Abstract
The substantia nigra pars compacta (SNc) and ventral tegmental area (VTA) contain the two largest populations of dopamine (DA)-releasing neurons in the mammalian brain. These neurons extend elaborate projections in striatum, a large subcortical structure implicated in motor planning and reward-based learning. Phasic activation of dopaminergic neurons in response to salient or reward-predicting stimuli is thought to modulate striatal output via the release of DA to promote and reinforce motor action1–4. Here we show that activation of DA neurons in striatal slices rapidly inhibits action potential firing in both direct-and indirect-pathway striatal projection neurons (SPNs) through vesicular release of the inhibitory transmitter γ-aminobutyric acid (GABA). GABA is released directly from dopaminergic axons but in a manner that is independent of the vesicular GABA transporter VGAT. Instead GABA release requires activity of the vesicular monoamine transporter VMAT2, which is the vesicular transporter for DA. Furthermore, VMAT2 expression in GABAergic neurons lacking VGAT is sufficient to sustain GABA release. Thus, these findings expand the repertoire of synaptic mechanisms employed by DA neurons to influence basal ganglia circuits, reveal a novel substrate whose transport is dependent on VMAT2, and demonstrate that GABA can function as a bona fide co-transmitter in monoaminergic neurons.
The striatum integrates inputs from cortex, hippocampus, thalamus, amygdala and VTA/SNc to instruct the selection of appropriate motor actions. Inputs from midbrain DA neurons play an important role in this process, as evidenced by the psychomotor deficits that arise following loss of these cells in Parkinson’s disease, or by the occurrence of compulsive and addictive behaviors upon potentiation of dopaminergic signaling5–7. Through release of DA, these neurons promote activation of direct pathway SPNs (dSPNs), which express Gαs/olf-coupled D1 receptors, and inhibit indirect pathway SPNs (iSPNs), which express Gαi/o-coupled D2 receptors3,5. However, midbrain DA neurons also express neuropeptides8 and a subset releases glutamate9–12, suggesting that the net effects of activity in these cells may not be limited to the actions of DA.
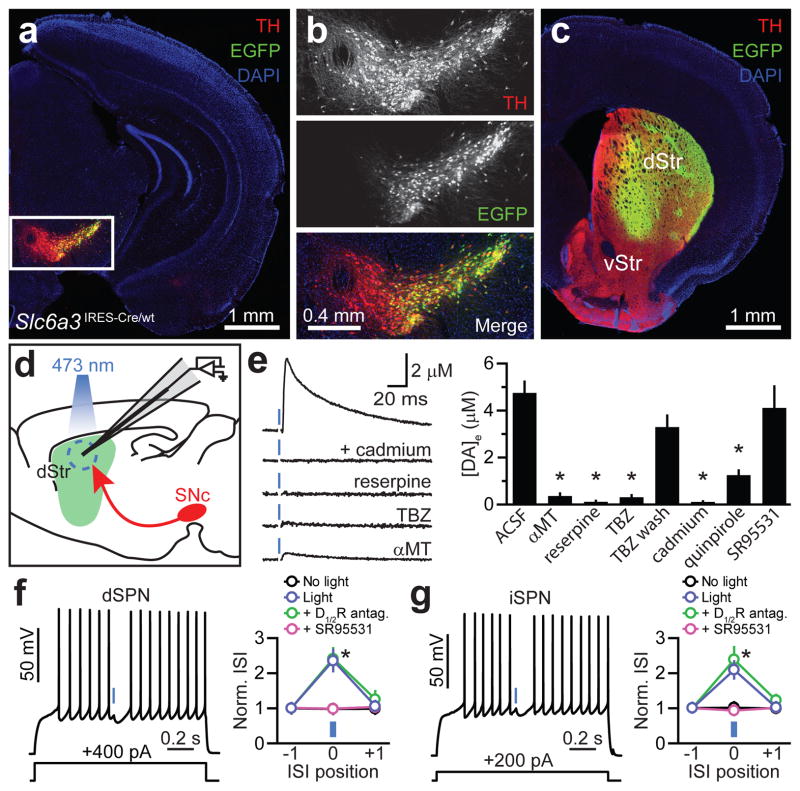
To investigate how DA neurons influence neuronal activity in striatum, we expressed the light-activated cation channel channelrhodopsin-2 (ChR2)13 inSNc neurons using Cre recombinase-dependent adeno-associated viruses(AAVs). In Slc6a3IRES-Cre/wt mice, Cre expression displays high penetrance and specificity for midbrain DA neurons14 (Supplementary Fig. 1). We validated SNc targeting using an AAV encoding Cre-dependent enhanced green fluorescent protein (EGFP): Fluorescence was restricted to Cre-containing neurons within SNc, and the vast majority of EGFP-expressing cells (97.9%, n=1587 cells; 3 mice) were immunopositive for the catecholamine biosynthetic enzyme tyrosine hydroxylase (TH), demonstrating specific expression in DA neurons (Fig. 1a,b; Supplementary Figs. 1 and 2). Moreover, EGFP+ axons densely innervated dorsal striatum (Fig. 1c), consistent with their nigrostriatal identity.
Figure 1. DA neuron stimulation inhibits SPNs.
a. Coronal midbrain section from a Slc6a3IRES-Cre/wt mouse transduced with a Cre-dependent EGFPAAV (green). DA neurons immunolabeled for TH (red). DAPI(blue), nuclear stain. b. Higher magnification of boxed area in a. Although expression levels vary greatly, the overwhelming majority of EGFP+ cells are TH+. c. EGFP+ SNc neurons densely innervate dorsal striatum (dStr). vStr; ventral striatum. d. Schematic of carbon-fiber recording configuration in a sagittal brain slice. ChR2+ DA neurons depicted red, laser illumination area blue. e. Left, light stimulation (blue) of DA terminals in dorsal striatum evoked cadmium-, reserpine-, TBZ-and αMT-sensitive DA release measured by amperometry. Stimulation artifacts blanked for clarity. Right, mean (n=4–19) peak extracellular DA concentration following optogenetic stimulation of nigrostriatal axons. DA transients recovered upon TBZ washout (wash). Asterisk, P<0.05vs. ACSF(Mann-Whitney). f, g. Left, membrane potential responses of a dorsal striatum dSPN(f) or iSPN(g) to current injection (1.3 s, bottom) and ChR2-mediated stimulation of DA axons(1 ms, blue). Right, average normalized duration of 3 consecutive interspike intervals (norm. ISI) that precede, straddle or follow the light flash(positions ‘−1’, ‘0’ and ‘+1’, respectively) upon ChR2 stimulation in ACSF (blue), DA receptor blockers (D1/2R antagonists = SCH23390 +SKF83566 + sulpiride +L-741,626; green) or SR95531(GABAA receptor blocker; purple). Black, light prevented from entering the sample. n=14dSPNs, 11iSPNs. Asterisk, P<0.05(two-way ANOVA). Data in e, f and g represent mean±s.e.m.
