Abstract
It seems to be a necessary need to develop an effective drug carrier system for targeted delivery of pharmaceuticals. Bacterial ghosts are emerging drug delivery platform that are capable of delivery of proteins, antigens, nucleic acids, and pharmaceuticals. Bacterial ghosts are generally produced by lysis of gram-negative bacteria. Pharmaceutically, these ghosts could be utilized to deliver proteins peptides, vaccines, drugs effectively. However, this technology is at initial stage and systematic studies are required to implement such system over humans.
Keywords: Bacterial ghost, biotherapeutics, delivery, drugs
INTRODUCTION
Modern recombinant proteins and deoxyribonucleic acid (DNA) vaccines are known to be poor immunogenic as well as weak to elicit proper response due to the fact that, they are in direct contact with biological fluids which leads to their degradation and lack of desired response whereas synthetic drugs also demonstrate poor therapeutic response due to improper maintenance of desired concentration at target organ Therefore, it becomes necessary to develop an effective carrier system that might be able to deliver proteins, antigens, nucleic acids, and pharmaceuticals. Such a delivery system is intended to offer delivery with high efficiency with reduced dosing and adequate safety.[1]
Live attenuated and inactivated whole-cell bacteria comprise of a useful means to have efficient delivery of antigens, nucleic acid, and drugs.[2] Bacterial ghost symbolize ‘empty non-denaturated envelopes derived from gram-negative bacteria by protein-E-mediated lysis, which retain all morphological and structural features of the natural cell.’ Due to presence of immune-stimulating compounds at their surface, they could be used as an ideal candidate for delivery of immunogenic biological as well as pharmaceuticals.
Bacterial ghost
Bacterial ghosts are created from gram-negative bacteria where they are formed by ‘the expression of the bacteriophage lysis gene E.’[3] Expression of ‘lysis gene E’ causes separation of cytoplasmic content leading to formation of a ‘transmembrane tunnel.’ Formation of this tunnel enables removal of cytoplasmic content of bacteria by osmosis.[4] The so formed internal empty shell has no cellular components but has ‘functional and antigenic determinants’ like their living homologues. These ghosts also have bioadhesive characters which enable them to interact with host cellular machinery and when the biomolecule or drug is entrapped in the tunnel, these ghosts offer an excellent means for delivery to the targeted site.[3]
Bacterial ghost as drug delivery carrier offers an excellent means to ‘elicit’ cellular and humoral response because of their immunogenic potential.[5] A number of ghost candidate have been utilized for immunization like Pasteurella multocida, Pasteurella haemolytica,[6] Vibrio cholera,[7] Pasteurella haemolytica,[8] Edwardsiella tarda,[9] Salmonella enteritidis,[10] Helicobacter pylori,[11] V. cholera,[12] and V. anguillarum.[13]
Bacterial ghosts are promising biomolecule and drug delivery system. One the one hand, they offer advantages as being nonliving carrier and capable of holding and delivering DNA, vaccines, and drugs [Figure 1]. On the other hand, they demonstrate promising role as being effectively recognized and uptake by antigen representing cells and could be targeted to different tissues.[14]
Figure 1.
