical Education. They were assigned to either the untrained (UT, n=10) or endurance trained (ET, n=10) group. Requirements in study participation included lack of smoking, chronic disease and current medication use. Students who did not participate in any physical activity except for compulsory classes at the university (approx. 4 h per week of low intensity exercise) were included in the UT group. Athletes in the ET group were long distance runners (average experience of 1.7–4.3 years, between 7 and 10 h per week). For each participant body composition was determined after overnight fasting using an Akern BIA-101 analyzer (Florence, Italy). The study was approved by the Ethical Committee for Human Studies of the Józef Piłsudski University of Physical Education. All subjects gave their informed consent prior to their inclusion in the study.
Exercise protocol
The study was conducted on two separate days over a 1-week period. In the first step (day one), peak oxygen uptake ( O2 max) was determined using a graded exercise test on a cycloergometer (Ergomedic 839E, Monark Exercise AB, Vansbro, Sweden) starting at 1 W · kg-1 of body weight for 3 min followed by increments of 0.75 W · kg-1 every 3 min until exhaustion. Oxygen consumption was monitored using an online system (Start 2000, MES, Cracow, Poland). In the second step (six or seven days later), the subjects performed either 30 (UT group) or 60 (ET group) minutes of exercise on the same cycloergometer at a workload estimated to elicit a load of 70% of O2 max. The period of exercise was chosen considering the differences in tolerance to exercise between the UT and ET groups (in a pilot study we observed that only 10% of UT subjects can complete the same 60 min of exercise to which the ET group was subjected in our experimental setting). Subjects were instructed to abstain from alcohol use during the 2 days preceding each visit to the laboratory. Both exercise bouts were performed in the morning, 1 h after a light carbohydrate-rich breakfast.
Blood collection
Blood samples were drawn through a catheter inserted into an antecubital vein into 4.3 ml plastic tubes containing 0.43 ml of 0.106 M sodium citrate solution as an anticoagulant (Sarstedt, Nümbrecht, Germany). Samples were taken just before exercise, after 30 and 60 (ET group only) minutes of pedaling, and after a 30-min rest. The catheter was kept patent by flushing it regularly with isotonic NaCl solution. Immediately after sampling, blood was centrifuged at 300 g for 10 min at room temperature and plasma was transferred to a fresh plastic tube. Supernatant was then transferred to a fresh plastic tube and re-centrifuged at 5000 g for 10 min to obtain platelet-free plasma. All samples were stored at -80°C until analysis. Plasma protein concentration was measured using the BCA protein assay kit (Sigma). Bovine serum albumin (fatty acid free, Sigma) was used as a standard. Three volunteers from the ET group were unable to complete the exercise protocol.
Quantitative immunoblotting
Plasma samples were boiled in the presence of sample buffer for 10 min and subjected to electrophoresis on 10% sodium dodecyl sulfate (SDS)-polyacrylamide. Samples loaded in each gel were accompanied by recombinant human plasma gelsolin standard (loaded in a concentration range comparable to the gelsolin concentration in the samples). After electrophoresis, proteins were transferred to PVDF membranes (Amersham Biosciences, Little Chalfont, UK). The membrane was then blocked by incubation in 5% (w/v) non-fat dry milk dissolved in TBS-T (150 mM NaCl, 50 mM TRIS, 0.05% Tween 20, pH=7.4). Transferred proteins were probed with a monoclonal anti-human gelsolin antibody (Sigma, St. Louis, MO, USA) used at a 1:10000 dilution in TBS-T. After incubation with HRP-conjugated secondary antibodies (1:20000 dilution), immunoblots were developed with the Fuji Film LAS-300 system using an ECL Plus HRP- targeted chemiluminescent substrate (Amersham Biosciences, Little Chalfont, UK). Western blots were quantified by densitometric analysis (Image Gauge - version 4.22 software; Fuji Photo Film Co, USA). The standard curve for determination of gelsolin concentration was prepared by loading 5, 10, 15, and 30 ng gelsolin in separate lanes of the gel. The intensity of each band on the Western blot minus the background signal was plotted versus the known amount of gelsolin and fitted to a straight line (R2≥0.9); the graph was used as a standard curve to determine unknown gelsolin levels in simultaneously assayed samples from UT and ET subjects [21].