Carbon-fiber amperometry confirmed the ability to evoke DA release from ChR2-expressing axons in slices of dorsal striatum. Brief flashes of blue light (1 ms) reliably evoked DA transients, which were sensitive to the TH antagonist α-methyl-tyrosine (αMT) and the VMAT2 antagonists reserpine and tetrabenazine (TBZ) (Fig. 1d,e). Moreover, DA release required Ca2+ channels, as it was blocked by cadmium, and activation of D2 receptors with quinpirole reduced DA release, consistent with the effect of DA autoreceptors in nigrostriatal axons15.
To determine the net effect of DA neuron stimulation on striatal output, we performed whole-cell current-clamp recordings from dSPNs and iSPNs in brain slices obtained from Slc6a3IRES-Cre/wt;Drd2-EGFP mice. Whereas action potentials evoked by current steps occurred at regular intervals under baseline conditions, light presentation reliably pausedfiring and rapidly hyperpolarized SPNs of both populations (dSPNs: 6.8±1.9 mV, n=10; iSPNs: 7.0±1.4 mV, n=7) (Fig. 1f,g). The light-evoked pause and hyperpolarization were unaffected by a cocktail of antagonists targeting DA receptors (dSPNs:6.6±2.2 mV, n=4; iSPNs:9.5±2.5 mV, n=4; both P>0.05 vs. light only, Mann-Whitney). Instead, they were abolished by the GABAA receptor antagonist SR95531 (n=4 dSPNs, 7 iSPNs) (Fig. 1f,g), which does not alter DA release (Fig. 1e), implicating the inhibitory transmitter GABA. These data indicate that DA neurons exert a rapid and strong inhibitory influence on SPNs through activation of GABAA receptors.
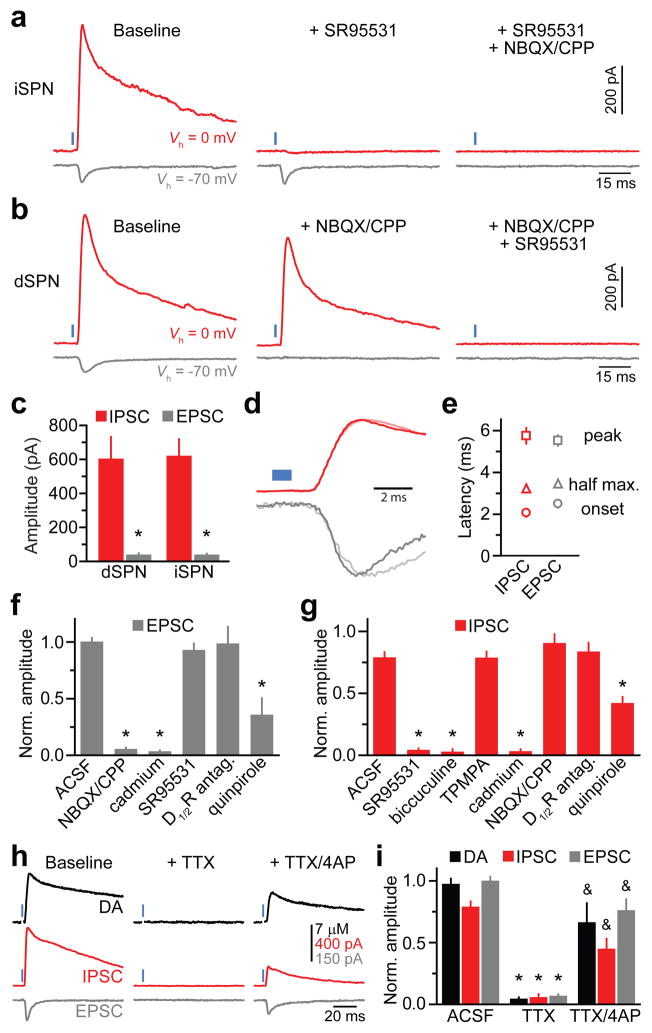
Previous attempts at characterizing DA receptor-independent effects of DA neurons on SPNs revealed a small, but rapid excitatory influence mediated by corelease of glutamate9–12. However, these experiments were performed in the presence of GABA receptor antagonists, precluding the detection of inhibitory influences. To reveal conductances recruited following DA neuron stimulation, we performed whole-cell voltage-clamp recordings from dSPNs and iSPNs in dorsal striatum without pharmacological blockers. When SPNs were held at −70 mV(ECl, the reversal potential for chloride), nigrostriatal fiber stimulation evoked fast inward currents in ~75% of dSPNs (n=6/8) and iSPNs (n=16/21) (Fig. 2a–e). These currents displayed similar properties in both cell types (Fig. 2a–c) and were consequently pooled for analysis: they exhibited peak amplitudesof40±5 pA (n=22), rise times of 2.6±0.4 ms and decay time constants of 6.8±1.5 ms. Moreover, they reversed at ~0 mV and were sensitive to the AMPA and NMDA receptor blockers NBQX and CPP(Fig. 2a,b,f), indicating that they are glutamatergic excitatory postsynaptic currents (EPSCs). Although glutamate release by midbrain DA neurons was proposed to be limited to mesolimbic fibers innervating ventral striatum12, the currents reported here in dorsal striatum display similar properties11,12, and are sensitive to the same pharmacological agents as DA release (Figs. 1e,2f), suggesting a dopaminergic origin. Moreover, EPSCs were unaffected by DA receptor antagonists (Fig. 2f), indicating that glutamate release is not secondary to DA receptor activation.
Figure 2. DA neurons directly release GABA onto SPNs.
a. Evoked current responses from an iSPN held at indicated potentials (Vh) to optogenetic activation of nigrostriatal axons(1 ms, blue)upon sequential bath application ofSR95531 and NBQX/CPP. b. As in a for a dSPN with antagonists applied in reverse order. c. Mean IPSC (red) and EPSC (gray) absolute amplitudes in dSPNs (n=8) and iSPNs (n=21). Asterisk, P<0.05 vs. IPSC (Mann-Whitney). d. Normalized IPSCs and EPSCs from a (dark) and b (light) shown on an expanded time scale. Blue, light presentation. e. Average (n=29 SPNs) IPSC(red) and EPSC (gray) latencies from light onset to current onset (circle), half maximal amplitude (triangle) and peak amplitude (square). IPSCs are not delayed compared to EPSCs. f, g. Mean(n=3–18) EPSC(f) and IPSC(g) amplitudes under control conditions (ACSF) or in indicated antagonists normalized to baseline. Asterisk, P<0.05vs. ACSF (Mann-Whitney). h. Amperometric DA transients (black) and current responses of a voltage-clamped iSPN (Vh=0 mV, red; Vh=−70 mV, gray) to DA neuron stimulation under baseline conditions (left), in TTX (middle), and following co-application of TTX and 4AP (right). i. Extracellular DA concentration (black), IPSC amplitude (red) and EPSC amplitude (gray) evoked by ChR2 stimulation across conditions normalized to baseline. Data from ACSF condition same as in Figs. 1e and 2f, g. Asterisk, P<0.05vs. ACSF; ampersand, P<0.05 vs. TTX (Mann-Whitney). Error bars denote s.e.m.