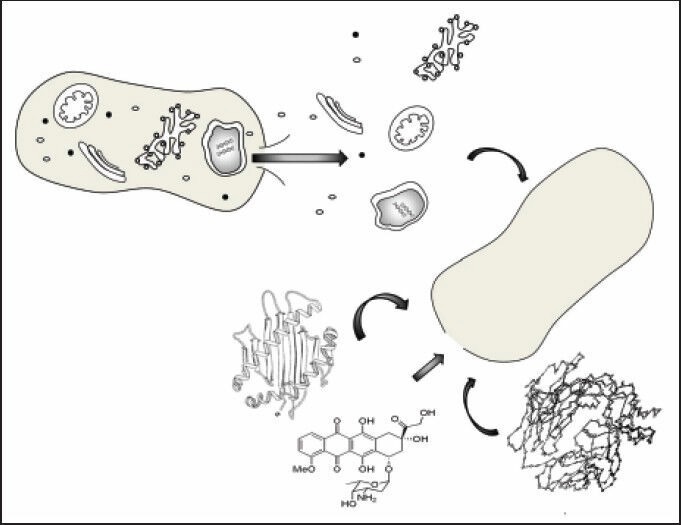
Role of bacterial ghost in drug delivery
Production of bacterial ghost
Bacterial ghosts are produced as a result of expression of cloned gene E from bacteriophage PhiX174 leading to ‘lysis’ of gram-negative bacteria. Gene E is responsible to code for membrane protein comprising of 91 amino acids, which fuse with inner and outer membrane of gram-negative bacteria. These results in membrane lysis and from where cytoplasmic contents are expelled.[4,15,16,17] Lysis of bacteria can be performed in different experimental conditions. New expression system causing mutation of EpR promotro/operator region causes stable E gene expression at temperature of 37°C.[15] Similarly, when Epr promoter/Ci repressor system with lacI/lacPO is utilized for gene expression control, the growth temperature can be lowered up to 28°C.[18]
General lysis of microbes is utilized for creation of bacterial ghost, which in turn prepares ‘intact’ cellular envelop.[19,20] Expression of plasmid-encoded gene E is utilized for lysis of bacteria. This is one of the easiest methods that does not affect any of the physical or chemical characteristics and has no detrimental effect on bacterial surface. Expression of gene E results in development of ‘a transmembrane tunnel structure’ by outer and inner bacterial membrane. The osmotic pressure inside the cell is released, leading to expulsion of protoplasm in neighbouring environment, thus resulting in the development of new ghost cell. During this process, periplasmatic space is sealed without affecting peptidoglycan.[20] The, so formed bacterial ghost contain desired cell structure along with memberane protein, pili, and adhesins, which exist as basic organization and thus pretends like a living bacteria. Bacterial ghosts are produced by fermentation process [batch fermentation]. As all the cytoplasmic material is released, residual bacterial DNA is deactivated by staphylococcal nuclease A by treatment with β-propiolactone. The whole process takes place within a day and thus rapid production of bacterial ghost is feasible.[21]
PHARMACEUTICAL APPLICATIONS OF BACTERIAL GHOST
Bacterial ghost as drug delivery system
Bacterial ghost can be targeted toward dendritic cells and macrophages,[22] microvascular endothelial cells,[23] ocular surface diseases[24] as well as in gene transfer to melanoma cells.[25] Bacterial ghosts are also studied for targeting potential toward human conjuctival epithelial cells using some in vitro models like Chang conjunctival epithelial cell line and primary human conjunctiva-derived epithelial cells. These studies concluded efficient internalization of bacterial ghost in corneal cells lines with no cytotoxicity.[24] Use of anticancer agents is often related with a large number of side effects. Delivery of cytotoxic agents via bacterial ghost provides a novel platform for cancer targeting. Bacterial ghosts from Mannheimia haemolytica loaded with doxorubicin are studied against human colorectal adenocarcinoma cells, whereby potent anticancer activity was observed with ghost loaded with doxorubicin as compared with plain drug in terms of higher antiproliferative effects.
