Abstract
Background
Gastroesophageal reflux disease (GERD) reduces sleep quality. Whether Barrett’s esophagus (BE) affects sleep differently is unknown. Obstructive sleep apnea (OSA) often coexists with GERD and may disrupt sleep; whether GERD reduces sleep quality independently of OSA is unknown. Our aims were to compare the effect of GERD and BE on sleep quality, and assess the impact of OSA on this association.
Methods
Validated questionnaires for GERD symptoms, sleep quality, and OSA risk were prospectively administered to subjects undergoing upper endoscopy. GERD was defined by erosive esophagitis and/or reflux symptoms >1/week. BE was defined histologically. Controls had normal endoscopy and were asymptomatic. Poor sleep quality was defined by a Pittsburgh Sleep Quality Index score >5. Risk of OSA was defined by a positive Berlin Questionnaire. The risk poor sleep quality in GERD, BE, and controls was evaluated in multivariate models.
Key Results
83 GERD, 63 BE, and 75 controls were included. OSA and poor sleep quality were significantly more frequent in GERD (65% and 60%) but not BE (52% and 46%) compared with controls (48% and 39%). Controlling for age, race, gender, smoking, BMI, and hypertension, the risk of poor sleep quality was significantly increased in GERD compared with controls (odds ratio [OR] = 2.79, 95% confidence interval [CI]: 1.08 – 6.80), significance was lost after adding OSA to the model (OR = 2.27, 95% CI: 0.87 – 5.85).
Conclusions and Inferences
GERD but not BE increases the risk of poor sleep quality. This association is not independent of OSA.
Keywords: Gastroesophageal Reflux Disease, Barrett’s Esophagus, Sleep Disturbance, Obstructive Sleep Apnea
INTRODUCTION
Gastroesophageal reflux disease (GERD) is a common disorder. Heartburn or acid regurgitation is experienced on a weekly basis by nearly 20% of the US population. A Gallup survey of 1000 heartburn sufferers found that heartburn resulted in disturbed sleep in 63% of respondents [2]. Subsequent studies have reported an association between GERD and measures of reduced sleep quality including insomnia [3], disrupted sleep induction and maintenance [4], and a low overall sleep quality as measured by the Pittsburgh Sleep Quality Index (PSQI) [5]. Furthermore, a recent randomized, double-blind, placebo-controlled trial showed that gastric acid suppression with a proton pump inhibitor significantly improves sleep quality in patients with moderate-to-severe nighttime heartburn and GERD-related sleep disturbances [6].
GERD patients with sleep-related complaints may have more severe reflux disease. This is as evident from a study that found greater esophageal acid exposure (% time pH<4) in patients with nighttime heartburn and sleep complaints compared to those without nighttime heartburn [7]. Approximately 10% of patients with GERD develop Barrett’s esophagus (BE) [8]; these patients have generally more severe reflux disease as documented by higher distal esophageal acid exposure, including greater acid exposure in the nighttime period [9], and larger hiatus hernia compared with GERD patients without BE [10]. Whether the association between GERD and sleep quality differs between patients with or without BE has not been studied.
It is important to account for obstructive sleep apnea (OSA) when examining the association between GERD and sleep quality; but this was not performed by several previous studies [3–6]. OSA can disrupt sleep, and treatment with continuous positive airway pressure (CPAP) can increase sleep quality in OSA patients [11]. Several studies have reported an association between OSA and GERD. On one hand, GERD may play a role in the development of OSA by causing upper airway inflammation and obstruction [12]. Acid suppression with proton pump inhibitors resulted in improvement of the apnea index in OSA patients in a small non-randomized study [13]. On the other hand, OSA may lead to GERD because of increased intra-thoracic pressure [14] and treatment with CPAP has been shown to improve nocturnal heartburn and regurgitation [15], as well as distal esophageal acid exposure and frequency of acid reflux episodes [16]. Irrespective of the nature of the association between GERD and OSA, it is important to account and adjust for the presence of OSA when studying sleep quality in GERD patients. Furthermore, it is unclear whether this association differs by the presence of BE. This information may help in clarifying the directionality or dose-response relationship of the association between GERD and OSA.