To isolate conductances that mediate SPN inhibition, the membrane potential was held at the reversal potential of ionotropic glutamate receptors (~0 mV). Under these conditions, DA neuron stimulation evoked large outward currents in all recorded dSPNs (n=8) and iSPNs (n=21) (Fig. 2a–c and Supplementary Fig. 3). Collectively, these currents had peak amplitudes of 617±78 pA (n=29; range=0.11–1.93 nA), rise times of 2.0±0.2 ms and decay time constants of 56±4 ms. They reversed at ECl and were blocked by SR95531 and bicuculine, but not by the GABAC receptor antagonist TPMPA (Fig. 2a,b,g), indicating that they represent GABAA receptor-mediated inhibitory postsynaptic currents (IPSCs). Similar observations were made in mice expressing Cre under control of a TH promoter (Supplementary Fig. 4). Consistent with previous reports11,12, no current remained with both glutamate and GABAA receptors blocked. Thus, these data show that 1) activation of dopaminergic terminals rapidly activates ionotropic glutamate and GABA receptors in SPNs, 2) the resulting currents do not differentially affect dSPNs and iSPNs, and 3) GABAergic conductances mediate the net inhibitory effect of DA neurons on striatal output.
DA neurons may activate GABAA receptors on SPNs either by recruiting a population of GABAergic interneurons – a mechanism akin to feed-forward inhibition – or by directly releasing GABA. Several lines of evidence suggest the latter. First, the latency between light and IPSC onset averaged 2.2±0.1 ms (n=29; Fig. 2d,e), which maybe too short to accommodate two synaptic transmission steps at 32–34°C16. Second, GABAergic conductances preceded or occurred synchronously with EPSCs (Fig. 2d,e), which arise following glutamate corelease from DA terminals10–12 (Fig. 2h,i). Third, a population of dopaminergic SNc neurons expresses mRNA for glutamic acid decarboxylase (GAD-65)17, indicating that they may synthesize GABA. We reasoned that if GABA originates from dopaminergic terminals, optically-evoked IPSCs, EPSCs and extracellular DA should be similarly affected by pharmacological agents and persist under conditions that prevent disynaptic transmission. Indeed, IPSCs in SPNs were reduced by quinpirole, eliminated by cadmium, but insensitive to glutamate and DA receptor inhibitors (Fig. 2g), indicating that they require Ca2+-dependent release of a transmitter other than glutamate or DA. Moreover, light-evoked IPSCs, EPSCs and DA release were abolished in the presence of the voltage-gated Na+ channel blocker tetrodotoxin (TTX), revealing that ChR2-mediated depolarization is not sufficient to trigger transmitter release from nigrostriatal axons (Fig. 2h,i). Neurotransmission can be rescued from directly-illuminated ChR2-expressing terminals in the presence of TTX by providing additional depolarization with the voltage-gated K+ channel blocker 4-aminopyridine (4AP)18. Accordingly, IPSCs, EPSCs and DA release recovered upon co-application of TTX and 4AP (Fig. 2h,i), indicating that GABA, glutamate and DA are directly released from dopaminergic terminals.
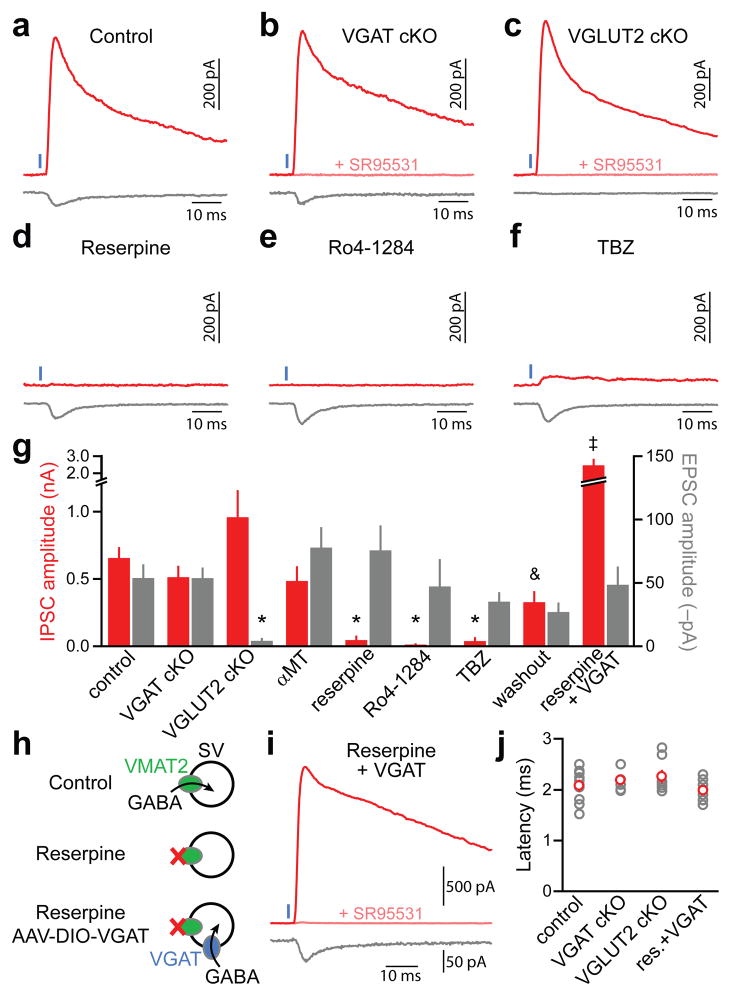
The vesicular GABA transporter VGAT (encoded by Slc32a1)is the only transporter known to package GABA into synaptic vesicles and is considered indispensable for inhibitory synaptic transmission19. We generated conditional knockout (cKO) mice in which VGAT is specifically deleted from DA neurons (Slc6a3IRES-Cre/wt;Slc32a1lox/lox mice), predicting that ChR2-evoked IPSCs would be abolished. However, light-evoked IPSCs and EPSCs were unaffected in these mice (Fig. 3a,b,g,j), indicating that VGAT is not responsible for vesicular loading of GABA in DA neurons. We instead hypothesized that GAD-65 may synthesize GABA from its metabolic precursor glutamate once inside synaptic vesicles. If correct, preventing glutamate loading into synaptic vesicles by genetically ablating the vesicular glutamate transporter 2 (VGLUT2; encoded by Slc17a6) from DA neurons (Slc6a3IRES-Cre/wt;Slc17a6lox/lox mice) should eliminate IPSCs and EPSCs10. However, conditional deletion of VGLUT2 abolished light-evoked EPSCs in SPNs without affecting IPSC amplitude or latency (Fig. 3c,g,j), excluding this possibility.