Statistical analysis
Data are reported as mean ± SD. Data analysis was performed using two-way analysis of variance (ANOVA) with appropriate post hoc test.
RESULTS
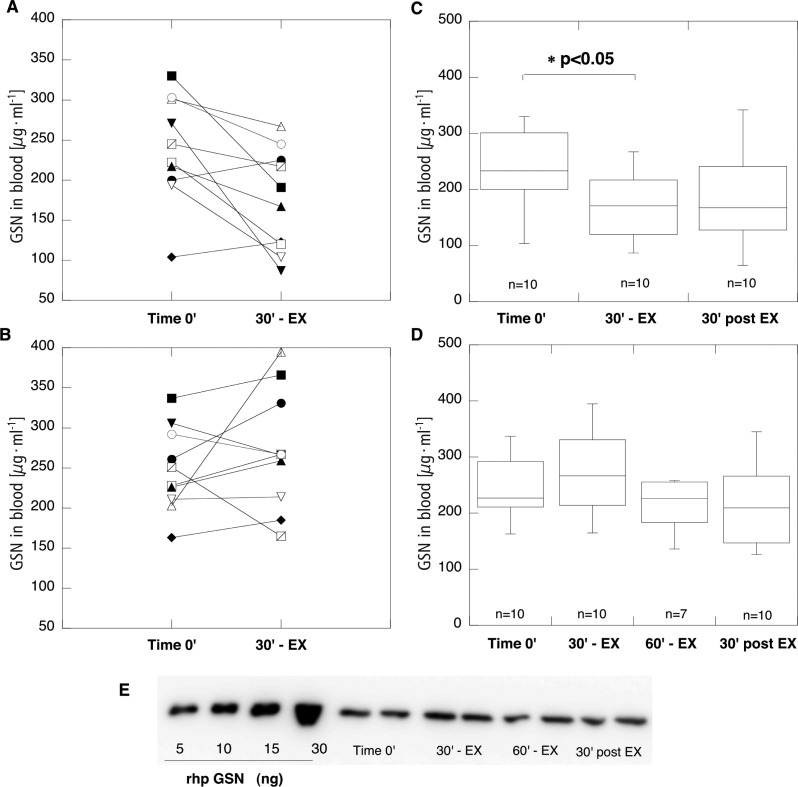
The average age of subjects (all male) included in the study for UT (n=10) and ET (n=10) groups was 20.4±0.7 and 21.1±0.9 years, and lean body mass was 64.6±7.6 and 58.4±2.9 kg respectively. Body mass index (kg · m-2) and% of body fat for the UT and ET groups were 22.6±2.3 versus 21±1.3 and 11.6±3.2 versus 10.6±1.4. There was a statistically significant difference (p<0.05) between O2 max (ml · kg -1 · min -1) in the UT and ET groups (47.3±3 versus 57.3±6.5). Total protein analysis in plasma samples did not differ between UT and ET groups. As indicated by data shown in Figure 1, Western blot analysis of gelsolin concentration in 8 of 10 subjects included in the UT group reveals a decrease after 30 minutes of exercise. At the same time point in 6 of 10 subjects included in the ET group, blood gelsolin concentration increased by more than 10% of the initial concentration (time 0’). On average, blood gelsolin concentration in the UT group for time 0’, 30’ - EX, and 30’ post EX was 238.7±66.5, 167.6±58.7 and 188±88.2 respectively. The difference between gelsolin concentration at time 0’ and 30’ - EX in the UT group reached statistical significance (p<0.05). In the ET group, average blood gelsolin concentration at time 0’, 30’ - EX, 60’ - EX and 30’ post EX was 244.2±53.6, 271.5±74.6, 213.9±49.6 and 213.8±68.4 respectively.
FIG. 1.