In summary, GERD is associated with poor sleep quality but whether BE - a more severe GERD presentation – affects sleep differently is not known. OSA often coexists with GERD and may also disrupt sleep and it remains unknown whether GERD with or without BE reduce sleep quality independently of OSA. Therefore, the aims of our study were: (1) to compare the effect of GERD and BE on sleep quality, and (2) to assess whether the association between sleep quality and GERD or its more severe form (i.e., BE), is independent of OSA.
METHODS
Study Population and Design
We conducted a case-control study nested within an ongoing cross-sectional study of BE risk factors at the Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, TX. We recruited all consecutive patients eligible for participation who were scheduled for an elective upper endoscopy at MEDVAMC. In addition, patients from the primary care clinic at MEDVAMC who were eligible for screening colonoscopy were recruited to undergo upper endoscopy in addition to their colonoscopy. We prospectively collected self-reported data regarding GERD symptoms, sleep quality, and risk of OSA, and endoscopy findings. Study groups were defined as cases with BE, cases with GERD but no BE, and controls without GERD or BE based upon GERD questionnaire data and endoscopy findings as described below.
Recruitment and eligibility criteria
The electronic medical records of all veterans with a scheduled upper endoscopy were screened to determine study eligibility based on the following criteria: 1) age between 40–80 years (50–80 for patients from the primary care clinic); 2) no previous esophageal or gastric surgery; 3) no esophageal or gastric cancer; 4) no active lung, liver, colon, or breast cancer; 5) not taking anticoagulants; 6) no significant liver disease indicated by platelet count below 70,000, ascites, or known gastroesophageal varices; 7) no history of major stroke or mental condition that would limit their ability to answer questions. The eligibility criteria were further checked among eligible patients on the day of the endoscopy. This research was approved by the Institutional Review Boards for the MEDVAMC and Baylor College of Medicine.
Data Collection
All study participants completed a research assistant administered computer assisted survey before the endoscopy. The survey contained demographic data, past medical history, medication use including proton pump inhibitor use, and validated questionnaires for GERD symptoms, sleep quality, and OSA that are explained in detail below. In addition, height and weight were measured in all subjects to calculate body mass index (BMI).
GERD Questionnaire
The questions were adapted and slightly modified from the GERQ [17], a validated questionnaire of GERD symptoms. Each question has 6 possible answers (never, <1/month, about 1/month, about 1/week. >1/week, daily). We defined GERD using 2 questions regarding the frequency of heartburn and acid regurgitation during the past one year; patients were considered as having GERD if they experienced heartburn or acid regurgitation in the past year occurring more than once per week.
Pittsburgh Sleep Quality Index (PSQI)
The PSQI [18] is a 19-item validated questionnaire designed to measure sleep quality during the previous month. Items are grouped into seven component scores: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. Each component score ranges from 0 (no difficulty) to 3 (severe difficulty). The component scores are summed to produce a global score (range of 0–21). Patients with a global PSQI score >5 are considered to have poor sleep quality as done in previous studies [18].
Berlin Questionnaire for Risk of Obstructive Sleep Apnea
This is a validated instrument [19] that includes 10 items grouped into 3 categories related to the risk of having OSA: snoring behavior, daytime sleepiness or fatigue, and presence of obesity or hypertension. For each of the three categories, patients are classified into high risk or low risk based on their responses to the individual items. Patients classified as high risk in two or more categories are considered to have a global high risk for OSA. In the present study, a global high risk of OSA was used as a surrogate marker for OSA. The Berlin questionnaire has good correlation with the respiratory disturbance index as measured by portable sleep monitoring [19].
Upper endoscopy
All study participants underwent an upper endoscopy with systematic recording of findings on erosive esophagitis based on the Los Angeles (LA) Classification [20] and suspected BE based on the Prague classification [21] and at least one targeted biopsy was obtained from the suspected BE area using Jumbo biopsy forceps.
Study Groups
GERD without BE (GERD Cases)
This group was defined by the presence of erosive esophagitis LA grade B or greater [20] and/or frequent GERD symptoms on the GERD Questionnaire as defined above[17] and the absence of suspected BE.
Barrett’s esophagus (BE Cases)
This group was defined by endoscopically suspected BE that was confirmed by the presence of specialized intestinal metaplasia in the histopathological examination of targeted biopsies. Two expert pathologists reviewed all slides for suspected BE to determine the presence of definitive BE.