Figure 3. GABA release from DA neurons requires VMAT2butnot VGAT.
a–c. Representative ChR2-evoked (1 ms, blue) IPSCs (red, Vh=0 mV) and EPSCs (gray, Vh=−70 mV) from SPNs in Slc6a3IRES-Cre/wt (control, a), Slc6a3IRES-Cre/wt;Slc32a1lox/lox (VGAT cKO, b), and Slc6a3IRES-Cre/wt;Slc17a6lox/lox (VGLUT2 cKO, c) mice. d–e. As in a for control Slc6a3IRES-Cre/wt mice treated in vivo and in vitro with the VMAT2 antagonists reserpine (d), Ro4-1284 (e) or TBZ (f). g. Mean IPSC (red) and EPSC (gray) amplitudes across conditions (n=4–33). Washout, slices obtained from mice treated in vivo with TBZ or Ro4-1284 and subsequently allowed to recover in vitro for >1 h in ACSF. Asterisk, P<0.05vs. ACSF; ampersand, P<0.05vs. TBZ/Ro4-1284; double dagger, P<0.05 vs. reserpine (Kruskal-Wallis). h. Schematic of working hypothesis: Provided DA neurons contain cytosolic GABA, viral expression of reserpine-resistant VGAT in DA neurons should rescue GABA transport into synaptic vesicles (SVs) and IPSCs in reserpine-treated mice. Note that VGAT might also incorporate into VMAT2− vesicles. i. Voltage-clamp recording (Vh=0 mV, red; Vh=−70 mV, gray) from a reserpine-treated iSPN upon optogenetic stimulation (1 ms, blue) of VGAT-expressing DA axons. j. IPSC onset latencies (gray, individual cells; red, average)did not differ across conditions. res, reserpine. GABAergic nature of outward currents (Vh=0 mV) in b, c and i was confirmed with SR95531 (pink). Error bars denote s.e.m.
These results indicate that GABA release originates in nigrostriatal terminals but is independent of VGAT and VGLUT2, suggesting the existence of an alternate vesicular transporter with previously unidentified activity for GABA. Consistent with this, IPSCs were eliminated in slices obtained from mice treated with the VMAT2 antagonists reserpine, Ro4-1284 or TBZ and largely recovered upon Ro4-1284 and TBZ washout (Fig. 3d–g). The same manipulation did not affect EPSCs or VGAT-dependent GABA release from SPNs (Supplementary Fig. 5), and we confirmed that DA itself does not function as a GABAA receptor agonist in SPNs (Supplementary Fig. 6). Moreover, DA depletion with αMT(Fig. 1e) had no effect on IPSCs or EPSCs (Fig. 3g). We therefore conclude that VMAT2, but not DA, is required for the release of GABA by DA neurons.
If GABAergic IPSCs depend on VMAT2solely for GABA transport into synaptic vesicles, IPSCs should be restored in reserpine-treated mice by expressing VGAT in DA neurons (Fig. 3h). Accordingly, in Slc6a3IRES-Cre/wt mice injected with AAV encoding Cre-dependent VGAT (AAV-DIO-VGAT) and treated with reserpine, optogenetic stimulation of nigrostriatal axons elicited large SR95531-sensitive IPSCs exhibiting synaptic latencies (Fig. 3g–j) and rise times (1.8±0.3, n=10; P>0.05 vs. control, Mann-Whitney) indistinguishable from IPSCs observed in untreated mice. Together, these data indicate that presynaptic DA terminals contain GABA, the synaptic packaging of which requiresVMAT2 but not VGAT.
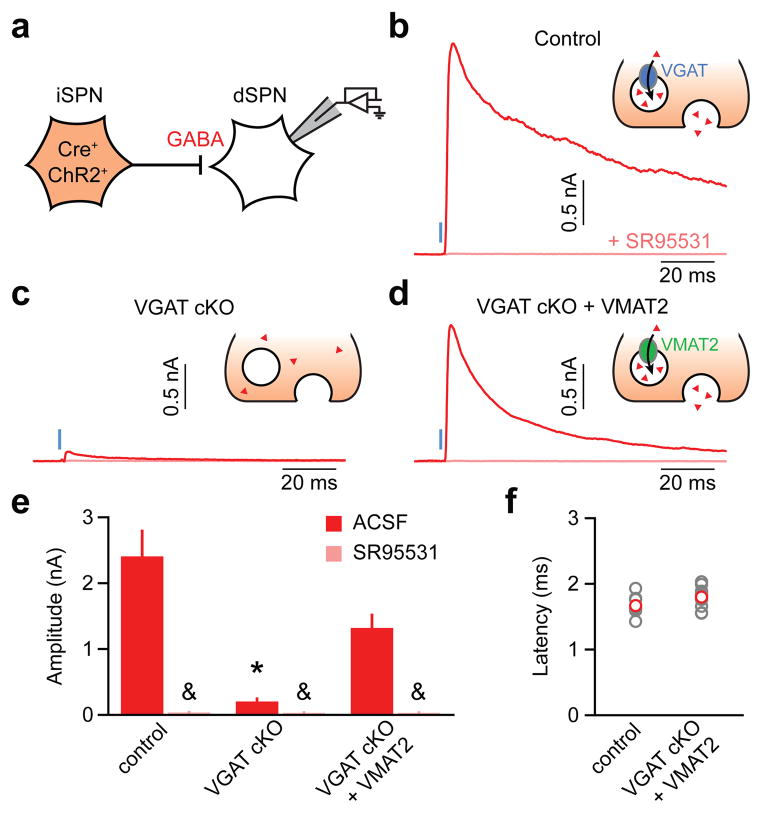
VMAT2 transportsa variety of substrates20, including catecholamines, serotonin and histamine. Although GABA does not bear structural resemblance to known VMAT2 substrates, our findings suggest that VMAT2 may function as a vesicular GABA transporter. To test this possibility, we asked if VMAT2 can substitute for VGAT to sustain GABA release in a population of non-monoaminergic GABAergic neurons. Specifically, we attempted to restore GABA release in iSPNs devoid of VGAT by exogenously expressing VMAT2. We conditionally excised the gene encoding VGAT in iSPNs and virally expressed ChR2 in these cells to allow monitoring of GABA release from iSPN axon collaterals onto neighboring dSPNs as light-evoked IPSCs21 (Fig 4a). Whereas optogenetic stimulation of iSPNs in control mice (Adora2a-Cre;Slc32a1lox/wt;Drd2-EGFP) reliably evoked large IPSCs in dSPNs, IPSCs were almost entirely abolished in dSPNs from VGAT cKO mice (Adora2a-Cre;Slc32a1lox/lox;Drd2-EGFP) (Fig. 4b,c,e), confirming the dependence of vesicular GABA transport in SPNs on VGAT19,21. By contrast, in VGAT cKO mice transduced with Cre-dependent VMAT2 (AAV-DIO-VMAT2), light reliably evoked large, short-latency IPSCs (Fig. 4d–f). Thus, VMAT2 expression in GABAergic neurons can functionally replace VGAT and sustain inhibitory synaptic transmission.