Use of bacterial ghost as an effective model to control fertility on animal model [Trichosurus vulpecula] has been documented. Ghosts encapsulated with possum zona pellucida protein-2 when subjected to be applied to the nostrils and eyes of female Trichosurus vulpecula, and its effects on fertility was determined by superovulation and artificial insemination. Ghosts provoked humoral and cell-mediated immune leading to fertilization of only few eggs. Effects of immunisation on fertility were assessed, following superovulation and artificial insemination. Both constructs evoked humoral [antibody] and cell-mediated immune responses in possums and significantly fewer eggs were fertilized in females immunized against zona pellucida protein-2C ghosts.[26]
Bacterial ghost for protein and peptide delivery
Escherichia coli ghost carrying hepatitis B virus core 149 (HBcAg-149) proteins anchored in inner and the outer membrane of E. coli was compared. Both these strategies demonstrated an excellent means to deliver HBcAg-149 as antigen to female BALB/c mice.[27] Vaccine with adequate spectrum of activity is often desired for population. V. cholera vaccine as whole-cell cholera and toxoid vaccines has only offered transient protection. V. cholera ghost were administered to reversible intestinal tie adult rabbit diarrhoea model. Vaccination by V. cholera ghost resulted in increased levels of serum vibriocidal titers.[28]
Bacterial ghost also offer a promising approach to immobilize plasmid DNA which offer a novel carrier along with intrinsic property of immunogenicity of gram-negative bacterial cell envelop.[29] Tumor cells and antigen presenting cells when transfected with bacterial ghost with plasmid DNA phagocytised them, bacterial ghost with antigens are capable of activating CD4+ and CD8+ T cells and thus elicit immune response against antigens which are expressed against target cells.[30]
Delivery of nucleic acid via bacterial ghost
Bacterial ghost technology could be an innovative approach in vaccine development due to their interaction and uptake by macrophages, monocytes, and dendriatic cells.[31] Nucleic acids can easily be incorporated inside bacterial ghost system. Lyophilized bacterial ghost are suspended in DNA solution and then washed to remove excess of DNA. This system could be easily be used for DNA and gene delivery. About 3000 minimum sized DNA plasmid copies could be encapsulated in ghost system.[32]
Immunization by bacterial ghost
Effective immunization against gram-negative bacteria can be achieved using bacterial ghost technology. Vaccination of pigs with Actinobacillus pleuropneumoniae resulted in prevention of colonization of pathogen and effective immunization without any adverse effect.[33] Bacterial ghost from P. multocida and M. haemolytica have been successfully evaluated for their immunogenic potential, where ghost effectively protected the animal models comparable to standard vaccines available in markets.[6,8] Preclinical testing on V. cholera ghost has been successfully completed, where mucosal administration of bacterial ghost has offered maximum protection due to humoral and cellular immune response.[31,34]
Immunostimulating components like pathogen-associated molecular patterns constituting peptidoglycan, lipopolysaccharides, monophosphoryl lipid A, and so on are present on ghost surface, which, on interaction with cellular components, evoke immune response.[31] Bacterial ghost also serve as an important means for enzymatic reactions. Although bacterial ghosts are produced when cytoplasmic material of bacteria is expelled out, but removal of cytoplasmic material do not confer total loss of enzymatic activity. Enzymes like membrane-bound β-galactosidase and chloramphenicol acetyl transferase are known to be present on bacterial surface.[35,36]
FUTURE PERSPECTIVE
Bacterial ghost technology is one of the challenging approaches for effective drug delivery. Recently, some ghost vaccines are under clinical studies. The future of the bacterial ghost vaccine seems to be promising, still some challenges like antigenecity of bacterial ghost, and their stability, site-specific drug delivery, and so on are required to be controlled. The future of bacterial ghost seems to be promising. Bacterial ghost system could be utilized for delivery of prophylactic and therapeutic vaccines to treat a number of infectious and non-infectious diseases. Current concepts and progress in the field of biotechnology, bacteriology, immunology, and genetics has aided in development of bacterial ghost system. The ‘inbuilt natural antigenic potential’ of bacterial ghost enhances its in vitro as well as in vivo performance for the delivery of drugs and biotherapeutics. Thus, bacterial ghost technology would definitely replace use of live or attenuated bacteria as vaccines.[37]
A number of reports support the effectiveness of bacterial ghost for the delivery of drugs, vaccines, and biotherapeutics in mammals.[38,39] The future of bacterial ghost would lie on their ability to deliver drug to target sites. However, a number of obstacles like immunogenicity of ghost system, lack of drug concentration at site of action, and sometimes poor invasion of ghost in cell may arise. Therefore, it is an immediate need to optimize, validate, and authenticate the overall production step of bacterial ghost. At the same time, it would also be necessary to evaluate the production variables for reproducible results. Safety issue is another matter of concern allied with bacterial ghost. The system seems to be unfit for immunocompromised individuals. Thus, over all a lot of work is required to be done.