No GERD or BE (Controls)
This group was defined by the absence of erosive esophagitis or suspected BE by endoscopy and no acid regurgitation or heartburn in the last one year in the GERD questionnaire [17], and no self-reported history of erosive esophagitis, BE, or proton pump inhibitor use.
Data Analysis
Characteristics of the two case groups (BE and GERD) were compared to controls without BE or GERD. We used the chi squared test to compare categorical variables, and unpaired t-tests to compare continuous measures. Multivariate logistic regression models were used to evaluate the risk of having poor sleep quality (defined by a PQSI>5) according to presence of GERD or BE; controls were the referent category for all analyses. Models were adjusted for race, gender, smoking (current vs. not current), recruitment source, BMI, hypertension (self-reported diagnosis or currently taking medication for hypertension), and OSA (defined by Berlin score as explained above). Results were computed with SAS software (SAS Institute, Cary, NC).
RESULTS
The study included 221 patients who participated in the cross-sectional study, underwent endoscopy and had complete information on the endoscopy report and all three of the study questionnaires to allow the definition of the case and control groups. Of these 221 subjects, 83 met study criteria for GERD, 63 met criteria for BE, and 75 met criteria for controls. PPI use was reported by 54% of GERD and 65% of BE patients, while controls were non-PPI users by definition. There was no statistically significant difference in prevalence of hiatus hernia for GERD compared to BE; however, hiatus hernia was significantly more prevalent in GERD compared to controls (72% vs 57%, p=0.01), and BE compared to controls (80% vs 57%, p=0.008). Erosive esophagitis was present in 34% of GERD patients (LA grades A, B, C and D were 7%, 18%, 7%, and 2% respectively). Of note, patients with LA grade A esophagitis were included in the study based upon their frequent symptoms rather than esophagitis, since the endoscopic definition of GERD was esophagitis LA grade B or greater. The length of BE was less than 3 cm in 74% of patients; 26% had a BE segment equal to or greater than 3 cm.
The demographic characteristics and prevalence of OSA and poor sleep quality for the three study groups are shown in Table 1. There were no significant differences in age, or BMI among the three study groups. Smoking was more frequent in BE but not GERD cases compared to controls. As expected, the proportions of Caucasian subjects were higher in GERD and BE compared with controls. The study included predominantly male subjects across all groups; however, the proportion of males was significantly lower in the GERD group than in the other two groups. The majority of GERD and BE patients were recruited from subjects scheduled for elective endoscopy; in contrast, the main recruitment source for the control group was the primary care clinic (i.e. subjects who were eligible for screening colonoscopy and were recruited to undergo upper endoscopy in addition to their colonoscopy).