Figure 4. VMAT2 functions as a vesicular GABA transporter.
a. Experimental setup: ChR2 was selectively expressed in Cre-containing iSPNs of mice with one (control; Adora2A-Cre;Slc32a1lox/wt;Drd2-EGFP mice) or both alleles of the gene encoding VGAT flanked by lox sites (VGAT cKO; Adora2A-Cre;Slc32a1lox/lox;Drd2-EGFP mice). VGAT cKO + VMAT2, an AAV encoding Cre-dependent VMAT2 (AAV-DIO-VMAT2) was co-injected with AAV-DIO-ChR2 in the striatum of VGAT cKO mice to rescue GABA release from iSPNs. b–d. Voltage-clamp recordings (Vh=0 mV) of axon-collateral IPSCs in dSPNs evoked by optogenetic stimulation (1 ms, blue) of iSPNs in the absence (red) or presence (pink) ofSR95531 in control (b), VGAT cKO (c) and VGAT cKO + VMAT2 (d) mice. Insets: iSPN presynaptic terminal schematic illustrating experimental conditions. Red triangles, GABA. e. Summary histogram (mean±s.e.m.) of experiments in b–d (n=10–15 dSPNs). Asterisk, P<0.05vs. control and VGAT cKO+VMAT2 (Kruskal-Wallis); ampersand, P<0.05 vs. IPSC without SR95531 (Mann-Whitney). f. IPSC onset latencies from light presentation onset (gray, individual cells; red, mean±s.e.m.) in control (Adora2A-Cre;Slc32a1lox/wt;Drd2-EGFP mice) and VGAT cKO+VMAT2 mice (Adora2A-Cre;Slc32a1lox/lox;Drd2-EGFP mice transduced with AAV-DIO-VMAT2 in striatum).
Midbrain DA neurons are critical for the initiation, selection and reinforcement of motor actions and have been implicated in motor, cognitive and addictive disorders1–7. Their effects are largely attributed to the slow neuromodulatory actions of DA receptors1–3. Our studies show that DA neurons additionally exert a rapid and potent inhibitory influence on the activity of SPNs by releasing another transmitter that activates GABAA receptors. Release of this transmitter depends on VMAT2activity, indicating that VMAT2 or a molecular complex requiring VMAT2 activity packages it in synaptic vesicles together with DA. The simplest model accounting for our data is that GABA is the transmitter packaged by VMAT2 and released onto SPNs. However, we cannot rule out that another molecule present in iSPNs and DA neurons, acting as a GABAA receptor agonist, and serving as a substrate for both VMAT2 and VGAT fulfills that role.
It is estimated that 5–10% of SNc DA neurons express GAD-65 and <1% express VGLUT2in rodents10,17,22. Therefore, distinct subpopulations of DA neurons may release GABA and glutamate, and reliable detection of IPSCs and EPSCs may result from innervation of SPNs by multiple DA neurons23. Alternatively, GABAergic transmission may be common to midbrain DA neurons as any means of acquiring GABA, such as plasma membrane uptake, would result in vesicular transport. GAD expression varies in response to neuronal activity24 and damage25, suggesting that GABAergic signaling from DA neurons may be altered by drug abuse or Parkinson’s disease.
Since all monoaminergic neurons express VMAT2, our results imply that GABA corelease may extend to adrenergic, noradrenergic, serotonergic, histaminergic, as well as other dopaminergic neurons. Indeed, GABA release was reported from dopaminergic amacrine cells in the retina26 and periglomerular cells in the olfactory bulb27, although dependence on VMAT2 remains to be determined. Moreover, a considerable fraction (up to 80%) of monoaminergic neurons in locus coeruleus28, hypothalamus29, and raphe nuclei30 contain GABA or express GAD. Together, these findings expand the repertoire of synaptic mechanisms available to monoaminergic cells and suggest that perturbations of GABA co-transmission might contribute to the etiology of monoaminergic pathologies or to the therapeutic efficacy of VMAT2 antagonists.