GELSOLIN CONCENTRATION IN PLASMA SAMPLES COLLECTED FROM UNTRAINED (PANELS A AND C) AND ENDURANCE TRAINED (PANELS B AND D) VOLUNTEERS. FROM ALL STUDY PARTICIPANTS, BLOOD SAMPLES WERE TAKEN BEFORE EXERCISE (TIME 0’), AT 30 MINUTES OF EXERCISE (30’ - EX) AND 30 MINUTES AFTER COMPLETION OF EXERCISE (30’ POST EX). ADDITIONALLY, IN THE ET GROUP BLOOD WAS ALSO COLLECTED AT 60 MINUTES OF EXERCISE (60’ - EX). PANEL A AND B SHOW CHANGES OF BLOOD GSN CONCENTRATION IN INDIVIDUAL SUBJECTS BETWEEN TIME 0’ AND 30 MINUTES OF EXERCISE IN UT AND ET GROUPS RESPECTIVELY. BOX PLOTS ON PANELS C AND D DISPLAY DIFFERENCES IN BLOOD GELSOLIN CONCENTRATION AT INDICATED TIME POINTS IN UT AND ET GROUPS RESPECTIVELY. THE HORIZONTAL LINE IN THE BOX INDICATES THE MEDIAN. PANEL E SHOWS A REPRESENTATIVE IMAGE OF WESTERN BLOT ANALYSIS OF 4 PLASMA SAMPLES TAKEN FROM 1 SUBJECT OF THE ET GROUP.
DISCUSSION
Exercise training elicits a number of adaptive changes in skeletal muscle that result in improved metabolic efficiency. Up to now the molecular mechanisms mediating the cellular adaptations to exercise training in human skeletal muscle have not been fully defined and several mediators, such as catecholamines, adrenocorticotrophic hormone, growth hormone, and proteins associated with skeletal muscle adaptation to the stress of contractile activity such as heat shock proteins (HSPs), have been proposed to govern this process [20, 24]. Since muscle tissue devotes ∼3% of its metabolic power to synthesis of extracellular gelsolin [14] it is plausible that increasing gelsolin production occurs in response to exercise. We found that training prevents a decrease of blood gelsolin associated with intense exercise, indicating that an increase of pGSN expression might occur as part of adaptive changes to exercise in skeletal muscle. However, a recent study in which alterations in protein abundance in skeletal muscle after one bout of exhaustive exercise using proteomic analysis was performed did not show any alteration in gelsolin expression [10]. Such an increase might not be easy to capture based on evaluation of blood gelsolin concentration if the increasing production is masked due to increasing demand by the extracellular actin buffering system. In a recent study, a decreased concentration of vitamin D binding protein which also contributes to the blood actin buffering system was observed after a one-hour exercise bout to exhaustion in obese subject [22]. However, no changes in blood gelsolin concentration were observed in those subjects. An observed decrease of blood gelsolin concentration in untrained subjects indicates that endurance training might increase the ability of muscle tissue to express plasma gelsolin, which might potentially compensate increasing demand for gelsolin by the extracellular actin buffering system. Administration of recombinant human plasma gelsolin was proposed as a treatment for different conditions in which hypogelsolinemia was observed [8]. A better understanding of factors that regulate gelsolin production might provide an alternative therapy in which stimulation of endogenous plasma gelsolin by muscle tissue might be used to maintain a healthy gelsolin concentration. A study of blood gelsolin changes during exercise correlated with hormonal and metabolic screening might provide a better strategy to recognize these factors.
CONCLUSIONS
It is concluded that training prevents a decrease of blood gelsolin associated with intense exercise. This may indicate that an increase of pGSN expression might occur as part of adaptive changes to exercise in skeletal muscle.
ACKNOWLEDGEMENTS
The study was supported by the Medical University of Białystok (grant 123-18809, 123-18810).
Conflict of interests
None declared.