Table 1.
Demographic Characteristics and Prevalence of Obstructive Sleep Apnea (OSA) and Poor Sleep Quality in Controls, GERD with no BE and BE patient groups.
| Variable | Controls (n=75) |
GERD (n=83) |
p | BE (n=63) |
p |
|---|---|---|---|---|---|
| Age (SD)* | 61.7 (6.7) | 59.2 (9.4) | 0.06 | 61.8 (70) | 0.91 |
| Males (%)# | 73 (97) | 73 (89) | 0.03 | 61 (97) | 0.85 |
| Caucasian (%)# | 36 (48) | 61 (73) | 0.004 | 52 (83) | <0.0001 |
| Current Smoker (%)# | 20 (27) | 25 (30) | 0.73 | 21 (33) | 0.01 |
| BMI (SD)* | 30.8 (6.1) | 30.1 (7.0) | 0.53 | 30.5 (5.7) | 0.50 |
| Recruitment Source: endoscopy† (%)# | 14 (19) | 68 (82) | <0.0001 | 49 (78) | <0.0001 |
| OSA by Berlin Questionnaire (%)# | 36 (48) | 54 (65) | 0.03 | 33 (52) | 0.61 |
| Pittsburgh Questionnaire Score* | 5.6 (4.1) | 6.9 (3.9) | 0.04 | 5.5 (3.1) | 0.80 |
| Poor sleep quality by Pittsburgh Questionnaire (%)# | 29 (39) | 50 (60) | 0.007 | 29 (46) | 0.38 |
GERD, Gastroesophageal reflux disease; BE, Barrett’s esophagus; SD, standard deviation; BMI, body mass index; OSA, obstructive sleep apnea
Means among cases and controls were compared using an unpaired student’s t-test
Prevalence among cases (GERD and BE) and controls was compared by the chi-squared test
Patients recruited from subjects scheduled for elective endoscopy, as opposed to those recruited from primary care clinic
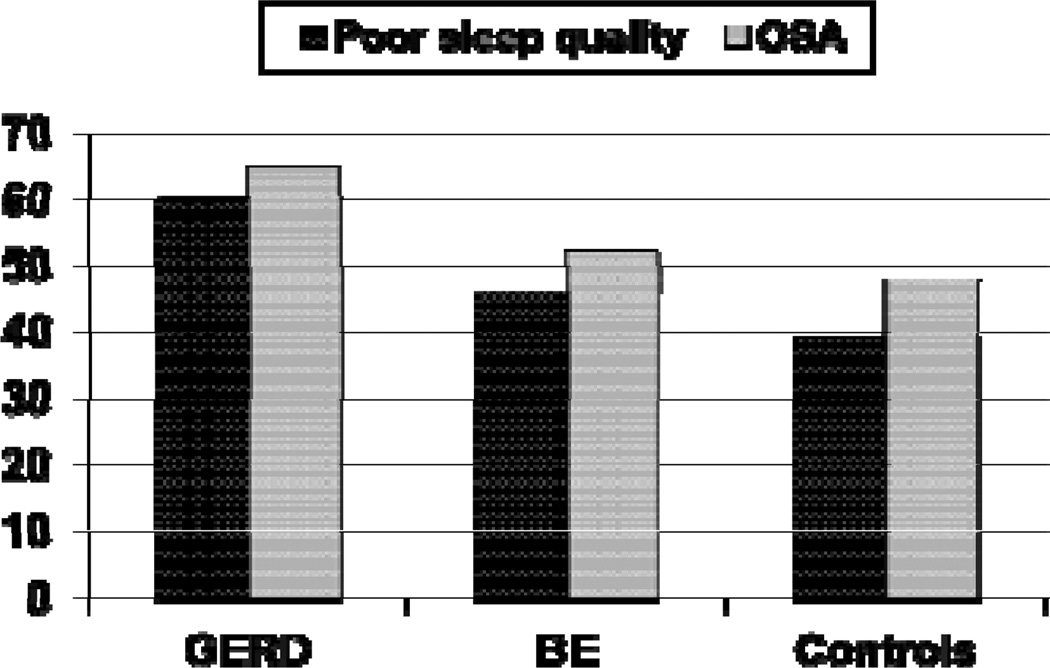
OSA and poor sleep quality were common in all three groups. However, the prevalence of OSA was significantly higher in the GERD case group but not in the BE case group compared with controls (Figure 1 and Table 1). The prevalence of poor sleep quality was also significantly higher in the GERD case group but not in the BE case group compared with controls (Figure 1 and Table 1).
Figure 1.
Prevalence of decreased sleep quality and obstructive sleep apnea (OSA) in patients with gastroesophageal reflux disease (GERD), Barrett’s esophagus (BE) and controls without GERD or BE. Decreased sleep quality was defined by a score > 5 on the Pittsburgh Sleep Quality Index18. OSA was defined by a global high risk of sleep apnea on the Berlin Questionnaire19.
Based on multivariate logistic regression models (Table 2) controlling for age, race, gender, smoking status, recruitment source, BMI, and hypertension, the risk of poor sleep quality remained significantly increased in patients with GERD compared with controls (odds ratio [OR] = 2.79, 95% confidence interval [CI]: 1.08 – 6.80). However, the risk of poor sleep quality was not significantly increased for GERD after adding the presence of OSA to the model, in fact it slightly attenuated the association (OR = 2.27, 95% CI: 0.87 – 5.85). Similar to the unadjusted analysis, there was no difference in the risk of poor sleep quality in BE patients compared with controls in the multivariate models (Table 2).
Table 2.