ONLINE METHODS
Mice
Knock-in mice bearing an internal ribosome entry site (IRES)-linked Cre recombinase gene downstream of the gene Slc6a3, which encodes the plasma membrane DA transporter DAT (referred to as Slc6a3IRES-Cre mice)31 were obtained from Jackson Labs (stock # 006660). Homozygous (Slc6a3IRES-Cre/IRES-Cre) and heterozygous (Slc6a3IRES-Cre/wt) animals were bred with Drd2-EGFPtransgenic mice (GENSAT, founder line S118), which express EGFP under control of a bacterial artificial chromosome (BAC) containing the D2 receptor genomic locus to permit distinction between direct and indirect pathway SPNs32. Alternatively, Slc6a3IRES-Cre mice were crossed with mice bearing a Cre-dependent TdTomato reporter transgene (Ai14; Jackson Labs, stock #007914; referred to as Rosa26lsl-tdtomato mice) to reveal the distribution of Cre+ cells33. Mice in which the second exon of Slc32a1, which encodes the vesicular GABA transporter (VGAT), or Slc17a6, which encodes the vesicular glutamate transporter 2 (VGLUT2) are flanked by lox sites (Slc32a1lox and Slc17a6lox, respectively) were kindly provided by Brad Lowell (Beth Israel Deaconess Medical Center)34,35. Conditional deletion of VGAT or VGLUT2 from Cre-expressing DA neurons was achieved by crossing Slc6a3IRES-Cre/wt;Slc32a1lox/wt and Slc32a1lox/lox;Drd2-EGFP mice, or Slc6a3IRES-Cre/wt;Slc17a6lox/wt and Slc17a6lox/lox mice, respectively. No differences were observed between control Slc6a3IRES-Cre mice and Slc6a3IRES-Cre mice carrying a single floxed allele of Slc32a1 or Slc17a6, so data from these animals were pooled. iSPNs were genetically targeted using Adora2a-Cre BAC transgenic mice (GENSAT, founder line KG139), which express Cre under transcriptional control of the adenosine A2A receptor genomic promoter36. Conditional deletion of VGAT in Cre-expressing iSPNs was achieved by crossing Adora2a-Cre;Slc32a1lox/wt and Slc32a1lox/lox;Drd2-EGFP mice. Other lines employed include Th-Cre transgenic mice (Jackson Labs, stock # 008601), Gad1IRES-EGFP knock-in mice37 and Drd1a-Cre BAC transgenic mice (GENSAT, fonder line EY262). With the exception of Slc6a3IRES-Cre and Rosa26lsl-tdtomato mice, which were maintained on a C57BL/6 background, all other strains were maintained on a mixed background of C57BL/6 and FVB. All experimental manipulations were performed in accordance with protocols approved by the Harvard Standing Committee on Animal Care following guidelines described in the US National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Virus preparation
Conditional expression of the light-gated non-selective cation channel channelrhodopsin-2 (ChR2, H134R variant) in Cre-containing neurons was achieved using a recombinant adeno-associated virus (AAV) encoding a double-floxed inverted open reading frame (DIO) of the ChR2-mCherry fusion protein under transcriptional control of the EF1α promoter (AAV-DIO-ChR2; http://www.stanford.edu/group/dlab/optogenetics/sequence_info.html#dio). Cre-dependent AAV vectors encoding EGFP (AAV-DIO-EGFP), VGAT (NM_009508.2;AAV-DIO-VGAT; Addgene plasmid39320) or VMAT2 (NM_172523.3; AAV-DIO-VMAT2; Addgeneplasmid39339) were generated by replacing the ChR2-mCherry coding sequence using Asc1 and Nhe1 restriction sites (Genscript). These viral vectors were subsequently packaged (serotype 8) by a commercial vector core facility (University of North Carolina). All AAVs were stored in undiluted aliquots at a working concentration >1012 genomic copies per ml at −80°C until intracranial injection.
Stereotaxic intracranial injections
Male and female mice (postnatal day 18–25) were anesthetized with isoflurane and placed in a small animal stereotaxic frame (David Kopf Instruments). After exposing the skull under aseptic conditions, a small burr hole was drilled and AAVs were injected (0.5–1 μl total volume) unilaterally through a pulled glass pipette at a rate of 100 nl·min−1 using a UMP3 microsyringe pump (World Precision Instruments). Injection coordinates were 0.8 mm anterior from Lambda, 1.3 mm lateral and 4.4 mm below pia for SNc, and 0.5 mm anterior from Bregma, 1.75 mm lateral, and 2.7 mm below pia for dorsal striatum. After surgical procedures, mice were returned to their home cage for >21 days to allow for maximal gene expression. To identify striatum-projecting neurons in ventral midbrain, Slc6a3IRES-Cre/wt;Rosa26lsl-tdtomato/wt mice were injected with 0.1–0.2 μl fluorescent latex microspheres (Green Retrobeads, Lumafluor) in dorsal striatum (same coordinates as above) and allowed to recover in their home cage for 7 days before processing their brain for TH immunolabeling.
Immunocytochemistry
Mice were deeply anesthetized with isoflurane and perfused transcardially with 4% paraformaldehyde in 0.1 M sodium phosphate buffer. Brains were post-fixed for 1–3 days, washed in phosphate buffered saline (PBS) and sectioned (40–50 μm) coronally (Vibratome). Free-floating sections were permeabilized/blocked with 5% normal goat serum in PBS with 0.3% Triton X-100 (PBST) for 2 h at room temperature and incubated with primary antibodies at 4°C overnight and with secondary antibodies for 2 h at room temperature in PBST supplemented with 1% normal goat serum. Brain sections were mounted on superfrost slides, dried and coverslipped with ProLong antifade reagent with DAPI (Molecular Probes). Primary antibodies used include: rabbit anti-TH (1:2000; AB152, Millipore), mouse anti-TH (1:1000; 22941, ImmunoStar) and rabbit anti-VMAT2 (1:2000; ab81855, Abcam). Alexa Fluor 488-and 647-conjugated secondary antibodies to rabbit and mouse (Invitrogen) were diluted 1:1000. Endogenous TdTomato and EGFP fluorescence were not immuno-enhanced. Whole sections were imaged with an Olympus VS110 slide scanning microscope. High resolution images of regions of interest were subsequently acquired with a Zeiss LSM 510 META confocal microscope (Harvard NeuroDiscovery Center). Images represent maximum intensity projections of 3–7 μm confocal stacks.
Slice preparation
Acute brain slices were obtained from 40–218 d old mice (median = 74 d) using standard techniques. Mice were anesthetized by isoflurane inhalation and perfused cardiacly with ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM) 125 NaCl, 2.5 KCl, 25 NaHCO3, 2 CaCl2, 1 MgCl2, 1.25 NaH2PO4 and 11 glucose (295 mOsm·kg−1). Cerebral hemispheres were removed, placed in cold choline-based cutting solution (consisting of (in mM): 110 choline chloride, 25 NaHCO3, 2.5 KCl, 7 MgCl2, 0.5 CaCl2, 1.25 NaH2PO4, 25 glucose, 11.6 ascorbic acid, and 3.1 pyruvic acid), blocked and transferred into a slicing chamber containing ice-cold choline-based cutting solution. Parasagittal slices of striatum (275 μm thick) were cut with a Leica VT1000s vibratome, transferred for 10–20 min to a holding chamber containing ACSF at 34°C and subsequently maintained at room temperature (20–22°C) until use. All recordings were obtained within 4 hours of slicing. Both cutting solution and ACSF were constantly bubbled with 95% O2/5% CO2.