CONCLUSION
Bacterial ghost represents a novel platform for delivery of various types of pharmaceuticals and biological. Such system may prove to be useful in terms of effective drug delivery. However, bacterial ghost system for delivery of drugs and biological is still a new concept and a series of detailed systematic studies are required to implement such system over humans.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Sesardic D, Dobbelaer R. European union regulatory developments for new vaccine adjuvants and delivery systems. Vaccine. 2004;22:2452–6. doi: 10.1016/j.vaccine.2003.11.071. [DOI] [PubMed] [Google Scholar]
- 2.Kang HY, Curtiss R., 3rd Immune responses dependent on antigen location in recombinant attenuated Salmonella typhimurium vaccines following oral immunization. FEMS Immunol Med Microbiol. 2003;37:99–104. doi: 10.1016/S0928-8244(03)00063-4. [DOI] [PubMed] [Google Scholar]
- 3.Huter V, Szostak MP, Gampfer J, Prethaler S, Wanner G, Gabor F, et al. Bacterial ghosts as drug carrier and targeting vehicles. J Control Release. 1999;61:51–63. doi: 10.1016/s0168-3659(99)00099-1. [DOI] [PubMed] [Google Scholar]
- 4.Witte A, Wanner G, Blasi U, Halfmann G, Szostak M, Lubitz W. Endogenous transmembrane tunnel formation mediated by phi X174 lysis protein E. J Bacteriol. 1990;172:4109–14. doi: 10.1128/jb.172.7.4109-4114.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medina E, Guzman CA. Use of live bacterial vaccine vectors for antigen delivery: Potential and limitations. Vaccine. 2001;19:1573–80. doi: 10.1016/s0264-410x(00)00354-6. [DOI] [PubMed] [Google Scholar]
- 6.Marchart J, Dropmann G, Lechleitner S, Schlapp T, Wanner G, Szostak MP, et al. Pasteurella multocida- and Pasteurella haemolytica-ghosts: New vaccine candidates. Vaccine. 2003;21:3988–97. doi: 10.1016/s0264-410x(03)00383-9. [DOI] [PubMed] [Google Scholar]
- 7.Eko FO, Mayr UB, Attridge SR, Lubitz W. Characterization and immunogenicity of Vibrio cholerae ghosts expressing toxin-coregulated pili. J Biotechnol. 2000;83:115–23. doi: 10.1016/s0168-1656(00)00315-1. [DOI] [PubMed] [Google Scholar]
- 8.Marchart J, Rehagen M, Dropmann G, Szostak MP, Alldinger S, Lechleitner S, et al. Protective immunity against pasteurellosis in cattle, induced by Pasteurella haemolytica ghosts. Vaccine. 2003;21:1415–22. doi: 10.1016/s0264-410x(02)00635-7. [DOI] [PubMed] [Google Scholar]
- 9.Lee DJ, Kwon SR, Zenke K, Lee EH, Nam YK, Kim SK, et al. Generation of safety enhanced Edwardsiella tarda ghost vaccine. Dis Aquat Organ. 2008;81:249–54. doi: 10.3354/dao01964. [DOI] [PubMed] [Google Scholar]
- 10.Peng W, Si W, Yin L, Liu H, Yu S, Liu S, et al. Salmonella enteritidis ghost vaccine induces effective protection against lethal challenge in specific-pathogen-free chicks. Immunobiology. 2011;216:558–65. doi: 10.1016/j.imbio.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Talebkhan Y, Bababeik M, Esmaeili M, Oghalaei A, Saberi S, Karimi Z, et al. Helicobacter pylori bacterial ghost containing recombinant Omp18 as a putative vaccine. J Microbiol Methods. 2010;82:334–7. doi: 10.1016/j.mimet.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Eko FO, Hensel A, Bunka S, Lubitz W. Immunogenicity of Vibrio cholerae ghosts following intraperitoneal immunization of mice. Vaccine. 1994;12:1330–4. doi: 10.1016/s0264-410x(94)80061-4. [DOI] [PubMed] [Google Scholar]