Risk of poor sleep quality among GERD and BE cases compared with controls. Poor sleep quality was defined by a score > 5 on the PSQI.
| Model | Poor Sleep Quality OR (95% CI) |
|
|---|---|---|
| GERD | Unadjusted | 2.33 (1.22–4.44) |
| Adjusted for age, race, gender, smoking, BMI, HTN, recruitment source | 2.79 (1.08–6.80) | |
| Adjusted for age, race, gender, smoking, BMI, HTN, recruitment source, OSA | 2.27 (0.88–5.85) | |
| BE | Unadjusted | 1.22 (0.61–2.45) |
| Adjusted for age, race, gender, smoking, BMI, HTN, and recruitment source | 1.33 (0.52–3.45) | |
| Age adjusted age, race, gender, smoking, BMI, HTN, recruitment source, OSA | 1.34 (0.52–3.48) |
GERD, Gastroesophageal reflux disease; BE, Barrett’s esophagus; PSQI, Pittsburgh Sleep Quality Index; BMI, body mass index; HTN, hypertension; OSA, obstructive sleep apnea; OR, odds ratio; CI confidence interval
We conducted additional multivariate models to assess the effect of PPI use on the risk of poor sleep quality in GERD and BE patients (controls were not on PPI by definition). The risk of poor sleep quality for the BE group compared to the GERD group was similar whether PPI was excluded (OR = 0.57, 95% CI: 0.26 – 1.27) or included (OR = 0.52, 95% CI: 0.23 – 1.17).
DISCUSSION
An association between GERD and reduced sleep quality was previously documented in several studies [3–6]. However, sleep quality has not been investigated in the subgroup of GERD patients with BE which is considered a more severe form of the disease. We present, for the first time, data regarding sleep quality in BE patients. Our study also evaluated the association between GERD and sleep quality in patients with GERD but no BE. Having a more severe form of GERD (i.e. BE) did not appear to increase the frequency of poor sleep quality. In fact, we found that sleep quality was reduced in GERD but not in BE patients compared with controls; the prevalence rates of poor sleep quality were 60%, 46%, and 39% for GERD, BE, and control patients, respectively. The lack of impact on sleep disturbance by BE may have been due to the fact that the majority of BE patients had a short segment (<3cm) and presumably less severe disease. Given the relatively small number of patients with long segment BE, an additional analysis of this issue was not feasible. On the other hand, our findings may suggest that the mechanisms underlying sleep disturbance may differ among these two groups of GERD patients (with and without BE). Since patients with BE may be less sensitive to acid reflux and may therefore experience fewer symptoms than patients with GERD but no BE [22–23], it is possible that this leads to less sleep disturbance in the BE group. However, these differences can only be determined by pathophysiological studies that should, among other things, evaluate reflux mechanisms, refluxate characteristics, and esophageal sensitivity while controlling for other risk factors that affect sleep.
A recent study that, similar to ours, defined poor sleep quality with a score >5 on the PSQI, found decreased sleep quality in 65% of patients with non-erosive reflux disease and 67% patients with erosive esophagitis; BE patients were not included and the use of PPIs was not reported [5]. In another study that defined poor sleep quality as a PSQI score > 5, Johnson and colleagues found that the prevalence of reduced sleep quality was 83% in untreated GERD patients with frequent heartburn or acid regurgitation, including nighttime symptoms at least twice per week [6]. The higher prevalence of poor sleep quality reported by that study may be explained by the fact that our study included patients regardless of whether they had frequent nighttime symptoms. In addition, although frequent heartburn or acid regurgitation – despite PPI therapy – was one of the inclusion criteria for our study, 53% of our patients were taking a PPI, in contrast to the untreated patients in Johnson’s study. The number of patients in our data set is not sufficiently large to enable further analysis after additional categorization of GERD patients by symptom severity or presence/absence of erosive esophagitis, and this will be important in future studies.
Our study is also the first one to account for the effect of OSA on sleep quality in patients with GERD, both with and without BE. Interestingly, the prevalence of OSA was significantly higher in GERD patients without BE compared to those with BE (65% vs. 52%). The relationship between gastroesophageal reflux and OSA is complex and the reasons for the higher prevalence of OSA in GERD patients without BE cannot be determined based upon our data. However, our findings suggest the possibility that the pathogenetic factors related to GERD that favor the development of BE in GERD patients may be different than those for OSA. Since the direction of the causality in the GERD-OSA relationship is not clear, an alternative hypothesis is that the type of reflux caused by OSA is less conducive to BE.