REFERENCES
- 1.Bucki R, Georges P.C, Espinassous Q, Funaki M, Pastore J.J, Chaby R, Janmey P.A. Inactivation of endotoxin by human plasma gelsolin. Biochemistry. 2005;44:9590–9597. doi: 10.1021/bi0503504. [DOI] [PubMed] [Google Scholar]
- 2.Bucki R, Janmey P.A. Interaction of the gelsolin-derived antibacterial PBP 10 peptide with lipid bilayers and cell membranes. Antimicrob. Agents Chemother. 2006;50:2932–2940. doi: 10.1128/AAC.00134-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bucki R, Pastore J.J. Bacterial endotoxin as inhibitor of the enzymatic activity of human thrombin. Eur. J. Haematol. 2006;76:510–515. doi: 10.1111/j.0902-4441.2005.t01-1-EJH2448.x. [DOI] [PubMed] [Google Scholar]
- 4.Bucki R, Byfield F.J, Kulakowska A, McCormick M.E, Drozdowski W, Namiot Z, Hartung T, Janmey P.A. Extracellular gelsolin binds lipoteichoic acid and modulates cellular response to proinflammatory bacterial wall components. J. Immunol. 2008;181:4936–4944. doi: 10.4049/jimmunol.181.7.4936. [DOI] [PubMed] [Google Scholar]
- 5.Bucki R, Levental I, Kułakowska A, Janmey P.A. Plasma gelsolin: function, prognostic value, and potential therapeutic use. Curr. Protein. Pept. Sci. 2008;9:541–551. doi: 10.2174/138920308786733912. [DOI] [PubMed] [Google Scholar]
- 6.Bucki R, Kułakowska A, Byfield F.J. Plasma gelsolin modulates cellular response to sphingosine 1-phosphate. Am. J. Physiol. Cell Physiol. 2011;299:C1516–1523. doi: 10.1152/ajpcell.00051.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou S.H, Lee P.S, Konigsberg R.G, Gallacci D, Chiou T, Arai K, Simmons S, Bauer D, Feske S.K, Lo E.H, Ning M. Plasma-type gelsolin is decreased in human blood and cerebrospinal fluid after subarachnoid hemorrhage. Stroke. 2011;42:3624–7. doi: 10.1161/STROKEAHA.111.631135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen T.S, Bucki R, Byfield F.J, Ciccarelli N.J, Rosenberg B, DiNubile M.J, Janmey P.A, Margulies S.S. Therapeutic potential of plasma gelsolin administration in a rat model of sepsis. Cytokine. 2011;54:235–238. doi: 10.1016/j.cyto.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erukhimov J.A, Tang Z.L, Johnson B.A. Actin-containing sera from patients with adult respiratory distress syndrome are toxic to sheep pulmonary endothelial cells. Am. J. Respir. Crit. Care Med. 2000;162:288–294. doi: 10.1164/ajrccm.162.1.9806088. [DOI] [PubMed] [Google Scholar]
- 10.Gandra P.G, Valente R.H, Perales J, Pacheco A.G, Macedo D.V. Proteomic analysis of rat skeletal muscle submitted to one bout of incremental exercise. Scand J. Med. Sci. Sports. 2012;22:207–16. doi: 10.1111/j.1600-0838.2010.01235.x. [DOI] [PubMed] [Google Scholar]
- 11.Haddad J.G, Harper K.D, Guoth M, Pietra G.G, Sanger J.W. Angiopathic consequences of saturating the plasma scavenger system for actin. Proc. Natl. Acad. Sci. USA. 1990;87:1381–1385. doi: 10.1073/pnas.87.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito H, Kambe H, Kimura Y, Nakamura H, Hayashi E, Kishimoto T, Kishimoto S, Yamamoto H. Depression of plasma gelsolin level during acute liver injury. Gastroenterology. 1992;102:1686–1692. doi: 10.1016/0016-5085(92)91731-i. [DOI] [PubMed] [Google Scholar]
- 13.Kulakowska A, Zajkowska J. M, Ciccarelli N. J, Mroczko B, Drozdowski W, Bucki R. Depletion of plasma gelsolin in patients with tick-borne encephalitis and Lyme neuroborreliosis. Neurodegener. Dis. 2011;8:375–380. doi: 10.1159/000324373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwiatkowski D, Mehl R, Izumo S, Nadal-Ginard B, Yin H.L. Muscle is the major source of plasma gelsolin. J. Biol. Chem. 1988;263:8239–8243. [PubMed] [Google Scholar]