Electrophysiology
Individual slices were transferred to a recording chamber mounted on an upright microscope (Olympus BX51WI) and continuously superfused (2–3 ml·min−1) with ACSF warmed to32–34°C by passing it through a feedback-controlled in-line heater (SH-27B; Warner Instruments). Cells were visualized through a 40X water-immersion objective with either infrared differential interference contrast optics or epifluorescence to identify EGFP+ iSPNs and striatal regions displaying the highest density of ChR2-mCherry+ axonal arbors. Epifluorescence was used sparingly to minimize ChR2 activation prior to recording. Whole-cell voltage-and current-clamp recordings were made from dSPNs or iSPNs in anterior dorsolateral and dorsomedial striatum within 300 μm of the callosal-striatal border. dSPNs and iSPNs were identified based on the respective absence or presence of EGFP fluorescence and their firing properties. Patch pipettes (2–4 MΩ) pulled from borosilicate glass (G150F-3, Warner Instruments) were filled either with a Cs+-based low Cl− internal solution containing (in mM) 135 CsMeSO3, 10 HEPES, 1 EGTA, 3.3 QX-314 (Cl− salt), 4 Mg-ATP, 0.3 Na-GTP, 8 Na2-Phosphocreatine (pH 7.3 adjusted with CsOH; 295 mOsm·kg−1)for voltage-clamp recordings, or with a K+-based low Cl− internal solution composed of (in mM) 135 KMeSO3, 3 KCl, 10 HEPES, 1 EGTA, 0.1 CaCl2, 4 Mg-ATP, 0.3 Na-GTP, 8 Na2-Phosphocreatine (pH 7.3 adjusted with KOH; 295 mOsm·kg−1)for current-clamp recordings. Bath solutions for whole-cell recordings did not contain drugs unless specified otherwise. For all voltage-clamp experiments, errors due to the voltage drop across the series resistance (<20 MΩ) were left uncompensated. Membrane potentials were corrected for a ~8 mV liquid junction potential. To activate ChR2-expressing fibers, light from a 473 nm laser (Optoengine) was focused on the back aperture of the microscope objective to produce wide-field illumination of the recorded cell. Brief pulses of light (1 ms duration; 6.5–10.0 mW·mm−2 under the objective) were delivered at the recording site at 30 s intervals under control of the acquisition software. For current-clamp recordings, depolarizing current steps evoking 10–20 Hz trains of action potentials were applied at regular intervals (10–15 s) either alone or in combination with a 1 ms flash of blue light.
Amperometric recordings
Constant-potential amperometry was carried out using homemade glass-encased carbon-fiber microelectrodes (7 μm diameter, 50–100 μm length) placed ~50 μm within dorsal striatum slices and held at a constant voltage of +400–600 mV vs. Ag/AgCl via a Multiclamp 700B amplifier (Molecular Devices). Electrodes were calibrated with fresh 5 μM dopamine standards in ACSF using fast-scanning cyclic voltammetry (from −0.5 V to 0.9 V, and back to −0.5 V at a rate of 280 mV·ms−1 every 100 ms, with the electrode held at 0 V between scans) to determine the optimal oxidation potential, followed by constant-potential amperometry of dopamine flow-in to permit conversion of current amplitude to extracellular dopamine concentration. Dopaminergic terminals surrounding the electrode were stimulated with 1 ms flashes of blue laser light (6.5–10.0 mW·mm−2)delivered at 2–3 min intervals.
Reagents
Drugs (all from Tocris, unless specified otherwise) were applied via bath perfusion: SR95531 (10 μM), (−)biccuculine (20 μM), (1,2,5,6-Tetrahydropyridin-4-yl)methylphosphinic acid (TPMPA; 20 μM), 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(f)quinoxaline (NBQX; 10 μM), R,S-3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid (CPP; 10 μM), tetrodotoxin (TTX; 1 μM), 4-aminopyridine (4AP; 0.1–1 mM), CdCl2 (Sigma, 30 μM) and(−)quinpirole (10 μM). The cocktail of antagonists used to broadly target D1 and D2 receptor families (D1/2R antagonists)consisted of SCH23390 (1 μM), SKF83566 (1 μM), (−)sulpiride (10 μM) and L-741,626 (1 μM). To inhibit monoaminergic vesicular transport and deplete transmitter-filled vesicles, Slc6a3IRES-Cre mice were injected intraperitoneally with either the irreversible VMAT inhibitor reserpine (5 mg·kg−1) 24 h prior to slicing, the reversible VMAT antagonist Ro4-1284 (Sigma, 15 mg·kg−1) 1 h prior to slicing, or the competitive and selective VMAT2 antagonist tetrabenazine (TBZ; 5 mg·kg−1) 2 h prior to slicing. To deplete presynaptic terminals of dopamine, Slc6a3IRES-Cre mice were administered the tyrosine hydroxylase antagonist α-methyl-DL-tyrosine methyl ester hydrochloride (αMT; Sigma, 250 mg·kg−1 i.p.) 3 h and 1 h before slicing. Brain sections from these animals were prepared as described above, but were recovered and incubated in ACSF containing 1 μM reserpine, 10 μM Ro4-1284, 50 μM TBZ, or 30 μM αMT, respectively. Half of the slices obtained from Ro4-1284 and TBZ-treated mice were kept >1 h in regular ACSF before recording to allow for drug washout and resumption of neurotransmitter transport into synaptic vesicles (washout condition in Figs. 1e and 3g).
Data acquisition and analysis
Membrane currents and potentials were amplified and low-pass filtered at 3 kHz using a Multiclamp 700B amplifier (Molecular Devices), digitized at 10 kHz and acquired using National Instruments acquisition boards and a custom version of ScanImage written in MATLAB (Mathworks)38. Amperometry, electrophysiology and imaging data were analyzed offline using Igor Pro (Wavemetrics) and ImageJ (NIH). In figures, amperometry and voltage-clamp traces represent the averaged waveform of 3–5 consecutive acquisitions. Detection threshold for IPSCs and EPSCs was set at 10 pA. Averaged waveforms were used to obtain current latency, peak amplitude, 10–90% rise time and decay time. Current onset was measured using a threshold set at 3 standard deviations of baseline noise. Peak amplitudes were calculated by averaging over a 2 ms window around the peak. For pharmacological analyses in Figs. 1e, 2f, 2g and 2i, the peak amplitude of 3 consecutive light-evoked responses 3–4 min following drug perfusion onset were averaged, normalized to baseline averages and compared statistically to values obtained at corresponding times in control preparations bathed in ACSF. Data (reported in text and figures as mean ± s.e.m.) were compared statistically using the following: Mann-Whitney rank sum test, Kruskal-Wallis analysis of variance (ANOVA) with Dunn’s multiple comparison test, and two-way ANOVA followed by Bonferroni post-hoc tests, as indicated in the text. P values smaller than0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
The authors thank A. Saunders and Y. Kozorovitskiy for generating and characterizing the AAV-DIO-EGFP and AAV-DIO-VGAT constructs, D. Sulzer and H. Zhang for assistance with amperometry, R. Shah and C. Johnson for technical support, and members of the laboratory for helpful discussions. This work was supported by grants from the National Institutes of Health (NS046579 to B.L.S. and 4R00NS075136 to J.B.D.).
Footnotes
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature.
AUTHORS CONTRIBUTIONS
N.X.T., J.B.D. and B.L.S. designed the experiments. N.X.T. performed the experiments described in the figures and text and analyzed the data. J.B.D. performed experiments that initiated this study, devised the injection coordinates, established amperometric recordings and participated in their acquisition. N.X.T. and B.L.S. wrote the manuscript with contributions from J.B.D.