An additional important finding in our study is a result of the multivariate models showing that the risk of poor sleep quality does not appear to be independent of OSA in GERD patients. From this we can infer that OSA explains some of the effect of GERD on sleep quality. No other study has looked at this specific issue and therefore these results need to be confirmed.
The study is limited by the inability of the case-control design to define the temporal association among GERD, OSA, and sleep quality and therefore causal inferences cannot be made. The study was limited to veteran patients who are mostly men and therefore caution must be used when generalizing these results to non veterans or women. The study did not account for psychiatric comorbidity, which may affect sleep quality and OSA. Lastly, we did not perform polysomnography or portable sleep monitoring to diagnose OSA; instead, a global high risk of OSA on the Berlin questionnaire was used as a surrogate marker for OSA (this questionnaire has good correlation with the respiratory disturbance index measured by portable sleep monitoring [10]). Thus, while we used validated questionnaires to define high risk of OSA and measure sleep quality, some misclassification is possible due to the absence of physiological assessments. However, there is no known reason for this misclassification to be differential among the three study groups.
Strengths of our study include the detailed characterization of the study groups by endoscopy and validated questionnaires, allowing us to account for multiple important variables that may affect sleep quality. Our sampling frame was consecutive patients referred to upper endoscopy irrespective of indication and therefore there is less likelihood of selection or ascertainment bias that would affect other studies of patients referred specifically for GERD or sleep disorders. Our study could have been strengthened by the addition of physiological measurements such as ambulatory reflux monitoring to document the amount of reflux in each participant, as an additional important variable. However, reflux monitoring was beyond the scope of this project.
In summary, we found that poor sleep quality is frequent in our VA population and the risk of poor sleep quality is increased in patients with GERD but not those with BE. In addition, the risk of poor sleep quality does not appear to be independent of OSA in GERD patients.
ACKNOWLEDGEMENTS
Financial support:
HES is supported by NCI R01 116845 and NIDDK K24-04-107. The work is supported in part by the Houston VA HSR&D Center of Excellence (HFP90-020), and by the Texas Digestive Disease Center NIH DK58338.
Footnotes
Guarantor of the article:
Marcelo F. Vela
Specific author contributions:
Marcelo F. Vela: Study concept, acquisition of data, analysis, study supervision, drafting and finalizing the manuscript
Jennifer R. Kramer: Acquisition of data, analysis, reviewing the manuscript
Peter A. Richardson; Acquisition of data, analysis
Rhiannon Dodge: Acquisition of data, analysis
Hashem B. El-Serag; Study concept, acquisition of data, analysis, study supervision, reviewing the manuscript
Conflict of interest:
Marcelo F. Vela [Given imaging -consulting]; no other conflicts for remaining authors (JRK, PAR, RD, HES).
REFERENCES
- 1.Locke GR, III, Talley NJ, Fett SL, et al. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448–1456. doi: 10.1016/s0016-5085(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 2.Shaker R, Castell DO, Schoenfeld PS, et al. Nighttime heartburn is an under-appreciated clinical problem that impacts sleep and daytime function: the results of a Gallup survey conducted on behalf of the American Gastroenterological Association. Am J Gastroenterol. 2003;98:1487–1493. doi: 10.1111/j.1572-0241.2003.07531.x. [DOI] [PubMed] [Google Scholar]
- 3.Janson C, Nordenstedt H, Wallander M, et al. A population-based study showing an association between gastroesophageal reflux disease and sleep problems. Clinical Gastroenterol Hepatol. 2009;7:960–965. doi: 10.1016/j.cgh.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Mody R, Bolge SC, Kannan H, Fass R. Effects of gastroesophageal reflux disease on sleep and outcomes. Clinical Gastroenterol Hepatol. 2009;7:953–959. doi: 10.1016/j.cgh.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Yi C-H, Hu C-T, Chen C-L. Sleep dysfunction in patients with GERD: erosive versus nonerosive reflux disease. Am J Med Sci. 2007;334:168–170. doi: 10.1097/MAJ.0b013e318141f4a5. [DOI] [PubMed] [Google Scholar]