- 13.Kwon SR, Kang YJ, Lee DJ, Lee EH, Nam YK, Kim SK, et al. Generation of Vibrio anguillarum ghost by coexpression of PhiX 174 lysis E gene and staphylococcal nuclease A gene. Mol Biotechnol. 2009;42:154–9. doi: 10.1007/s12033-009-9147-y. [DOI] [PubMed] [Google Scholar]
- 14.Tabrizi CA, Walcher P, Mayr UB, Stiedl T, Binder M, McGrath J, et al. Bacterial ghosts-biological particles as delivery systems for antigens, nucleic acids and drugs. Curr Opin Biotechnol. 2004;15:530–7. doi: 10.1016/j.copbio.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Jechlinger W, Szostak MP, Lubitz W. Cold-sensitive E-lysis systems. Gene. 1998;218:1–7. doi: 10.1016/s0378-1119(98)00405-3. [DOI] [PubMed] [Google Scholar]
- 16.Witte A, Wanner G, Sulzner M, Lubitz W. Dynamics of PhiX174 protein E-mediated lysis of Escherichia coli. Arch Microbiol. 1992;157:381–8. doi: 10.1007/BF00248685. [DOI] [PubMed] [Google Scholar]
- 17.Witte A, Brand E, Schrot G, Lubitz W. Pathway of PhiX174 Protein E Mediated Lysis of Escherichia coli. In: Hfltje JV, Lfffelhardt W, editors. Bacterial Growth and Lysis. New York: Plenum Press; 1993. pp. 277–83. [Google Scholar]
- 18.Jechlinger W, Szostak MP, Witte A, Lubitz W. Altered temperature induction sensitivity of the lambda PR/cI857 system for controlled gene E-expression in Escherichia coli. FEMS Microbiol Lett. 1999;173:347–52. doi: 10.1111/j.1574-6968.1999.tb13524.x. [DOI] [PubMed] [Google Scholar]
- 19.Witte A, Lubitz W. Biochemical characterization of PhiX174-protein-E-mediated lysis of Escherichia coli. Eur J Biochem. 1989;180:393–8. doi: 10.1111/j.1432-1033.1989.tb14661.x. [DOI] [PubMed] [Google Scholar]
- 20.Witte A, Wanner G, Lubitz W, Holtje JV. Effect of phi x174 E-mediated lysis on murein composition of Escherichia coli. FEMS Microbiol Lett. 1998;164:149–57. doi: 10.1111/j.1574-6968.1998.tb13080.x. [DOI] [PubMed] [Google Scholar]
- 21.Szostak MP, Mader H, Truppe M, Kamal M, Eko FO, Huter V, et al. Bacterial ghosts as multifunctional vaccine particles. Behring Inst Mitt. 1997:191–6. [PubMed] [Google Scholar]
- 22.Felnerova D, Kudela P, Bizik J, Haslberger A, Hensel A, Saalmuller A, et al. T cell-specific immune response induced by bacterial ghosts. Med Sci Monit. 2004;10:BR362–70. [PubMed] [Google Scholar]
- 23.Gröger M, Holnthoner W, Maurer D, Lechleitner S, Wolff K, Mayr BB, et al. Dermal microvascular endothelial cells express the 180-kDa macrophage mannose receptor in situ and in vitro. J Immunol. 2000;165:5428–34. doi: 10.4049/jimmunol.165.10.5428. [DOI] [PubMed] [Google Scholar]
- 24.Kudela P, Koller VJ, Mayr UB, Nepp J, Lubitz W, Barisani-Asenbauer T. Bacterial Ghosts as antigen and drug delivery system for ocular surface diseases: Effective internalization of Bacterial Ghosts by human conjunctival epithelial cells. J Biotechnol. 2011;153:167–75. doi: 10.1016/j.jbiotec.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 25.Kudela P, Paukner S, Mayr UB, Cholujova D, Kohl G, Schwarczova Z, et al. Effective gene transfer to melanoma cells using bacterial ghosts. Cancer Lett. 2008;262:54–63. doi: 10.1016/j.canlet.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 26.Walcher P, Cui X, Arrow JA, Scobie S, Molinia FC, Cowan PE, et al. Bacterial ghosts as a delivery system for zona pellucida-2 fertility control vaccines for brushtail possums (Trichosurus vulpecula) Vaccine. 2008;26:6832–8. doi: 10.1016/j.vaccine.2008.09.088. [DOI] [PubMed] [Google Scholar]