- 15.Kwiatkowski D.J, Mehl R, Yin H. L. Genomic organization and biosynthesis of secreted and cytoplasmic forms of gelsolin. J. Cell Biol. 1988;106:375–384. doi: 10.1083/jcb.106.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee P.S, Waxman A.B, Cotich K.L, Chung S.W, Perrella M.A, Stossel T.P. Plasma gelsolin is a marker and therapeutic agent in animal sepsis. Crit. Care Med. 2007;35:849–855. doi: 10.1097/01.CCM.0000253815.26311.24. [DOI] [PubMed] [Google Scholar]
- 17.Lee W.M, Galbraith R.M. The extracellular actin-scavenger system and actin toxicity. N. Engl. J. Med. 1992;326:1335–1341. doi: 10.1056/NEJM199205143262006. [DOI] [PubMed] [Google Scholar]
- 18.Li G.H, Arora P.D, Chen Y, McCulloch C.A, Liu P. Multifunctional roles of gelsolin in health and diseases. Med. Res. Rev. 2012;32:999–1025. doi: 10.1002/med.20231. [DOI] [PubMed] [Google Scholar]
- 19.Meerschaert K, De Corte V, De Ville Y, Vandekerckhove J, Gettemans J. Gelsolin and functionally similar actin-binding proteins are regulated by lysophosphatidic acid. Embo. J. 1998;17:5923–5932. doi: 10.1093/emboj/17.20.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morton J.P, Kayani A.C, McArdle A, Drust B. The exercise-induced stress response of skeletal muscle, with specific emphasis on humans. Sports Med. 2009;39:643–662. doi: 10.2165/00007256-200939080-00003. [DOI] [PubMed] [Google Scholar]
- 21.Mounzer K.C, Moncure M, Smith Y.R, Dinubile M.J. Relationship of admission plasma gelsolin levels to clinical outcomes in patients after major trauma. Am. J. Respir. Crit. Care Med. 1999;160:1673–1681. doi: 10.1164/ajrccm.160.5.9807137. [DOI] [PubMed] [Google Scholar]
- 22.Oberbach A, Bluher M, Wirth H, Till H, Kovacs P, Kullnick Y, Schlichting N, Tomm J.M, Rolle-Kampczyk U, Murugaiyan J, Binder H, Dietrich A, von Bergen M. Combined proteomic and metabolomic profiling of serum reveals association of the complement system with obesity and identifies novel markers of body fat mass changes. J. Proteome Res. 2011;10:4769–4788. doi: 10.1021/pr2005555. [DOI] [PubMed] [Google Scholar]
- 23.Osborn T. M, Dahlgren C, Hartwig J. H, Stossel T. P. Modifications of cellular responses to lysophosphatidic acid and platelet-activating factor by plasma gelsolin. Am. J. Physiol. Cell Physiol. 2007;292:C1323–1330. doi: 10.1152/ajpcell.00510.2006. [DOI] [PubMed] [Google Scholar]
- 24.Pestell R.G, Hurley D.M, Vandongen R. Biochemical and hormonal changes during a 1000 km ultramarathon. Clin. Exp. Pharmacol. Physiol. 1989;16:353–361. doi: 10.1111/j.1440-1681.1989.tb01571.x. [DOI] [PubMed] [Google Scholar]
- 25.Smith D.B, Janmey P.A, Sherwood J.A, Howard R. J, Lind S. E. Decreased plasma gelsolin levels in patients with Plasmodium falciparum malaria: a consequence of hemolysis? Blood. 1998;72:214–218. [PubMed] [Google Scholar]
- 26.Vouyiouklis D.A, Brophy P.J. A novel gelsolin isoform expressed by oligodendrocytes in the central nervous system. J. Neurochem. 1997;69:995–1005. doi: 10.1046/j.1471-4159.1997.69030995.x. [DOI] [PubMed] [Google Scholar]
- 27.Xu J.F, Liu W.G, Dong X.Q, Yang S.B, Fan J. Change in plasma gelsolin level after traumatic brain injury. J. Trauma. 2011 doi: 10.1097/ta.0b013e318226ec39. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Yin H.L, Kwiatkowski D.J, Mole J.E, Cole F.S. Structure and biosynthesis of cytoplasmic and secreted variants of gelsolin. J. Biol Chem. 1984;259:5271–5276. [PubMed] [Google Scholar]