- 6.Johnson D, Crawley JA, Hwang C, Brown K. Clinical trial: esomeprazole for moderate-to-severe nighttime heartburn and gastro-oesophageal reflux disease-related sleep disturbances. Alimentary Pharmacol Ther. 2010;32:182–190. doi: 10.1111/j.1365-2036.2010.04339.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen C-L, Robert JJT, Orr WC. Sleep symptoms and gastroesophageal reflux disease. J Clin Gastroenterol. 2008;42:13–17. doi: 10.1097/MCG.0b013e31802fc1bc. [DOI] [PubMed] [Google Scholar]
- 8.Cameron AJ. Barrett's esophagus: prevalence and size of hiatal hernia. Am J Gastroenterol. 1999;94:2054–2059. doi: 10.1111/j.1572-0241.1999.01277.x. [DOI] [PubMed] [Google Scholar]
- 9.Dickman R, Parthasarathy S, Malagon IB, et al. Comparisons of the distribution of oesophageal acid exposure throughout the sleep period among the different gastro-oesophageal reflux disease groups. Aliment Pharmacol Ther. 2007;26:41–48. doi: 10.1111/j.1365-2036.2007.03347.x. [DOI] [PubMed] [Google Scholar]
- 10.Avidan B, Sonnenberg A, Schnell TG, Sontag SJ. Hiatal hernia and acid reflux frequency predict presence and length of Barrett’s esophagus. Dig Dis Sci. 2002;47:256–264. doi: 10.1023/a:1013797417170. [DOI] [PubMed] [Google Scholar]
- 11.Loredo JS, Ancoli-Israel S, Kim E-J, et al. Effect of continuous positive airway pressure versus supplemental oxygen on sleep quality in obstructive sleep apnea: a placebo-CPAP-controlled study. Sleep. 2006;29:565–571. doi: 10.1093/sleep/29.4.564. [DOI] [PubMed] [Google Scholar]
- 12.Zanation AM, Senior BA. The relationship between extreaesophageal reflux and obstructive sleep apnea. Sleep Med Rev. 2005;9:453–458. doi: 10.1016/j.smrv.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Senior BA, Kahn M, Schwimmer C, et al. Gastroesophageal reflux and obstructive sleep apnea. Laryngoscope. 2001;111:2144–2146. doi: 10.1097/00005537-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Kerr P, Shoenut JP, Millar, et al. Nasal CPAP reduces gastroesophageal reflux in obstructive sleep apnea syndrome. Chest. 1992;101:1539–1544. doi: 10.1378/chest.101.6.1539. [DOI] [PubMed] [Google Scholar]
- 15.Green BT, Broughton WA, O’Connor JB. Marked improvement in nocturnal gastroesophageal reflux in a large cohort of patients with obstructive sleep apnea treated with continues positive airway pressure. Arch Intern Med. 2003;163:41–45. doi: 10.1001/archinte.163.1.41. [DOI] [PubMed] [Google Scholar]
- 16.Tawk M, Goodrich S, Kinaswitz G, Orr W. The effect of 1 week of continuous positive airway pressure treatment in obstructive sleep apnea patients with concomitant gastroesophageal reflux. Chest. 2006;130:1003–1008. doi: 10.1378/chest.130.4.1003. [DOI] [PubMed] [Google Scholar]
- 17.Locke GR, Talley NJ, Weaver AL, Zinsmeister AR. A new questionnaire for gastroesophageal reflux disease. Mayo Clin Proc. 1994;69:539–547. doi: 10.1016/s0025-6196(12)62245-9. [DOI] [PubMed] [Google Scholar]
- 18.Buysse DJ, Reynolds CF, III, Monk TH, et al. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 19.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 20.Lundell LR, Dent J, Bennet JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172–180. doi: 10.1136/gut.45.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma P, Dent J, Armstrong D, Bergman JJ, et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C & M criteria. Gastroenterology. 2006;131:1392–1399. doi: 10.1053/j.gastro.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 22.Brandt MG, Darling GE, Miller L. Symptoms, acid exposure and motility in patients with Barrett’s esophagus. Can J Surg. 2004;47:4751. [PMC free article] [PubMed] [Google Scholar]
- 23.Byrne PJ, Mulligan ED, O’Riordan J, Keeling PW, Reynolds JV. Impaired visceral sensitivity to acid reflux in patients with Barrett’s esophagus. The role of esophageal motility. Dis Esophagus. 2003;16:199–203. doi: 10.1046/j.1442-2050.2003.00328.x. [DOI] [PubMed] [Google Scholar]