- 27.Jechlinger W, Haller C, Resch S, Hofmann A, Szostak MP, Lubitz W. Comparative immunogenicity of the hepatitis B virus core 149 antigen displayed on the inner and outer membrane of bacterial ghosts. Vaccine. 2005;23:3609–17. doi: 10.1016/j.vaccine.2004.11.078. [DOI] [PubMed] [Google Scholar]
- 28.Eko FO, Schukovskaya T, Lotzmanova EY, Firstova VV, Emalyanova NV, Klueva SN, et al. Evaluation of the protective efficacy of Vibrio cholerae ghost (VCG) candidate vaccines in rabbits. Vaccine. 2003;21:3663–74. doi: 10.1016/s0264-410x(03)00388-8. [DOI] [PubMed] [Google Scholar]
- 29.Mayrhofer P, Tabrizi CA, Walcher P, Haidinger W, Jechlinger W, Lubitz W. Immobilization of plasmid DNA in bacterial ghosts. J Control Release. 2005;102:725–35. doi: 10.1016/j.jconrel.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 30.Trombetta ES, Ebersold M, Garrett W, Pypaert M, Mellman I. Activation of lysosomal function during dendritic cell maturation. Science. 2003;299:1400–3. doi: 10.1126/science.1080106. [DOI] [PubMed] [Google Scholar]
- 31.Riedmann EM, Kyd JM, Cripps AW, Lubitz W. Bacterial ghosts as adjuvant particles. Expert Rev Vaccines. 2007;6:241–53. doi: 10.1586/14760584.6.2.241. [DOI] [PubMed] [Google Scholar]
- 32.Paukner S, Kudela P, Kohl G, Schlapp T, Friedrichs S, Lubitz W. DNA-loaded bacterial ghosts efficiently mediate reporter gene transfer and expression in macrophages. Mol Ther. 2005;11:215–23. doi: 10.1016/j.ymthe.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 33.Huter V, Hensel A, Brand E, Lubitz W. Improved protection against lung colonization by Actinobacillus pleuropneumoniae ghosts: Characterization of a genetically inactivated vaccine. J Biotechnol. 2000;83:161–72. doi: 10.1016/s0168-1656(00)00310-2. [DOI] [PubMed] [Google Scholar]
- 34.Walcher P, Mayr UB, Azimpour-Tabrizi C, Eko FO, Jechlinger W, Mayrhofer P, et al. Antigen discovery and delivery of subunit vaccines by nonliving bacterial ghost vectors. Expert Rev Vaccines. 2004;3:681–91. doi: 10.1586/14760584.3.6.681. [DOI] [PubMed] [Google Scholar]
- 35.Buckley KJ, Hayashi M. Lytic activity localized to membrane-spanning region of phi X174 E protein. Mol Gen Genet. 1986;204:120–5. doi: 10.1007/BF00330198. [DOI] [PubMed] [Google Scholar]
- 36.Maratea D, Young K, Young R. Deletion and fusion analysis of the phage phi X174 lysis gene E. Gene. 1985;40:39–46. doi: 10.1016/0378-1119(85)90022-8. [DOI] [PubMed] [Google Scholar]
- 37.Lubitz W. Bacterial ghosts as carrier and targeting systems. Expert Opin Biol Ther. 2001;1:765–71. doi: 10.1517/14712598.1.5.765. [DOI] [PubMed] [Google Scholar]
- 38.Szostak MP, Hensel A, Eko FO, Klein R, Auer T, Mader H, et al. Bacterial ghosts: Non-living candidate vaccines. J Biotechnol. 1996;44:161–70. doi: 10.1016/0168-1656(95)00123-9. [DOI] [PubMed] [Google Scholar]
- 39.Eko FO, Witte A, Huter V, Kuen B, Fürst-Ladani S, Haslberger A, et al. New strategies for combination vaccines based on the extended recombinant bacterial ghost system. Vaccine. 1999;17:1643–9. doi: 10.1016/s0264-410x(98)00423-x. [DOI] [PubMed] [Google Scholar]