AUTHOR INFORMATION
Reprints and permissions information is available atwww.nature.com/reprints. The authors declare no competing financial interests. Readers are welcome to comment on the online version of this article atwww.nature.com/nature. Correspondence and requests for materials should be addressed to B.L.S. (bsabatini@hms.harvard.edu).
References
- 1.Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80 (1):1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Wickens JR, Reynolds JN, Hyland BI. Neural mechanisms of reward-related motor learning. Curr Opin Neurobiol. 2003;13 (6):685–690. doi: 10.1016/j.conb.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmiter RD. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: lessons from dopamine-deficient mice. Ann N Y Acad Sci. 2008;1129:35–46. doi: 10.1196/annals.1417.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12 (10):366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 6.Dagher A, Robbins TW. Personality, addiction, dopamine: insights from Parkinson’s disease. Neuron. 2009;61 (4):502–510. doi: 10.1016/j.neuron.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 7.Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69 (4):628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bentivoglio M, Morei M. Handbook of Chemical Neuroanatomy -Dopamine. Vol. 21. Elsevier; Amsterdam: 2005. The organization and circuits of mesencephalic dopaminergic neurons and the distribution of dopamine receptors in the brain; pp. 1–107. [Google Scholar]
- 9.Chuhma N, et al. Dopamine neurons mediate a fast excitatory signal via their glutamatergic synapses. J Neurosci. 2004;24 (4):972–981. doi: 10.1523/JNEUROSCI.4317-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hnasko TS, et al. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 2010;65 (5):643–656. doi: 10.1016/j.neuron.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tecuapetla F, et al. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J Neurosci. 2010;30 (20):7105–7110. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2010;30 (24):8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8 (9):1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 14.Backman CM, et al. Characterization of a mouse strain expressing Cre recombinase from the 3′ untranslated region of the dopamine transporter locus. Genesis. 2006;44 (8):383–390. doi: 10.1002/dvg.20228. [DOI] [PubMed] [Google Scholar]
- 15.Schmitz Y, Benoit-Marand M, Gonon F, Sulzer D. Presynaptic regulation of dopaminergic neurotransmission. J Neurochem. 2003;87 (2):273–289. doi: 10.1046/j.1471-4159.2003.02050.x. [DOI] [PubMed] [Google Scholar]
- 16.Sabatini BL, Regehr WG. Timing of synaptic transmission. Annu Rev Physiol. 1999;61:521–542. doi: 10.1146/annurev.physiol.61.1.521. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Hernandez T, Barroso-Chinea P, Acevedo A, Salido E, Rodriguez M. Colocalization of tyrosine hydroxylase and GAD65 mRNA in mesostriatal neurons. Eur J Neurosci. 2001;13 (1):57–67. [PubMed] [Google Scholar]
- 18.Cruikshank SJ, Urabe H, Nurmikko AV, Connors BW. Pathway-specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron. 2010;65 (2):230–245. doi: 10.1016/j.neuron.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wojcik SM, et al. A shared vesicular carrier allows synaptic corelease of GABA and glycine. Neuron. 2006;50 (4):575–587. doi: 10.1016/j.neuron.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 20.Yelin R, Schuldiner S. The pharmacological profile of the vesicular monoamine transporter resembles that of multidrug transporters. FEBS Lett. 1995;377 (2):201–207. doi: 10.1016/0014-5793(95)01346-6. [DOI] [PubMed] [Google Scholar]
- 21.Kozorovitskiy Y, Saunders A, Johnson CA, Lowell BB, Sabatini BL. Recurrent network activity drives striatal synaptogenesis. Nature. 2012;485 (7400):646–650. doi: 10.1038/nature11052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berube-Carriere N, et al. The dual dopamine-glutamate phenotype of growing mesencephalic neurons regresses in mature rat brain. J Comp Neurol. 2009;517 (6):873–891. doi: 10.1002/cne.22194. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda W, et al. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J Neurosci. 2009;29 (2):444–453. doi: 10.1523/JNEUROSCI.4029-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramirez M, Gutierrez R. Activity-dependent expression of GAD67 in the granule cells of the rat hippocampus. Brain Res. 2001;917 (2):139–146. doi: 10.1016/s0006-8993(01)02794-9. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Hernandez T, Barroso-Chinea P, Rodriguez M. Response of the GABAergic and dopaminergic mesostriatal projections to the lesion of the contralateral dopaminergic mesostriatal pathway in the rat. Mov Disord. 2004;19 (9):1029–1042. doi: 10.1002/mds.20206. [DOI] [PubMed] [Google Scholar]
- 26.Hirasawa H, Puopolo M, Raviola E. Extrasynaptic release of GABA by retinal dopaminergic neurons. J Neurophysiol. 2009;102 (1):146–158. doi: 10.1152/jn.00130.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maher BJ, Westbrook GL. Co-transmission of dopamine and GABA in periglomerular cells. J Neurophysiol. 2008;99 (3):1559–1564. doi: 10.1152/jn.00636.2007. [DOI] [PubMed] [Google Scholar]
- 28.Iijima K. Chemocytoarchitecture of the rat locus ceruleus. Histol Histopathol. 1993;8 (3):581–591. [PubMed] [Google Scholar]
- 29.Trottier S, et al. Co-localization of histamine with GABA but not with galanin in the human tuberomamillary nucleus. Brain Res. 2002;939 (1–2):52–64. doi: 10.1016/s0006-8993(02)02546-5. [DOI] [PubMed] [Google Scholar]
- 30.Broadbelt KG, Paterson DS, Rivera KD, Trachtenberg FL, Kinney HC. Neuroanatomic relationships between the GABAergic and serotonergic systems in the developing human medulla. Auton Neurosci. 2010;154 (1–2):30–41. doi: 10.1016/j.autneu.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Backman CM, et al. Characterization of a mouse strain expressing Cre recombinase from the 3′ untranslated region of the dopamine transporter locus. Genesis. 2006;44 (8):383–390. doi: 10.1002/dvg.20228. [DOI] [PubMed] [Google Scholar]
- 32.Gong S, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425 (6961):917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- 33.Madisen L, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13 (1):133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tong Q, et al. Synaptic glutamate release by ventromedial hypothalamic neurons ispart of the neurocircuitry that prevents hypoglycemia. Cell Metab. 2007;5 (5):383–393. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. NatNeurosci. 2008;11 (9):998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Durieux PF, et al. D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nat Neurosci. 2009;12 (4):393–395. doi: 10.1038/nn.2286. [DOI] [PubMed] [Google Scholar]
- 37.Tamamaki N, et al. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467 (1):60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- 38.Pologruto TA, Sabatini BL, Svoboda K. ScanImage: flexible software for operating laser scanning microscopes. Biomed Eng Online. 2003;2:13. doi: 10.1186/1475-925X-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.